Abstract
Obesity has increased dramatically in the last few decades and affects over one third of the adult US population. The economic effect of obesity in 2005 reached a staggering sum of $190.2 billion in direct medical costs alone. Obesity is a major risk factor for a wide host of diseases. Historically, little was known regarding adipose and its major and essential functions in the body. Brown and white adipose are the two main types of adipose but current literature has identified a new type of fat called brite or beige adipose. Research has shown that adipose depots have specific metabolic profiles and certain depots allow for a propensity for obesity and other related disorders. The goal of this protocol is to provide researchers the capacity to identify and excise adipose depots that will allow for the analysis of different factorial effects on adipose; as well as the beneficial or detrimental role adipose plays in disease and overall health. Isolation and excision of adipose depots allows investigators to look at gross morphological changes as well as histological changes. The adipose isolated can also be used for molecular studies to evaluate transcriptional and translational change or for in vitro experimentation to discover targets of interest and mechanisms of action. This technique is superior to other published techniques due to the design allowing for isolation of multiple depots with simplicity and minimal contamination.
Keywords: Medicine, Issue 94, adipose, surgical, excision, subcutaneous adipose tissue (SQ), perivascular adipose tissue (PVAT), visceral adipose tissue (VAT), brown adipose tissue (BAT), white adipose tissue (WAT)
Introduction
Adipose made a notable appearance in the media spotlight, due to obesity’s dramatic increase during the last few decades of the 20th century. Obesity currently affects more than one-third of adults and 17% of children and adolescents in the United States (US)1. Spanning all ethnic groups, statistical research surrounding the obesity epidemic has shown that non-Hispanic blacks have the highest age-adjusted rate of obesity (49.5%) compared with Mexican Americans (40.4%), all Hispanics (39.1%), and non-Hispanic whites (34.3%)2. The economic effect of obesity is also a growing concern for the healthcare system. In 2012, it was estimated that the annual medical cost of care for obesity in the US in 2005 was $190.2 billion, nearly 21% of the overall medical spending budget. Sadly, childhood obesity was estimated to be responsible for $14 billion in direct medical costs alone. Statistically, it was determined that the average medical cost of individuals with obesity was $2,741 higher a year than those without this morbidity3-5.
Obesity is a major risk factor for a variety of conditions such as: type 2 diabetes, dyslipidemia, cardiovascular disease, cancer, muscular skeletal disorders and chronic inflammation. Obesity is deeply tied to the pathogenesis of metabolic syndrome and other chronic diseases6-8. With such drastic and negative connections between obesity and other comorbidities, scientific research has focused attention to better understand the current epidemic and the diverse and pivotal roles played by adipose.
Historically, adipose tissue was considered inconsequential and was viewed merely as a simple filling tissue. Currently, adipose has been shown to play many essential roles in the body’s function in: metabolism, hormone regulation, inflammation, protection and insulation9. Adipose tissue is composed primarily of adipocytes but also contains pericytes, endothelial cells, monocytes, macrophages and pluripotent stem cells8. Adipose tissue is distributed throughout the body in distinct depots. The principal depots can be found subdermally, subcutaneously, intramuscularly, and viscerally10. Adipose depots have been shown to have depot specific metabolic profiles, which have shown a depot specific susceptibility to obesity and related disorders8.
Traditionally, adipose tissue has been classified into two major types: white adipose tissue (WAT) and brown adipose tissue (BAT); although recent literature indicates the presences of a third group christened brite or beige adipose11. Adipose tissue has been shown to have different colors, morphologies, metabolic functions, biochemical features and genetic patterns of expression10. Adipocytes in WAT have a single, large lipid droplet and variable amounts of mitochondria. WAT is dominantly found in subcutaneous and visceral localities of the body. WAT functions primarily as a site of energy storage and organ protection. Adipocytes in BAT have a multilocular morphology and abundant mitochondria. BAT is located primarily in the neck and large blood vessels of the thorax, as well as the scapulae12. BAT primarily functions in energy-expending behaviors that regulate thermogenesis7. Brite or beige adipose has been shown to share an analogous morphology and expression to BAT but has been found to be originated from white adipocytes11.
The described surgical method in this manuscript provides researchers with the capacity to analyze different effects that factors such as: environment, pharmaceuticals, and genetics, have on adipose; as well as the beneficial or detrimental role adipose plays in disease and overall health. Also, providing a way to identify and isolate different types of adipose tissue to allow for better understand of the biochemical relationships and differences between depots. This can aid in determining the relationship between location, function, and types of fat within the body. This described method accomplishes this by providing the means for gross visualization, gene expression analysis, protein expression analysis, histological examination, and isolation of primary cell lines for in vitro studies. Currently there are many articles that provide insight into the metabolic behavior of different adipose depots, as well as their anatomic locations; but do not provide an in-depth method on how to specifically localize, identify, and isolate these depots. This surgical method provides a precise technique that allows for isolation of multiple depots with a minimal quantity of dissection and contamination compared to others methods designed for the isolate of one or two depots13-14.
The goal of this protocol is to provide a precise method for the identification and isolation of different types of fat depots from multiple anatomic locations.
Protocol
NOTE: All animal procedures were performed with the approval of the Institutional Animal Care and Use Committee (IACUC) of the University of Cincinnati and in accordance with the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health (NIH Publication No. 85-23, Revised 1996).
1. Euthanize and Sterilize the Mouse
Place the mouse in a dropbox containing a supratheraputic dose of isoflurane and allow to inhale to effect. Once the mouse is euthanized, remove from the box.
Cervically dislocate as a second means of euthanasia.
Sterilize the external surface of the mouse by cleansing the animal with 70% ethanol.
2. Identification and Isolation of Three Different Adipose Depots
- Brown Adipose Tissue (BAT) Isolation:
- Make sure the fur is wet from the alcohol cleansing to aid in cutting through the epidermal and dermal layers.
- Position the mouse in a supine position with its back against the table.
- Grab the skin just below the diaphragm with forceps, lift and incise with scissors.
- Cut transversely around the circumference of the mouse to expose the peritoneum.
- Reveal the butterfly-shaped BAT depot by degloving the top half of the mouse. Hold the lower appendages and abdomen in one hand and pulling the skin up towards the head.
- Orient the mouse, so that it is positioned prone on the table. Be careful not to contaminate the exposed depot with hair.
- Clean surgical instruments and change to a fresh pair of gloves.
- Locate the scapulae and corresponding depot. Carefully remove any superficial white adipose atop the butterfly and then dissect the butterfly of interscapular brown fat. Be careful to avoid the muscle closely associated with the brown fat. NOTE: The use of a dissection microscope is recommended for removal of the white adipose, as well as in the separation of the brown adipose from the scapulae.
- Remove fat depot and transfer to a 2 ml microcentrifuge tube.
- If RNA or protein is to be extracted, freeze the tissue by immersion in liquid nitrogen and store at -80 °C. Freeze the sample at once to prevent degradation. If culturing, cover the tissue in DMEF-12 and set on ice till all samples are collected for culture (supplemental info).
- Isolation of Subcutaneous Adipose Tissue (SQ), a White Adipose Tissue Depot (WAT):
- Put on a fresh pair of gloves as to not cross-contaminate between fat depots.
- Reveal the inguinal, triangular SQ depots by de-gloving the bottom half of the mouse. Hold the upper appendages and thorax in one hand and pulling the skin down towards the feet with the other hand.
- Orient the mouse in a supine position while being careful not to contaminate the exposed depot with hair.
- Clean surgical instruments and change to a fresh pair of gloves.
- Carefully dissect the triangles of SQ fat. Be careful not to contaminate the sample with muscle, neighboring fat, mammary glands or by cutting any vessels and contaminate the sample with blood. NOTE: The use of a dissection microscope is recommended if borders are not clearly defined.
- Remove fat depots and transfer to 2 ml microcentrifuge tubes.
- If RNA or protein is to be extracted, freeze the tissue by immersion in liquid nitrogen and store at -80 °C. Freeze the sample at once to prevent degradation.
- For staining, fix or embed in OCT. If culturing, cover the tissue in DMEF-12 and set on ice till all samples are collected for culture (supplemental info).
- Isolation of Gonadal Fat a Visceral Adipose Tissue (VAT) and White Adipose Tissue (WAT) Depot:
- Put on a fresh pair of gloves as to not cross-contaminate between fat depots.
- With scissors, cut the peritoneum transversely, directly below the diaphragm. Cut the peritoneum from the diaphragm to the rectum mid coronally to expose abdominal organs.
- Locate the testes or ovaries and identify the attached white adipose tissue, known as the epididymal adipose in males or gonadal adipose in females.
- Clean surgical instruments and change to a fresh pair of gloves
- Carefully dissect both epididymal fat depots from the testes, epididymides, and vasa deferentia. Or if female, carefully dissect both gonadal fat pads from the ovaries.
- Remove fat depots and transfer them to 2 ml microcentrifuge tubes.
- If RNA or protein is to be extracted, freeze the tissue by immersion in liquid nitrogen and store at -80 °C. Freeze the sample at once to prevent degradation.
- For staining, fix or embed in OCT. If culturing, cover the tissue in DMEF-12 and set on ice till all samples are collected for culture (supplemental info).
3. Isolation of Perivascular Adipose Tissue (PVAT)
- Isolation of the Heart:
- Lay the mouse out in a supine position with upper and lower appendages extended outward.
- Secure appendages using surgical tape.
- After positioning the mouse as listed above, create tension by lifting up on the xiphoid process with forceps. Cut horizontally through the diaphragm, exposing the lower portion of the thoracic cavity.
- While maintaining tension, by lifting up on the xiphoid process, cut through the ribcage superiorly towards the head, just to the side of the sternum.
- Pick up the rib cage just inferior to the clavicle, and cut along the inferior length of the clavicle out toward the axilla in both directions. The thoracic cavity and its contents (heart, lungs, etc.) should now be clearly visible.
- Clean the thoracic cavity of extraneous blood and fluid by using sterile gauze to absorb the fluid. If collecting organs or vessels be sure to profuse (supplemental info).
- Once the area is cleared of fluid, cut the bronchi and attaching vessels to remove the lungs, allowing better exposure of the heart.
- Localization and Excision of Aortic Perivascular Adipose Tissue (PVAT):
- Remove the following organs as to better identify the inferior portions of the aorta: liver, stomach, spleen, pancreas, intestines, and colon.
- First start with identification of the stomach and the esophagus. Cut the esophagus at the gastro-esophageal junction to free the stomach from the body.
- Next, identify the intestines and the surrounding mesentery. Cut superficially through mesentery, as it lies very closely to the renal portion of the aorta, then “run the bowel.”
- Cut the colon free as close to the rectum as possible. Thus, freeing the stomach, intestine and colon from the mouse.
- Remove the stomach, intestine, colon, pancreas and spleen by cutting through the attaching mesentery and vessels. The pancreas and spleen should come free with the stomach, intestine and colon.
- Remove the liver by cutting through the hepatic veins and attaching mesentery, remove all lobes.
- Cut away the visceral layer and fat surround the kidneys. Leave the kidneys attached to the aorta in vivo to serve as geographic markers for different segments of the aorta.
- Rinse the area with sterile 1x PBS and remove all fluid by absorption with a sterile gauze.
- Using micro-scissors and micro-forceps, separate the aorta from its dorsal attachment to the spine and its ventral attachment to the esophagus.
- Isolate the aorta by following and detaching the aorta the length of the descending aorta from the origin in the heart to the bifurcation in the iliac region.
- Identify and isolate the subclavian vessels. Isolate these vessels from the neck to the aortic root to better expose the aortic junction and root in the heart.
- Remove the thymus then cut the brachiocephalic artery, the left common carotid artery, and the left subclavian artery, allowing movement of the heart.
- With the assistance of a dissection microscope, view the perivascular adipose tissue (PVAT) layer surrounding the aorta.
- Taking great care to not pinch or squeeze the PVAT, gently pull the PVAT way from the aorta with micro-forceps. Gently cut the attachment of the PVAT to the aorta with micro-scissors starting at the thoracic region just superior to where the diaphragm is located. NOTE: A dissection microscope is recommended.
- Repeat this process the entire length of the aorta, finishing at the infrarenal aortic region, which is located just superior to the iliac bifurcation of the aortic vessel.
- If aortic arch PVAT is desired, used the same method to remove the PVAT from the lesser curvature of the arch.
- Place PVAT samples into 2 ml microcentrifuge tube(s).
- If RNA or protein is to be extracted, freeze the tissue by immersion in liquid nitrogen and store at -80 °C. Freeze the sample at once to prevent degradation. If culturing, cover the tissue in DMEF-12 and set on ice till all samples are collected for culture (supplemental info). NOTE: Additional adipose depots to consider if interested in a comprehensive adipose depot analysis include: retroperitoneal, mesenteric, omental, pericardial and popliteal.
Representative Results
Identification, and localization of inguinal subcutaneous adipose, interscapular brown adipose, visceral epididymal adipose (Figure 1), as well as the aortic arch perivascular adipose, thoracic aortic adipose, suprarenal aortic adipose and the infrarenal aortic adipose (Figure 2) was achieved successfully using the described surgical method. Histological examination and differentiation between BAT and WAT samples were positively evaluated using Hematoxylin and Eosin (H&E) staining (Figure 3). Analysis of RNA levels of adiponectin (AdipoQ), peroxisome proliferator-activated receptor gamma (PPAR-γ), cell death-inducing DFFA-like effector a (CIDEA), and other fat specific markers were measured for all the above isolated and excised depots (data not shown).
Primary cell lines of subcutaneous adipocytes and perivascular adipocytes were cultured and differentiated from preadipocytes to adipocytes successfully for microarray analysis. The cultured preadipocytes converted to adipocytes were confirmed with Oil Red O staining (Figure 4). Successful isolation, culture and differentiation of adipocytes was achieved for use in in vitro studies, and protein activity was successfully measured. Enzymatic activity of matrix metaloprotease-2 (MMP2) was measured in a treatment group compared to the control. The activity of MMP2 was measured in situ in a primary perivascular adipocyte line via zymography (Figure 5).
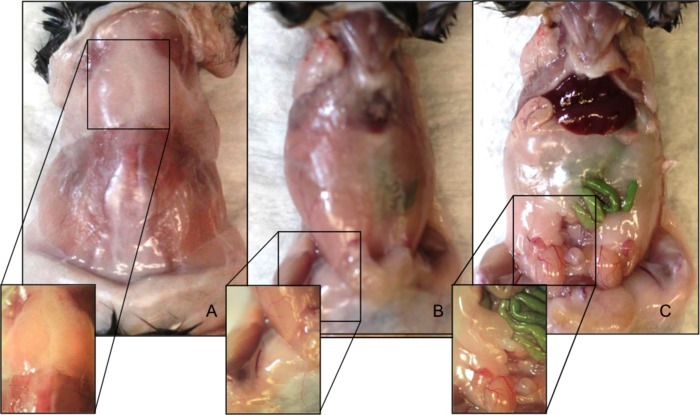 Figure 1. Anatomical locations of C57BL/6 male mouse adipose depots. (A) Interscapular brown adipose fat depot. (B) Inguinal subcutaneous adipose fat depot. (C) Visceral epididymal adipose fat depot. Please click here to view a larger version of this figure.
Figure 1. Anatomical locations of C57BL/6 male mouse adipose depots. (A) Interscapular brown adipose fat depot. (B) Inguinal subcutaneous adipose fat depot. (C) Visceral epididymal adipose fat depot. Please click here to view a larger version of this figure.
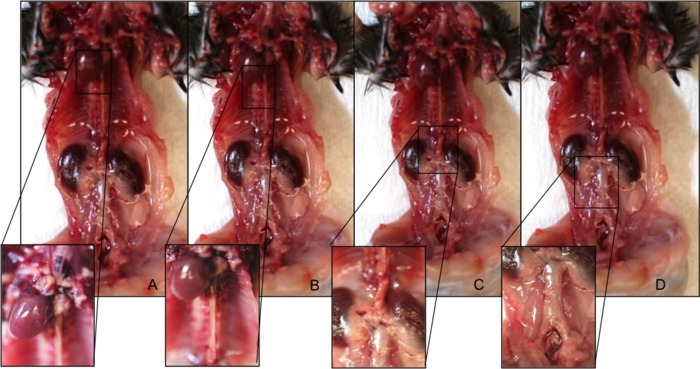 Figure 2. Anatomical locations of PVAT depots in a C57BL/6 male mouse. (A) Aortic arch perivascular adipose depot. (B) Thoracic aortic perivascular adipose depot. (C) Suprarenal aortic perivascular adipose depot. (D) Infrarenal aortic perivascular adipose depot. Please click here to view a larger version of this figure.
Figure 2. Anatomical locations of PVAT depots in a C57BL/6 male mouse. (A) Aortic arch perivascular adipose depot. (B) Thoracic aortic perivascular adipose depot. (C) Suprarenal aortic perivascular adipose depot. (D) Infrarenal aortic perivascular adipose depot. Please click here to view a larger version of this figure.
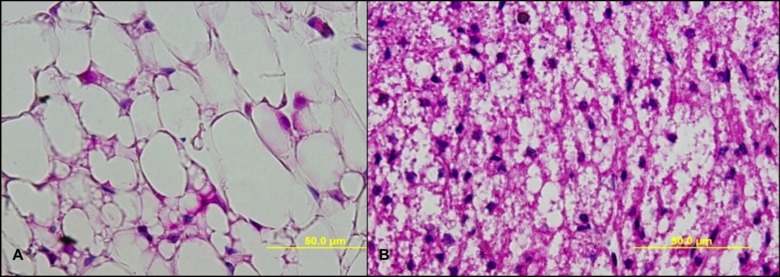 Figure 3. H&E staining of BAT and WAT adipose. (A) H&E staining of paraformaldehyde fixed, paraffin embedded C57BL/6 male mouse sample of WAT adipose at a 40X magnification. (B) H&E staining of paraformaldehyde fixed, paraffin embedded C57BL/6 male mouse sample of BAT at a 40X magnification. Please click here to view a larger version of this figure.
Figure 3. H&E staining of BAT and WAT adipose. (A) H&E staining of paraformaldehyde fixed, paraffin embedded C57BL/6 male mouse sample of WAT adipose at a 40X magnification. (B) H&E staining of paraformaldehyde fixed, paraffin embedded C57BL/6 male mouse sample of BAT at a 40X magnification. Please click here to view a larger version of this figure.
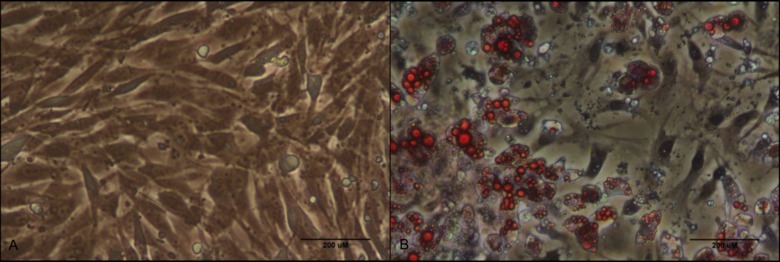 Figure 4. Oil Red O staining of cultured PVAT preadipocytes and adipocytes. (A) Oil Red O staining of cultured aortic perivascular preadipocytes at baseline of differentiation at 20X magnification with phase contrast. (B) Oil Red O staining of cultured aortic perivascular adipocytes after 5 days of differentiation at 20X magnification with phase contrast. Please click here to view a larger version of this figure.
Figure 4. Oil Red O staining of cultured PVAT preadipocytes and adipocytes. (A) Oil Red O staining of cultured aortic perivascular preadipocytes at baseline of differentiation at 20X magnification with phase contrast. (B) Oil Red O staining of cultured aortic perivascular adipocytes after 5 days of differentiation at 20X magnification with phase contrast. Please click here to view a larger version of this figure.
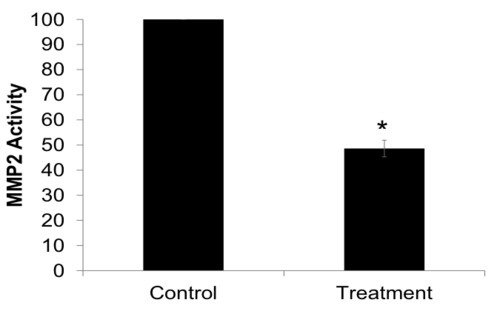 Figure 5. Zymography of differentiated adipocytes, isolated from perivascular adipose tissue, demonstrated a decreased activity of MMP2 released after treatment compared to untreated (control) cells, *P < 0.01 compared to control.
Figure 5. Zymography of differentiated adipocytes, isolated from perivascular adipose tissue, demonstrated a decreased activity of MMP2 released after treatment compared to untreated (control) cells, *P < 0.01 compared to control.
Discussion
Obesity can lead to a large host of morbidities and the full understanding of the role that adipose plays is not fully understood; therefore continued research in the field of adipose is necessary. Animal models, specifically murine models are ideal for initial research in the progression of diseases and testing of potential pharmaceutical treatments. In using these models, precise isolation and excision of adipose depots is an extremely important and necessary tool in the study of pathology of adipose affected diseases.
In current literature regarding adipose depots, there is a substantial quantity of literature regarding metabolic behavior and variation between adipose depots, as well as their anatomic locations. However, there are few that provide an in-depth method on how to specifically localize, identify, and isolate these depots. Based on the review of current isolation methods of adipose, there is a small subset of protocols that provide methodology on how to isolate one or two depots at a time. However, a precise technique that allows for isolation of multiple depots with a minimal quantity of dissection and contamination, as well as addresses various methods of studying the samples collected is distinctive to this protocol13-14.
Within this methodology, there are several steps that are vitally important to the isolation and purity of the sample. Cleaning tools, gloves and surfaces often to remove hair and contaminants is an imperative step to avoiding depot contamination. When cutting the skin transversely around the circumference of the mouse to expose the peritoneum for degloving, it is vital to avoid cutting too deeply. Cutting the peritoneum will make degloving very difficult and will raise the potential for contamination of the sample. When excising the SQ adipose depots it is vital to identify the triangular boundaries of the depot before excising any of the adipose. Also, careful cuts should be made to avoid muscle, and adjacent vessels, glands and adipose. This will prevent contamination of the sample from alternate adipose, glandular tissue, muscle or blood.
The major limitation to isolation and excision of adipose depots, in this method and other comparable methods can be found in the defining of the boundaries of certain depots. Due to the poorly defined borders in depots, such as the subcutaneous depots, isolation lacking a small amount of contamination from neighboring adipose can be challenging. Another limitation can be found in ensuring enough tissue is collected for supplemental experimentation in vascular associated depots. This limitation sometimes requires pooling of samples, although this is dependent on the site of isolation and the diet associated with the animal.
After the adipose depots are isolated, they can be utilized for a variety of assays. The adipose can be used for molecular studies such as protein expression, enzyme activity and gene expression analysis. Additionally, one can isolate adipocytes for primary cell line in vitro studies. Immortalized cells lines can also be used for in vitro studies, however immortal cells are not as credible as primary isolated cell lines. Finally, the adipose can be fixed or frozen in OCT for histological examination to identify leukocyte infiltration, protein localization, as well as characterization of adipocyte morphology.
Disclosures
The authors declare that they have no competing financial interests or other conflicts of interest.
Acknowledgments
The authors have no acknowledgements.
References
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief. 2012;82:1–8. [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. Journal of Health Economics. 2012;31(1):219–230. doi: 10.1016/j.jhealeco.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Obesity accounts for 21 percent of U.S. health care costs, study finds. Cornell University; 2012. Available from: http://www.sciencedaily.com/releases/2012/04/120409103247.htm. [Google Scholar]
- Marder W, Chang S. Childhood Obesity: Costs, Treatment Patterns, Disparities in Care, and Prevalent Medical Conditions. Thomson Medstat Research Brief. 2006.
- Cortez M, Carmo LS, Rogero MM, Borelli P, Fock RA. A high-fat diet increases IL-1, IL-6, and TNF-α production by increasing NF-κB and attenuating PPAR-γ expression in bone marrow mesenchymal stem cells. Inflammation. 2013;36(2):379–386. doi: 10.1007/s10753-012-9557-z. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J, Hung CM, Sparks CA, Tang Y, Li H, Guertin DA. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab. 2012;16(3):348–362. doi: 10.1016/j.cmet.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi JK, Vidal-Puig AJ. Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007;48(6):1253–1262. doi: 10.1194/jlr.R700005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarsland A, Chinkes D, Wolfe RR. Hepatic and whole-body fat synthesis in humans during carbohydrate overfeeding. Am J Clin Nutr. 1997;65(6):1774–1782. doi: 10.1093/ajcn/65.6.1774. [DOI] [PubMed] [Google Scholar]
- Park A, Kim WK, Bae KH. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J. Stem Cells. 2014;6(1):33–42. doi: 10.4252/wjsc.v6.i1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and. 2012;150(2):366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giralt M, Villarroya F. White, brown, beige/brite: different adipose cells for different functions. Endocrinology. 2013;154(9):2992–3000. doi: 10.1210/en.2013-1403. [DOI] [PubMed] [Google Scholar]
- Casteilla L, Pénicaud L, Cousin B, Calise D. Choosing an adipose tissue depot for sampling: factors in selection and depot specificity. Methods Mol. Biol. 2008;456:23–38. doi: 10.1007/978-1-59745-245-8_2. [DOI] [PubMed] [Google Scholar]
- Grant R, Youm YH, Ravussin A, Dixit VD. Quantification of adipose tissue leukocytosis in obesity. Methods Mol. Biol. 2013;1040:195–209. doi: 10.1007/978-1-62703-523-1_15. [DOI] [PMC free article] [PubMed] [Google Scholar]


