Abstract
Collagen is a major structural component of the extracellular matrix that supports tissue formation and maintenance. Although collagen remodeling is an integral part of normal tissue renewal, excessive amount of remodeling activity is involved in tumors, arthritis, and many other pathological conditions. During collagen remodeling, the triple helical structure of collagen molecules is disrupted by proteases in the extracellular environment. In addition, collagens present in many histological tissue samples are partially denatured by the fixation and preservation processes. Therefore, these denatured collagen strands can serve as effective targets for biological imaging. We previously developed a caged collagen mimetic peptide (CMP) that can be photo-triggered to hybridize with denatured collagen strands by forming triple helical structure, which is unique to collagens. The overall goals of this procedure are i) to image denatured collagen strands resulting from normal remodeling activities in vivo, and ii) to visualize collagens in ex vivo tissue sections using the photo-triggered caged CMPs. To achieve effective hybridization and successful in vivo and ex vivo imaging, fluorescently labeled caged CMPs are either photo-activated immediately before intravenous injection, or are directly activated on tissue sections. Normal skeletal collagen remolding in nude mice and collagens in prefixed mouse cornea tissue sections are imaged in this procedure. The imaging method based on the CMP-collagen hybridization technology presented here could lead to deeper understanding of the tissue remodeling process, as well as allow development of new diagnostics for diseases associated with high collagen remodeling activity.
Keywords: Bioengineering, Issue 83, collagen remodeling, triple helix, near infrared fluorescence, bioimaging, tissue staining
Introduction
Collagen, the most abundant protein in mammal, plays a critical role in tissue development and regeneration by supporting proliferation and differentiation of cells. While fibrous collagens (e.g. type I, II), in connective tissues give mechanical strength to the tissues, the network-like collagen (type IV) form basic scaffold of the basement membrane where cells attach and form organized tissues. Collagen remodeling involves both collagen degradation (by proteases) and synthesis (promoted by growth factors). Although collagen remodeling, for example in bone, is part of the normal tissue renewal process, excess remodeling activity, or its occurrence in abnormal locations, typically indicates wound healing response to an injury or chronic pathological conditions such as cancer, osteoporosis, arthritis, and fibrosis1-4. The ability to directly image collagens undergoing remodeling in vivo could lead to understanding of the progression of these diseases, as well as new diagnostics and therapeutics. For example, live imaging can provide information about the severity and the location of the diseases, and can also be used to assess the efficacy of new therapeutic agents. Multiphoton laser scanning microscopy and second harmonic generation have been applied to image fibrillar collagens for monitoring extracellular matrix remodeling in tumor in live mice5. However, this technique requires animals to be mounted with transparent dorsal skinfold chambers, which is an invasive procedure. Direct and noninvasive imaging of collagen remodeling will benefit from a probe that specifically targets collagen undergoing remodeling. Such probe is difficult to prepare since it needs to distinguish the remodeling collagens from the intact and mature collagens, which are abundant in normal tissues6.
Collagen is made up of extremely rare protein structure called triple helix, which is cleaved by proteases such as matrix metalloproteinases (MMP) during collagen remodeling. The cleaved collagen fragments lose their triple helical structure and become unfolded strands (gelatin), which are further digested by nonspecific proteases1. It was recently discovered that the collagen mimetic peptide (CMP) which has the propensity to fold into triple helical structure can specifically target collagen strands which are dissociated from its triple helical state by either heat denaturation or by enzymatic degradation1,7. The binding is primarily driven by triple-helix hybridization between monomeric CMPs and the denatured collagen strands. Because CMPs self-assemble into homotrimeric triple helices at room temperature with little driving force for collagen hybridization, a caged CMP [(GPO)4NBGPO(GPO)4, designated as NB(GPO)9, O: hydroxyproline], was developed, which contains a photo-cleavable nitrobenzyl group (NB) attached to the central glycine of the peptide. The NB cage group sterically prevents the CMP from folding into triple helix; yet, removal of the cage group by UV irradiation immediately triggers the triple helical folding and collagen hybridization1. When monomeric CMPs labeled with near infrared (NIR) fluorophores are systemically delivered to model mice, they can specifically target and allow in vivo imaging of denatured collagens in tissues undergoing normal (e.g. in bone and cartilage) and pathological (e.g. in tumors) remodeling1.
Fluorescently labeled CMPs can also be used for imaging collagens in histological tissue sections. In histological study, harvested tissues are often preserved by fixation to keep the cellular components and overall tissue morphology from deterioration. The fixing procedures, which include heat, and treatment with organic solvents, and chemical cross-linking reagents (e.g. paraformaldehyde), denature the triple helical structure of collagen8. This denaturation generates sites for CMP hybridization. It has been shown that fluorescently labeled CMPs can specifically bind to collagens in fixed tissue sections (e.g. skin, cornea, and bone) even more effectively than an anti-collagen antibody, which allowed facile identification of pathologic conditions in fibrotic liver tissues9. The CMP targets denatured collagen strands containing amino acid sequence of triple helical motifs, which is common to all types of collagens. Therefore CMP can be considered a broad-spectrum collagen staining agent. Here, we present detailed experimental procedures for i) imaging denatured collagen strands in vivo and ii) visualizing collagens in ex vivo tissue sections using fluorescently labeled caged CMPs. A NIR tag, IR680, was conjugated to the caged CMP for live imaging, while carboxyfluorescein (CF) was used in tissue staining work for its compatibility with standard fluorescence microscopes. This protocol focuses on the imaging application of CMPs as related to collagen remodeling. Methods for CMP synthesis can be found in previous reports1,7,9-15. In this video report, imaging skeletal tissues in normal mice and tissue sections of mouse cornea were chosen for demonstration purpose; however the methods presented here can be readily applied to many pathologies and biological models involving collagen remodeling (e.g. tumors, wound healing), as well as to almost any prefixed tissue sample that contains collagens.
Protocol
All animal studies were undertaken in compliance with the regulations of the Johns Hopkins Animal Care and Use Committee.
1. Near Infrared (NIR) Fluorescence Imaging of Skeletal Collagen Remodeling In vivo
- Photo-activation of the caged CMP and tail vein injection
- Prepare 110 µl of caged CMP solution for each mouse (20-30 g mouse weight): 100 µl of solution for dosing, with an extra 10 µl cushion for the possible loss during transfer and injection. Mix 11 µl of IR680-Ahx-NB(GPO)9 stock solution (400 µM) with 11 µl of 100 µM cysteine in sterile PBS solution; then bring the total volume to 110 µl with 1x PBS buffer. The final peptide solution contains 4 nmol of IR680-Ahx-NB(GPO)9 and 1 nmol of cysteine in 100 µl of 1x PBS solution.
- Slowly withdraw the peptide solution into a 0.5 ml insulin syringe with a transparent barrel and a small gauge (28-30 G) needle, and carefully remove the air bubbles. Turn on the UV lamp to allow the lamp to warm up for 2-5 min.
- Place the peptide-containing syringe directly under the UV lamp (365 nm, >25 mW/cm2) for 5 min to allow photo-activation.
- Meanwhile, position the mouse in a restrainer under a heated lamp; label the mouse with a permanent marker on the tail or other identification methods such as ear tag or toe tattoo, and disinfect the tail with 70% alcohol.
- Immediately after the UV activation, inject 100 µl of UV-activated IR680-Ahx-NB(GPO)9 solution into the tail vein, preferentially at the posterior part of the tail. Place the mouse back to the cage after injection.
- NIR fluorescence imaging of collagen remodeling activity
- Set the heater plate of the imaging bed of the impulse imager to 37 °C using Pearl Impulse 2.0 software.
- Put the nude or shaved mouse into the anesthesia induction chamber containing 2% isoflurane in oxygen. Verify depth of anesthesia plane by lack of mouse movement during handling and by monitoring the frequency of respirations (~1/sec).
- After the mouse is anesthetized, open the Pearl imager drawer, and place the animal on the imaging bed. Quickly remove the nose cone plug, and slide the mouse's muzzle inside the nose cone to keep the mouse under anesthesia (by 2% isoflurane in oxygen delivered at 2 L/min through the nose cone) during the imaging process. Once the mouse is in place, close the drawer.
- When the instrument indicates "ready" on the Image software panel, click the "700 channel," "white light" and "85 micron" boxes followed by the "acquire image" button. NIR (excitation 685 nm, emission 720 nm) and white light photographs will then be taken using automatic focus and exposure.
- When the recording is completed, open the drawer, turn the mouse, and acquire images from another angle. The mouse can be placed with its front (ventral), back (dorsal) or sides facing the camera. Narrow strips of tape can be used as restraints to stretch its limbs to get the best view of the region of interest (e.g. rib cage, ankles, and wrists).
- A proper time to observe skeletal uptake of IR680-CMP is between 24-96 hr post injection (h.p.i.), based on the dosage (~4 nmol/mouse). To acquire high resolution images, sacrifice the mouse by cervical dislocation while under deep anesthesia after 72 h.p.i, remove the skin (and hair) using surgical forceps and scissors, and image it by NIR fluorescence as described above.
- Analyze the acquired NIR fluorescence images.
2. Visualization of Collagens in Ex vivo Tissue Sections
- Material preparation
- Prepare 1 ml of 10% (w/v) BSA solution in deionized water. Thaw 1 ml of goat serum in a water bath at room temperature. Cut a few pieces of Parafilm in the size of approximately 2 cm x 5 cm.
- Prepare 10 ml of blocking buffer by mixing 500 µl of goat serum, 1 ml of 10x PBS and 8.5 ml of deionized water. Prepare 5 ml of CMP dilution buffer by diluting 50 µl of 10% (w/v) BSA with 4.45 ml of deionized water and 500 µl 10x PBS.
- Dilute stock solutions of carboxyfluorescein-labeled caged CMP, CFNB(GPO)9 (480 µM), to 5 µM using the CMP dilution buffer. Optimal CMP concentration varies from 2.5-30 µM, depending on the type of tissues and the nature of tissue samples. A volume of 100 µl is recommended for staining one tissue section slide. Store the formulated CMP solutions in dark.
- Let the mouse cornea tissue slides equilibrate to room temperature. Incubate the slides in 1x PBS buffer for 5 min. The mouse cornea tissues used in this demonstration have been prefixed with 4% paraformaldehyde in PBS solution for 1 hr, cryopreserved in Tissue-Tek O.C.T. medium, cryosectioned to 8 µm thickness, and mounted on charged glass slides.
- Tissue staining and imaging
- Place the slides in a humidified chamber. To each slide, apply 0.5 ml of blocking solution and incubate the slides for 30 min at room temperature. Remove the blocking solution by blotting the slides on a paper towel.
- Apply approximately 100 µl of CFNB(GPO)9 solutions to cover the tissue sections of each slide. Incubate the slides for 2 min to allow the CMP solution to permeate the tissues.
- Turn on the UV lamp. When the lamp is warmed up, expose the CMP-covered tissue sections to the UV light for 6 min (365 nm, ~8 mW/cm2) to deprotect the caged CMPs. After UV exposure, cover the tissue sections of each slide with a piece of Parafilm to prevent drying. Place the slides in the humidified chamber and incubate them at 4 °C for 2 hr.
- Prepare a 1:3,000 dilution of DAPI solution in 1x PBS. After CMP staining, gently remove the Parafilm with forceps, and blot the slides on a paper towel to remove excess CMP solution. Apply approximately 100 µl of diluted DAPI solutions to each tissue slide and incubate for 1 min at room temperature.
- After staining, immerse the slides in 1x PBS buffer in a staining jar for 5 min. Repeat this 3x in fresh 1x PBS to wash off unbound staining agents.
- Add a drop of mounting medium on the tissue section and cover it with a glass cover slip while avoiding trapping air bubbles. Put the slides in a cardboard slide tray to protect them from light.
- Image the collagen strands (FITC channel) and cell nuclei (DAPI channel) in the tissue slides using a fluorescence microscope.
Representative Results
Figure 1 shows a typical result of how photo-triggered IR680-Ahx-(GPO)9 distributes in a healthy female SKH1 nude mouse after 24-96 hr post injection. It shows apparent CMP accumulation in the skeleton after 24 h.p.i., at which point most of unbound peptides have been largely cleared out. The process of CMP hybridizing with the remodeling collagen strands seems relatively slow, especially in contrast to other peptide imaging probes (e.g. RGD16). This most likely is because of the slow folding rate of the collagen triple helix17,18. However, once hybridized, the CMP strands are strongly bound to the collagenous tissues, as only slight signal reduction is seen after 24 h.p.i. (Figure 1). At 96 h.p.i., the NIR fluorescence image of the mouse after skin removal clearly demonstrates the skeletal uptake of IR680-Ahx-(GPO)9 in spine and ribs, as well as within the knees, ankles, wrists, and lower mandibles (Figure 2A).
In ex vivo tissue staining, photo-triggered fluorescently-labeled caged CMPs specifically hybridize to denatured collagen strands in tissue sections. In Figure 3, CMP staining clearly reveals the fine parallel collagen fibrils in the corneal stroma, which demonstrates its use as a collagen specific staining agent.
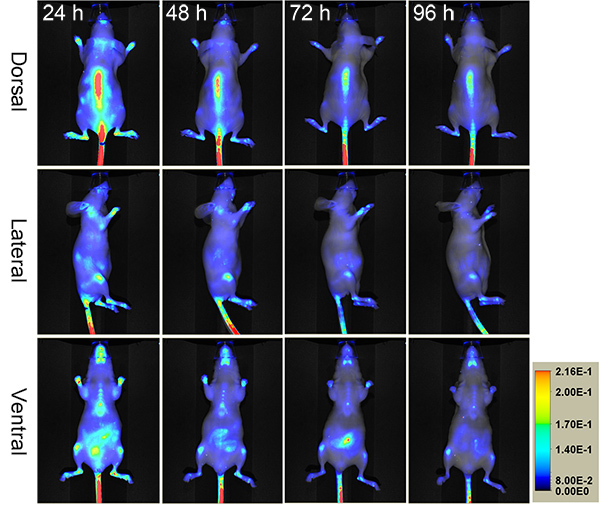 Figure 1.
Serial NIR fluorescence images of a nude mouse administered intravenously with photo-decaged IR680-Ahx-(GPO)9 showing skeletal uptake over four days.
Click here to view larger image.
Figure 1.
Serial NIR fluorescence images of a nude mouse administered intravenously with photo-decaged IR680-Ahx-(GPO)9 showing skeletal uptake over four days.
Click here to view larger image.
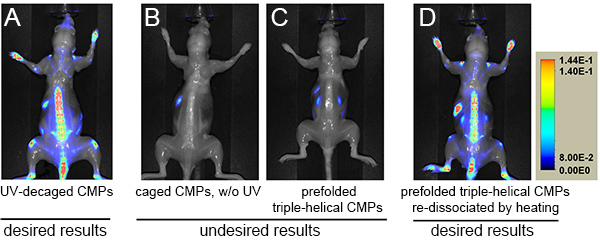 Figure 2. NIR fluorescence images of mice administered with photo-decaged IR680-Ahx-(GPO)9 (A), caged IR680-Ahx-NB(GPO)9 without UV exposure (B), prefolded triple-helical [IR680-Ahx-(GPO)9]3 (C), or heat-dissociated single-strand IR680-Ahx-(GPO)9 (D) at 96 h.p.i. after skin removal. Skeletal targeting by the CMP can only be observed in A and D. NIR fluorescence signals are shown in rainbow scale. Click here to view larger image.
Figure 2. NIR fluorescence images of mice administered with photo-decaged IR680-Ahx-(GPO)9 (A), caged IR680-Ahx-NB(GPO)9 without UV exposure (B), prefolded triple-helical [IR680-Ahx-(GPO)9]3 (C), or heat-dissociated single-strand IR680-Ahx-(GPO)9 (D) at 96 h.p.i. after skin removal. Skeletal targeting by the CMP can only be observed in A and D. NIR fluorescence signals are shown in rainbow scale. Click here to view larger image.
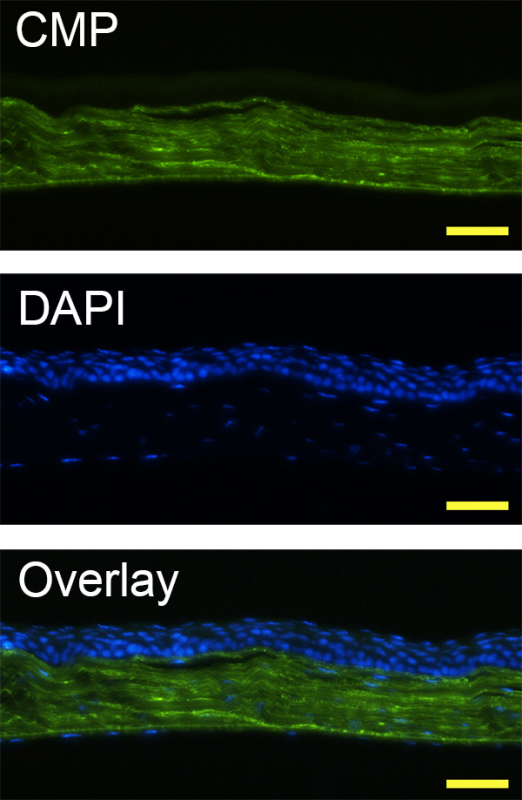 Figure 3. Fluorescence micrographs of a mouse corneal tissue section prefixed in paraformaldehyde, and stained with DAPI and photo-triggered CF(GPO)9 using Protocol 2, displaying dense collagenous stroma (stained by CMP, in green) as well as cellular epithelium and endothelium (stained by DAPI, in blue) (scale bar: 100 µm).
Figure 3. Fluorescence micrographs of a mouse corneal tissue section prefixed in paraformaldehyde, and stained with DAPI and photo-triggered CF(GPO)9 using Protocol 2, displaying dense collagenous stroma (stained by CMP, in green) as well as cellular epithelium and endothelium (stained by DAPI, in blue) (scale bar: 100 µm).
Discussion
As can be seen in this protocol, the delivery of CMP is straightforward since the UV-activation of the caged CMPs is the only additional step to the common tail vein injection protocols19. The key is to inject the peptide probes in an uncaged and metastable single-strand state. The cage group prevents CMP from self-assembly and binding to collagens (Figure 2B) until it is removed by UV light at which point the CMP starts to fold into triple helix. The injection formulation contains cysteine, which can quickly react with the cleaved cage group during UV irradiation to minimize toxicity. Numerous mice with different strains (e.g. SKH-1 and DR-1) have been tested using this formulation, and we did not detect any behavioral or other health defects in these mice up to six months. If the injection does not follow immediately after UV exposure, the CMPs will fold into homotrimeric triple helices, which have no hybridization capacity. Figure 2C shows an extreme case where prior to injection the CMPs were fully reassembled into triple helices by long delay time (>2 days). To circumvent this problem, CMP solution should be prepared in relatively low concentration (e.g. ~40 µM), and injected into the mice immediately after UV-decaging. Because of the slow triple helix folding rate of the CMPs in dilute conditions (half time of refolding: >50 min) and the short UV exposure and injection time (5~7 min total), most of the CMPs enter the bloodstream in single-strand form when this protocol is followed. Once injected, CMPs are expected to remain as single-strands, as the concentration is reduced by a factor of 20 in the blood pool, leading to dramatic reduction in the reassembling rate18. If injection is delayed (e.g. due to animal handling) and homotrimeric refolding is suspected, the partially assembled peptides can be reactivated by heating to above 70 °C to dissociate the CMPs to single-strands, followed by prompt cooling and injection. Although less convenient, such heat-dissociated CMPs also show expected results of in vivo targeting (Figure 2D).
In this demonstration, nude mice were used for NIR fluorescence imaging because up to 50% of the NIR signal can be blocked by hair. When working with haired mouse models (especially the ones with black hairs), we recommend shaving the mouse in the region of interest prior to imaging using an electric shaver or hair remover. In Figure 1, there are false positive signals from the animal's abdominal region, which is caused by auto-fluorescence of chlorophylls in the animal diet. Such background signals can be significantly lowered by switching the mouse to purified diets approximately 4 days prior to imaging. As seen in Figures 1 and 2A, there are strong CMP uptakes in the tail spine. Therefore, to avoid artifacts resulting from imperfect tail vein injections, we recommend injecting at the posterior part of the tail so that the injection site is not captured in the fluorescence image.
The labeled CMP enables targeting and imaging of specific locations of collagen remodeling in vivo that are difficult to detect using other methods. For instance, the ELISA-based blood and urine assays are sensitive for cleaved collagen telopeptide fragments, but the method cannot localize the source of the collagen turnover, because it targets soluble antigens. The main limitations of the in vivo imaging technique using NIR labeled CMPs are depth-of-penetration attenuation and quenching by proximal pooled red cells as hemoglobin is an endogenous quencher of 680/710 nm NIR dyes. Future applications of CMP in vivo imaging include SPECT or PET imaging using CMPs labeled with direct gamma-emitting radionuclides or with positron emitters, for diagnostic of disease states involving collagen remodeling (e.g. osteoporosis, arthritis, atherosclerosis, and fibrosis). These radio-labeled CMP analogs will eliminate the limitations of signal loss due to tissue depth and presence of pooled red cells, and will allow quantitative measurements of diseased tissues. In addition, the relatively late imaging time (e.g. 48-96 h.p.i.) resulting from the slow binding and clearance of CMPs could make it difficult to follow rapid changes in collagen remodeling. Such limitation could be overcome by further engineering of the binding kinetics and affinity of CMP imaging agents. Since CMP binds to denatured collagens which have little structure, the CMP-based collagen imaging is complementary to second harmonic generation microscopy which can only image collagen fibers5.
When staining tissue sections with fluorescently labeled CMPs, we found it beneficial to incubate the samples in the UV-activated peptide solutions at 4 °C, because the triple helical hybridization is facilitated at lower temperature1,20. It was also found that immersing the slides in fresh PBS buffer in a staining jar is the most effective way to wash off unbound CMPs, which works much better than merely pipetting washing buffers over the samples. The CMP-collagen strand hybridization is very specific and robust; therefore the simple staining protocol presented in this video can be readily modified for costaining additional biomarkers, and for identifying degraded collagens in unfixed tissue sections. This is an effective and convenient alternative to using anti-collagen antibodies for detecting fibrous collagens in various histological samples.
Disclosures
The authors declare no competing financial interests.
Acknowledgments
The authors thank Gilbert Green for technical assistance. This work was supported by grants from NIAMS/NIH (R01-AR060484) and DOD (W81XWH-12-1-0555) awarded to S.M.Y., and from NIH (U24 CA92871 and U54 CA151838) awarded to M.G.P.
References
- Li Y, et al. Targeting collagen strands by photo-triggered triple-helix hybridization. Proc. Natl. Acad. Sci. U.S.A. 2012;109:14767–14772. doi: 10.1073/pnas.1209721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa AG, Cusano NE, Silva BC, Cremers S, Bilezikian JP. Cathepsin K: its skeletal actions and role as a therapeutic target in osteoporosis. Nat. Rev. Rheumatol. 2011;7:447–456. doi: 10.1038/nrrheum.2011.77. [DOI] [PubMed] [Google Scholar]
- Perentes JY, et al. et al. In vivo imaging of extracellular matrix remodeling by tumor-associated fibroblasts. Nat. Methods. 2009;6:143–145. doi: 10.1038/nmeth.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breurken M, Lempens EHM, Meijer EW, Merkx M. Semi-synthesis of a protease-activatable collagen targeting probe. Chem. Commun. 2011;47:7998–8000. doi: 10.1039/c1cc11964h. [DOI] [PubMed] [Google Scholar]
- Wang AY, et al. Spatio-temporal modification of collagen scaffolds mediated by triple helical propensity. Biomacromolecules. 2008;9:1755–1763. doi: 10.1021/bm701378k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bächinger HP, Morris NP. Analysis of the thermal stability of type II collagen in various solvents used for reversed-phase high performance chromatography. Matrix. 1990;10:331–338. doi: 10.1016/s0934-8832(11)80189-7. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. Direct detection of collagenous proteins by fluorescently labeled collagen mimetic peptides. Bioconjugate Chem. 2013;24:9–16. doi: 10.1021/bc3005842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Mo X, Kim D, Yu SM. Template-tethered collagen mimetic peptides for studying heterotrimeric triple-helical interactions. Biopolymers. 2011;95:94–104. doi: 10.1002/bip.21536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl PJ, Cruz JC, Li Y, Yu SM, Hristova K. On-the-resin N-terminal modification of long synthetic peptides. Anal. Biochem. 2012;424:137–139. doi: 10.1016/j.ab.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AY, Mo X, Chen CS, Yu SM. Facile modification of collagen directed by collagen mimetic peptides. J. Am. Chem. Soc. 2005;127:4130–4131. doi: 10.1021/ja0431915. [DOI] [PubMed] [Google Scholar]
- Wang AY, et al. Immobilization of growth factors on collagen scaffolds mediated by polyanionic collagen mimetic peptides and its effect on endothelial cell morphogenesis. Biomacromolecules. 2008;9:2929–2936. doi: 10.1021/bm800727z. [DOI] [PubMed] [Google Scholar]
- Mo X, An YJ, Yun CS, Yu SM. Nanoparticle-assisted visualization of binding interactions between collagen mimetic peptide and collagen fibers. Angew. Chem. Int. Ed. 2006;45:2267–2270. doi: 10.1002/anie.200504529. [DOI] [PubMed] [Google Scholar]
- Chan TR, Stahl PJ, Yu SM. Matrix-bound VEGF mimetic peptides: design and endothelial cell activation in collagen scaffolds. Adv. Funct. Mater. 2011;21:4252–4262. doi: 10.1002/adfm.201101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hagen PM, et al. Evaluation of a radiolabelled cyclic DTPA-RGD analogue for tumour imaging and radionuclide therapy. Int. J. Cancer. 2000;90:186–198. [PubMed] [Google Scholar]
- Boudko S, et al. Nucleation and propagation of the collagen triple helix in single-chain and trimerized peptides: transition from third to first order kinetics. J. Mol. Biol. 2002;317:459–470. doi: 10.1006/jmbi.2002.5439. [DOI] [PubMed] [Google Scholar]
- Ackerman MS, et al. Sequence dependence of the folding of collagen-like peptides. J. Biol. Chem. 1999;274:7668–7673. doi: 10.1074/jbc.274.12.7668. [DOI] [PubMed] [Google Scholar]
- Machholz E, Mulder G, Ruiz C, Corning BF, Pritchett-Corning KR. Manual restraint and common compound administration routes in mice and rats. J. Vis. Exp. 2012. [DOI] [PMC free article] [PubMed]
- Brodsky B, Persikov AV. Molecular structure of the collagen triple helix. Adv. Prot. Chem. 2005;70:301–339. doi: 10.1016/S0065-3233(05)70009-7. [DOI] [PubMed] [Google Scholar]


