Abstract
Malignant gliomas constitute a heterogeneous group of highly infiltrative glial neoplasms with distinct clinical and molecular features. Primary orthotopic xenografts recapitulate the histopathological and molecular features of malignant glioma subtypes in preclinical animal models. To model WHO grades III and IV malignant gliomas in transplantation assays, human tumor cells are xenografted into an orthotopic site, the brain, of immunocompromised mice. In contrast to secondary xenografts that utilize cultured tumor cells, human glioma cells are dissociated from resected specimens and transplanted without prior passage in tissue culture to generate primary xenografts. The procedure in this report details tumor sample preparation, intracranial transplantation into immunocompromised mice, monitoring for tumor engraftment and tumor harvesting for subsequent passage into recipient animals or analysis. Tumor cell preparation requires 2 hr and surgical procedure requires 20 min/animal.
Keywords: Medicine, Issue 83, Glioma, Malignant glioma, primary orthotopic xenograft, isocitrate dehydrogenase
Introduction
Malignant gliomas are primary glial tumors of the central nervous system that occur in the brain and occasionally the spinal cord. Gliomas are classified by the World Health Organization (WHO) according to histologic resemblance to astrocytes, oligodendrocytes or ependymal cells and then numerically graded (I to IV) for pathologic features of malignancy. The most common histologic subtypes are astrocytomas, oligodendrogliomas and mixed oligoastrocytomas. Malignant gliomas encompassing WHO grades II to IV are characterized by invasive growth and recalcitrance to current therapies. Each year in the United States, approximately 15,750 individuals are diagnosed with a malignant glioma and an estimated 12,740 patients succumb to this disease. These statistics highlight the particularly lethal nature of malignant gliomas and important need for enhanced therapeutic efficacy.
Cancer models are essential for investigating tumor biology and therapies. Human cancer cell lines represent an important first step for in vitro manipulations and in vivo xenografting studies (secondary xenografts)1. However, standard cancer cell cultures undergo phenotypic and genotypic transformation2-4 that may not be restored in secondary xenografts5. Furthermore, genetic alterations such as isocitrate dehydrogenase (IDH) mutations6, distinct stem cell populations7 and dependency on key signaling pathways8 can be lost in cancer cell cultures. Genomic profiles can be maintained to better extent in cancer sphere culture, though still fail to mirror fully the genotype of primary tumors2,3. Direct orthotopic transplantation negates the influences of in vitro culture, provides a proper microenvironment, and preserves the integrity of tumor-initiating cells9,10. Therefore, primary xenografts represent a powerful and relevant preclinical model for rigorously testing targeted agents to aid in the rational design of future clinical trials5,11,12.
Protocol
1. Preparation of Tumor Cell Suspension
Note: Appropriate institutional approvals for the use of patient material and animals are required to establish and maintain primary orthotopic glioma xenografts. At Vanderbilt University Medical Center, resected tumor material that is in excess of that required for diagnostic purposes is collected with patient consent for a research tissue repository. Specimens are labeled with a randomized 5-digit REDcap database number and all patient-specific identifiers are removed. The REDcap database contains deidentified clinical data for each specimen in the tissue repository that includes gender, age (in years), race, survival status, pathology, cancer treatments, year of resection, and time to progression. To maintain sterility and optimize success of the primary xenograft method, all of the reagents, steam-autoclaved surgical instruments, and the surgical site should be assembled or prepared in advance.
Note: Glioma specimens often contain large amounts of myelin and debris. Depending on specimen size, it may be necessary to use more than 4 discontinuous gradient tubes for adequate removal of myelin and debris.
Note: The process is completed when there is no, or little, clearly visible undissociated tissue remaining. Avoid the introduction of bubbles during the trituration process.
Note: Cell viability following dissociation is typically >90%. Lower viabilities may reflect many variables including suboptimal tissue handling or transport time from the operating room, cryopreservation method, or papain dissociation technique. Lower cellular viabilities may still be adequate, however, as long as a sufficient number of viable may be transplanted in the appropriate volume. For each mouse, 50,000-200,000 viable cells are implanted in volume of 2 µl. Due to the beveled tip of the syringe needle, an additional 5 µl of cell suspension should be included in the final volume to ensure that adequate material can be drawn into the syringe for each injection. For example, to inject 2 µl/mouse for 5 mice the final volume should be 15 µl (15 µl = (5 x 2 µl) + 5 µl).
Place freshly resected, deidentified patient material in a sterile, capped container on ice for transport to the laboratory. Process the specimen directly for transplantation or cryopreserve the specimen in liquid nitrogen (see below) for storage in liquid nitrogen and later use.
- Prepare the papain dissociation solutions (papain solution, wash/protease inhibitor solution and discontinuous gradient solution) in a sterile hood with the kit components and instructions supplied by the manufacturer. Label sterile 15 ml polystyrene conical tubes and distribute the solutions as follows:
- Tube 1: 5 ml of papain solution
- Tube 2: 3 ml of wash/protease inhibitor solution
- Tubes 3-6: 5 ml each of discontinuous gradient solution
Place the glioma specimen in tube 1 with 5 ml of papain solution and triturate with a 5 ml pipette over a 10-20 min time period.
Pipette the material up and down 10 times and then incubate the material for 2-3 min at room temperature before initiating the next cycle of trituration.
Position the tip of the 5 ml pipette closer to the bottom of the conical tube with each cycle of trituration such that the tip is touching the bottom of the tube for the last 2 cycles.
Centrifuge at 300 x g for 5 min at room temperature. Remove the supernatant and pipette the pellet up and down 10x in 3 ml of the wash/protease inhibitor solution.
Carefully layer an equal volume of the suspension over each of the 5 ml discontinuous gradient solutions (tubes 3-6), with a pipettor set at the “gravity” dispense speed.
Place the tubes in a centrifuge equipped with a swinging-bucket rotor, with the acceleration speed reduced to the lowest setting and the brake turned off. Centrifuge at 76 x g for 12 min at room temperature. With the brake turned off, centrifugation is generally completed after 20 min.
Remove the supernatant, resuspend and combine each pellet in 5 ml of sterile balanced salt solution and place the cells on ice.
Remove a portion (10-100 µl) of the cell suspension and determine the density of viable cells by trypan blue exclusion with a hemocytometer.
Centrifuge at 300 x g for 5 min. Remove the supernatant and resuspend the cell pellet at a density of 25,000-125,000 viable cells per µl.
Transfer an adequate volume of the cell suspension to a 200 μl microcentrifuge tube (PCR tube) and place on ice.
2. Preparation of Surgical Site and Instruments
Note: Sterile surgical gloves are worn during the procedure. Depending upon requirements of each institution, surgical gowns, caps and face guards may be required.
Prepare a disinfected benchtop for rodent surgery with separated adjoining areas for animal preparation, operating field and animal recovery.
Sterilize surgical instruments in a steam autoclave. Subsequently, use a hot bead sterilizer to flash-sterilize instruments when transitioning from one animal subject to the next.
3. Induction, Anesthesia, and Analgesia
Note: Surgeries exceeding 15 min from the time the mice is anesthetized require a contact heat source. To prevent hypothermia, the mice are provided with a heat source (circulating hot water blanket) during the pre- and post-operative periods.
Prepare ketamine/xylazine cocktail for induction anesthesia such that each mouse receives ketamine 100 mg/kg and xylazine 10 mg/kg by injecting 10 µl of the cocktail per gram of body weight.
- Table 1. Anesthesia cocktail.
Stock concentration Volume to prepare for one mouse five mice ten mice Ketamine 100 mg/ml 25 µl 125 µl 250 µl Xylazine 100 mg/ml 2.5 µl 12.5 µl 25 µl Isotonic saline 223 µl 1,113 µl 2,225 µl Total 250 ul 1,250 µl 2,500 µl Prepare ketoprofen solution for analgesia by diluting the stock solution (10 mg/ml) 1:20 in sterile, pyogen-free water such that each mouse receives a dose of 5 mg/kg by injecting 10 µl of the solution per gram of body weight.
Weigh and record presurgical weight. Individual mouse weights vary, but generally range from 18-22 g.
Inject 10 µl of the ketamine/xylazine cocktail per gram of mouse body weight into the intraperitoneal space with an insulin syringe. For example, inject 200 µl for a 20 g mouse.
Determine whether mouse is fully anesthetized by toe pinch of the hind leg. It generally takes 3-5 min for the mouse to no longer withdraw from the pinch.
Inject 10 µl of the ketoprofen solution per gram of mouse body weight subcutaneously in the loose skin of the flank with an insulin syringe. For example, inject 200 µl for a 20 g mouse.
Shave hair over the skull with clippers, scrub the exposed scalp with povidone-iodine using a cotton tip applicator and then wipe an alcohol pad. Repeat these steps, povidone-iodine scrub and alcohol wipe, two more times.
Apply ophthalmic ointment to the eyes of the mouse.
Make a 1 cm midline incision extending from behind the ears to just in front of the eyes with a sterile scalpel blade.
Place the mouse under a dissecting scope and expose the skull to identify the suture lines. Using circular motions with a drill, center a burr hole 2.5 mm lateral to the bregma and 1 mm posterior to the coronal suture.
4. Intracranial Transplantation
Note: Injection volumes exceeding 2.5 µl may be lethal to mice.
Position the mouse on a stereotactic frame by hooking the upper incisors over the incisor bar and applying the nose clamp so that the skull is firmly held in a neutral position.
Draw 5-10 µl of the cells in a microcentrifuge tube up and down several times into a 10 µl Hamilton syringe clamped in the probe holder of the stereotactic manipulator to mix the suspension and eliminate bubbles. Depress the plunger so that the syringe is loaded with 2 µl (the adequate volume to inject one animal).
- Pull the lateral edge of the skin incision to expose the burr hole and, with the micromanipulator, introduce the needle into the burr hole so that the beveled portion is just below the skull surface.
- Advance the needle 3 mm and then withdraw the needle 0.5 mm to create a space for injection.
- Advance the plunger slowly over 30 sec and then allow the needle to remain in the brain for an additional 2 min. Withdraw the needle gradually by ascending 0.5 mm and waiting for an additional 30 sec before removing the needle from the brain.
Remove the mouse from the stereotactic frame and close the wound with an autoclip.
Place the mouse on a warming pad to maintain body temperature and monitor continuously the respiration pattern and color of mucous membranes and exposed tissues (soles of feet).
Place the mouse in a sterile microisolator cage once it recovers fully from anesthesia (sternal and ambulatory), and return it to the animal housing facility.
5. Post-implantation Animal Care and Observation
Note: The most reliable indication of tumor engraftment and infiltrative growth is gradual, sustained weight loss accompanied by development of hunched posture and rough hair coat. On rare occasions, neurological deficits are noted that include ataxia, paresis, and seizures. Orthotopic primary xenografts are highly invasive and develop over a long period of time. Mice typically manifest symptoms of tumor growth 3-6 months after transplantation.
Observe mice with orthotopic xenografts daily for food and water intake, behavior, grooming, and signs of infection at the incision site.
Administer ketoprofen (5 mg/kg subcutaneously, 1-2x a day) if pain or distress is observed postoperatively.
Remove autoclips 8-10 days after surgery.
Weigh mice weekly.
Monitor for signs and symptoms of tumor engraftment.
6. Tumor Harvesting
Note: By this method, the first coronal slice (#1) contains the posterior aspect of the olfactory bulbs and the anterior aspect of the frontal lobes. Engrafted tumor is most abundant in the right hemisphere of the subsequent 3 coronal slices (slice #2, #3, and #4) and the injection sight is routinely contained in coronal slice #3. Depending upon experimental needs, the material may be used for tumor passage, frozen tissue sections, formalin fixed paraffin embedded tissue sections, flow cytometry of dissociated tissue, cell culture or lysates for DNA, RNA, or protein analysis. Portions of the tumor may be cryopreserved for future needs with cell viability ranging from of 80-95%.
Euthanize mice by prolonged exposure to carbon dioxide followed by cervical dislocation.
Decapitate euthanized mice at the base of the brain (foramen magnum level) with a larger pair of surgical scissors, and pull the skin from incision forward to fully expose the skull.
- Remove the brain.
- Insert one tip of pair of fine surgical scissors into the lateral aspect of the foramen magnum to level of the left external auditory meatus to cut the occipital bone along the left side of the skull. Perform the same cut on the right side.
- Cut the occipital bone along a coronal plane from one external auditory meatus to the other and remove the bone to expose the cerebellum.
- Cut the nasal bone plates between the eyes.
- Cut the sagittal suture by cutting posteriorly along the sagittal suture from the nasal bone plates to the bregma. Complete the midline incision along the sagittal suture by cutting anteriorly from the lambda to the bregma.
- Carefully insert the tip of a fine pair of forceps along the sagittal opening on each side to tear any dural adhesion of the skull to the brain and then peel the two skull halves laterally to expose the brain and olfactory bulbs dorsally.
- Slide the forceps between the ventral aspect of the olfactory bulbs and the skull to free any adhesions.
- Tilt the skull back to allow the brain to be retracted so that the optic and other cranial nerves are exposed. Sever the cranial nerves to release the brain into a dish containing sterile PBS.
Transfer the brain with a weighing spatula to a matrix slicer and generate 2 mm coronal slices with razor blades.
Arrange the coronal slices in a dish containing sterile PBS for further visual inspection and further tissue processing.
7. Tumor Cryopreservation
- Prepare a stock solution of cryopreservation medium.
- Add 50 ml of proliferation supplement, 5 ml of 100x penicillin and streptomycin solution, and 450 µl of 0.2% heparin to 450 ml of basal medium.
- Store stock solution at 4 °C protected from light.
- Prepare a working solution of cryopreservation medium.
- Combine 47.5 ml of cryopreservation medium and 2.5 ml of sterile DMSO in a 50 ml polypropylene conical tube and mix well.
- Aliquot 1.0 ml of working solution to each cryovial and store at 4 °C protected from light.
Place one 2 mm coronal slice of xenografted brain into a cryovial containing cryopreservation medium on ice.
Tightly cap the vial and place it in a freezing container at -80 °C. Transfer the cryovial the following day to a liquid nitrogen tank for longer storage.
Representative Results
Dissociated glioma cells are transplanted directly into the brains of immunocompromised mice to obtain primary orthotopic xenograft lines. Each tumor specimen is assigned a randomized number prior to transplantation, as part of the deidentification process to remove protected health information. We use a 5-digit REDcap database number for this purpose. Figure 1 illustrates the process and nomenclature for establishing a xenograft line from a glioblastoma (GBM 17182) with isocitrate dehydrogenase 1 (IDH1) mutation arginine 132 to histidine (R132H). Following transplantation an "X" is added to the tumor identification number to denote xenografted tumor, followed by a "P" to track the passage number. P0 designates the initial transplantation recipient and P1 designates recipient mice of the first xenograft passage. Typically, 3-5 mice are initially transplanted to establish a line and xenografted tumor is then passaged into 10 recipient mice (P1) to expand the line for future studies.
Following orthotopic transplantation, recipient mice are monitored for signs and symptoms of tumor engraftment and growth. The most sensitive indication of tumor engraftment is significant and sustained weight loss. As mice are transplanted at a young age, post-operative weight gain is expected. Mouse weights may fluctuate in seemingly dramatic fashion from week to week, however sustained loss of greater than 3 g is generally a good indication of tumor engraftment. Typically, most of the mice in a xenograft cohort develop weight loss over a similar period of time ranging from as short as 60 days to as long as 350 days depending on the tumor. Median survival times for a given tumor are generally similar from passage to passage when the same number of tumor cells are transplanted each time. Illustrated in Figure 2 is a cohort of 5 mice transplanted with a GBM (17182XP2) that had been previously engrafted and passaged once before. Four of the five 17182XP2 mice demonstrated significant weight loss 17 weeks after transplantation. The remaining mouse (mouse 3) did not exhibit weight loss and no tumor was detected at post-mortem examination, indicating tumor engraftment in only 80% of the recipients.
When the brain of a symptomatic mouse is harvested, a brain slicer matrix is an extremely valuable tool for tissue processing. The generation of multiple, anatomically defined sections from each brain allows for multiple modes of analysis with some portions and cryopreservation of other portions of the tissue. For example, mutational status of IDH1 may be assessed by Sanger sequencing with material from one portion or immunohistochemistry with another (Figure 3). Tumor engraftment may be readily apparent by visual inspection of the coronal sections, however, histology with immunohistochemistry may be required for some xenografts (Figures 4 and 5). When dissociating a xenograft for serial passage or other studies, it is important start with a small and defined portion of material so that myelin and debris may be adequately removed (Figure 6). The discontinuous gradient of the Papain Dissociation System is best suited for processing small portions of an adult mouse brain. We recommend cutting each coronal section along the midline and using two discontinuous gradients for each half.
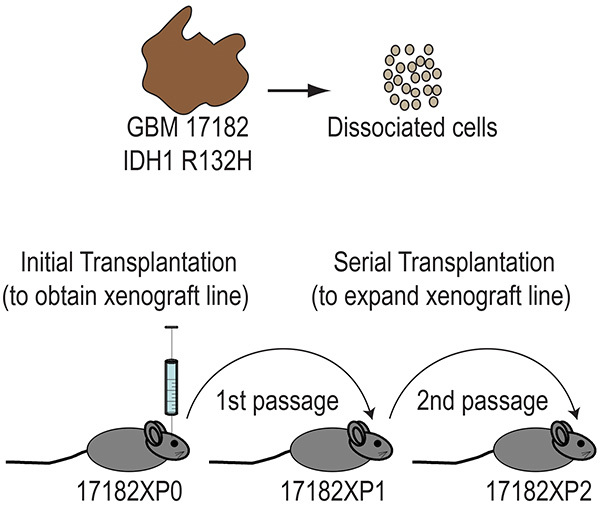 Figure 1. Primary orthotopic xenograft lines are established by transplanting dissociated glioma cells directly into the brains of immunocompromised mice and then expanded by serial transplantation. Heterozygous somatic mutations at arginine 132 of the IDH1 gene occur in more than 70% of diffuse astrocytomas, oligodendrogliomas, oligoastrocytomas and secondary glioblastomas, and less than 7% of primary glioblastomas. GBM 17182 contains the most common IDH1 mutation, arginine 132 to histidine (R132H). Click here to view larger image.
Figure 1. Primary orthotopic xenograft lines are established by transplanting dissociated glioma cells directly into the brains of immunocompromised mice and then expanded by serial transplantation. Heterozygous somatic mutations at arginine 132 of the IDH1 gene occur in more than 70% of diffuse astrocytomas, oligodendrogliomas, oligoastrocytomas and secondary glioblastomas, and less than 7% of primary glioblastomas. GBM 17182 contains the most common IDH1 mutation, arginine 132 to histidine (R132H). Click here to view larger image.
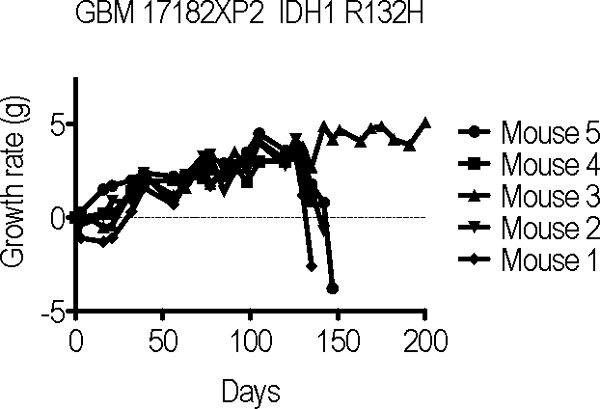 Figure 2. Growth curves of serial transplant recipients of GBM 17182XP1 demonstrate significant weight loss 130-150 days after surgery in 4 of 5 GBM 17182XP2 mice.Click here to view larger image.
Figure 2. Growth curves of serial transplant recipients of GBM 17182XP1 demonstrate significant weight loss 130-150 days after surgery in 4 of 5 GBM 17182XP2 mice.Click here to view larger image.
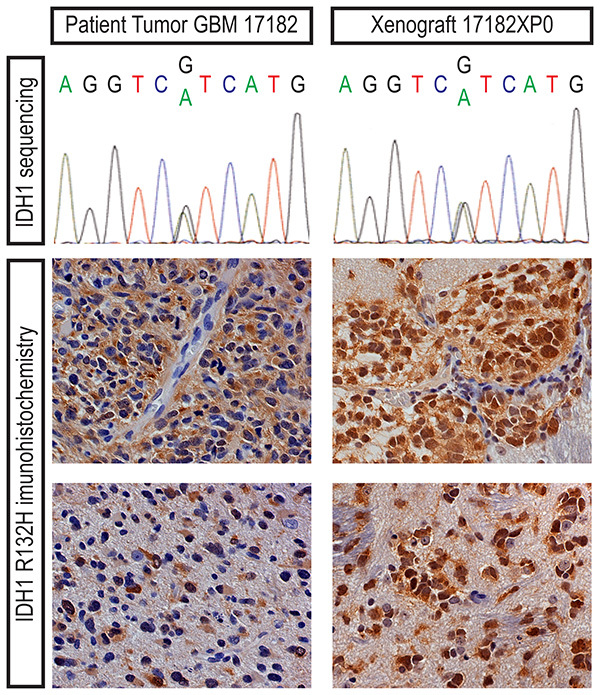 Figure 3. Heterozygous, somatic IDH1 R132H mutation in the patient tumor (GBM 17182) and primary xenograft (17182XP0) can be detected by Sanger sequencing or antibody staining. Immunohistochemistry with an IDH1 R132H-specific antibody demonstrates mutant glioma cells densely clustered around blood vessels or in more diffusely infiltrated regions of the patient specimen and mouse brain. Click here to view larger image.
Figure 3. Heterozygous, somatic IDH1 R132H mutation in the patient tumor (GBM 17182) and primary xenograft (17182XP0) can be detected by Sanger sequencing or antibody staining. Immunohistochemistry with an IDH1 R132H-specific antibody demonstrates mutant glioma cells densely clustered around blood vessels or in more diffusely infiltrated regions of the patient specimen and mouse brain. Click here to view larger image.
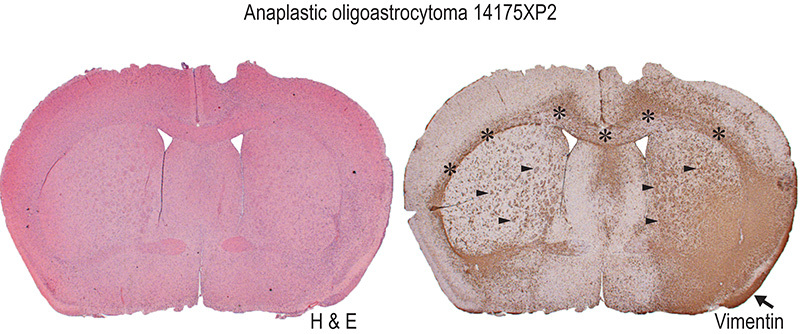 Figure 4. Primary orthotopic xenografts display highly infiltrative growth that may be difficult to fully appreciate by hematoxylin and eosin (H & E) staining alone. Immunohistochemistry with a human-specific antibody is a particularly useful method for identifying engrafted human cells. Anti-human vimentin staining of an analplastic oligoastrocytoma xenograft (14175XP2) reveals typical features of an infiltrative glioma with a diffuse tumor mass in the injected right hemisphere and invasive growth along myelinated tracts of the corpus callosum (asterisks) and the striatopallidal fibers (black arrowheads) and leptomeningeal dissemination (black arrow). Not appreciated at this magnification (1.25X) is clustering of human tumor cells around mouse neurons (perineuronal satellitosis) in the invaded cortex. Click here to view larger image.
Figure 4. Primary orthotopic xenografts display highly infiltrative growth that may be difficult to fully appreciate by hematoxylin and eosin (H & E) staining alone. Immunohistochemistry with a human-specific antibody is a particularly useful method for identifying engrafted human cells. Anti-human vimentin staining of an analplastic oligoastrocytoma xenograft (14175XP2) reveals typical features of an infiltrative glioma with a diffuse tumor mass in the injected right hemisphere and invasive growth along myelinated tracts of the corpus callosum (asterisks) and the striatopallidal fibers (black arrowheads) and leptomeningeal dissemination (black arrow). Not appreciated at this magnification (1.25X) is clustering of human tumor cells around mouse neurons (perineuronal satellitosis) in the invaded cortex. Click here to view larger image.
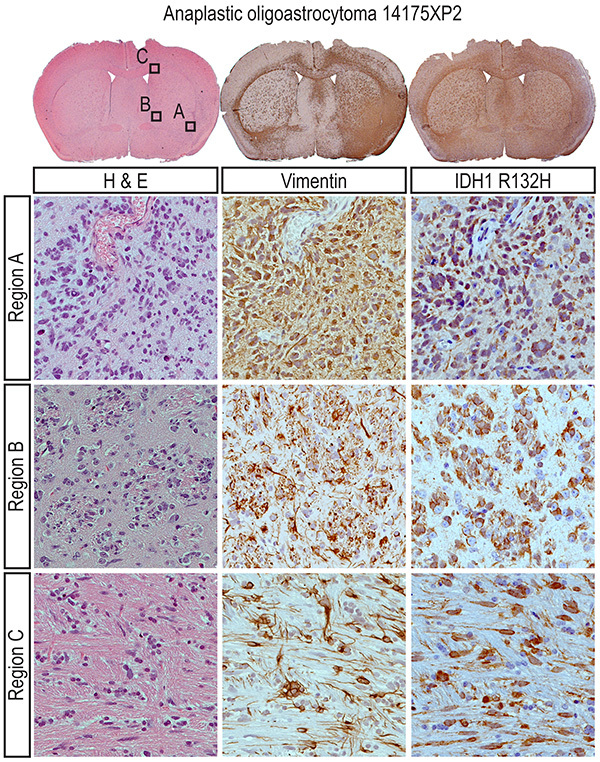 Figure 5. Analysis of analplastic oligoastrocytoma 14175XP2 coronal sections at higher power (40X) in boxed regions A-C reveals IDH mutant human cells around blood vessels (region A), in striatopallidal tracts ("pencil fibers;" region B) and the corpus callosum (region C). As with GBM 17182XP2, IDH1 R132H staining of anaplastic oligoastrocytoma 14175XP2 demonstrates that IDH mutation is maintained in a primary orthotopic xenograft following serial passages in recipient mice. Click here to view larger image.
Figure 5. Analysis of analplastic oligoastrocytoma 14175XP2 coronal sections at higher power (40X) in boxed regions A-C reveals IDH mutant human cells around blood vessels (region A), in striatopallidal tracts ("pencil fibers;" region B) and the corpus callosum (region C). As with GBM 17182XP2, IDH1 R132H staining of anaplastic oligoastrocytoma 14175XP2 demonstrates that IDH mutation is maintained in a primary orthotopic xenograft following serial passages in recipient mice. Click here to view larger image.
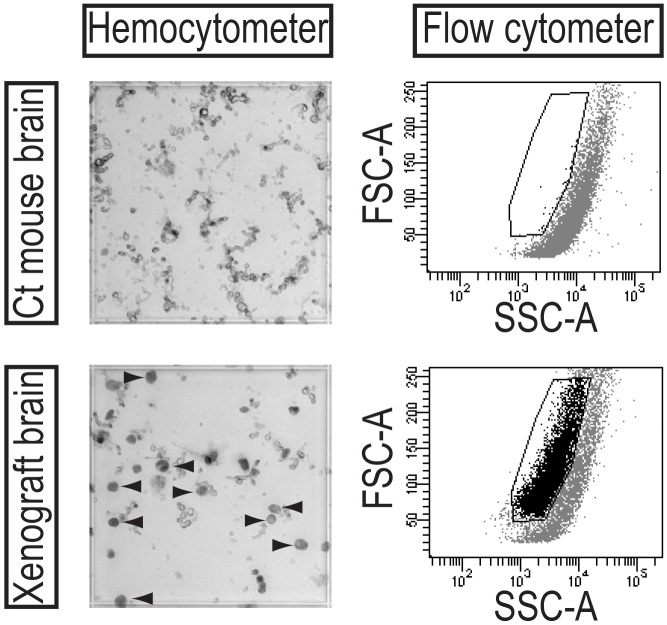 Figure 6. Human tumor cells can be distinguished from mouse cells in dissociated xenografts based upon size. To quantify human tumor cells in a dissociated xenograft for serial passage, human cells are identified in a hemocytometer under light microscopy by their large size and dark green luminescence (arrowheads). By flow cytometry, human cells have distinct scatter properties from mouse material and can be gated for further analysis. Click here to view larger image.
Figure 6. Human tumor cells can be distinguished from mouse cells in dissociated xenografts based upon size. To quantify human tumor cells in a dissociated xenograft for serial passage, human cells are identified in a hemocytometer under light microscopy by their large size and dark green luminescence (arrowheads). By flow cytometry, human cells have distinct scatter properties from mouse material and can be gated for further analysis. Click here to view larger image.
Discussion
Cultured cell lines, xenografts and genetically engineered mice are the most common methods for modeling gliomas, and there are distinct benefits and limitations for each model system3,13,14. Relevant benefits of primary orthotopic glioma xenografts include infiltrative growth that typifies diffuse gliomas and the retention of genetic alterations and important signaling mechanisms that can be exceedingly difficult to maintain in cultured glioma cells. For example, isocitrate dehydrogenase mutations and Sonic hedgehog ligand production can be maintained in primary orthotopic xenografts15,16 but only rarely in cultured glioma cells14,17-19. It is important to consider significant disadvantages of primary orthotopic xenografts, however, depending upon experimental needs. Notwithstanding access to adequate patient material, it is more difficult to measure glioma growth over time in the brain than in a heterotopic site. Additionally, primary orthotopic xenografts grow at a significantly slower rate than heterotopic xenografts. Thus investigators may prefer to transplant cells into the flank of an immunocompromised mouse, where important histological and molecular features may also be retained4.
Two important considerations for establishing primary orthotopic xenografts are the type of immunocompromised mouse recipient and the grade of glioma malignancy. We have had severely limited success establishing primary xenografts in athymic (nu-nu) mice and equally good success with NOD/SCID and NSG (NOD/SCID/Interleukin-2 receptor gamma chain deficient) mice. NSG mice are preferable recipients because of increased engraftment rate of tumorigenic cells and longer lifespan as compared to NOD/SCID mice20,21. With regard to WHO tumor grade, we have observed glioma engraftment of grade III and IV tumors but not grades I and II.
For primary orthotopic glioma xenografts, dissociated tumor cells may be transplanted directly into the brains of immunocompromised mice9 or tumor-initiating cells may be prospectively isolated (for example by selecting CD133-expressing cells) for transplantation22. Either source of cells is suitable for successful tumor engraftment in our experience15,16, though the use of CD133-enriched cells requires more starting material. Whether preparing sorted or unsorted cells for transplantation, it is important to adequately remove myelin and debris from dissociated glioma specimens. Failure to adequately remove myelin and debris markedly hampers the ability to draw the suspension into a Hamilton syringe and transplant a uniform number of cells into each recipient. Following papain dissociation, it is helpful to evaluate a small aliquot using a hemocytometer in order to determine the number of discontinuous gradient tubes that will be required to sufficiently eliminate myelin and debris. Complete removal is not feasible, but one can gain an appreciation for the difficulty quantifying cells in a dissociated specimen prior to myelin removal and the relative ease following removal with discontinuous gradients.
The yield of viable cells from dissociated patient specimens can be highly variable. Optimally, 105 cells are transplanted into each of five recipient mice though we have obtained engraftment with as few as 3,000 cells/animal. Approximately half of the primary malignant glioma specimens (WHO grades III and IV) that we have transplanted have engrafted in 40-100% of the initial recipient mice. Following orthotopic transplantation, recipient mice must be weighed weekly and monitored regularly for signs and symptoms of tumor engraftment and growth. Primary orthotopic xenografts demonstrate diffuse, infiltrative growth and focal neurological symptoms such as hemiparesis or seizures are observed only on rare occasions. The most sensitive indication of tumor engraftment is significant and sustained weight loss that may be accompanied by development of hunched posture, rough hair coat or domed skull. The rate at which mice develop sustained weight loss is highly variable depending upon the patient specimen and can range from 60-350 days. NSG mice typically weigh 18-25 g at transplantation and post-operatively achieve adult weights of 28-33 g. Bedding material should be changed regularly as cage conditions may alter animal weights to a considerable extent. An animal's weights may fluctuate by as much as a gram from one week to the next, and determining whether this represents tumor engraftment can be difficult. A good indication of tumor engraftment in NSG mice that have achieved adult weights is sustained weight loss of greater than 3 g. Although engrafted mice typically develop gradual weight loss that can be measured over a several week period, occasionally an animal's health may decline rapidly due to tumor growth, following the development of hydrocephalus for example, or from unrelated causes such as infection.
Following euthanasia, tumor engraftment may not be readily apparent by gross inspection of the brain and histological confirmation is essential. For initial tumor recipients, we process each brain in the same fashion so that one portion is evaluated histologically and other portions are cryopreserved for subsequent tumor passage if engraftment is verified. As discussed above, the brain is placed in a matrix slicer to generate 2 mm coronal slices with each slice representing a defined region of the brain along the anterior to posterior axis. Coronal slice #3, which routinely contains the injection site, is fixed in formalin and each of the remaining slices is placed in a separate, labeled cryovial containing 1 ml of cryopreservation medium. Formalin fixed paraffin embedded (FFPE) slides are stained with hematoxylin and eosin to evaluate the histology and with antibodies against Ki67 and human vimentin to visualize engrafted cells.
Once engraftment is verified, the tumor may be passaged and engraftment rates in secondary recipients are typically higher at 80-100%. We recommend passaging a tumor the first time in a larger number of recipient mice so that adequate cryopreserved material may be generated to maintain a given tumor line at low-passage (2-5) for future studies. Median survival times for a given tumor are generally similar from passage to passage when the same number of tumor cells are transplanted each time.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
We are particularly indebted to patients at Vanderbilt University Medical Center who provided invaluable research material for the Molecular Neurosurgical Tissue Bank. We thank those who established and maintain the Tissue Bank, Reid C. Thompson MD (principal investigator), Cherryl Kinnard RN (research nurse) and Larry A. Pierce MS (manager). Histological services were performed, in part, by the Vanderbilt University Medical Center (VUMC) Translational Pathology Shared Resource (supported by award 5P30 CA068485 to the Vanderbilt-Ingram Cancer Center). This work was supported by grants to MKC from the NINDS (1R21NS070139), the Burroughs Wellcome Fund and VMC development funds. MKC is supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development through grant 1 I01 BX000744-01. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- Johnson JI, et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br. J. 2001;84:1424–1431. doi: 10.1054/bjoc.2001.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt Hamer De, C P, et al. The genomic profile of human malignant glioma is altered early in primary cell culture and preserved in spheroids. Oncogene. 2008;27:2091–2096. doi: 10.1038/sj.onc.1210850. [DOI] [PubMed] [Google Scholar]
- Lee J, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Pandita A, Aldape KD, Zadeh G, Guha A, James CD. Contrasting in vivo and in vitro fates of glioblastoma cell subpopulations with amplified EGFR. Genes Chromosomes Cancer. 2004;39:29–36. doi: 10.1002/gcc.10300. [DOI] [PubMed] [Google Scholar]
- Daniel VC, et al. A primary xenograft model of small-cell lung cancer reveals irreversible changes in gene expression imposed by culture in vitro. Cancer Res. 2009;69:3364–3373. doi: 10.1158/0008-5472.CAN-08-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaskowski S, et al. Glioma cells showing IDH1 mutation cannot be propagated in standard cell culture conditions. Br. J. Cancer. 2011;104:968–970. doi: 10.1038/bjc.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat. Rev. 2006;6:425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- Sasai K, et al. Shh pathway activity is down-regulated in cultured medulloblastoma cells: implications for preclinical studies. Cancer Res. 2006;66:4215–4222. doi: 10.1158/0008-5472.CAN-05-4505. [DOI] [PubMed] [Google Scholar]
- Shu Q, et al. Direct orthotopic transplantation of fresh surgical specimen preserves CD133+ tumor cells in clinically relevant mouse models of medulloblastoma and glioma. Stem Cells. 2008;26:1414–1424. doi: 10.1634/stemcells.2007-1009. [DOI] [PubMed] [Google Scholar]
- Suggitt M, Bibby MC. 50 years of preclinical anticancer drug screening: empirical to target-driven approaches. Clin. Cancer Res. 2005;11:971–981. [PubMed] [Google Scholar]
- Kerbel RS. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived-but they can be improved. Cancer Biol. Ther. 2003;2:134–139. [PubMed] [Google Scholar]
- Park CY, Tseng D, Weissman IL. Cancer stem cell-directed therapies: recent data from the laboratory and clinic. Mol. Ther. 2009;17:219–230. doi: 10.1038/mt.2008.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson BL, Pokorny JL, Schroeder MA, Sarkaria JN. Establishment, maintenance and in vitro and in vivo applications of primary human glioblastoma multiforme (GBM) xenograft models for translational biology studies and drug discovery. Curr. Protoc. Pharmacol. Chapter. 2011;14 doi: 10.1002/0471141755.ph1416s52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambardzumyan D, Parada LF, Holland EC, Charest A. Genetic modeling of gliomas in mice: new tools to tackle old problems. Glia. 2011;59:1155–1168. doi: 10.1002/glia.21142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarangi A, et al. Targeted inhibition of the Hedgehog pathway in established malignant glioma xenografts enhances survival. Oncogene. 2009;28:3468–3476. doi: 10.1038/onc.2009.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadez JG, et al. Identification of Hedgehog pathway responsive glioblastomas by isocitrate dehydrogenase mutation. Cancer Lett. 2013;328:297–306. doi: 10.1016/j.canlet.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar EE, et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehtesham M, et al. Ligand-dependent activation of the hedgehog pathway in glioma progenitor cells. Oncogene. 2007;26:5752–5761. doi: 10.1038/sj.onc.1210359. [DOI] [PubMed] [Google Scholar]
- Kelly JJ, et al. Oligodendroglioma cell lines containing t(1;19)(q10;p10. Neuro-oncology. 2010;12:745–755. doi: 10.1093/neuonc/noq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana E, et al. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz LD, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- Singh SK, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]


