Abstract
Objective
The number of patients who require prolonged mechanical ventilation increased during the last decade, which generated a large population of chronically ill patients. This study established the incidence of prolonged mechanical ventilation in four intensive care units and reported different characteristics, hospital outcomes, and the impact of costs and services of prolonged mechanical ventilation patients (mechanical ventilation dependency ≥ 21 days) compared with non-prolonged mechanical ventilation patients (mechanical ventilation dependency < 21 days).
Methods
This study was a multicenter cohort study of all patients who were admitted to four intensive care units. The main outcome measures were length of stay in the intensive care unit, hospital, complications during intensive care unit stay, and intensive care unit and hospital mortality.
Results
There were 5,287 admissions to the intensive care units during study period. Some of these patients (41.5%) needed ventilatory support (n = 2,197), and 218 of the patients met criteria for prolonged mechanical ventilation (9.9%). Some complications developed during intensive care unit stay, such as muscle weakness, pressure ulcers, bacterial nosocomial sepsis, candidemia, pulmonary embolism, and hyperactive delirium, were associated with a significantly higher risk of prolonged mechanical ventilation. Prolonged mechanical ventilation patients had a significant increase in intensive care unit mortality (absolute difference = 14.2%, p < 0.001) and hospital mortality (absolute difference = 19.1%, p < 0.001). The prolonged mechanical ventilation group spent more days in the hospital after intensive care unit discharge (26.9 ± 29.3 versus 10.3 ± 20.4 days, p < 0.001) with higher costs.
Conclusion
The classification of chronically critically ill patients according to the definition of prolonged mechanical ventilation adopted by our study (mechanical ventilation dependency ≥ 21 days) identified patients with a high risk for complications during intensive care unit stay, longer intensive care unit and hospital stays, high death rates, and higher costs.
Keywords: Respiration, artificial/methods; Prognosis; Mortality
Abstract
Objetivo
Na última década ocorreu um aumento no número de pacientes que necessitam manutenção de ventilação mecânica prolongada, resultando no surgimento de uma grande população de pacientes crônicos criticamente enfermos. Este estudo estabeleceu a incidência de ventilação mecânica prolongada em quatro unidades de terapia intensiva e relatou as diferentes características, desfechos hospitalares e impacto nos custos e serviços de pacientes com ventilação mecânica prolongada (dependência de ventilação mecânica por 21 dias ou mais) em comparação a pacientes sem ventilação mecânica prolongada (dependência de ventilação mecânica inferior a 21 dias).
Métodos
Este foi um estudo multicêntrico de coorte que envolveu todos os pacientes admitidos em quatro unidades de terapia intensiva. As principais avaliações de desfechos incluíram o tempo de permanência na unidade de terapia intensiva e no hospital, a incidência de complicações durante a permanência na unidade de terapia intensiva, e a mortalidade na unidade de terapia intensiva e no hospital.
Resultados
Durante o período do estudo, ocorreram 5.287 admissões às unidades de terapia intensiva. Alguns desses pacientes (41,5%) necessitaram de suporte ventilatório (n = 2.197), e 218 dos pacientes (9,9%) cumpriram os critérios de ventilação mecânica prolongada. Algumas complicações se desenvolveram durante a permanência na unidade de terapia intensiva como fraqueza muscular, úlceras de pressão, sepse nosocomial bacteriana, candidemia, embolia pulmonar, e delirium hiperativo; estas se associaram com um risco significantemente maior de ventilação mecânica prolongada. Os pacientes de ventilação mecânica prolongada tiveram um aumento significante da mortalidade na unidade de terapia intensiva (diferença absoluta = 14,2%; p < 0,001) e da mortalidade hospitalar (diferença absoluta = 19,1%; p < 0,001). O grupo com ventilação mecânica prolongada permaneceu mais dias no hospital após receber alta da unidade de terapia intensiva (26,9 ± 29,3 versus 10,3 ± 20,4 dias; p < 0,001) e acarretou custos mais elevados.
Conclusão
A classificação de pacientes crônicos criticamente enfermos segundo a definição de ventilação mecânica prolongada adotada em nosso estudo (dependência de ventilação mecânica por período igual ou superior a 21 dias) identificou pacientes com risco elevado de complicações durante a permanência na unidade de terapia intensiva, permanência mais longa na unidade de terapia intensiva e no hospital, taxas de mortalidade maiores e custos mais elevados.
INTRODUCTION
Advances in intensive care have enabled more patients to survive an acute critical illness. However, these advances also created a large and growing population of patients with prolonged dependence on mechanical ventilation (MV) and other intensive care therapies.(1-3) Most patients require short periods of respiratory support, but a minority require prolonged MV (PMV), which is defined as a period of ≥ 6 hours/day on MV for 21 days.(4) The requirement for a period of MV usually mandates admission to an intensive care unit (ICU), and PMV is included in the term ‘‘chronically critically ill’’ described in a 1985 study by Girard and Raffin.(1) This study focused on patients who survived an initial episode of critical illness but remained dependent on intensive care. These patients neither died during the acute period of ICU treatment nor recovered.(5)
The hallmark of chronic critical illness (CCI) is respiratory failure that requires prolonged dependence on MV, and the number of patients who require ventilatory support is predicted to increase, particularly in the elderly or patients with comorbidities (e.g., chronic obstructive pulmonary disease [COPD], cancer and sepsis). These factors will likely increase the incidence of PMV.(6) CCI is a devastating condition for patients, their families, and the entire health care system.(3,6-8) Trends in the numbers of patients requiring PMV are of interest to health service planners because these patients consume a disproportionate amount of health care resources and impose high illness costs.(9-11)
The limited available data on patient outcomes indicate considerable variations between the populations studied.(12) Single-center studies(6,13-17) indicate that approximately 3 to 11% of patients receiving MV meet the criteria for ventilatory dependency for more than 21 days. Nevertheless, multicenter studies using this stricter definition of PMV have not been performed previously.(18) Therefore, this cohort study established the incidence of PMV in four ICUs and reported different characteristics, hospital outcomes, and the impact of costs and services of PMV patients (MV dependency ≥ 21 days) compared to non-PMV patients (MV dependency < 21 days).
METHODS
We performed a retrospective cohort study using an ICU database prospectively and routinely collected by the ICU staff of four institutions. The Research Ethics Committees of four institutions waived the need for formal ethical review. Patient confidentiality was ensured because the dataset was fully anonymized.
Each hospital has a “closed” adult general mixed medical/surgical ICU with specialist intensive care staff. The number of ICU beds included in this study was 93 (ICU-1 [HospitalMoinhos de Vento - 31 beds], ICU-2 [Central-ICU of Hospital Santa Casa - 18 beds], ICU-3 [Hospital Santa Rita - 10 beds], and ICU-4 [Hospital Mãe de Deus - 34 beds]), and data were collected for 26 months (between June 2008 and July 2010). VM strategies in the 4 participating hospitals followed the recommendations of the current ventilatory support guidelines.
All data were entered prospectively by clinical staff and include demographics (gender, age, body mass index [BMI]); cause of ICU admission; admission source; disease severity scores (Acute Physiology and Chronic Health Evaluation [APACHE] II scoring at 24 hours, Sepsis-related Organ Failure Assessment [SOFA], Therapeutic Intervention Scoring System [TISS], and Glasgow Coma Score [GCS] at admission and discharge). Documented preexisting chronic diseases and severity of underlying medical conditions were recorded and classified using the Charlson Index, number of comorbidities, and McCabe score (as nonfatal - score 1, ultimately fatal - score 2, or fatal - score 3], the; presence of infection and sepsis. Organ support data during ICU stay (e.g., renal replacement therapy [RRT], vasoactive therapy, and need for parenteral nutrition) and the need for unscheduled surgery or tracheotomy were entered on a daily basis during an admission episode. Complications during ICU hospitalization, ICU length of stay (LOS), hospital LOS, days on MV, survival status at ICU and hospital discharge were also recorded.
All patients on MV received respiratory and motor physiotherapy during the follow-up. All units used daily interruption of sedation and a weaning protocol based on a tolerance at spontaneous breathing trial. Tracheotomy was performed according to the attending physician. Non-invasive ventilation was available in all units. RRT in the four units could be performed by continuous hemofiltration or intermittent hemodialysis in more stable patients.
We calculated an average daily cost per capita in a patient sample (non-PMV = 425 and PMV = 28 at ICU-4, where cost data were disclosed and collected in all patients) to evaluate resource utilization. The obtained value was multiplied by ICU LOS (ICU cost) and hospital LOS (hospital cost).
Statistical analysis
Patient characteristics are presented as number and percentage, mean and standard deviation (SD). Characteristics are described for PMV and non-PMV groups and compared using the following tests: t-test for normally distributed data, Mann-Whitney U test for non-normally distributed data, and chi-squared test for categorical variables. Trends were analyzed using chi-squared test for categorical variables. The association between PMV status and diagnostic category was assessed using odds ratio (OR). A multivariate analysis was performed to determine the risk factors for evolution to chronic critical illness using backward stepwise multiple logistic regression. Factors with a value of p ≤ 0.01 (as determined by univariate analysis) were selected for the model. Confidence intervals (CI) for incidence rates were derived using the Poisson distribution. A significance level of 1% was used for analyses, and 95% CI are presented (unless stated). All analyses were performed using Statistical Package for the Social Science (SPSS) v18.
RESULTS
There were 5,287 admissions to the four ICUs during study period (ICU-1 = 3,345, ICU-2 = 603, ICU-3 = 886, and ICU-4 = 453). Readmissions were excluded from the analysis. Some of these patients (41.5%) needed ventilatory support (n = 2,197), and 218 (9.9%) met the criteria for PMV (ICU-1 = 112 (9.1%), ICU-2 = 40 (6.7%), ICU-3 = 38 (10.2%), and ICU-4 = 28 (13.7%)). Tables 1 and 2 show patient baseline characteristics.
Table 1.
Characteristics of the study subjects at admission in the intensive care unit
| Characteristics | PMV | Non-PMV | p value | OR (95% CI) |
|---|---|---|---|---|
| (N = 218) | (N = 1,979) | |||
| Male gender | 103 (47.2) | 1,088 (55) | 0.03 | 0.73 (0.55 - 0.97) |
| Age (years) | 66.6 ± 17.4 | 65.7 ± 17.3 | 0.47 | |
| Age group ≥ 65 years | 123 (56.4) | 1,163 (58.8) | 0.55 | |
| BMI (Kg/m2) | 25.4 ± 5.6 | 25.4 ± 4.9 | 1.00 | |
| At ICU admission | ||||
| Source | ||||
| Ward | 78 (35.7) | 619 (31.3) | 0.20 | 1.22 (0.91 - 1.64) |
| Emergency room | 56 (25.6) | 466 (23.5) | 0.53 | 1.12 (0.91 - 1.64) |
| Operating room | 36 (16.5) | 543 (27.4) | < 0.0001 | 0.52 (0.36 - 0.76) |
| Transfer | 26 (11.9) | 230 (11.6) | 0.98 | 1.03 (0.66 - 1.58) |
| Others | 12 (5.5) | 121 (6.2) | 0.83 | 0.89 (0.48 - 1.64) |
| Preexisting chronic diseases and severity of underlying medical conditions | ||||
| Comorbidities | ||||
| Heart failure | 33 (15.1) | 264 (13.3) | 0.52 | 1.16 (0.78 - 1.71) |
| COPD | 30 (16.9) | 241 (14.4) | 0.57 | 1.15 (0.76 - 1.73) |
| End-stage renal failure | 8 (4.5) | 84 (5) | 0.82 | 0.86 (0.41 - 1.79) |
| AIDS | 16 (7.3) | 89 (4.5) | 0.09 | 1.68 (0.97 - 2.92) |
| Cancer | 79 (36.2) | 703 (35.5) | 0.89 | 1.03 (0.77 - 1.38) |
| Peripheral vascular disease | 30 (13.8) | 227 (11.5) | 0.37 | 1.23 (0.82 - 1.85) |
| Cirrhosis | 2 (1.6) | 34 (3.5) | 0.54 | 0.53 (0.12 - 2.22) |
| Neuromuscular disease | 8 (6.6) | 37 (3.8) | 0.12 | 1.99 (0.92 - 4.35) |
| Number of preexisting diseases | ||||
| None | 65 (29.8) | 712 (26) | 0.08 | 0.75 (0.56 - 1.02) |
| One | 109 (50) | 931 (47) | 0.45 | 1.12 (0.85 - 1.49) |
| Two | 39 (17.9) | 288 (14.6) | 0.22 | 1.28 (0.88 - 1.85) |
| More than two | 5 (2.3) | 48 (2.4) | 0.91 | 0.94 (0.37 - 2.39) |
| Charlson index | 1.67 ± 1.84 | 1.45 ± 1.66 | 0.07 | |
| McCabe score | ||||
| 3 | 13 (8.6) | 88 (6.1) | 0.40 | 1.36 (0.75 - 2.48) |
| 2 | 26 (17.1) | 172 (12) | 0.15 | 1.42 (0.92 - 2.20) |
| 1 | 113 (74.3) | 1168 (81.6) | 0.06 | 0.76 (0.57 - 1.00) |
| Reason for ICU admission | ||||
| Surgical* | 41 (18.8) | 582 (29.4) | 0.001 | 0.54 (0.38 - 0.77) |
| Medical | 177 (81.2) | 1397 (70.6) | 0.001 | 1.80 (1.26 - 2.56) |
| Respiratory | 90 (41.3) | 547 (27.6) | ns** | |
| Neurological | 39 (17.8) | 321 (16.2) | ns** | |
| Cardiovascular | 10 (4.6) | 212 (10.7) | ns** | |
| Trauma | 24 (11.1) | 162 (7.7) | ns** | |
| Gastrointestinal | 10 (4.6) | 114 (5.8) | ns** | |
| Renal | 4 (1.8) | 41 (2.1) | ns** |
PMV - prolonged mechanical ventilation; OR - odds ratio; 95% CI - 95% confidence interval; BMI - body mass index; ICU - intensive care unit; COPD - chronic obstructive pulmonary disease; AIDS - acquired immune deficiency syndrome; ns - not significant. The results are expressed as number (percentage) or mean ± standard deviation. Data obtained by univariate analysis.
Not ontly at admission;
comparisons within medical group (chi square with residue analysis).
Table 2.
Characteristics and main outcomes of the study patients during admission to the intensive care unit
| Characteristic | PMV | Non-PMV | p value | OR (95% CI) |
|---|---|---|---|---|
| (N = 218) | (N = 1,979) | |||
| Severity at ICU: day 1 | ||||
| APACHE II*,** | 21.4 ± 7.2 | 19.4 ± 8.3 | 0.001 | |
| GCS | 12.0 ± 4.0 | 11.5 ± 4.5 | 0.12 | |
| SOFA | 5.3 ± 3.7 | 5.1 ± 3.5 | 0.42 | |
| TISS | 26.5 ± 7.4 | 26.1 ± 7.8 | 0.47 | |
| Presence of infection | 134 (61.4) | 1004 (50.7) | < 0.001 | 1.94 (1.40 - 2.69) |
| Diagnosis of severe sepsis | 48 (22.0) | 354 (17.9) | 0.01 | 1.54 (1.07 - 2.21) |
| During ICU stay | ||||
| Organ support during ICU stay | ||||
| Vasoactive drug use | 183 (83.9) | 1332 (67.3) | < 0.001 | 2.54 (1.74 - 3.69) |
| RRT | 54 (24.8) | 235 (11.9) | < 0.001 | 2.44 (1.74 - 3.42) |
| Parenteral nutrition support | 27 (12.4) | 131 (6.7) | 0.002 | 1.98 (1.27 - 3.07) |
| Tracheotomy need | 151 (69.2) | 168 (8.5) | < 0.001 | 24.3 (17.4 - 33.7) |
| Need for unscheduled surgery | 62 (28.4) | 291 (14.7) | < 0.001 | 2.3 (1.67 - 3.17) |
| DNR decision | 15 (6.9) | 138 (7.0) | 0.93 | 0.98 (0.56 - 1.71) |
| At ICU discharge | ||||
| Severity at ICU discharge | ||||
| GCS | 13.5 ± 2.52 | 14.2 ± 1.79 | 0.01 | |
| SOFA | 3.01 ± 4.03 | 2.64 ± 4.18 | 0.21 | |
| TISS | 10.8 ± 11.07 | 10.6 ± 10.12 | 0.78 | |
| ICU death | 113 (51.8) | 744 (37.6) | < 0.001 | 1.78 (1.35 - 2.36) |
| Hospital death | 142 (65) | 911 (44.9) | < 0.001 | 2.23 (1.67 - 2.99) |
PMV - prolonged mechanical ventilation; OR - odds ratio; 95% CI - 95% confidence interval; APACHE - Acute Physiology and Chronic Health Evaluation; GCS - Glasgow Coma Score; SOFA - Sepsis-related Organ Failure Assessment; TISS - Therapeutic Intervention Scoring System; ICU - intensive care unit; RRT - renal replacement therapy; DNR - do not resuscitate. Results are expressed as number (percentage) or mean ± standard deviation. Data obtained by univariate analysis;
only in patients without sedation;
score applied and calculated in neurological and no neurological patients.
Comparison of PMV and non-PMV groups: at ICU admission
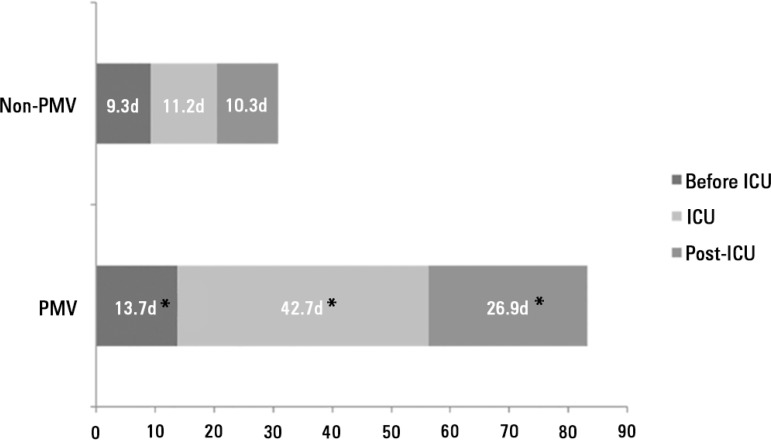
PMV patients were more likely to have ICU admissions for respiratory (41.3% versus 27.6%, p < 0.001) and infection (61.4% versus 50.7%, p < 0.001) causes (Table 1) and more prolonged hospital LOS before ICU admission (13.7 ± 44.3 days versus 9.3 ± 21.6 days, p = 0.01) (Figure 1). No differences were found in age, BMI, preexisting chronic diseases and severity of underlying medical conditions.
Figure 1.
Mean of length of stay in the intensive care unit, in the hospital before intensive care unit, and in the hospital post-intensive care unit stay of non-prolonged mechanical ventilation and prolonged mechanical ventilation patients.
d - days; PMV - prolonged mechanical ventilation; ICU - intensive care unit. * p < 0.001 compared to non-PMV.
Comparison of PMV and non-PMV groups: during ICU stay and at ICU discharge
PMV patients had higher APACHE II scores with 24 hours of ICU admission. PMV patients were more likely to receive organ support (vasoactive therapy [83.9% versus 67.3%, p < 0.001], RRT [24.8% versus 11.9%, p < 0.001] and parenteral nutrition [12.4% versus 6.7%, p < 0.001]). These patients underwent more unscheduled surgery (28.4 versus 14.7%, p < 0.001) and tracheotomy (69.2% versus 8.5%, p < 0.001) (Table 2).
Several complications developed during ICU stay, such as pressure ulcers (OR 9.18, 95% CI 6.64 - 12.7), muscle weakness (OR 6.99, 95% CI 4.94-9.82), infection (bacterial sepsis (OR 5.18, 95% CI 3.75 - 7.15), candidemia (OR 3.82, 95% CI 1.31 - 11.09) and pulmonary embolism (OR 3.71, 95% CI 1.43 - 9.63), and these complications were associated with a significantly higher risk of PMV (Table 3).
Table 3.
Complications during intensive care unit stay
| Complications | PMV | Non-PMV | p value | OR (95% CI) |
|---|---|---|---|---|
| (N = 218) | (N = 1979) | |||
| Myocardial infarction | 5 (2.6) | 41 (2.3) | 0.97 | 1.1 (0.43 - 2.83) |
| Cerebrovascular disease | 3 (2) | 37 (2.6) | 0.73 | 0.73 (0.22 - 2.39) |
| Pulmonary embolism | 6 (5.4) | 17 (1.5) | 0.004 | 3.71 (1.43 - 9.63) |
| Muscle weakness | 71 (37.6) | 144 (7.9) | < 0.001 | 6.99 (4.97 - 9.82) |
| ARDS | 37 (17.1) | 124 (6.3) | < 0.001 | 3.07 (2.06 - 4.57) |
| Bacterial nosocomial sepsis | 129 (68.3) | 533 (29.3) | < 0.001 | 5.18 (3.75 - 7.15) |
| Candidemia | 5 (5.4) | 12 (1.5) | 0.008 | 3.82 (1.31 - 11.09) |
| Upper gastrointestinal bleeding | 19 (10.1) | 59 (3.2) | < 0.001 | 3.33 (1.94 - 5.71) |
| Hyperactive delirium | 27 (22.3) | 147 (15) | 0.03 | 1.62 (1.02 - 2.57) |
| Pressure ulcer | 86 (39.4) | 131 (6.6) | < 0.001 | 9.18 (6.64 - 12.7) |
PMV - prolonged mechanical ventilation; OR - odds ratio; 95% CI - 95% confidence interval; ARDS - acute respiratory distress syndrome. The results are expressed as number (percentage) or mean ± standard deviation. Data obtained by univariate analysis.
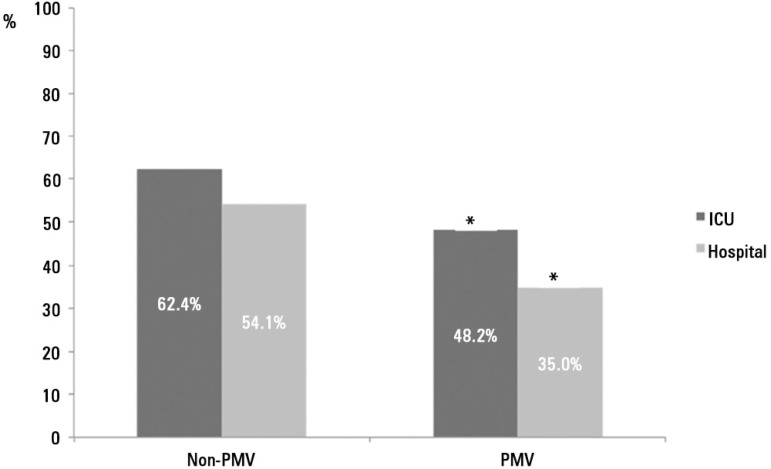
PMV patients spent more days in the ICU (42.7 ± 25.9 versus 11.2 ± 15.3, p < 0.001) and hospital after ICU discharge (26.9 ± 29.3 versus 10.3 ± 20.4, p < 0.001) than non-PMV patients (Figure 1). Prolonged MV patients had a significant increase in ICU mortality (absolute difference = 14.2%, 95% CI 1.78 (1.35 - 2.36), p < 0.001) and hospital mortality (absolute difference = 19.1%, 95% CI 2.23 (1.67 - 2.99), p < 0.001) (Table 2 and Figure 2).
Figure 2.
Intensive care unit and hospitalization of non-prolonged mechanical ventilation and prolonged mechanical ventilation patients.
PMV - prolonged mechanical ventilation; ICU - intensive care unit.* p < 0.001 compared to non-prolonged mechanical ventilation.
Patterns of discharge from the ICU were worse in the PMV group, based on GCS evaluation (13.5 ± 2.52 versus 14.2 ± 1.79, p = 0.01).
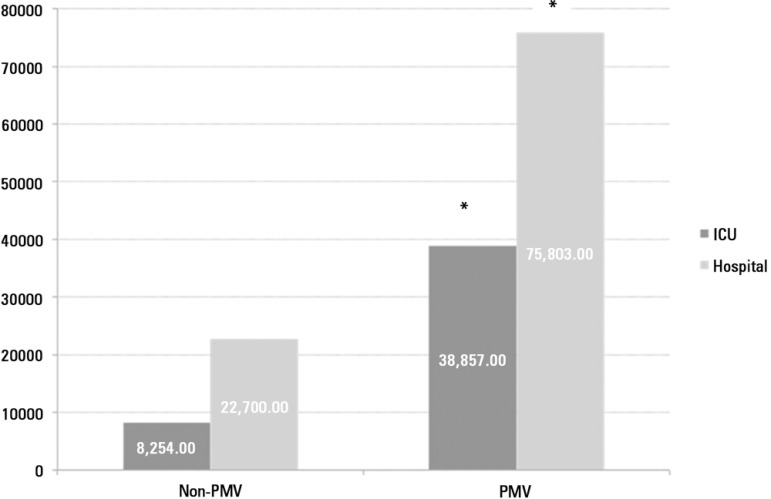
The average daily cost per capita was US$737.00 for non-PMV and US$910.00 for PMV patients. The length of stay was greater in the PMV group, and the hospital cost was 3.3 times higher in PMV patients than non-PMV patients. Figure 3 shows the average daily cost in the ICU and hospital stay between PMV and non-PMV patients.
Figure 3.
The average cost in intensive care unit and hospital stay comparing prolonged mechanical ventilation and non-prolonged mechanical ventilation patients.
PMV - prolonged mechanical ventilation; ICU - intensive care unit. * p < 0.001 compared to non-prolonged mechanical ventilation.
DISCUSSION
This analysis of a large, multicenter and retrospective cohort of MV patients found that patients who required PMV (MV support ≥ 21 days) had higher risk for complications during ICU stays, higher death rates during ICU and hospital LOS, and higher illness costs.
Between 3 to 10% of patients who require MV for acute conditions develop chronic critical illness.(3,13-16) Generalization of outcomes from published reports is complicated by variations in the study populations that are described as CCI, definitions of outcomes of interest, and post-acute care practices that affect hospital use. A threshold period of MV, ranging from 2 to 30 days, was used to define the majority of cohorts in longitudinal studies.(8,18,19) Martin et al.(7) evaluated patients who required ≥ 10 days of MV. Combes et al.(20) studied the outcome and health-related quality of life of patients who required ≥ 14 days of MV. Carson et al.(21) and Cox et al.(22) evaluated the long-term prognosis of patients who required ≥ 21 days of MV. A consensus conference established a formal definition for PMV to limit the heterogeneity of these cohorts and improve the comparability of outcomes across different studies. PMV is defined as at least 21 consecutive days on a ventilator for more than 6 hours/day.(4) Our data demonstrated a 9.92% incidence of CCI using this criterion, and this incidence varied according to patient characteristics in each ICU study (ICU-1 = 8.8%; ICU-2 = 12%; ICU-3 = 9%; ICU-4 = 15%). However, other authors identified chronically critically ill patients by elective placement of a tracheotomy to facilitate prolonged ventilation and weaning efforts.(14,22-25) Referral for tracheotomy reflects a clinician’s judgment that the patient will neither wean nor die in the immediate future, which provides a point of demarcation between acute and chronic critical illness that is clinically meaningful and practical.(26,27) Cox et al.(22) revealed that these two suggested definitions for PMV, Diagnosis-Related Groups (DRGs) 541/542 (Medicare’s definition: tracheotomy and MV ≥ 96 hours) and MV ≥ 21 days, select cohorts with similar baseline clinical characteristics and trends in survival, disposition and resource utilization. However, PMV defined by ventilation for ≥ 21 days can more specifically identify patients who are outliers in resource consumption among ventilated patients, which is why we analyzed patients based on this definition.(3,4)
Physicians and scoring systems are inaccurate for the predicting of morbidity and mortality at the time of ICU admission.(28) However, it is important to identify patients with a high risk of becoming chronic early enough to manage these patients. Preexisting chronic diseases and the severity of underlying medical conditions were not associated with CCI, age, BMI and the source of admitted patients in our study. Notably, the operating room as the source of admission seemed protective in our study. However, longer hospital stay before ICU admission and non-surgical admission (OR 1.8 (1.26 - 2.56), p < 0.001) were associated with the evolution to CCI. The admission of patients for nonsurgical reasons was identified previously as a factor that is associated with the evolution to chronic critical illness.(3,23) Severity scores are considered predictors of prolonged MV.(3,17,23,29) Our data at admission are not consistent with this assumption. However, multi-organ involvement is also related with mortality. Recently, Carson et al.(21) developed the ProVent score (need for a vasopressor and hemodialysis, presence of thrombocytopenia, and age ≥ 50 years), which showed good discriminatory power to predict mortality in PMV patients.
Regardless of PMV definition, the main characteristics of this population are repeated episodes of shock and infection during ICU stays.(6) This condition is associated with persistent inflammation, immunosuppression and catabolism syndrome or persistent inflammation and catabolism syndrome (PICS).(30) Therefore, CCI cannot be viewed as a simple extension of an acute critical illness, but rather as a complex syndrome that is characterized by metabolic, neuroendocrine, neuropsychiatric and immunological changes.(3,31) These changes include profound weakness attributed to myopathy, neuropathy, and alterations in body composition (loss of lean body mass, increased adiposity, and anasarca),(32) increased vulnerability to infection, often with multi-resistant microbial organisms,(33,34) brain dysfunction (coma or delirium),(35) neuroendocrine changes (loss of pulsatile secretion of anterior pituitary hormones, which contributes to low target organ hormone levels and impaired anabolism)(36-38) and skin breakdown (pressure ulcers).(39,40) Some of these conditions were evaluated and confirmed in our study (Tables 2 and 3).
Some previous authors found higher mortality in PMV patients,(7,17,20,22,23,40,41) but other authors did not observe this effect.(6,22) The reason for these discrepancies is likely related to the heterogeneity and intrinsic characteristics of the study populations. However, most studies show that CCI and PMV patients have worse disease courses and higher mortality. The ICU and hospital mortality in PMV patients was 14.2% and 19.1%, respectively, in our study, which was absolutely higher than non-PMV patients.
Critical ill survivors have an ongoing morbidity, and actual evidence suggests that quality of life after intensive care admission is generally poor compared to population data.(42-47) These morbidities include a high incidence of psychological problems,(47-50) cognitive dysfunction,(51) impairments in pulmonary function,(47,52,53) and development of neuromuscular complications of critical illness,(54) which may be long-lasting despite slow improvement over time. These problems are more prevalent in chronic critical patients, and the need for PMV may also affect the patient’s prognosis for the ability to perform activities of daily living.(20) Some authors report that 5% to 20% of ICU patients receive MV, and 25% of these patients require MV for more than seven days.(55) Nearly all patients with CCI leave the hospital with profound impairments in physical function, cognitive status, or both. Therefore, most of these patients require institutional care.(56,57) Hospital readmission rates during the year after hospital discharge exceed 40% in these subjects. Patients who are discharged to extended care facilities and cannot be sufficiently rehabilitated to return home within 6 months usually remain institutionalized until death.(3,56,57) Multiple studies demonstrated that fewer than 12% of CCI patients were alive and independent one year after their acute illness.(58) The prolonged need for ventilatory support reduces life quality and life expectancy in the long term.(20,58,59) Functional limitations are common even in patients who recover sufficiently to permit discharge from an inpatient facility, but these patients typically require paid care giving as outpatients, or family members must leave their jobs to provide ongoing care. Indeed, some patients may benefit from these efforts, but other patients may not.(24) In this scenario, the identification of the predictors of a poor prognosis might help doctors choose more aggressive treatments or treatments that prioritize comfort.(3,4,41)
CCI is growing, and it is becoming a serious problem for the health care system in many countries.(3) CCI accounts for fewer than 10% of patients who receive MV, but these patients consume 20 to 40% of ICU bed-days and other critical care resources.(16,27) PMV patients represented 10% of all ICU admissions and 71% of the total cost of all ICU admissions during our study period. Our results indicate that the PMV patients imposed a cost of more than three times non-PMV patients. The overall cost to the health care system for the management of CCI already exceeds an estimated $20 billion annually, and this number is expected to climb with increases in the incidence of this syndrome and overall expenditures for critical care. These expenditures nearly doubled between 1985 and 2000 and represented 13% of all hospital costs in the United States.(2,60)
This study has a few limitations. This study was an observational study without long-term outcomes, such as evaluations of quality of life and cognitive impairment. This was a retrospective study, and it was not possible to describe and compare the different mechanical ventilation strategies (including ventilatory mode and flow/pressure adjustments) in patients who developed acute respiratory distress syndrome. All PMV patients were a group with more than 21 days on MV, whereas non-PMV patients were a heterogeneous group with variation in the total number of MV days until 20 MV days. However, the study provides an important contribution to CCI with a large patient sample that was obtained from various centers and the utilization of a uniform definition.
CONCLUSION
Patients in our study with prolonged mechanical ventilation constituted a distinct group of patients, who were sicker on admission and exhibited higher mortality, longer hospital stays and higher costs. Patients in respiratory failure at admission who experience a period of pre-intensive care unit hospitalization longer than 12 days, are non-surgical and have sepsis exhibit a higher risk to become dependent on prolonged mechanical ventilation support.
Footnotes
Conflicts of interest: None.
Responsible editor: Carmen Valente Barbas
REFERENCES
- 1.Girard K, Raffin TA. The chronically critically ill: to save or let die? Respir Care. 1985;30(5):339–347. [PubMed] [Google Scholar]
- 2.Halpern NA, Pastores SM, Greenstein RJ. Critical care medicine in the United States 1985-2000: an analysis of bed numbers, use, and costs. Crit Care Med. 2004;32(6):1254–1259. doi: 10.1097/01.ccm.0000128577.31689.4c. [DOI] [PubMed] [Google Scholar]
- 3.Nelson JE, Cox CE, Hope AA, Carson SS. Chronic critical illness. Am J Respir Crit Care Med. 2010;182(4):446–454. doi: 10.1164/rccm.201002-0210CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacIntyre NR, Epstein SK, Carson S, Scheinhorm D, Christopher K, Muldoon S, National Association for Medical Direction of Respiratory Care Management of patients requiring prolonged mechanical ventilation: report of a NAMDRC consensus conference. Chest. 2005;128(6):3937–3954. doi: 10.1378/chest.128.6.3937. [DOI] [PubMed] [Google Scholar]
- 5.Zilberberg MD, de Wit M, Pirone JR, Shorr AF. Growth in adult prolonged acute mechanical ventilation: implications for healthcare delivery. Crit Care Med. 2008;36(5):1451–1455. doi: 10.1097/CCM.0b013e3181691a49. [DOI] [PubMed] [Google Scholar]
- 6.Estenssoro E, Reina R, Canales HS, Saenz MG, Gonzalez FE, Aprea MM, et al. The distinct clinical profile of chronically critically ill patients: a cohort study. Crit Care. 2006;10(3):R89. doi: 10.1186/cc4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin CM, Hill AD, Burns K, Chen LM. Characteristics and outcomes for critically ill patients with prolonged intensive care unit stays. Crit Care Med. 2005;33(9):1922–1927. doi: 10.1097/01.ccm.0000178184.97813.52. quiz 1936. [DOI] [PubMed] [Google Scholar]
- 8.Daly BJ, Douglas SL, Kelley CG, O’Toole E, Montenegro H. Trial of a disease management program to reduce hospital readmissions of the chronically critically ill. Chest. 2005;128(2):507–517. doi: 10.1378/chest.128.2.507. [DOI] [PubMed] [Google Scholar]
- 9.Robson V, Poynter J, Lawler PG, Baudouin SV. The need for a regional weaning centre, a one-year survey of intensive care weaning delay in the Northern Region of England. Anaesthesia. 2003;58(2):161–165. doi: 10.1046/j.1365-2044.2003.02964_1.x. [DOI] [PubMed] [Google Scholar]
- 10.Zilberberg MD, Luippold RS, Sulsky S, Shorr AF. Prolonged acute mechanical ventilation, hospital resource utilization, and mortality in the United States. Crit Care Med. 2008;36(3):724–730. doi: 10.1097/CCM.0B013E31816536F7. [DOI] [PubMed] [Google Scholar]
- 11.Kahn JM, Benson NM, Appleby D, Carson SS, Iwashyna TJ. Long-term acute care hospital utilization after critical illness. JAMA. 2010;303(22):2253–2259. doi: 10.1001/jama.2010.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mauri T, Pivi S, Bigatello LM. Prolonged mechanical ventilation after critical illness. Minerva Anestesiol. 2008;74(6):297–301. [PubMed] [Google Scholar]
- 13.Gracey DR, Viggiano RW, Naessens JM, Hubmayr RD, Silverstein MD, Koenig GE. Outcomes of patients admitted to a chronic ventilator-dependent unit in an acute-care hospital. Mayo Clin Proc. 1992;67(2):131–136. doi: 10.1016/s0025-6196(12)61313-5. [DOI] [PubMed] [Google Scholar]
- 14.Engoren M, Arslanian-Engoren C, Fenn-Buderer N. Hospital and long-term outcome after tracheostomy for respiratory failure. Chest. 2004;125(1):220–227. doi: 10.1378/chest.125.1.220. [DOI] [PubMed] [Google Scholar]
- 15.Seneff MG, Zimmerman JE, Knaus WA, Wagner DP, Draper EA. Predicting the duration of mechanical ventilation. The importance of disease and patient characteristics. Chest. 1996;110(2):469–479. doi: 10.1378/chest.110.2.469. [DOI] [PubMed] [Google Scholar]
- 16.Wagner DP. Economics of prolonged mechanical ventilation. Pt 2Am Rev Respir Dis. 1989;140(2):S14–S18. doi: 10.1164/ajrccm/140.2_Pt_2.S14. [DOI] [PubMed] [Google Scholar]
- 17.Loss SH, Marchese CB, Boniatti MM, Wawrzeniak IC, Oliveira RP, Nunes LN, et al. Prediction of chronic critical illness in a general intensive care unit. Rev Assoc Med Bras. 2013;59(3):241–247. doi: 10.1016/j.ramb.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Chelluri L, Im KA, Belle SH, Schulz R, Rotondi AJ, Donahoe MP, et al. Long-term mortality and quality of life after prolonged mechanical ventilation. Crit Care Med. 2004;32(1):61–69. doi: 10.1097/01.CCM.0000098029.65347.F9. [DOI] [PubMed] [Google Scholar]
- 19.Gracey DR, Naessens JM, Krishan I, Marsh HM. Hospital and posthospital survival in patients mechanically ventilated for more than 29 days. Chest. 1992;101(1):211–214. doi: 10.1378/chest.101.1.211. [DOI] [PubMed] [Google Scholar]
- 20.Combes A, Costa MA, Trouillet JL, Baudot J, Mokhtari M, Gibert C, et al. Morbidity, mortality, and quality-of-life outcomes of patients requiring >or=14 days of mechanical ventilation. Crit Care Med. 2003;31(5):1373–1381. doi: 10.1097/01.CCM.0000065188.87029.C3. [DOI] [PubMed] [Google Scholar]
- 21.Carson SS, Garret J, Hanson LC, Lanier J, Govert J, Brake MC, et al. A prognostic model for one-year mortality in patients requiring prolonged mechanical ventilation. Crit Care Med. 2008;36(7):2061–2069. doi: 10.1097/CCM.0b013e31817b8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox CE, Carson SS, Lindquist JH, Olsen MK, Govert JA, Chelluri L, Quality of Life After Mechanical Ventilation in the Aged (QOL-MV) Investigators Differences in one-year health outcomes and resource utilization by definition of prolonged mechanical ventilation: a prospective cohort study. Crit Care. 2007;11(1):R9. doi: 10.1186/cc5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boniatti MM, Friedman G, Castilho RK, Vieira SR, Fialkow L. Characteristics of chronically critically ill patients: comparing two definitions. Clinics (Sao Paulo) 2011;66(4):701–704. doi: 10.1590/S1807-59322011000400027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carson SS, Bach PB, Brzozowski L, Leff A. Outcomes after long-term acute care. An analysis of 133 mechanically ventilated patients. Pt 1Am J Respir Crit Care Med. 1999;159(5):1568–1573. doi: 10.1164/ajrccm.159.5.9809002. [DOI] [PubMed] [Google Scholar]
- 25.Engoren M, Arslanian-Engoren C. Hospital and long-term outcome of trauma patients with tracheostomy for respiratory failure. Am Surg. 2005;71(2):123–127. [PubMed] [Google Scholar]
- 26.Kahn JM, Carson SS, Angus DC, Linde-Zwirble WT, Iwashyna TJ. Development and validation of an algorithm for identifying prolonged mechanical ventilation in administrative data. Health Serv Outcomes Res Method. 2009;9:117–132. [Google Scholar]
- 27.Cox CE, Carson SS, Holmes GM, Howard A, Carey TS. Increase in tracheostomy for prolonged mechanical ventilation in North Carolina, 1993-2002. Crit Care Med. 2004;32(11):2219–2226. doi: 10.1097/01.ccm.0000145232.46143.40. [DOI] [PubMed] [Google Scholar]
- 28.Sinuff T, Adhikari NK, Cook DJ, Schünemann HJ, Griffith LE, Rocker G, et al. Mortality predictions in the intensive care unit: comparing physicians with scoring systems. Crit Care Med. 2006;34(3):878–885. doi: 10.1097/01.CCM.0000201881.58644.41. [DOI] [PubMed] [Google Scholar]
- 29.Honarmand A, Safavi M, Moradi D. The use of infection probability score and sequential organ failure assessment scoring systems in predicting mechanical ventilation requirement and duration. Ulus Travma Acil Cerrahi Derg. 2009;15(5):440–447. [PubMed] [Google Scholar]
- 30.Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72(6):1491–1501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nierman DM. A structure of care for the chronically critically ill. Crit Care Clin. 2002;18(3):477-91, v. doi: 10.1016/s0749-0704(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 32.Hollander JM, Mechanick JI. Nutrition support and the chronic critical illness syndrome. Nutr Clin Pract. 2006;21(6):587–604. doi: 10.1177/0115426506021006587. [DOI] [PubMed] [Google Scholar]
- 33.Kalb TH, Lorin S. Infection in the chronically critically ill: unique risk profile in a newly defined population. Crit Care Clin. 2002;18(3):529–552. doi: 10.1016/s0749-0704(02)00009-x. [DOI] [PubMed] [Google Scholar]
- 34.Scheinhorn DJ, Hassenpflug MS, Votto JJ, Chao DC, Epstein SK, Doig GS, Knight EB, Petrak RA, Ventilation Outcomes Study Group Post-ICU mechanical ventilation at 23 long-term care hospitals: a multicenter outcomes study. Chest. 2007;131(1):85–93. doi: 10.1378/chest.06-1081. [DOI] [PubMed] [Google Scholar]
- 35.Nelson JE, Tandon N, Mercado AF, Camhi SL, Ely EW, Morrison RS. Brain Dysfunction: another burden for the chronically critically ill. Arch Intern Med. 2006;166(18):1993–1999. doi: 10.1001/archinte.166.18.1993. [DOI] [PubMed] [Google Scholar]
- 36.Van den Berghe G, de Zegher F, Veldhuis JD, Wouters P, Awouters M, Verbruggen W, et al. The somatotropic axis in critical illness: effect of continuous growth hormone (GH)-releasing hormone and GH-releasing peptide-2 infusion. J Clin Endocrinol Metab. 1997;82(2):590–599. doi: 10.1210/jcem.82.2.3736. [DOI] [PubMed] [Google Scholar]
- 37.Van den Berghe G, de Zegher F, Veldhuis JD, Wouters P, Gouwy S, Stockman W, et al. Thyrotrophin and prolactin release in prolonged critical illness: dynamics of spontaneous secretion and effects of growth hormone-secretagogues. Clin Endocrinol (Oxf) 1997;47(5):599–612. doi: 10.1046/j.1365-2265.1997.3371118.x. [DOI] [PubMed] [Google Scholar]
- 38.Van den Berghe G. Growth hormone secretagogues in critical illness. Horm Res. 1999;51(Suppl 3):21–28. doi: 10.1159/000053158. Review. [DOI] [PubMed] [Google Scholar]
- 39.Carasa M, Polycarpe M. Caring for the chronically critically ill patient: establishing a wound- healing program in a respiratory care unit. Am J Surg. 2004;188(1A) Suppl:18–21. doi: 10.1016/S0002-9610(03)00286-1. [DOI] [PubMed] [Google Scholar]
- 40.Brem H, Nierman DM, Nelson JE. Pressure ulcers in the chronically critically ill patient. Crit Care Clin. 2002;18(3):683–694. doi: 10.1016/s0749-0704(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 41.Heyland DK, Konopad E, Noseworthy TW, Johnston R, Gafni A. Is it ‘worthwhile’ to continue treating patients with a prolonged stay (>14 days) in the ICU? An economic evaluation. Chest. 1998;114(1):192–198. doi: 10.1378/chest.114.1.192. [DOI] [PubMed] [Google Scholar]
- 42.Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med. 2011;39(2):371–379. doi: 10.1097/CCM.0b013e3181fd66e5. [DOI] [PubMed] [Google Scholar]
- 43.Fildissis G, Zidianakis V, Tsigou E, Koulenti D, Katostaras T, Economou A, et al. Quality of life outcome of critical care survivors eighteen months after discharge from intensive care. Croat Med J. 2007;48(6):814–821. doi: 10.3325/cmj.2007.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cuthbertson BH, Hull A, Strachan M, Scott J. Post-traumatic stress disorder after critical illness requiring general intensive care. Intensive Care Med. 2004;30(3):450–455. doi: 10.1007/s00134-003-2004-8. [DOI] [PubMed] [Google Scholar]
- 45.Cuthbertson BH, Roughton S, Jenkinson D, Maclennan G, Vale L. Quality of life in the five years after intensive care: a cohort study. Crit Care. 2010;14(1):R6. doi: 10.1186/cc8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oeyen SG, Vandijck DM, Benoit DD, Annemans L, Decruyenaere JM. Quality of life after intensive care: a systematic review of the literature. Crit Care Med. 2010;38(12):2386–2400. doi: 10.1097/CCM.0b013e3181f3dec5. [DOI] [PubMed] [Google Scholar]
- 47.Dowdy DW, Eid MP, Sedrakyan A, Mendez-Tellez PA, Pronovost PJ, Herridge MS, et al. Quality of life in adult survivors of critical illness: a systematic review of the literature. Intensive Care Med. 2005;31(5):611–620. doi: 10.1007/s00134-005-2592-6. [DOI] [PubMed] [Google Scholar]
- 48.Azoulay E, Pochard F, Kentish-Barnes N, Chevret S, Aboab J, Adrie C, Annane D, Bleichner G, Bollaert PE, Darmon M, Fassier T, Galliot R, Garrouste-Orgeas M, Goulenok C, Goldgran-Toledano D, Hayon J, Jourdain M, Kaidomar M, Laplace C, Larché J, Liotier J, Papazian L, Poisson C, Reignier J, Saidi F, Schlemmer B, FAMIREA Study Group Risk of post-traumatic stress symptoms in family members of intensive care unit patients. Am J Respir Crit Care Med. 2005;171(9):987–994. doi: 10.1164/rccm.200409-1295OC. [DOI] [PubMed] [Google Scholar]
- 49.Flaatten H. Mental and physical disorders after ICU discharge. Curr Opin Crit Care. 2010;16(5):510–515. doi: 10.1097/MCC.0b013e32833cc90b. [DOI] [PubMed] [Google Scholar]
- 50.de Miranda S, Pochard F, Chaize M, Megarbane B, Cuvelier A, Bele N, et al. Postintensive care unit psychological burden in patients with chronic obstructive pulmonary disease and informal caregivers: A multicenter study. Crit Care Med. 2011;39(1):112–118. doi: 10.1097/CCM.0b013e3181feb824. [DOI] [PubMed] [Google Scholar]
- 51.Hopkins RO, Jackson JC. Long-term neurocognitive function after critical illness. Chest. 2006;130(3):869–878. doi: 10.1378/chest.130.3.869. Review. [DOI] [PubMed] [Google Scholar]
- 52.Quinnell TG, Pilsworth S, Shneerson JM, Smith IE. Prolonged invasive ventilation following acute ventilatory failure in COPD: weaning results, survival, and the role of noninvasive ventilation. Chest. 2006;129(1):133–139. doi: 10.1378/chest.129.1.133. [DOI] [PubMed] [Google Scholar]
- 53.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS, Canadian Critical Care Trials Group One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 54.Hough CL. Neuromuscular sequelae in survivors of acute lung injury. Clin Chest Med. 2006;27(4):691–703. doi: 10.1016/j.ccm.2006.07.002. abstract x. [DOI] [PubMed] [Google Scholar]
- 55.Esteban A, Anzueto A, Frutos F, Alía I, Brochard L, Stewart TE, Benito S, Epstein SK, Apezteguía C, Nightingale P, Arroliga AC, Tobin MJ, MechanicalVentilation International Study Group Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002;287(3):345–355. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 56.Rimachi R, Vincent JL, Brimioulle S. Survival and quality of life after prolonged intensive care unit stay. Anaesth Intensive Care. 2007;35(1):62–67. doi: 10.1177/0310057X0703500108. [DOI] [PubMed] [Google Scholar]
- 57.Euteneuer S, Windisch W, Suchi S, Köhler D, Jones PW, Schönhofer B. Health-related quality of life in patients with chronic respiratory failure after long-term mechanical ventilation. Respir Med. 2006;100(3):477–486. doi: 10.1016/j.rmed.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 58.Lipsett PA, Swoboda SM, Dickerson J, Ylitalo M, Gordon T, Breslow M, et al. Survival and functional outcome after prolonged intensive care unit stay. Ann Surg. 2000;231(2):262–268. doi: 10.1097/00000658-200002000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tonnelier A, Tonnelier JM, Nowak E, Gut-Gobert C, Prat G, Renault A, et al. Clinical relevance of classification according to weaning difficulty. Respir Care. 2011;56(5):583–590. doi: 10.4187/respcare.00842. [DOI] [PubMed] [Google Scholar]
- 60.Cox CE, Carson SS, Govert JA, Chelluri L, Sanders GD. An economic evaluation of prolonged mechanical ventilation. Crit Care Med. 2007;35(8):1918–1927. doi: 10.1097/01.CCM.0000275391.35834.10. [DOI] [PMC free article] [PubMed] [Google Scholar]