Abstract
Objective
This study aimed to evaluate Brazilian physicians’ perceptions regarding the diagnosis, severity assessment, treatment and risk stratification of severe community-acquired pneumonia patients and to compare those perceptions to current guidelines.
Methods
We conducted a cross-sectional international anonymous survey among a convenience sample of critical care, pulmonary, emergency and internal medicine physicians from Brazil between October and December 2008. The electronic survey evaluated physicians’ attitudes towards the diagnosis, risk assessment and therapeutic interventions for patients with severe community-acquired pneumonia.
Results
A total of 253 physicians responded to the survey, with 66% from Southeast Brazil. The majority (60%) of the responding physicians had > 10 years of medical experience. The risk assessment of severe community-acquired pneumonia was very heterogeneous, with clinical evaluation as the most frequent approach. Although blood cultures were recognized as exhibiting a poor diagnostic performance, these cultures were performed by 75% of respondents. In contrast, the presence of urinary pneumococcal and Legionella antigens was evaluated by less than 1/3 of physicians. The vast majority of physicians (95%) prescribe antibiotics according to a guideline, with the combination of a 3rd/4th generation cephalosporin plus a macrolide as the most frequent choice.
Conclusion
This Brazilian survey identified an important gap between guidelines and clinical practice and recommends the institution of educational programs that implement evidence-based strategies for the management of severe community-acquired pneumonia.
Keywords: Community-acquired infections, Sepsis, Antimicrobial agents, Risk assessment, Diagnosis, Intensive care units, Questionnaires, Brazil
Abstract
Objetivo
Avaliar a percepção dos médicos brasileiros quanto ao diagnóstico, à avaliação de gravidade, ao tratamento e à estratificação de risco em pacientes com pneumonia grave adquirida na comunidade, e compará-la com as diretrizes atuais.
Métodos
Estudo transversal realizado por meio da aplicação de um questionário anônimo a uma amostra de médicos brasileiros especialistas em cuidados intensivos, medicina de emergência, medicina interna e pneumologia. Entre outubro e dezembro de 2008, foram avaliadas as atitudes dos médicos no diagnóstico, a avaliação de risco e as intervenções terapêuticas para pacientes com pneumonia grave adquirida na comunidade.
Resultados
Responderam ao questionário 253 médicos, sendo 66% da Região Sudeste do Brasil. A maioria (60%) dos médicos que responderam tinha mais de 10 anos de experiência. Verificou-se que a avaliação de risco de pneumonia grave adquirida na comunidade foi muito heterogênea, sendo a avaliação clínica a forma de avaliação de risco mais frequente. As hemoculturas foram habitualmente realizadas por 75% dos médicos, entretanto, foi reconhecido seu fraco desempenho diagnóstico. Por outro lado, a pesquisa de antígenos urinários de Pneumococo e Legionella foi solicitada por menos de um terço dos médicos. A maioria (95%) prescreveu antibióticos de acordo com as diretrizes. A combinação de uma cefalosporina de terceira ou quarta geração com um macrolídeo foi a escolha mais comum.
Conclusão
Este inquérito brasileiro demonstrou diferenças entre as diretrizes publicadas e a prática clínica. Isso leva à necessidade de se desenvolverem programas educacionais e de adoção de protocolos para implementar estratégias baseadas em evidências no manejo da pneumonia grave adquirida na comunidade.
INTRODUCTION
Community-acquired pneumonia (CAP) is an important public health problem and a significant cause of mortality and morbidity in all age groups.(1,2) High mortality rates have been reported, especially in underdeveloped and developing countries, such as Brazil, Argentina and India.(3,4) Despite substantial progress in the detection of pathogens and in therapeutic options for the management of CAP, several issues remain controversial.(1,5) Although different models have been used to predict pneumonia severity,(5-9) there is a gap between the recommended guidelines and current practice for the management of CAP. The association of clinical scores and biomarkers appears to be better for predicting short-and long-term morbidity and mortality.(10,11) The major challenge for physicians is to translate the recommended guidelines into clinical practice.(12,13) This study aims to answer some questions that remain controversial and help in the decision-making process.
We conducted a secondary analysis of Brazilian data from an international survey that evaluated physicians’ perceptions regarding practice in the context of the diagnosis, severity assessment, treatment and risk stratification of severe CAP.(14)
METHODS
In this study, we analyzed 253 (54% of respondents) questionnaires that were extracted from a previous international survey and corresponded to the Brazilian cohort. The Local Ethics Committee from Instituto Nacional de Câncer approved the study (Nº 105/08). A full description of the survey development is detailed elsewhere.(14)
We performed a detailed description of current practices and performed comparisons to evaluate clinicians’ adherence to guidelines and the current implementation of guidelines in the treatment of CAP patients.
In Brazil, an invitation to answer the survey and the associated web link were sent by email to a convenience sample of intensive care unit (ICU) and pulmonary care physicians listed in the Brazilian Research in Intensive Care Network (BRICNet), the Brazilian Society of Pulmonary Diseases, the Associación Latino Americana del Tórax (ALAT) and the personal mailing lists of the investigators.
Data and statistical analysis
The survey results were exported into a Microsoft Excel template and analyzed using the Statistical Package for Social Science (SPSS) 13.0 software package (Chicago, Illinois, USA). Standard descriptive statistics were used, as appropriate. The variables are reported as the number (%). As the number of respondents varied across the questions, the proportions displayed in the results section and tables were not constant. Fischer’s exact test was used to compare the variables. A two sided p-value of < 0.05 was considered to be significant.
RESULTS
Demographics
A total of 253 questionnaires were available for analysis, with 66% of the questionnaires from Southeast Brazil, 12% of the questionnaires from South Brazil, 10% of the questionnaires from Northeast Brazil, 4% of the questionnaires from North Brazil and 1% of the questionnaires from Midwest Brazil. The demographic characteristics of the respondents are described in table 1.
Table 1.
Characteristics of the responding physicians
| Characteristics | N (%) |
|---|---|
| Duration of experience (years) | N = 253 |
| < 5 | 41 (16) |
| 5 - 10 | 61 (24) |
| > 10 | 151 (60) |
| Primary specialty | N = 253 |
| Pneumology | 66 (26) |
| Intensive care | 97 (38) |
| Other | 90 (36) |
| Experience in critical care (years) | N = 185 |
| < 5 | 50 (27) |
| 5 - 10 | 49 (26) |
| > 10 | 86 (47) |
| Hospital size (beds) | N = 239 |
| < 250 | 124 (52) |
| 250 - 500 | 81 (34) |
| > 500 | 34 (14) |
| Intensive care unit size (beds) | N = 220 |
| < 10 | 89 (40) |
| 10 - 20 | 74 (34) |
| > 20 | 57 (26) |
| Documented CAP patient volume | N = 207 |
| < 10 | 18 (9) |
| 10 - 50 | 125 (60) |
| > 50 | 64 (31) |
ICU - intensive care unit; CAP - community-acquired pneumonia. Results are expressed as the N (%).
The majority (60%) of the responding physicians had > 10 years of medical experience. A total of 18% of physicians worked primarily in university-affiliated hospitals, and 43% worked primarily in private hospitals. The physicians’ medical experience in critical care was quite high, with 47% of the respondents reporting > 10 years and 27% of the respondents reporting between 5 and 10 years of practice in this specialty. A total of 193 respondents reported an average ICU occupancy rate > 75%, with 150 respondents reporting a rate above 85%.
Risk assessment and diagnostic practices
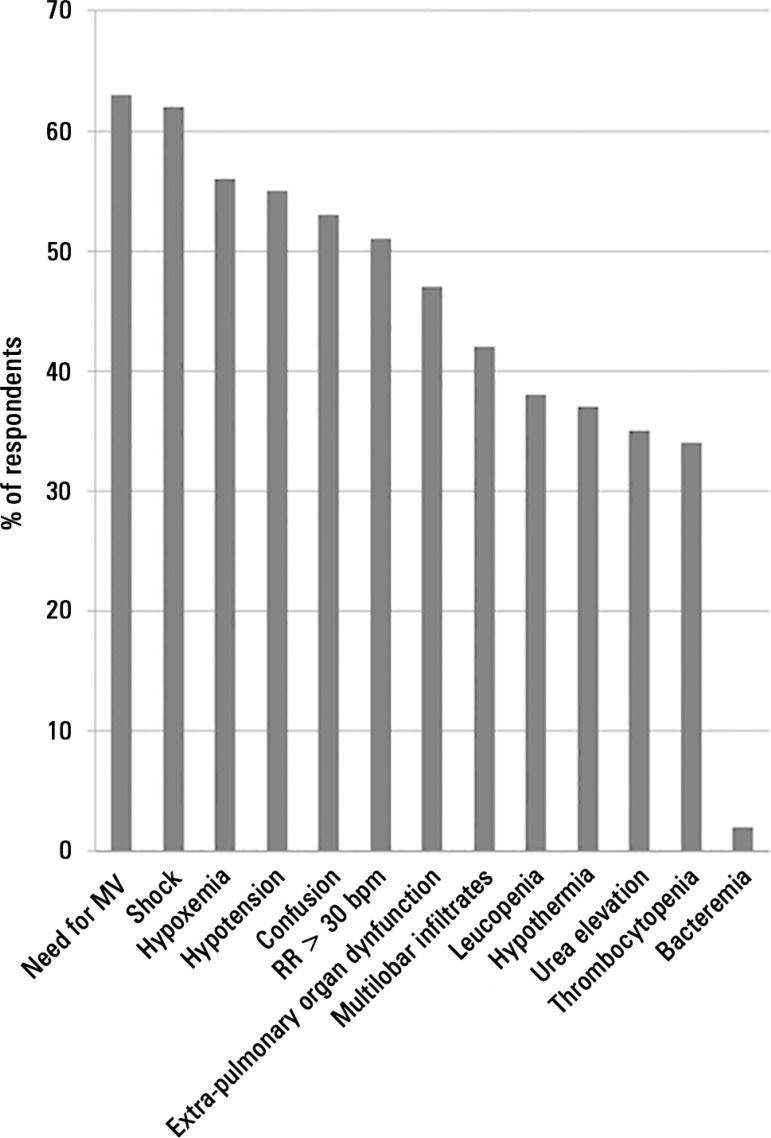
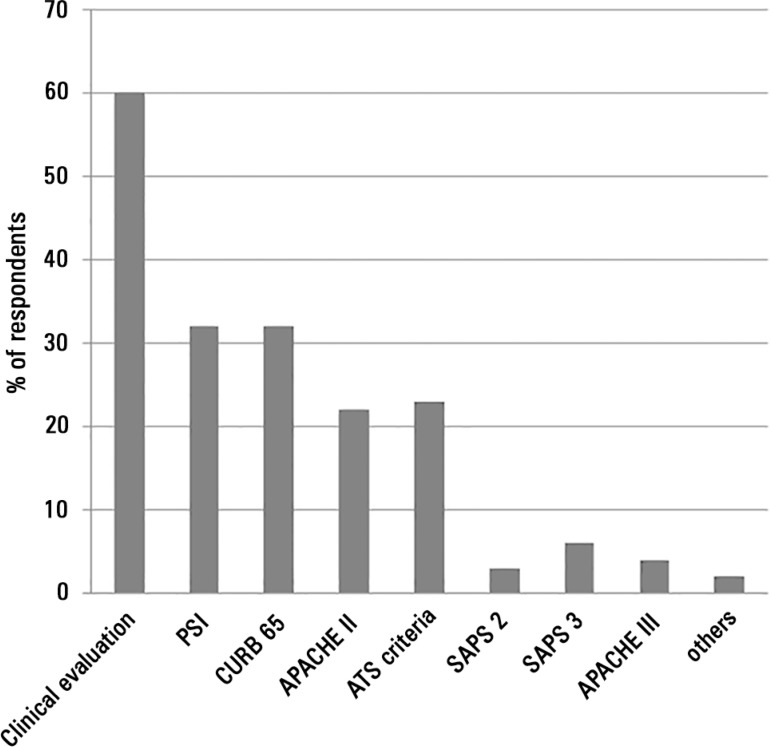
The criteria used by the physicians to define the severity of CAP were heterogeneous (Figure 1), and the presence of shock, the need for mechanical ventilation (MV) and ICU admission were the most frequently reported measures for classifying CAP. Physicians regularly performed risk stratification in CAP patients using clinical evaluation (Figure 2). Structured severity assessment tools were used systematically in less than 40% of cases.A total of 52% of physicians reported using severity scores to support decisions concerning whether CAP patients should be admitted to the ICU or the medical ward.
Figure 1.
Variables used by physicians to define the severity of community-acquired pneumonia.
MV - mechanical ventilation; RR - respiratory rate; CAP - community-acquired pneumonia.
Figure 2.
Risk assessment of community-acquired pneumonia.
PSI - Pneumonia Severity Index; APACHE - acute physiology and chronic health evaluation; ATS - American Thoracic Society; SAPS - simplified acute physiology score; CAP - community-acquired pneumonia.
The use of biomarkers to assess CAP severity was also evaluated. Seventy-one percent of the respondents routinely used laboratory tests or biomarkers, specifically C-reactive protein (CRP) (47%) and procalcitonin (PCT) (8%).
In the emergency department, > 75% of patients with CAP undergo SaO2 measurement, as reported by 53% of responding physicians.
We also assessed the diagnostic workflow. For CAP patients admitted to the hospital, the majority of respondents performed blood cultures (75%). However, the collection of respiratory samples was performed less frequently: sputum cultures were collected in 32% of cases, tracheal aspirates were collected in 39% of cases and bronchoalveolar lavage was performed (BAL) in 34% of cases. With respect to these samples, 33% of respondents reported performing a Gram stain. In addition, 14% and 20% of physicians reported asking for pneumococcal and Legionella urinary antigen tests, respectively. Overall, 15% of respondents required routine serology for atypical pathogens. C-reactive protein was used routinely to support the clinical diagnosis by 29% of respondents.
In this survey, we investigated the perceived rate of pneumonia caused by atypical pathogens. Concerning the prevalence of Legionella, 62 respondents (33%) had no data and 42% of respondents reported that this pathogen occurred in less than 10% of cases. For patients receiving invasive mechanical ventilation, the diagnostic workflow was similar to the workflow described above. The answers concerning the microbiological documentation of CAP varied widely: 18% of clinicians reported that microbiological documentation occurred in less than 10% of patients, 38% of clinicians reported that microbiological documentation occurred in 10 - 25% of patients, 34% of clinicians reported that microbiological documentation occurred in 25-50% of patients, and 10% of clinicians reported that > 50% of patients have microbiological documentation.
As expected, 42% of the respondents reported lung biopsy as an unusual practice (< 3 biopsies/year) and half of the respondents never used lung biopsy as a diagnostic practice.
Therapeutic management of severe community-acquired pneumonia
Regarding the prescription of antibiotics for patients with CAP, nearly all (97%) respondents used a guideline for the initial choice of an antibiotic regimen: 32% used the American Thoracic Society/Infectious Diseases Society of America, 36% used national guidelines, and 30% used local guidelines. Some physicians used other guidelines from the Centers for Disease Control and Prevention (CDC) or recommendations present in classic handbooks, such as the “Sanford Manual of Antimicrobial Therapy.”
The primary antibiotic regimen was a β-lactam plus a macrolide in 38% of cases, a 3rd/4th generation cephalosporin plus a macrolide in 38% of cases, a β-lactam plus a quinolone in 10% of cases, a quinolone in 7% of cases, an anti-pseudomonal agent plus a macrolide in 5% of cases, a 3rd/4th generation cephalosporin in 2% of cases and a β-lactam in 0.5% of cases.
The approximate duration of antibiotic therapy was up to 8 days in 29% of cases, 9 - 14 days in 64% of cases and > 14 days in 6% of cases. In CAP patients, antibiotics were primarily stopped using clinical criteria (68%). A CRP course was used in 28% of cases, and a PCT course was used in 2% of cases. Only 5% of respondents reported that they did not use any biomarker in the assessment of clinical course.
To assess the response of CAP to antibiotics, 72% of respondents relied on different clinical criteria: the improvement of hypoxemia was used by 59% of respondents, radiologic improvement was used by 41% of respondents, apyrexia was used by 62% of respondents, the resolution of shock was used by 55% of respondents, and a decrease in the amount and purulence of tracheobronchial secretions was used by 49% of respondents.
The monitoring of biomarkers as surrogate markers of the response to treatment in patients with severe CAP was also reported, with CRP decreasing in 42% of patients, the CRP-ratio decreasing in 15% of patients and cytokines decreasing in 2% of patients. Other methods were mentioned by only 4% of physicians (i.e., respiratory rate, white cell count, the ratio of arterial oxygen concentration to the fraction of inspired oxygen, and the level of consciousness).
The time from the prescription of an antibiotic to the first assessment of clinical response was also heterogeneous: 8% of physicians assessed the clinical response after 24 hours, 55% of physicians assessed the clinical response after 48 hours, 35% of physicians assessed the clinical response after 72 hours, 2% of physicians assessed the clinical response on the 5th day and 0.5% of physicians assessed the clinical response on the 7th day. Only 5% of the physicians reported that they never prescribe steroids. Among the physicians who prescribed steroids, the main reasons for steroid administration were adrenal insufficiency (30%), refractory shock (30%) and acute respiratory distress syndrome (ARDS) (18%).
The criteria for weaning steroids were also variable. A quarter of the physicians stopped steroids according to a pre-determined schedule, other physicians stopped steroids after the resolution of shock (56%) or hypoxemia (18%) and 1% of the physicians stopped steroids at the time of ICU discharge. The assessment of adrenal function was performed by 25% of physicians for CAP patients with septic shock and by 4% of physicians during the treatment of CAP patients with ARDS.
Non-invasive ventilation (NIV) was never used in CAP patients by 8% of physicians. Among the physicians that considered using NIV, 23% used NIV in less than 10% of patients, 24% used NIV in 10 - 25% of patients, 27% used NIV in > 25 - 50% of patients, and 12% used NIV in > 50 - 75% of patients. In addition, 15% of the physicians used NIV in over 75% of CAP patients.
No differences in clinical practice were observed when the physicians were compared based on professional experience, when specialists were compared to non-specialists and when physicians working in a university hospital were compared to physicians working in other types of hospitals.
DISCUSSION
We conducted a secondary analysis of an international survey that evaluated the perceived management of the diagnosis, risk assessment and treatment of severe CAP in Brazil. The main purpose was to establish physicians’ perceptions regarding the care of patients with severe CAP in Brazilian ICUs. No differences in clinical practices were observed when we evaluated professional experience, being board certified in the specialty or working in a university hospital.
The risk assessment of severe CAP was very heterogeneous in our sample, and clinical evaluation was the most frequent method of severity assessment for patients with severe CAP. Structured severity assessment tools were used systematically in less than 40% of cases. These results are consistent with British Thoracic Society guidelines,(15) but the CURB65 score (or another validated scoring system) was not routinely applied in conjunction with clinical judgment.(15) Brazilian guidelines suggest the use of the American Thoracic Society’s criteria for severe CAP; these guidelines describe major and minor criteria and define the presence of 1 major or 2 minor criteria as sufficient for the assessment of severity.(16) The major criteria are invasive mechanical ventilation and septic shock, which are represented by the most frequent physician answers.(16,17) The minor criteria are PaO2/FiO2 < 250, multilobar involvement, systolic blood pressure < 90mmHg and diastolic blood pressure < 60mmHg.(16,17)
The accuracy of scoring systems, such as the Pneumonia Severity Index (PSI) and CURB-65, for predicting outcomes is questionable.(18) Thus, it is essential to identify new tools to help physicians assess patient outcomes and stratify the risk of CAP. Novel biomarkers, such as cortisol, pro-adrenomedullin and endothelin-1, have been shown to be associated with disease severity and short-term outcomes in patients with CAP.(18)
Pneumococcal and Legionella urine antigen tests are recommended for all patients with severe CAP by the British Thoracic Society guidelines.(15) In our population, only 14% and 20% of physicians asked for these tests, respectively. A secondary analysis of an international database reported an incidence of atypical pathogens of 21% among CAP patients in Latin America.(19) Better outcomes, such as a shorter time to clinical stability, a shorter length of hospital stay and lower hospital mortality, were demonstrated in the subgroup of patients who were treated with atypical coverage.(19)
The vast majority of the physicians (95%) stated that the prescription of antibiotics is performed according to the available guidelines. The combination of a β-lactam or a 3rd/4th generation cephalosporin plus a macrolide was the most frequent choice (76%); this approach is consistent with the British Thoracic Society and the Infectious Diseases Society of America guidelines for the treatment of SCAP. A total of 64% of the Brazilian physicians stated that antibiotic therapy was prescribed for between 8 and 14 days of treatment, although the international recommendation considers a seven-day course to be sufficient and safe.(20) This finding can be explained by the use of clinical criteria to determine the therapy duration and the low use of biomarkers for monitoring the response.
In developing countries, the implementation of clinical protocols could improve compliance with best practices in sepsis management and improve outcomes.(21) A recent study that was performed in ten private hospitals from Brazil involved the implementation of a multifaceted sepsis education program. That study reported that compliance with the resuscitation bundle was associated with a lower risk of hospital mortality and was also cost-effective.(21)
Concerning the use of steroids, the British Thoracic Society guidelines do not recommend steroid use for the routine treatment of severe CAP. In this survey, only 5% of the physicians reported never prescribing steroids. The main reasons for the use of steroids were refractory septic shock and adrenal insufficiency, which occur in severely ill patients with severe sepsis and septic shock. This practice could be a reflection of the Surviving Sepsis Campaign guidelines (SSC 2004 and 2008) that recommended adjunctive therapy with low-dose steroids in septic shock patients.
Guidelines differ concerning the use of NIV in severe CAP patients. The British Thoracic Society guidelines state that neither NIV nor continuous positive airway pressure (CPAP) should be used routinely in the management of patients with respiratory failure due to CAP. Additionally, these guidelines state that an NIV trial can be performed, but this trial should only be conducted in a critical care area, with the possibility for a rapid switch to invasive ventilation. In this survey, NIV was never used by 8% of physicians. We found that NIV is used in a large percentage of patients; this finding should be evaluated in a secondary analysis. In a future study, the questions concerning NIV should focus on the co-existence of other respiratory diseases, such as chronic obstructive pulmonary disease, or end-stage diseases, such as lung cancer, which might determine the choice of NIV in these patients.
We acknowledge that our study has some limitations. One of the main limitations is that this study is a retrospective study in which a secondary analysis of a survey was performed. There is a temporal limitation because the survey was performed in 2008; thus, the data were evaluated with an important time delay. The sample choice (i.e., a convenience sample of physicians) is also a limitation and might generate an important bias in the results. The fact that this survey focuses on physicians’ perceptions may create another bias because the perceptions of the clinicians may not accurately represent the real clinical practice scenario. In addition, data concerning viral pneumonia were beyond the scope of this study. In spite of these limitations, our study sheds light on the knowledge of clinical practice for the management of CAP in Brazilian ICUs.
CONCLUSION
In conclusion, this survey presented valuable data related to the management of severe community-acquired pneumonia in Brazilian intensive care units. Although heterogeneous approaches were reported, we observed an incomplete application of the current literature recommendations in clinical practice in all evaluated domains. This study identified a gap between guidelines and clinical practice and suggests that the implementation of educational programs and protocols that include evidence-based strategies is needed to improve the management of severe community-acquired pneumonia.
ACKNOWLEDGMENTS
This study was supported by the infrastructure of the Brazilian Research in Intensive Care Network (BRICNet).
Footnotes
Conflicts of interest: None.
Responsible editor: Gilberto Friedman
REFERENCES
- 1.Brown SM, Jones BE, Jephson AR, Dean NC, Infectious Disease Society of America/American Thoracic Society 2007 Validation of the Infectious Disease Society of America/American Thoracic Society 2007 guidelines for severe community-acquired pneumonia. Crit Care Med. 2009;37(12):3010–3016. doi: 10.1097/CCM.0b013e3181b030d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silva E, Dalfior L, Junior, Fernandes HD, Moreno R, Vincent JL. Prevalence and outcomes of infections in Brazilian ICUs: a subanalysis of EPIC II study. Rev Bras Ter Intensiva. 2012;24(2):143–150. [PubMed] [Google Scholar]
- 3.Conde KA, Silva E, Silva CO, Ferreira E, Freitas FG, Castro I, et al. Differences in sepsis treatment and outcomes between public and private hospitals in Brazil: a multicenter observational study. PloS One. 2013;8(6):e64790. doi: 10.1371/journal.pone.0064790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozza FA, Salluh JI. An urban perspective on sepsis in developing countries. Lancet Infect Dis. 2010;10(5):290–291. doi: 10.1016/S1473-3099(10)70074-8. [DOI] [PubMed] [Google Scholar]
- 5.Ewig S, Ruiz M, Mensa J, Marcos MA, Martinez JA, Arancibia F, et al. Severe community-acquired pneumonia. Assessment of severity criteria. Am J Respir Crit Care Med. 1998;158(4):1102–1108. doi: 10.1164/ajrccm.158.4.9803114. [DOI] [PubMed] [Google Scholar]
- 6.Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 7.Community-acquired pneumonia in adults in British hospitals in 1982-1983: a survey of aetiology, mortality, prognostic factors and outcome. The British Thoracic Society and the Public Health Laboratory Service. Q J Med. 1987;62(239):195–220. [PubMed] [Google Scholar]
- 8.Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ewig S, de Roux A, Bauer T, García E, Mensa J, Niederman M, et al. Validation of predictive rules and indices of severity for community acquired pneumonia. Thorax. 2004;59(5):421–427. doi: 10.1136/thx.2003.008110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krüger S, Ewig S, Giersdorf S, Hartmann O, Suttorp N, Welte T, German Competence Network for the Study of Community Acquired Pneumonia (CAPNETZ) Study Group Cardiovascular and inflammatory biomarkers to predict short- and long-term survival in community-acquired pneumonia: Results from the German Competence Network, CAPNETZ. Am J Respir Crit Care Med. 2010;182(11):1426–1434. doi: 10.1164/rccm.201003-0415OC. [DOI] [PubMed] [Google Scholar]
- 11.Schuetz P, Christ-Crain M, Thomann R, Falconnier C, Wolbers M, Widmer I, Neidert S, Fricker T, Blum C, Schild U, Regez K, Schoenenberger R, Henzen C, Bregenzer T, Hoess C, Krause M, Bucher HC, Zimmerli W, Mueller B, ProHOSP Study Group Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059–1066. doi: 10.1001/jama.2009.1297. [DOI] [PubMed] [Google Scholar]
- 12.Silveira CD, Ferreira CS, Corrêa R de A. Adherence to guidelines and its impact on outcomes in patients hospitalized with community-acquired pneumonia at a university hospital. J Bras Pneumol. 2012;38(2):148–157. doi: 10.1590/s1806-37132012000200002. [DOI] [PubMed] [Google Scholar]
- 13.Wunderink RG, Waterer GW. Community-acquired pneumonia. N Engl J Med. 2014;370(19):1863. doi: 10.1056/NEJMc1402692. [DOI] [PubMed] [Google Scholar]
- 14.Salluh JIF, Lisboa T, Bozza FA, Soares M, Póvoa P. Management of severe community-acquired pneumonia: a survey on the attitudes of 468 physicians in Iberia and South America. J Crit Care. 2014;29(5):743–747. doi: 10.1016/j.jcrc.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Lim WS, Woodhead M, British Thoracic Society British Thoracic Society adult community acquired pneumonia audit 2009/10. Thorax. 2011;66(6):548–549. doi: 10.1136/thoraxjnl-2011-200081. [DOI] [PubMed] [Google Scholar]
- 16.Corrêa R de A, Lundgren FL, Pereira-Silva JL, Frare e Silva RL, Cardoso AP, Lemos AC, Rossi F, Michel G, Ribeiro L, Cavalcanti MA, de Figueiredo MR, Holanda MA, Valery MI, Aidê MA, Chatkin MN, Messeder O, Teixeira PJ, Martins RL, da Rocha RT, Comissão de Infecções Respiratórias e Micoses - Sociedade Brasileira de Pneumologia e Tisiologia Brazilian guidelines for community-acquired pneumonia in immunocompetent adults - 2009. J Bras Penumol. 2009;35(6):574–601. doi: 10.1590/s1806-37132009000600011. [DOI] [PubMed] [Google Scholar]
- 17.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Jr, Musher DM, Niederman MS, Torres A, Whitney CG, Infectious Diseases Society of America; American Thoracic Society Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabello LS, Pitrowsky MT, Soares M, Póvoa P, Salluh JI. Novel biomarkers in severe community-acquired pneumonia. Rev Bras Ter Intensiva. 2011;23(4):499–506. [PubMed] [Google Scholar]
- 19.Arnold FW, Summersgill JT, Lajoie AS, Peyrani P, Marrie TJ, Rossi P, Blasi F, Fernandez P, File TM, Jr, Rello J, Menendez R, Marzoratti L, Luna CM, Ramirez JA, Community-Acquired Pneumonia Organization (CAPO) Investigators A worldwide perspective of atypical pathogens in community-acquired pneumonia. Am J Respir Crit Care Med. 2007;175(10):1086–1093. doi: 10.1164/rccm.200603-350OC. [DOI] [PubMed] [Google Scholar]
- 20.Torres A, Blasi F, Peetermans WE, Viegi G, Welte T. The aetiology and antibiotic management of community-acquired pneumonia in adults in Europe: a literature review. Eur J Clin Microbiol Infect Dis. 2014;33(7):1065–1079. doi: 10.1007/s10096-014-2067-1. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noritomi DT, Ranzani OT, Monteiro MB, Ferreira EM, Santos SR, Leibel F, et al. Implementation of a multifaceted sepsis education program in an emerging country setting: clinical outcomes and cost-effectiveness in a long-term follow-up study. Intensive Care Med. 2014;40(2):182–191. doi: 10.1007/s00134-013-3131-5. [DOI] [PubMed] [Google Scholar]