Abstract
Objective
To evaluate the immediate effects of red blood cell transfusion on central venous oxygen saturation and lactate levels in septic shock patients with different transfusion triggers.
Methods
We included patients with a diagnosis of septic shock within the last 48 hours and hemoglobin levels below 9.0g/dL Patients were randomized for immediate transfusion with hemoglobin concentrations maintained above 9.0g/dL (Group Hb9) or to withhold transfusion unless hemoglobin felt bellow 7.0g/dL (Group Hb7). Hemoglobin, lactate, central venous oxygen saturation levels were determined before and one hour after each transfusion.
Results
We included 46 patients and 74 transfusions. Patients in Group Hb7 had a significant reduction in median lactate from 2.44 (2.00 - 3.22) mMol/L to 2.21 (1.80 - 2.79) mMol/L, p = 0.005, which was not observed in Group Hb9 [1.90 (1.80 - 2.65) mMol/L to 2.00 (1.70 - 2.41) mMol/L, p = 0.23]. Central venous oxygen saturation levels increased in Group Hb7 [68.0 (64.0 - 72.0)% to 72.0 (69.0 - 75.0)%, p < 0.0001] but not in Group Hb9 [72.0 (69.0 - 74.0)% to 72.0 (71.0 - 73.0)%, p = 0.98]. Patients with elevated lactate or central venous oxygen saturation < 70% at baseline had a significant increase in these variables, regardless of baseline hemoglobin levels. Patients with normal values did not show a decrease in either group.
Conclusion
Red blood cell transfusion increased central venous oxygen saturation and decreased lactate levels in patients with hypoperfusion regardless of their baseline hemoglobin levels. Transfusion did not appear to impair these variables in patients without hypoperfusion.
Keywords: Erythrocyte transfusion; Ischemia; Shock, septic; Sepsis; Oxygenation
Abstract
Objetivo
Avaliar os efeitos imediatos da transfusão de hemácias nos níveis de saturação venosa central de oxigênio e de lactato em pacientes com choque séptico usando diferentes níveis gatilho de hemoglobina para indicar transfusão.
Métodos
Incluímos pacientes com diagnóstico de choque séptico nas últimas 48 horas e níveis de hemoglobina abaixo de 9,0g/dL. Os pacientes foram randomizados para receber imediatamente transfusão se as concentrações se mantivessem acima de 9,0g/dL (Grupo Hb9) ou adiar a transfusão até que a hemoglobina caísse abaixo de 7,0g/dL (Grupo Hb7). Os níveis de hemoglobina, lactato e saturação venosa central de oxigênio foram determinados antes e 1 hora após cada transfusão.
Resultados
Incluímos 46 pacientes, totalizando 74 transfusões. Os pacientes do Grupo Hb7 tiveram uma redução significante nos níveis medianos de lactato de 2,44 (2,00 - 3,22) mMol/L para 2,21 (1,80 - 2,79) mMol/L; p = 0,005. Isto não foi observado no Grupo Hb9 [1,90 (1,80 - 2,65) mMol/L para 2,00 (1,70 - 2,41) mMol/L; p = 0,23]. A saturação venosa central de oxigênio aumentou no Grupo Hb7 [68,0 (64,0 - 72,0)% para 72,0 (69,0 - 75,0)%; p < 0,0001], mas não no Grupo Hb9 [72,0 (69,0 - 74,0)% para 72,0 (71,0 - 73,0)%; p = 0,98]. Pacientes com elevados níveis de lactato ou saturação venosa central de oxigênio menor que 70% na avaliação basal tiveram um aumento significante nessas variáveis, independentemente dos níveis basais de hemoglobina. Pacientes com valores normais não demonstraram diminuição em quaisquer dos grupos.
Conclusão
A transfusão de hemácias aumentou a saturação venosa central de oxigênio e diminuiu os níveis de lactato em pacientes com hipoperfusão, independentemente de seus níveis basais de hemoglobina. A transfusão não pareceu influenciar essas variáveis em pacientes sem hipoperfusão.
INTRODUCTION
Sepsis is a common condition associated with both high costs and mortality and is usually related to multiple organ dysfunction.(1-8) One of the primary mechanisms for organ dysfunction is inadequate cellular metabolism due to alterations in oxygen supply and consumption.(9) Adequate hemoglobin (Hb) levels could theoretically increase arterial oxygen content and thus improve tissue oxygen delivery.(10) However, there are no conclusive data regarding the optimal level of Hb in septic shock patients.(11)
Although it is not clear that alterations in the central venous oxygen saturation (ScvO2) and lactate levels actually represent improvements in the oxygen delivery/oxygen consumption ratio these alterations may be a better indication for transfusion than the absolute Hb value. Studies on the effects of transfusion on oxygen transport variables have yielded conflicting results, with some trials showing an increase in oxygen delivery/oxygen consumption ratio while others show no increase.(12-17) Some authors have recently suggested that these variables could help to identify surgical patients who would benefit from transfusion.(18) Others have shown that muscle tissue oxygenation, oxygen consumption and microvascular reactivity as assessed by near-infrared spectroscopy improve after transfusion only in patients with alterations in these variables at baseline.(19)
Rivers et al. demonstrated a significant reduction in hospital mortality rates among patients who were randomized to undergo early goal-directed therapy that included red blood cell (RBC) transfusion.(20) However, a clear relationship between blood transfusion and improved outcomes could not be demonstrated with this type of study. Recently, Holst et al. randomized 998 septic shock patients to a restrictive or liberal transfusion strategy;(21) they could not find any difference between those patients assigned to be transfused only if Hb levels were below 7.0 and those with Hb 9.0g/dL as a trigger. However, those patients were transfused throughout their intensive care unit (ICU) stay, regardless of their tissue oxygenation variables, which reduces the relevance of the study’s findings. The primary controversy concerns the potential benefits of transfusion in patients with signs of hypoperfusion and Hb levels over 7.0g/dL.
Therefore, we designed a physiological study to determine the effects of RBC transfusion on ScvO2 and lactate levels in septic shock patients who were randomized into two groups with different Hb levels. Although increased Hb levels will hypothetically increase oxygen content and oxygen delivery on a mathematical basis, we hypothesized that this would increase SvcO2 only if Hb levels were below 7.0g/dL. It is possible that with higher Hb levels, this contribution differs from lower levels, which supports a restrictive approach to transfusion. The goal of this study was to test this hypothesis in random populations of patients in whom transfusion was indicated as Hb fell below 9.0g/dL and in those for whom transfusion was withheld until Hb fell below 7.0g/dL.
METHODS
Patients
This multi-center, prospective, randomized study was conducted in three Brazilian general ICU with a total of 55 beds. Patients who were admitted to one of the participant ICUs with a diagnosis of septic shock(22) between March 1st and August 31st 2008 were included on a non-consecutive basis if they fulfilled the following inclusion criteria: age over 18 years old, a shock diagnosis that was made less than 48 hours prior to participation in the study, Hb levels less than 9.0g/dL and a central venous catheter in the superior vena cava. The exclusion criteria were as follows: pregnancy, known coronary disease, active bleeding and previous participation in the study. The Ethical Committee of the coordinating center approved the study under number 1,177/04, and the trial was registered at ClinicalTrials.gov (NCT01611753). All patients or their legal representatives signed informed consents.
Red blood cell transfusion characteristics
Packed RBCs were obtained from the blood bank at each participating site. None of the RBC units transfused in this study was leukoreduced. Storage solution (citrate phosphate dextrose adenine - CPDA-1) was routinely added to the RBCs before storage. The storage period can be extended up to 35 days, and there is no blood bank policy for preferentially transfusing fresh RBCs in ICU patients.
Study protocol
We registered only transfusions received while the patients were in shock using vasopressors. We randomized all patients into two groups: patients in Group 1 received transfusions immediately so that their Hb levels were maintained above 9.0g/dL; in Group 2, transfusion was withheld until the patients’ Hb levels fell below 7.0g/dL. We used sealed envelopes with blocks of 10 in each of the participating hospitals for randomization. For practical reasons, the attending physicians and protocol staff were not blinded to the group assignments. Demographic data and the Acute Physiology And Chronic Health Evaluation II (APACHE II)(23) and Sequential Organ Failure Assessment (SOFA)(24) severity scores were determined on the day of inclusion.
Each time a patient received a transfusion, we collected a set of laboratory tests, including Hb levels, ScvO2 and lactate at two time points, once immediately before transfusion and once one hour after the end of transfusion. Laboratory tests were collected through a central venous access after discarding 5mL of blood. The correct position of the central venous catheter was assessed using chest radiography. Blood gas data and lactate levels were both measured using a microtechnique in a blood gas analyzer (ABL 700 Radiometer, Copenhagen, Denmark). Hb levels were measured by spectrophotometry (Cell-din® 3700/Abbott®, Illinois, USA).
Immediately prior to each transfusion, we verified that patients had an adequate volemic state, which was defined by a central venous pressure (CVP) above 12mmHg and the decision of the assistant physician that patients did not require another fluid challenge. If the CVP was less than this value, patients received lactated Ringer’s solution or normal saline. Vasopressors were used to maintain the mean arterial pressure above 70mmHg. Only if clinically required, ventilator parameters, vasopressors or dobutamine infusion could be adjusted during the study period. In such a case, the specific transfusion would not be considered in the final analysis.
We assessed Hb levels daily to evaluate the need for transfusion, and as soon as the results became available, patients received an RBC transfusion if required. For each transfusion, only one unit of RBC was transfused. Every transfusion during the study period was registered. We followed the patients until death or the resolution of shock, which was defined as the withdrawal of vasopressors for at least 24 hours. Thereafter, any transfusion was conducted at the discretion of the attending physician.
Statistical analysis
The primary endpoint of the study was the effect of transfusion on lactate and ScvO2. The pre-specified subgroups were those patients with signs of hypoperfusion detected using lactate levels at least 1.5 times the normal values and those with ScvO2 below 70%. In addition, we also analyzed patients without signs of hypoperfusion based on normal lactate and ScvO2 levels.
The sample size was calculated assuming that ScvO2 would increase in 80% of transfusions for patients in Group Hb7, in whom transfusion was withheld until Hb fell below 7.0g/dL compared to only 45% of those in the group transfused to maintain Hb above 9.0g/dL with an alpha error of 0.05 and a power of 80%. Improvement was defined as an increase of 5% over the pre-transfusion ScvO2.(18) According to our calculations, 28 transfusions would be necessary in each group; however, to correct for the potential non-parametric distribution of the primary variables, the number was adjusted to 35 transfusions in each group.
Categorical variables were compared using the Pearson chi-squared test corrected by the Mantel-Haenszel method. The distribution of continuous variables was assessed using the Shapiro-Wilk test, and the homogeneity of variance was assessed using the Bartlett test. Normally distributed variables with homogeneous variance were expressed as the mean ± standard deviation, and non-parametric variables were expressed as the median (interquartile 25% - interquartile 75%). Between groups, Student’s t-test and the Mann-Whitney test were used. For comparison within each group, the Wilcoxon paired test was used because Hb, lactate and ScvO2 were not normally distributed. We also performed a receiver operating characteristic (ROC) curve analysis to assess the accuracy of pre-transfusion Hb levels, pre-transfusion lactate and pre-transfusion ScvO2 in predicting patients whose ScvO2 would increase more than 5% with transfusion. We did not consider patients with levels above 75% in this analysis, as the physiological interpretation of this situation is controversial.
In all tests, the results were considered significant if the p value was less than 0.05. Statistical analyses were conducted using Statistical Package for Social Science (SPSS) 17.0 and GraphPad Prism 5.0.
RESULTS
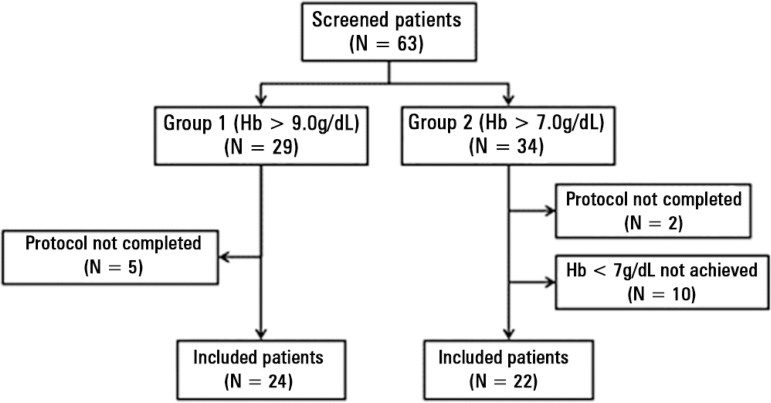
Sixty-three patients were included in this study. Overall, there were 74 RBC transfusions with 39 in Group Hb9 and 35 in Group Hb7 (Figure 1); 19 patients received more than one transfusion. There were no significant differences between groups regarding demographic variables, severity scores (Table 1) or hemodynamic characteristics immediately before transfusion (Table 2). There were no changes in respiratory parameters or vasoactive drug infusions, and no patient received additional fluids during the transfusion period.
Figure 1.
Study flowchart.
Hb - hemoglobin.
Table 1.
Baseline characteristics of the patients
| Variable | Global | Liberal | Restrictive | p value |
|---|---|---|---|---|
| N = 46 | N = 24 | N = 22 | ||
| Male gender | 25 (54.3) | 14 (58.3) | 11 (50.0) | 0.76 |
| Age (years) | 57.5 ± 1.3 | 57.4 ± 1.3 | 57.6 ± 1.4 | 0.95 |
| Comorbidities | ||||
| Arterial hypertension | 24 (52.2) | 12 (50.0) | 12 (54.5) | 0.77 |
| Coronary artery disease | 17 (37.0) | 10 (41.7) | 7 (31.8) | 0.55 |
| Congestive heart failure | 14 (30.4) | 5 (20.8) | 9 (40.9) | 0.20 |
| Diabetes | 15 (32.6) | 9 (37.5) | 6 (27.3) | 0.53 |
| Chronic renal disease | 22 (47.8) | 13 (54.2) | 9 (40.9) | 0.39 |
| Chronic pulmonary disease | 15 (32.6) | 8 (33.3) | 7 (31.8) | 1.00 |
| Chronic hepatic disease | 8 (17.4) | 4 (16.7) | 4 (18.2) | 1.00 |
| Immunosuppression | 5 (10.9) | 2 (8.3) | 3 (13.6) | 0.65 |
| Neoplasm | 10 (21.7) | 6 (25.0) | 4 (18.2) | 0.72 |
| Cerebral vascular disease | 6 (13.0) | 2 (8.3) | 4 (18.2) | 0.40 |
| Source of infection | ||||
| Urinary | 17 (37.0) | 9 (37.5) | 8 (36.4) | 0.80 |
| Pulmonary | 16 (34.8) | 7 (29.2) | 9 (40.9) | 0.61 |
| Abdominal | 9 (19.6) | 5 (20.8) | 4 (18.2) | 0.73 |
| Other | 4 (8.7) | 3 (12.5) | 1 (4.5) | 0.31 |
| APACHE II | 14.0 ± 3.6 | 13.9 ± 3.7 | 14.2 ± 3.6 | 0.77 |
| SOFA | 6.8 ± 0.9 | 6.7 ± 0.7 | 6.9 ± 1.1 | 0.46 |
| Admission Hb (g/dL) | 10 (9.1 - 11.0) | 10 (9.5 - 12.0) | 9.8 (8.8 - 9.8) | 0.15 |
| Mortality | 24 (52.1) | 13 (54) | 11 (50) | 0.87 |
APACHE - Acute Physiologic and Chronic Health Evaluation; SOFA - Sequential Organ Failure Assessment; Hb - hemoglobin. The results are expressed as numbers (%), mean ± standard deviation or median (25 - 75%). Chi-square, t-student or Mann-Whitney test used as appropriate.
Table 2.
Hemodynamic profile immediately prior to transfusion
| Variable | Global | Liberal | Restrictive | p value |
|---|---|---|---|---|
| N = 74 | N = 39 | N = 35 | ||
| CVP (cmH2O) | 17.4 ± 2.6 | 17.0 ± 2.4 | 17.9 ± 2.9 | 0.15 |
| Dobutamine use | 55 (74.3) | 29 (52.7) | 26 (47.3) | 1.00 |
| Dobutamine dose (μg/kg/min) | 5.6 ± 2.1 | 5.1 ± 2.2 | 5.8 ± 2.0 | 0.20 |
| Norepinephrine dose (μg/kg/min) | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.98 |
CVP - central venous pressure. The results are expressed as numbers (%) or mean ± standard deviation. Chi square test, t-student or Mann-Whitney test.
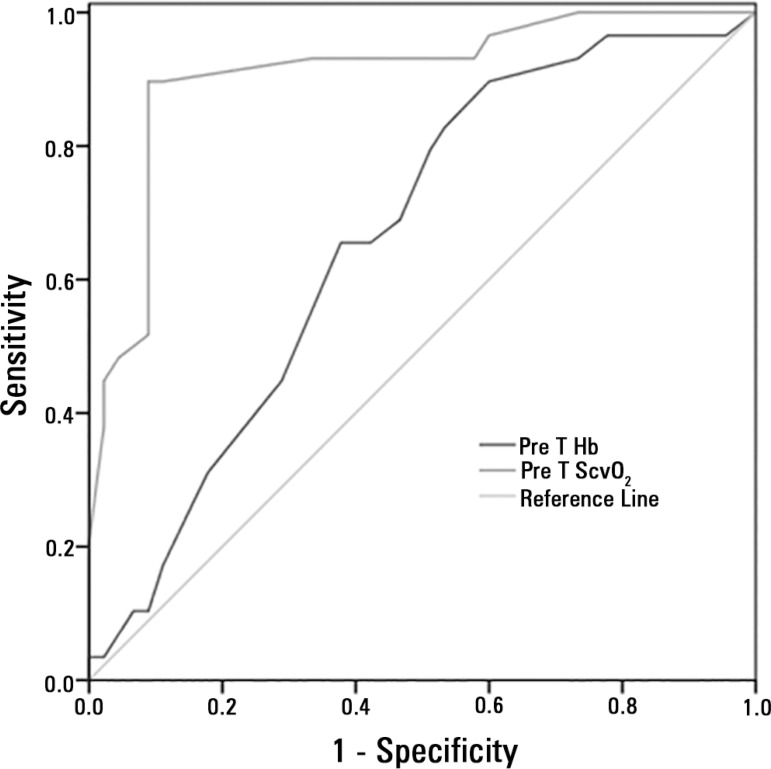
Transfusion increased global Hb levels (p < 0.0001) and, as expected, the pre-transfusion Hb levels were higher in the Group Hb9) (p < 0.0001, Table 3), and pre-transfusion lactate levels were higher in Group Hb7. A significant reduction in lactate levels after transfusion could be found only in Group Hb7 (Group Hb9: p = 0.23, Group Hb7: p = 0.005). Additionally, as expected, ScvO2 was significantly lower at baseline in Group Hb7, and an increase could be detected only in this group (Group Hb9: p = 0.96 and Group Hb7: p < 0.0001). The ROC curves showed that pre-transfusion ScvO2 levels were better than the pre-transfusion Hb or lactate levels (AUC: 0.733 ± 0.064; 95%CI: 0.607 - 0.858) in predicting which patients would respond to transfusion with an increase in ScvO2 greater than 5% (Figure 2).
Table 3.
Pre- and post-transfusion comparison of lactate, central venous oxygen saturation and hemoglobin levels
| Variables | Liberal | Restrictive | p value* |
|---|---|---|---|
| (N = 39) | (N = 35) | ||
| Hemoglobin (g/dL) | |||
| PreT Hb | 8.5 (8.2 - 8.7) | 6.8 (6.6 - 6.9) | < 0.0001 |
| PosT Hb | 9.4 (9.1 - 9.5) | 7.6 (7.4 - 7.8) | < 0.0001 |
| Difference | 0.9 (0.8 - 1.1) | 0.9 (0.8 - 1.1) | |
| p value# | < 0.0001 | < 0.0001 | |
| ScvO2 (%) | |||
| PreT ScvO2 | 72.0 (69.0- 74.0) | 68.0 (64.0 - 72.0) | 0.008 |
| PosT ScvO2 | 72.0 (71.0 - 73.0) | 72.0 (69.0 - 75.0) | 0.45 |
| Difference | -1.0 (-3.0 - 3.0) | 4.0 (2.0 - 6.0) | |
| p value# | 0.96 | 0.0001 | |
| Lactate (mMol/L) | |||
| PreT lactate | 1.90 (1.80 - 2.65) | 2.44 (2.00 - 3.22) | 0.02 |
| PosT lactate | 2.00 (1.70 -- 2.41) | 2.21 (1.80 - 2.79) | 0.07 |
| Difference | - 0.09 (- 0.50 - 0.20) | - 0.19 (-0.40 - 0.10 | |
| p value# | 0.23 | 0.005 |
Hb - hemoglobin; ScvO2 - central venous oxygen saturation; PreT - pre-transfusion; PosT - post-transfusion. The results are expressed as the median values (25 - 75%).
p value refers to comparison within the same group (Wilcoxon rank test).
p value refers to differences between groups (Mann-Whitney test).
Figure 2.
Receiver operating characteristic curves comparing the ability of pre-transfusion hemoglobin levels and central venous oxygen saturation to discriminate responders and non-responders to transfusion in septic shock patients with pre-transfusion central venous oxygen saturation levels below 75%. Responders were defined as patients with an increase ≥ 5%. The ROC curve areas were as follows: ScvO2: 0.879 ± 0.048; 95%CI: 0.785 - 0.973, p < 0.0001); Hb levels: 0.680 ± 0.067; 95%CI: 0.548 - 0.812, p = 0.014).
Hb - hemoglobin; PreT Hb - pre-transfusion hemoglobin levels; PreT ScvO2 - pre-transfusion central venous oxygen saturation.
In 34 transfusions, high lactate levels were present at baseline in 14 cases (35.9%) in Group Hb9 and 20 cases (57.1%) in Group Hb7 (p = 0.06 for difference between groups). In both groups, transfusion was associated with a significant reduction in lactate levels (p = 0.02 and p = 0.001 for Groups Hb9 and Hb7, respectively; Table S1 in the electronic supplementary materials (136.7KB, pdf) ). We found an improvement of at least 10% in 64.3% and 45.0% of the transfusions in Group Hb9 and Hb7, respectively (p = 0.516). However, only patients in Group Hb7 had low ScvO2 levels at baseline, which increased after transfusion.
Baseline ScvO2 levels were less than 70% for 10 (25.6%) cases in Group Hb9 and for 20 (57.1%) cases in Group Hb7 (p = 0.005 between groups). In both groups, ScvO2 increased significantly after transfusion (p = 0.007 and p < 0.0001 for Groups Hb9 and Hb7, respectively; Table S2 and Figure S1 in the electronic supplementary materials (136.7KB, pdf) ). We found an increase of at least 5% or to values above 70% in 66.7% and 90% of the transfusions in Group Hb9 and Group Hb7, respectively (p = 0.095).
We also analyzed the data by exploring only the first transfusion episode for each patient; these results were similar with only slight changes in the p levels (Table S3 in the electronic supplementary material (136.7KB, pdf) ).
In the transfusions for which the pre-transfusion lactate levels were normal (n = 40), a decrease was detected on only six occasions (4 in Group Hb9 and 2 in Group Hb7 (p = 0.81). In 33 transfusions, baseline ScvO2 was between 70% and 75%. Although all four cases in which ScvO2 decreased after transfusion were from Group Hb9, this difference in distribution was not significant (p = 0.17; Table S4 in the electronic supplementary material (136.7KB, pdf) ).
DISCUSSION
In this physiological prospective randomized study of septic shock patients, we demonstrate that RBC transfusion leads to an increase in lactate and ScvO2 levels in patients with altered baseline levels, even if their Hb levels were between 7.0 and 9.0g/dL. No signs of worsened perfusion, as assessed by these variables, could be found, even in patients with normal and higher levels of Hb. Moreover, we found that pre-transfusion ScvO2 was a better predictor of an increase in ScvO2 with transfusion than pre-transfusional Hb or lactate levels.
Hemoglobin level is one of the determinants of oxygen delivery. As expected, patients randomized to the Group Hb7, who consequently had lower levels of Hb, had a higher frequency of lower levels of ScvO2 and higher lactate levels. However, the threshold at which oxygen supply is impaired due to Hb in critically ill patients is not known. Maintaining Hb levels at 7.0g/dL has been considered safe, primarily as a result of a previous randomized study conducted in a general population of critically ill but stable patients.(25) However, there has been no randomized study comparing transfusion triggers in cases of impaired oxygen delivery/oxygen consumption ratio status, as found in septic patients. The recent published study of Holst et al., in which septic shock patients were randomized to a restrictive group (transfusion only if Hb < 7.0g/dl) or a liberal group (transfusion if Hb < 9.0g/dL) did not show differences between the groups, favoring the restrictive approach.(21) However, the study design did not focus on the presence of hypoperfusion because patients were transfused when Hb fell below each group trigger during their entire ICU stay regardless the presence of shock or signs of hypoperfusion.
Although this study is small with only physiological endpoints, its results suggests that the presence of hyperlactatemia or low ScvO2 should be considered in future clinical studies that address this problem. It might be possible that a liberal approach is superior to a restrictive one when considering only patients who actually need to improve their oxygen delivery. Thus, if only patients with Hb levels ≤ 9.0g/dL were included and the presence of signs of hypoperfusion were required to indicate transfusion, we would be able to properly compare two strategies of transfusion: an early transfusion strategy initiated when Hb levels drop below 9.0g/dL; and a late transfusion strategy, when transfusions are indicated only when Hb drops below 7.0g/dL.
A first step to such a randomized interventional study aimed at establishing the best Hb levels in septic patients would be to determine if transfusion is able to lower lactate and increase ScvO2 levels, even in patients with intermediate Hb levels, as shown in this study. The effect of Hb on hemodynamic variables has been previously demonstrated by Adamczyk et al.(18) in postoperative patients for whom an improvement in ScvO2 could be found only in patients with lower ScvO2 levels when their Hb levels were between 7.0g/dL and 9.0g/dL. However, these authors did not analyze patients with the lower Hb levels currently used as an indication for transfusion (i.e., below 7.0g/dL), which precludes any attempt to relate responses to Hb levels. This improvement in tissue oxygenation may have been related to an improvement in oxygen content; however, a possible mechanism would have been the improvement of cardiac output secondary to an increase in preload. This cannot be ruled out because this variable was not assessed in these patients. The absence of this measurement is one of this study’s limitations because it might compromise the quality of the hemodynamic assessments presented. Although all patients were stabilized before inclusion, and CVP levels were above 12mmHg, we did not measure fluid responsiveness; thus, we cannot be certain that transfusion would not have led to an increase in cardiac output.
However, this study has numerous strengths. First, the studied population is homogeneous, only including septic shock patients. The majority of previous studies included a heterogeneous population of critically ill individuals, such as surgical patients without hemodynamic instability,(26) shock patients with multiple etiologies(16) or septic patients without shock.(12,14,27-29) Studies evaluating only septic shock patients were not randomized and included only a small number of transfusions (e.g., 15 to 35 transfusions); as a result, they lacked statistical power to demonstrate slight differences in these variables.(15,17) Second, the number of transfusions in this study allowed us to analyze subgroups of patients with and without tissue oxygenation abnormalities. This type of analysis was not emphasized in many previous studies.(12,26) Third, the patients used in this study were randomized into two different transfusion threshold levels. This strategy allows a comparison between groups without any difference in disease severity, and a clear separation regarding pre-transfusion Hb levels could be established.
Some limitations are also noteworthy. First, the evaluation of a transfusion’s beneficial and deleterious effects based only on ScvO2 and lactate is limited. The use of these variables to assess perfusion is also a limitation. We did not assess any clinical outcomes. Second, patients were selected on a non-consecutive basis, and more than one episode of transfusion per patient was registered, which could have resulted in a selection bias. However, the transfusions were not given at the same time point, and only one red blood cell unit was given at each time. This resulted in different hemodynamic conditions and baseline Hb levels for each transfusion. We recalculated data using only the first transfusion for each patient, and the results were similar. Third, we might interpret the unknown storage duration as a limitation because it precludes an eventual association of this variable with the transfusion outcome. However, there are contradictory data regarding the relevance of storage time to clinical, microcirculatory and physiological variables after transfusion.(30-32) Although we did not measure the storage period of the RBCs transfused in this study, a previous report demonstrated that the median storage time of RBCs in blood banks was 14 (7-21) days, suggesting that at least half of this study’s patients received fresh blood.(33) Fourth, as already mentioned, the absence of cardiac output measurements and hemodynamic data after transfusion prevents analysis of the correlation between tissue oxygenation improvement and a possible increase in cardiac output secondary to increased preload after transfusion. Fifth, although we collected post-transfusion blood samples immediately after transfusion and there was no change in ventilator parameters or arterial oxygenation, we cannot eliminate the possibility that the observed changes in ScvO2 and lactate could be influenced by modifications in oxygen saturation, changes in oxygen consumption ratio or as a consequence of aerobic glycolysis. Finally, we did not directly measure tissue perfusion.
CONCLUSION
In conclusion, red blood cell transfusion in septic shock patients with low central venous oxygen saturation or high lactate levels can result in an increase in central venous oxygen saturation and a decrease in lactate levels in patients with hemoglobin levels below 7.0g/dL and in patients with hemoglobin levels between 7.0 and 9.0g/dL. In patients with normal lactate and central venous oxygen saturation levels, transfusion did not change these variables, even in individuals with higher hemoglobin levels.
ACKNOWLEDGMENTS
We are grateful to the healthcare professionals from the participating intensive care units in the following institutions: Anesthesiology, Pain and Critical Care Discipline - Universidade Federal de São Paulo, São Paulo, Brazil; Santa Casa de Misericordia, Cruzeiro (SP), Brazil and Hospital Antoninho da Rocha Marmo, São José dos Campos - SP, Brazil.
Footnotes
Conflicts of interest None.
Authors’ contributions
B. F. Mazza and F. R. Machado designed and coordinated this study; B. F. Mazza, F. G. R. Freitas, M. M. O. Barros, L. C. P. Azevedo and F. R. Machado contributed to data collection; B. F. Mazza and F. R. Machado drafted the manuscript; and B. F. Mazza, L. C. P. Azevedo, F. G. R. Freitas and F. R. Machado revised the article. All authors have read and approved the final manuscript.
Responsible editor: Gilberto Friedman
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Finfer S, Bellomo R, Lipman J, French C, Dobb G, Myburgh J. Adult-population incidence of severe sepsis in Australian and New Zealand intensive care units. Intensive Care Med. 2004;30(4):589–596. doi: 10.1007/s00134-004-2157-0. Erratum in Intensive Care Med. 2004;30(6):1252. [DOI] [PubMed] [Google Scholar]
- 4.Moss M, Martin GS. A global perspective on the epidemiology of sepsis. Intensive Care Med. 2004;30(4):527–529. doi: 10.1007/s00134-004-2182-z. [DOI] [PubMed] [Google Scholar]
- 5.Padkin A, Goldfrad C, Brady AR, Young D, Black N, Rowan K. Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care units in England, Wales, and Northern Ireland. Crit Care Med. 2003;31(9):2332–2338. doi: 10.1097/01.CCM.0000085141.75513.2B. [DOI] [PubMed] [Google Scholar]
- 6.Salvo I, de Cian W, Musicco M, Langer M, Piadena R, Wolfler A, et al. The Italian SEPSIS study: preliminary results on the incidence and evolution of SIRS, sepsis, severe sepsis and septic shock. Intensive Care Med. 1995;21(Suppl 2):S244–S249. doi: 10.1007/BF01740762. [DOI] [PubMed] [Google Scholar]
- 7.Silva E, Pedro Mde A, Sogayar AC, Mohovic T, Silva CL, Janiszewski M, Cal RG, de Sousa EF, Abe TP, de Andrade J, de Matos JD, Rezende E, Assunção M, Avezum A, Rocha PC, de Matos GF, Bento AM, Corrêa AD, Vieira PC, Knobel E, Brazilian Sepsis Epidemiological Study Brazilian Sepsis Epidemiological Study (BASES study) Crit Care. 2004;8(4):R251–R260. doi: 10.1186/cc2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sogayar AM, Machado FR, Rea-Neto A, Dornas A, Grion CM, Lobo SM, Tura BR, Silva CL, Cal RG, Beer I, Michels V, Safi J, Kayath M, Silva E, Costs Study Group - Latin American Sepsis Institute A multicentre, prospective study to evaluate costs of septic patients in Brazilian intensive care units. Pharmacoeconomics. 2008;26(5):425–434. doi: 10.2165/00019053-200826050-00006. [DOI] [PubMed] [Google Scholar]
- 9.Hotchkiss RS, Karl IE. Reevaluation of the role of cellular hypoxia and bioenergetic failure in sepsis. JAMA. 1992;267(11):1503–1510. [PubMed] [Google Scholar]
- 10.Weg JG. Oxygen transport in adult respiratory distress syndrome and other acute circulatory problems: relationship of oxygen delivery and oxygen consumption. Crit Care Med. 1991;19(5):650–657. doi: 10.1097/00003246-199105000-00011. Review. [DOI] [PubMed] [Google Scholar]
- 11.Hébert PC, Tinmouth A, Corwin H. Anemia and red cell transfusion in critically ill patients. Crit Care Med. 2003;31(12) Suppl:S672–S677. doi: 10.1097/01.CCM.0000117291.73810.5a. [DOI] [PubMed] [Google Scholar]
- 12.Lorente JA, Landín L, De Pablo R, Renes E, Rodríguez-Díaz R, Liste D. Effects of blood transfusion on oxygen transport variables in severe sepsis. Crit Care Med. 1993;21(9):1312–1318. doi: 10.1097/00003246-199309000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Silverman HJ, Tuma P. Gastric tonometry in patients with sepsis. Effects of dobutamine infusions and packed red blood cell transfusions. Chest. 1992;102(1):184–188. doi: 10.1378/chest.102.1.184. [DOI] [PubMed] [Google Scholar]
- 14.Marik PE, Sibbald WJ. Effect of stored-blood transfusion on oxygen delivery in patients with sepsis. JAMA. 1993;269(23):3024–3029. [PubMed] [Google Scholar]
- 15.Conrad SA, Dietrich KA, Hebert CA, Romero MD. Effect of red cell transfusion on oxygen consumption following fluid resuscitation in septic shock. Circ Shock. 1990;31(4):419–429. [PubMed] [Google Scholar]
- 16.Dietrich KA, Conrad SA, Hebert CA, Levy GL, Romero MD. Cardiovascular and metabolic response to red blood cell transfusion in critically ill volume-resuscitated nonsurgical patients. Crit Care Med. 1990;18(9):940–944. doi: 10.1097/00003246-199009000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Mazza BF, Machado FR, Mazza DD, Hassmann V. Evaluation of blood transfusion effects on mixed venous oxygen saturation and lactate levels in patients with SIRS/sepsis. Clinics (Sao Paulo) 2005;60(4):311–316. doi: 10.1590/s1807-59322005000400009. [DOI] [PubMed] [Google Scholar]
- 18.Adamczyk S, Robin E, Barreau O, Fleyfel M, Tavernier B, Lebuffe G, et al. Contribution of central venous oxygen saturation in postoperative blood transfusion decision. Ann Fr Anesth Reanim. 2009;28(6):522–530. doi: 10.1016/j.annfar.2009.03.013. French. [DOI] [PubMed] [Google Scholar]
- 19.Creteur J, Neves AP, Vincent JL. Near-infrared spectroscopy technique to evaluate the effects of red blood cell transfusion on tissue oxygenation. Crit Care. 2009;13(Suppl 5):S11. doi: 10.1186/cc8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M, Early Goal-Directed Therapy Collaborative Group Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 21.Holst LB, Haase N, Wetterslev J, Wernerman J, Guttormsen AB, Karlsson S, Johansson PI, Aneman A, Vang ML, Winding R, Nebrich L, Nibro HL, Rasmussen BS, Lauridsen JR, Nielsen JS, Oldner A, Pettilä V, Cronhjort MB, Andersen LH, Pedersen UG, Reiter N, Wiis J, White JO, Russell L, Thornberg KJ, Hjortrup PB, Müller RG, Møller MH, Steensen M, Tjäder I, Kilsand K, Odeberg-Wernerman S, Sjøbø B, Bundgaard H, Thyø MA, Lodahl D, Maerkedahl R, Albeck C, Illum D, Kruse M, Winkel P, Perner A, TRISS Trial Group. Scandinavian Critical Care Trials Group Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371(15):1381–1391. doi: 10.1056/NEJMoa1406617. [DOI] [PubMed] [Google Scholar]
- 22.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 23.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 24.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 25.Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–417. doi: 10.1056/NEJM199902113400601. Erratum in N Engl J Med 1999;340(13):1056. [DOI] [PubMed] [Google Scholar]
- 26.Babineau TJ, Dzik WH, Borlase BC, Baxter JK, Bistrian BR, Benotti PN. Reevaluation of current transfusion practices in patients in surgical intensive care units. Am J Surg. 1992;164(1):22–25. doi: 10.1016/s0002-9610(05)80640-3. [DOI] [PubMed] [Google Scholar]
- 27.Steffes CP, Bender JS, Levison MA. Blood transfusion and oxygen consumption in surgical sepsis. Crit Care Med. 1991;19(4):512–517. doi: 10.1097/00003246-199104000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Sakr Y, Chierego M, Piagnerelli M, Verdant C, Dubois MJ, Koch M, et al. Microvascular response to red blood cell transfusion in patients with severe sepsis. Crit Care Med. 2007;35(7):1639–1644. doi: 10.1097/01.CCM.0000269936.73788.32. [DOI] [PubMed] [Google Scholar]
- 29.Fernandes CJ, Jr, Akamine N, De Marco FV, De Souza JA, Lagudis S, Knobel E. Red blood cell transfusion does not increase oxygen consumption in critically ill septic patients. Crit Care. 2001;5(6):362–367. doi: 10.1186/cc1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frenzel T, Westphal-Varghese B, Westphal M. Role of storage time of red blood cells on microcirculation and tissue oxygenation in critically ill patients. Curr Opin Anaesthesiol. 2009;22(2):275–280. doi: 10.1097/ACO.0b013e328323f7c4. [DOI] [PubMed] [Google Scholar]
- 31.Lelubre C, Piagnerelli M, Vincent JL. Association between duration of storage of transfused red blood cells and morbidity and mortality in adult patients: myth or reality? Transfusion. 2009;49(7):1384–1394. doi: 10.1111/j.1537-2995.2009.02211.x. [DOI] [PubMed] [Google Scholar]
- 32.Vamvakas EC. Meta-analysis of clinical studies of the purported deleterious effects of "old" (versus "fresh") red blood cells: are we at equipoise? Transfusion. 2010;50(3):600–610. doi: 10.1111/j.1537-2995.2009.02465.x. [DOI] [PubMed] [Google Scholar]
- 33.Assuncao M, Paula I, Falcao L, Mazza B, Barros M, Jackiu M, et al. Transfusion profile in intensive care units from a university hospital. Critical Care. 2007;11(Suppl 2):P412. [Google Scholar]