Abstract
Objective
To assess the adherence to Infectious Disease Society of America/American Thoracic Society guidelines and the causes of lack of adherence during empirical antibiotic prescription in severe pneumonia in Latin America.
Methods
A clinical questionnaire was submitted to 36 physicians from Latin America; they were asked to indicate the empirical treatment in two fictitious cases of severe respiratory infection: community-acquired pneumonia and nosocomial pneumonia.
Results
In the case of communityacquired pneumonia, 11 prescriptions of 36 (30.6%) were compliant with international guidelines. The causes for non-compliant treatment were monotherapy (16.0%), the unnecessary prescription of broad-spectrum antibiotics (40.0%) and the use of non-recommended antibiotics (44.0%).
In the case of nosocomial pneumonia, the rate of adherence to the Infectious Disease Society of America/American Thoracic Society guidelines was 2.8% (1 patient of 36). The reasons for lack of compliance were monotherapy (14.3%) and a lack of dual antibiotic coverage against Pseudomonas aeruginosa (85.7%). If monotherapy with an antipseudomonal antibiotic was considered adequate, the antibiotic treatment would be adequate in 100% of the total prescriptions.
Conclusion
The compliance rate with the Infectious Disease Society of America/American Thoracic Society guidelines in the community-acquired pneumonia scenario was 30.6%; the most frequent cause of lack of compliance was the indication of monotherapy. In the case of nosocomial pneumonia, the compliance rate with the guidelines was 2.8%, and the most important cause of non-adherence was lack of combined antipseudomonal therapy. If the use of monotherapy with an antipseudomonal antibiotic was considered the correct option, the treatment would be adequate in 100% of the prescriptions.
Keywords: Community-acquired pneumonia/drug therapy, Pneumonia ventilator-associated/drug therapy, Anti-bacterial agents/therapeutic use, Advance directive adherence
Abstract
Objetivo
Valorar tasa de adherencia y causas de no adherencia a las guías terapéuticas internacionales para la prescripción antibiótica empírica en la neumonía grave en Latinoamérica.
Métodos
Encuesta clínica realizada a 36 médicos de Latinoamérica donde se pedía indicar el tratamiento empírico en 2 casos clínicos ficticios de pacientes con infección respiratoria grave: neumonía adquirida en la comunidad y neumonía nosocomial.
Resultados
En el caso de la neumonía comunitaria el tratamiento fue adecuado en el 30,6% de las prescripciones. Las causas de no adherencia fueron monoterapia (16,0%), cobertura no indicada para multirresistentes (4,0%) y empleo de antibióticos con espectro inadecuado (44,0%). En el caso de la neumonía nosocomial el cumplimiento de las guías terapéuticas Infectious Disease Society of America/American Thoracic Society fue del 2,8%. Las causas de falta de adherencia fueron monoterapia (14,3%) y la falta de doble tratamiento antibiótico frente a Pseudomonas aeruginosa (85,7%). En caso de considerar correcta la monoterapia con actividad frente a P. aeruginosa, el tratamiento sería adecuado en el 100% de los casos.
Conclusión
En la neumonía comunitaria la adherencia a las guías terapéuticas Infectious Disease Society of America/American Thoracic Society fue del 30,6%; la causa más frecuente de incumplimiento fue el uso de monoterapia. La adherencia en el caso de la neumonía nosocomial fue del 2,8% y la causa más importante de incumplimiento fue la falta de doble tratamiento frente a P. aeruginosa, considerando adecuada monoterapia con actividad frente a P. aeruginosa la adherencia sería del 100%.
INTRODUCTION
Acute respiratory infection is associated with high morbidity and social costs,(1,2) which significantly increase in complicated cases with septic shock.(3,4) Antibiotic therapy is one of the most effective tools for reducing mortality.(4) The association between the administration of inadequate antibiotics in the case of respiratory septic shock and a significant increase in both morbimortality(5-7) and multi-drug resistance has been extensively described.(8)
Several scientific societies have published therapeutic and clinical management recommendations, with the guidelines published by the Infectious Disease Society of America/American Thoracic Society (IDSA/ATS) serving as a reference in Latin America.(9,10)
Various studies conducted in Europe,(11,12) the United States(13) and Australia(14) have analyzed adherence to therapeutic guidelines in empirical antibiotic prescription. However, no similar studies have been performed in Latin America.
In the present study, adherence to IDSA/ATS indications for the treatment of severe pneumonia and the causes of non-compliance with the recommendations were analyzed.
METHODS
A survey was administered to 36 Latin American physicians with extensive experience in the intensive care unit (ICU). The survey was administered during a course on antibiotic politics for critical care patients, which took place in the Hospital Vall d’ Hebron, Barcelona, Spain, in May and July of 2013. The same questionnaire was utilized to conduct an Australian study published by Dulhunty et al.(14) and is composed of fictitious clinical cases of patients with severe infection. In the present study, only cases of respiratory infection were analyzed: community-acquired pneumonia and nosocomial pneumonia. Both settings are described in the electronic supplementary materials.
All participants were asked to indicate their medical specialty, their years of experience in the ICU and the characteristics of the hospital/ICU at which they worked. In addition, for each clinical case, they were requested to indicate how many and which antibiotics they would prescribe, their dose and duration. For these cases, the presence of empyema or any other complication that required surgical intervention or an invasive procedure was discarded. The weight of the patient was indicated as being 80kg, and their renal and liver function was listed as normal.
They were asked to choose between one and three antibiotics without including antivirals, antifungals or tuberculostatic drugs. The dose of medication was calculated and expressed in g per day.
The two settings were bilateral community-acquired pneumonia with secondary septic shock (case 1, available in the electronic supplementary materials (50.6KB, pdf) ); and nosocomial pneumonia in the postoperative period following cholecystectomy (case 2, available in the electronic supplementary materials (50.6KB, pdf) ).
The number of antibiotics indicated, along with their dose and duration, was recorded. The indicated regimen and the dose of antibiotic were then consulted according to the indications from the respective therapeutic guides. As specific indications for Latin America did not exist, we decided to utilize the IDSA/ATS recommendations.(9,10) In table S1 of the electronic supplementary materials (50.6KB, pdf) , the recommended antibiotic regimens are indicated. In the case of prescription of antibiotics that were not indicated in the recommendations or where the dose of antibiotic established by the IDSA/ATS was omitted, the dose suggested by the manuals with the greatest frequency of clinical use was considered to be valid.(15)
Because this was a spontaneous survey conducted using fictitious clinical cases, informed consent was not solicited to perform this study. The survey participants were informed about the purpose of the survey and notified that their compliance was not a condition for obtaining the certificate of course participation.
The results are expressed as the medians and interquartile ranges for continuous variables or as an absolute frequency and percentage frequency for categorical variables. The data management and the statistical analysis were conducted using Statistical Package for the Social Sciences (SPSS) version 15.
RESULTS
Thirty-six physicians responded to the survey: 56% (20 physicians) were ICU specialists with > 5 years of experience in the ICU; 33% (12) were specialists in infectious diseases; and 3% (1) were ICU specialists with < 5 years of experience. Less than 10% (3 physicians) identified with another specialty: anesthesia, internal medicine or cardiology (Table 1). Seventeen physicians (47%) worked in an academic institution; 31 of 36 (86%) practiced in a medical-surgical ICU. The provenance was primarily from Brazil (25 physicians; 69%), followed by Venezuela (4; 11%), Mexico (2; 6%) and Chile (2; 6%).
Table 1.
Information from the 36 survey participants
| Frequency | |
|---|---|
| Specialization | |
| Intensive care for more than 5 years | 20 (55.6) |
| Intensive care for less than 5 years | 1 (2.8) |
| Infectious diseases | 12 (33.3) |
| Other specialties | 3 (8.3) |
| Type of hospital | |
| University | 17 (47.2) |
| Non-university | 5 (13.9) |
| Did not answer | 14 (38.9) |
| Hospital funding | |
| Public | 18 (50.0) |
| Private | 11 (30.6) |
| Did not answer | 7 (19.4) |
| Type of ICU | |
| Mixed medical-surgical | 31 (86.1) |
| Medical | 2 (5.6) |
| Surgical | 0 |
| Did not answer | 3 (8.3) |
| Level of care | |
| Third level | 27 (75.0) |
| Second level | 1 (2.8) |
| Did not answer | 8 (22.2) |
| Participant origin | |
| Brazil | 25 (69.4) |
| Venezuela | 4 (11.1) |
| Mexico | 2 (5.6) |
| Chile | 2 (5.6) |
| Did not answer | 3 (8.3) |
Results are expressed as the absolute values and percentages: n (%). ICU - intensive care unit.
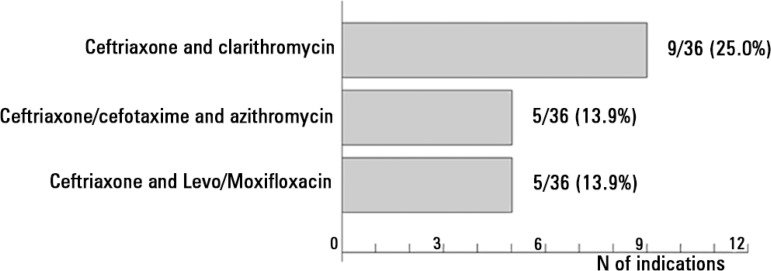
In the two clinical cases, a total of 135 antibiotics were detailed (Tables 2 and 3): 68 in the first case and 67 in the second. In case 1, the most employed group of antibiotics was beta-lactams (29 of 68 prescriptions; 42.7%), with ceftriaxone being prescribed in 29.4% of cases. Macrolides (clarithromycin and azithromycin) were indicated in 19 of 68 prescriptions (27.9%), and quinolones (levofloxacin and moxifloxacin) were indicated in 8 (11.8%). The most prescribed antibiotic patterns were as follows (Figure 1): ceftriaxone and clarithromycin (9 patients of 36; 25.0%), ceftriaxone/cefotaxime and azithromycin (5 patients; 13.9%), and ceftriaxone and levofloxacin/moxifloxacin (5; 13.9%). In 32 of 36 cases (88.9%), combined treatment was indicated. Active anti-Pseudomonas treatment was prescribed in 15 of 36 patients (41.7%) while treatment for methicillin-resistant Staphylococcus aureus (MRSA) was indicated in 6 of 36 patients (16.7%).
Table 2.
Antibiotic prescription, dose and duration in the case of community-acquired pneumonia
| Antibiotic | N indications | Dose* | Indicated dose ≥ recommendation | Duration < 7 days | Duration 7 - 10 days | Duration > 10 days |
|---|---|---|---|---|---|---|
| Beta-lactams | 29/68 (42.7) | |||||
| Ceftriaxone | 20/68 (29.4) | 2.0 (2.0 - 3.5) | 19/20 (95.0) | 0/20 (0) | 17/20 (85.0) | 3/20 (15.0) |
| Cefepime | 5/68 (7.4) | 6.0 (5.0 - 6.0) | 4/5 (80.0) | 0/5 (0) | 3/5 (60.0) | 2/5 (40.0) |
| Meropenem | 4/68 (5.9) | 3.0 (1.9 - 3.0) | 3/4 (75.0) | 0/4 (0) | 3/4 (75.0) | 1/4 (25.0) |
| Macrolides | 19/68 (27.9) | |||||
| Clarithromycin | 10/68 (14.7) | 1.0 (0.9 - 1.0) | 8/10 (80.0) | 0/10 (0) | 8/10 (80.0) | 2/10 (20.0) |
| Azithromycin | 9/68 (13.2) | 0.5 (0.5 - 1.0) | 9/9 (100) | 0/9 (0) | 7/9 (77.8) | 2/9 (22.2) |
| Quinolones | 8/68 (11.8) | |||||
| Levofloxacin | 4/68 (5.9) | 0.8 (0.6 - 0.8) | 3/4 (75.0) | 0/4 (0) | 4/4 (100) | 0/4 (0) |
| Moxifloxacin | 4/68 (5.9) | 0.4 (0.4 - 1.3) | 4/4 (100) | 0/4 (0) | 4/4 (100) | 0/4 (0) |
| Glycopeptides | 4/68 (5.9) | |||||
| Vancomycin | 4/68 (5.9) | 2.0 (2.0 - 2.0) | 4/4 (100) | 0/4 (0) | 3/4 (75.0) | 1/4 (25.0) |
| Others | 8/68 (11.8) |
Results are expressed as the absolute values and percentages: n (%);
result is expressed as the median and interquartile range.
Table 3.
Antibiotic prescription, dose and duration in the case of nosocomial pneumonia
| Antibiotic | N indications | Dose* | Indicated dose ≥ recommendation | Duration < 7 days | Duration 7 - 10 days | Duration > 10 days |
|---|---|---|---|---|---|---|
| Beta-lactams | 33/67 (49.3) | |||||
| Meropenem | 20/67 (29.9) | 3.0 (3.0 - 6.0) | 18/20 (90.0) | 0/20 (0) | 13/20 (65.0) | 7/20 (35.0) |
| Piperacillin-tazobactam | 13/67 (19.4) | 18.0 (15.8 - 18.0) | 12/13 (92.3) | 0/13 (0) | 10/13 (76.9) | 3/13 (23.1) |
| Glycopeptides | 14/67 (20.9) | |||||
| Vancomycin | 14/67 (20.9) | 2.0 (2.0 - 2.0) | 14/14 (100) | 0/14 (0) | 12/14 (85.7) | 2/14 (14.3) |
| Oxazolidinones | 14/67 (20.9) | |||||
| Linezolid | 14/67 (20.9) | 1.2 (1.2 - 1.2) | 14/14 (100) | 0/14 (0) | 9/14 (64.3) | 5/14 (35.7) |
| Others | 6/67 (8.9) |
Results are expressed as the absolute values and percentages: n (%);
result is expressed as the median and interquartile range.
Figure 1.
Antibiotic regimens most frequently indicated in the case of community-acquired pneumonia.
Treatment was adequate according to the IDSA/ATS recommendations in 11 of 36 prescriptions (30.6%) (Table 4). The causes of non-compliance were monotherapy (4 of 25; 16.0%); unnecessary coverage for P. aeruginosa/MRSA in 10 prescriptions (40.0%); and administration of double antibiotic treatment with medications that were not indicated in 11 of 25 prescriptions (44.0%). The most employed antibiotics were clarithromycin (10 prescriptions), clindamycin (1 prescription) and amoxicillin clavulanic (1 prescription).
Table 4.
Fulfillment of Infectious Disease Society of America/American Thoracic Society recommendations and reasons for non-adherence
| Clinical case | Adherence to recommendations | Case 1 - Reasons for non-adherence | Case 2 - Reasons for non-adherence | ||||
|---|---|---|---|---|---|---|---|
| Complied | Not complied | Monotherapy | multiR coverage | Non-indicated AB | Monotherapy | Without double PA coverage | |
| Community-acquired pneumonia | 11/36 (30.6) | 25/36 (69.4) | 4/25 (16.0) | 10/25 (40.0) | 11/25 (44.0) | --- | |
| Case 1 | |||||||
| Nosocomial pneumonia | 1/36 (2.8) | 35/36 (97.2) | --- | 5/35 (14.3) | 30/35 (85.7) | ||
| Case 2 | |||||||
Results are expressed as the absolute values and percentages: n (%). multiR - multi-resistant; AB - antibiotic; PA - Pseudomonas aeruginosa.
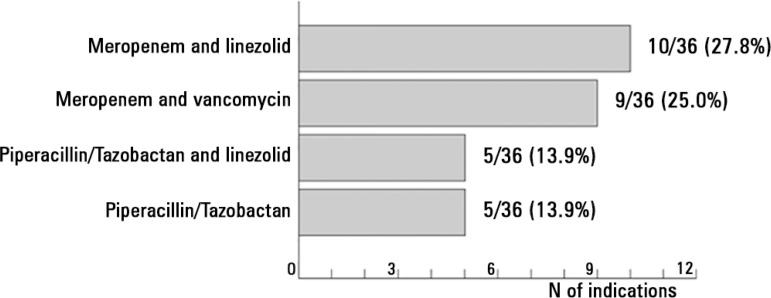
In case 2, the most indicated antibiotics were meropenem (20 prescriptions of 67; 29.9%), vancomycin (14; 20.9%), linezolid (14; 20.9%) and piperacillin-tazobactam (13; 19.4%) (Table 3). The most highly employed regimens are shown in figure 2: meropenem and linezolid (10 patients of 36; 27.8%), meropenem and vancomycin (9 patients of 36; 25.0%), piperacillin-tazobactam and linezolid (5 of 36; 13.9%) and piperacillin-tazobactam in monotherapy (5; 13.9%). Monotherapy was indicated in 5 of 36 patients (13.9%), and combined antibiotic treatment was prescribed in the 31 remaining patients (86.1%). In all cases, active treatment was indicated for P. aeruginosa. Active treatment for MRSA was indicated in 30 of 36 patients (83.3%).
Figure 2.
Antibiotic regimens most frequently indicated in the case of nosocomial pneumonia.
Adherence to the IDSA/ATS indications occurred in 3% of patients (1 patient of 36, with meropenem and levofloxacin being indicated) (Table 4). The causes of non-compliance were monotherapy in 5 prescriptions of 35 (14.3%) and lack of double antibiotic coverage for P. aeruginosa in 30 indications of 35 (85.7%). If the administration of only one active antibiotic for P. aeruginosa had been considered adequate, as indicated in other recommendations,(16) whether it was paired with an active antibiotic for MRSA, the rate of appropriate treatment would have been 100%.
DISCUSSION
The most noteworthy conclusion from this study population is the limited adherence to IDSA/ATS recommendations when prescribing empirical antibiotics for severe pneumonia. In line with the results obtained from studies conducted on other continents,(11-14) this conclusion carries strong implications because a low adherence to therapeutic recommendations is associated with greater morbidity and mortality as well as with an increase in health costs.(4,8,17)
Studies conducted in Europe report adherence rates of between 20 and 100%. The proposed causes of non-adherence were differences between the patient being treated and the condition described in the guidelines, the presence of kidney or liver failure, the unavailability or excessive costs of specific antibiotics, and differences between local flora and international recommendations.(11,12,18)
In the case of community-acquired pneumonia, treatment was adequate in 31% of patients (11 of 36). Although the therapeutic guidelines recommend initiation of broad-spectrum treatment in high-suspect cases of multi-resistant pathogens, this was not considered a correct option because in the setting presented, the patient did not have risk factors for nosocomial pathogens. There were various causes of non-adherence. Prescription of monotherapy (16%) was a reason for non-adherence, as various studies have shown that in patients with respiratory shock, combination antibiotic treatment reduces mortality.(19-21) In addition, a clinical trial conducted in patients with severe community-acquired pneumonia concluded that mortality is higher in patients who received fluoroquinolone versus bitherapy, although statistical significance was not achieved.(22) In general, every patient admitted to the ICU with severe CAP should receive treatment for pneumococcus and Legionella pneumophila, using an anti-pneumococcal bactericide, with the first option being a beta-lactam and an active agent for Legionella spp., such as levofloxacin or azithromycin.(23,24)
Another cause for the lack of adherence was the extensive coverage for P. aeruginosa and MRSA. A total of 40% of patients received active multi-resistant treatment. It has been previously shown that the prevalence of multi-resistant organisms and MRSA is higher in Latin America than in other countries.(25,26) However, the extensive use of broad-spectrum antibiotics leads to an increase in the appearance of opportunistic pathogens.(27-29) Because of this fact, it is essential to monitor the local bacterial flora.(9)
The third reason for the lack of adherence was the prescription of antibiotics not indicated in the recommendations. A total of 44% of patients received treatment with a non-antipseudomonal cephalosporin in addition to clarithromycin. In the 2003 IDSA guidelines, the association of a beta-lactam with a macrolide (erythromycin, clarithromycin or azithromycin) is indicated,(30) while in the latest update,(9) azithromycin is recommended.
The utility of macrolides has been essential in the treatment of pneumonia, due to their activity against pneumococcus and atypical pathogens.(31) The utility of erythromycin, the first macrolide available, has diminished due to its gastrointestinal adverse effects, lack of efficacy against Haemophilus influenzae and the emergence of pneumococcus resistance.
The main advantages of azithromycin and clarithromycin compared to erythromycin are less adverse effects and greater tissue penetration, stability against gastric pH, greater half-life, few pharmacological interactions and a greater post-antibiotic effect. The tissue concentration of azithromycin can be between 10 and 100 times greater than that obtained in blood.(32) Although the maximum concentrations reached by azithromycin and clarithromycin are close to the maximum values of the minimum inhibitory concentration for the important pathogens, their rapid collection in the intra-cellular compartment and their slow liberation make them efficient for this purpose. This phenomenon is more marked with azithromycin due to a more prolonged antimicrobial exposure as a result of its post-antibiotic effect, which allows 3-5-day treatment cycles to be sufficient.(33) However, a limitation of azithromycin is its high intra-cell/interstitial concentration quotient, which could justify its bad behavior against extracellular microorganisms.(34)
In the case of nosocomial pneumonia, an adherence of only 3% of the recommendations is noteworthy, with the only adequate regimen being meropenem and levofloxacin. The IDSA/ATS recommendations indicate using a beta-lactam paired with an aminoglycoside or an active quinolone for P. aeruginosa. In the case of MRSA infection risk, its coverage is indicated. However, in the present case, it was not considered necessary to provide coverage for MRSA, although providing coverage for this infection was not classified as inadequate.
Of the 35 inadequate regimens, monotherapy was indicated in five cases, always ensuring Pseudomonas aeruginosa coverage; on the other hand, combined therapy was indicated in 30 cases. In all the 30 cases of combined therapy, at least one antibiotic was active against P. aeruginosa and another was active against MRSA. The IDSA/ATS recommendations suggest initiating a double antipseudomonal treatment with the purpose of minimizing the risk of not covering the pathogen due to an antibiotic resistance pattern.
Garnacho-Montero et al. addressed monotherapy versus bitherapy in ventilator-associated pneumonia due to P. aeruginosa. In their conclusions, they confirm that combination treatment reduces the risk of inadequate empirical treatment. However, there were no differences in mortality between monotherapy and combination therapy. In addition, the cases of Pseudomonas with reduced sensitivity to carbapenems that received 6g of meropenem per day did not increase mortality.(35) In this sense, in a hospital institution at which the resistance of P. aeruginosa to carbapenem is not a problem, the use of monotherapy with high-dose of meropenem could be an acceptable option. Similarly, the utilization of a beta-lactam in monotherapy in an environment with very low risk of antibiotic resistance could be an adequate option. Despite this, the lack of randomized clinical trials does not allow this finding to be generalized. To support this assertion, according to the European recommendations,(16) a patient with nosocomial pneumonia that is acquired within the first four days of admittance should receive only one antibiotic. Considering the administration of an anti P. aeruginosa to be adequate in our population, the rate of adherence would be 100%.
The present analysis has several limitations. Most importantly, this is a clinical survey based on fictitious cases, and therefore, the data do not come from real clinical practice. However, the high rate of intensive care physicians or infectious disease specialists with extensive work experience in the ICU in a third-level university setting confers a high validity and reliability to the study results.
Another important limitation is that the majority of those surveyed came from Brazil, and there was not, therefore, a proportional representation of the different Latin American countries. An analysis was conducted to compare the responses of physicians from and not from Brazil, and no significant differences were obtained. In addition, the physicians came from different cities in Brazil and from other Latin American countries. Due to confidentiality considerations and to not bias the responses, the authors decided not to communicate the survey participants’ cities of origin. Finally, a higher number of survey participants may have supplied more representative data. The conduction of a similar study with a greater sample size could be useful for confirming the findings obtained and guaranteeing a higher reproducibility and representation of all Latin American states.
CONCLUSION
In the present survey on empirical antibiotic prescription in severe pneumonia, which was conducted using a small sample of physicians from Latin America, adherence to therapeutic Infectious Disease Society of America/American Thoracic Society guidelines was relatively low. However, these findings were in line with results from studies conducted on other continents. In the case of community-acquired pneumonia, the causes of non-adherence were the high indication of monotherapy, coverage for multi-resistant pathogens made when it was not indicated and the employment of antibiotics that were not indicated. In the case of nosocomial pneumonia, the most important cause of non-compliance was the use of only one active antibiotic for P. aeruginosa.
Footnotes
Conflicts of interest: Dr. Rello is a member of the Advisory Board and a Speaker for Pfizer. The other authors have no conflicts of interest to declare.
Investigators from the CRIPS
Roser Anglès, Joan Balcells, Bárbara Borgatta, Candido Diaz, Elisabeth Gallart, Simone Gattarello, Nuria Masnou, Elisabeth Papiol, Ana Parra, Teresa Pont, Maria Alba Riera, Jordi Riera and Jordi Rello.
Responsible editor: Thiago Costa Lisboa
REFERENCIAS
- 1.Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67(1):71–79. doi: 10.1136/thx.2009.129502. [DOI] [PubMed] [Google Scholar]
- 2.Diaz E, Muñoz E, Agbaht K, Rello J. Management of ventilator-associated pneumonia caused by multiresistant bacteria. Curr Opin Crit Care. 2007;13(1):45–50. doi: 10.1097/MCC.0b013e3280121816. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, Marrie TJ, Obrosky DS, Clermont G, Dremsizov TT, Coley C, et al. Severe community-acquired pneumonia: use of intensive care services and evaluation of American and British Thoracic Society Diagnostic criteria. Am J Respir Crit Care Med. 2002;166(5):717–723. doi: 10.1164/rccm.2102084. [DOI] [PubMed] [Google Scholar]
- 4.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale J, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 6.Menendez R, Torres A. Treatment failure in community-acquired pneumonia. Chest. 2007;132(4):1348–1355. doi: 10.1378/chest.06-1995. Review. [DOI] [PubMed] [Google Scholar]
- 7.Menéndez R, Torres A, Zalacaín R, Aspa J, Martín Villasclaras JJ, Borderías L, Benítez Moya JM, Ruiz-Manzano J, Rodríguez de Castro F, Blanquer J, Pérez D, Puzo C, Sánchez Gascón F, Gallardo J, Alvarez C, Molinos L, Neumofail Group Risk factors of treatment failure in community acquired pneumonia: implications for disease outcome. Thorax. 2004;59(11):960–965. doi: 10.1136/thx.2003.017756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kollef MH. Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin Infect Dis. 2000;31(Suppl 4):S131–S138. doi: 10.1086/314079. [DOI] [PubMed] [Google Scholar]
- 9.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Jr, Musher DM, Niederman MS, Torres A, Whitney CG, Infectious Diseases Society of America. American Thoracic Society Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Thoracic Society. Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 11.Rello J, Lorente C, Bodí M, Diaz E, Ricart M, Kollef MH. Why do physicians not follow evidence-based guidelines for preventing ventilator-associated pneumonia? A survey based on the opinions of an international panel of intensivists. Chest. 2002;122(2):656–661. doi: 10.1378/chest.122.2.656. [DOI] [PubMed] [Google Scholar]
- 12.Bassetti M, De Gaudio R, Mazzei T, Morace G, Petrosillo N, Viale P, et al. A survey on infection management practices in Italian ICUs. Crit Care. 2012;16(6):R221. doi: 10.1186/cc11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bochicchio G, Smit PA, Moore R, Bochicchio K, Auwaerter P, Johnson SB, Scalea T, Bartlett JG, POC-IT Group Pilot study of a web-based antibiotic decision management guide. J Am Coll Surg. 2006;202(3):459–467. doi: 10.1016/j.jamcollsurg.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Dulhunty JM, Webb SA, Paterson DL, Bellomo R, Myburgh J, Roberts JA, et al. A survey of antibiotic prescribing practices in Australian and New Zealand intensive care units. Crit Care Resusc. 2010;12(3):162–170. [PubMed] [Google Scholar]
- 15.Gilbert DN, Moellering RC, Jr, Eliopoulos GM, Chambers HF, Saag MS. The Sanford guide to antimicrobial therapy 2013. 43rd ed. Sperryville, VA: Antimicrobial Therapy, Inc.; 2013. [Google Scholar]
- 16.Torres A, Ewig S, Lode H, Carlet J, European HAP working group Defining, treating and preventing hospital acquired pneumonia: European perspective. Intensive Care Med. 2009;35(1):9–29. doi: 10.1007/s00134-008-1336-9. [DOI] [PubMed] [Google Scholar]
- 17.Ferrer M, Menendez R, Amaro R, Torres A. The impact of guidelines on the outcomes of community-acquired and ventilator-associated pneumonia. Clin Chest Med. 2011;32(3):491–505. doi: 10.1016/j.ccm.2011.06.002. Review. [DOI] [PubMed] [Google Scholar]
- 18.Sierra R, Benítez E, León C, Rello J. Prevention and diagnosis of ventilator- associated pneumonia: a survey on current practices in Southern Spanish ICUs. Chest. 2005;128(3):1667–1673. doi: 10.1378/chest.128.3.1667. [DOI] [PubMed] [Google Scholar]
- 19.Gattarello S, Borgatta B, Solé-Violán J, Vallés J, Vidaur L, Zaragoza R, Torres A, Rello J, Community-Acquired Pneumonia en la Unidad de Cuidados Intensivos II Study Investigators Decrease in mortality in severe community-acquired pneumococcal pneumonia: impact of improving antibiotic strategies (2000-2013) Chest. 2014;146(1):22–31. doi: 10.1378/chest.13-1531. [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez A, Mendia A, Sirvent JM, Barcenilla F, de la Torre-Prados MV, Solé-Violán J, Rello J, CAPUCI Study Group Combination antibiotic therapy improves survival in patients with community-acquired pneumonia and shock. Crit Care Med. 2007;35(6):1493–1498. doi: 10.1097/01.CCM.0000266755.75844.05. [DOI] [PubMed] [Google Scholar]
- 21.Baddour LM, Yu VL, Klugman KP, Feldman C, Ortqvist A, Rello J, Morris AJ, Luna CM, Snydman DR, Ko WC, Chedid MB, Hui DS, Andremont A, Chiou CC, International Pneumococcal Study Group Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170(4):440–444. doi: 10.1164/rccm.200311-1578OC. [DOI] [PubMed] [Google Scholar]
- 22.Leroy O, Saux P, Bédos JP, Caulin E. Comparison of levofloxacin and cefotaxime combined with ofloxacin for ICU patients with community-acquired pneumonia who do not require vasopressors. Chest. 2005;128(1):172–183. doi: 10.1378/chest.128.1.172. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz M, Ewig S, Torres A, Arancibia F, Marco F, Mensa J, et al. Severe community-acquired pneumonia. Risk factors and follow-up epidemiology. Am J Respir Crit Care Med. 1999;160(3):923–929. doi: 10.1164/ajrccm.160.3.9901107. [DOI] [PubMed] [Google Scholar]
- 24.Torres A, Serra-Batlles J, Ferrer A, Jiménez P, Celis R, Cobo E, et al. Severe community-acquired pneumonia. Epidemiology and prognostic factors. Am Rev Respir Dis. 1991;144(2):312–318. doi: 10.1164/ajrccm/144.2.312. [DOI] [PubMed] [Google Scholar]
- 25.Garza-González E, Dowzicky MJ. Changes in Staphylococcus aureus susceptibility across Latin America between 2004 and 2010. Braz J Infect Dis. 2013;17(1):13–19. doi: 10.1016/j.bjid.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones RN, Guzman-Blanco M, Gales AC, Gallegos B, Castro AL, Martino MD, et al. Susceptibility rates in Latin American nations: report from a regional resistance surveillance program (2011) Braz J Infect Dis. 2013;17(6):672–681. doi: 10.1016/j.bjid.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luna CM, Vujacich P, Niederman MS, Vay C, Gherardi C, Matera J, et al. Impact of BAL data on the therapy and outcome of ventilator-associated pneumonia. Chest. 1997;111(3):676–685. doi: 10.1378/chest.111.3.676. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez-Lerma F. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU-Acquired Pneumonia Study Group. Intensive Care Med. 1996;22(5):387–394. doi: 10.1007/BF01712153. [DOI] [PubMed] [Google Scholar]
- 29.Kollef MH, Ward S. The influence of mini-BAL cultures on patient outcomes: implications for the antibiotic management of ventilator-associated pneumonia. Chest. 1998;113(2):412–420. doi: 10.1378/chest.113.2.412. [DOI] [PubMed] [Google Scholar]
- 30.Mandell LA, Bartlett JG, Dowell SF, File TM, Jr, Musher DM, Whitney C, Infectious Diseases Society of America Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin Infect Dis. 2003;37(11):1405–1433. doi: 10.1086/380488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katzung Bertram G. Katzung BG. Basic & clinical pharmacology. 9th ed. McGraw-Hil; 2004. Chapter 44. Chloramphenicol, tetracyclines, macrolides, clindamycin, & streptogramins. [Google Scholar]
- 32.Foulds G, Shepard RM, Johnson RB. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother. 1990;25(Suppl. A):73–82. doi: 10.1093/jac/25.suppl_a.73. [DOI] [PubMed] [Google Scholar]
- 33.Giner Almaraz S, Canós Cabedo M, Rodilla Calvelo F, Ferrer Gómez C. Nuevos macrolidos: superan a eritromicina? Farm Hosp (Valencia) 1995;19(5):259–265. [Google Scholar]
- 34.Pahissa A. Los macrólidos en el tratamiento de la infección respiratoria. Enferm Infecc Microbiol Clin. 1994;12:423–425. [PubMed] [Google Scholar]
- 35.Garnacho-Montero J, Sa-Borges M, Sole-Violan J, Barcenilla F, Escoresca-Ortega A, Ochoa M, et al. Optimal management therapy for Pseudomonas aeruginosa ventilator-associated pneumonia: an observational, multicenter study comparing monotherapy with combination antibiotic therapy. Crit Care Med. 2007;35(8):1888–1895. doi: 10.1097/01.CCM.0000275389.31974.22. [DOI] [PubMed] [Google Scholar]