Abstract
The corticospinal tract (CST) can be completely severed unilaterally in the medullary pyramids of the rodent brainstem. The CST is a motor tract that has great importance for distal muscle control in humans and, to a lesser extent, in rodents. A unilateral cut of one pyramid results in loss of CST innervation of the spinal cord mainly on the contralateral side of the spinal cord leading to transient motor disability in the forelimbs and sustained loss of dexterity. Ipsilateral projections of the corticospinal tract are minor. We have refined our surgical method to increase the chances of lesion completeness. We describe postsurgical care. Deficits on the Montoya staircase pellet reaching test and the horizontal ladder test shown here are detected up to 8 weeks postinjury. Deficits on the cylinder rearing test are only detected transiently. Therefore, the cylinder test may only be suitable for detection of short term recovery. We show how, electrophysiologically and anatomically, one may assess lesions and plastic changes. We also describe how to analyse fibers from the uninjured CST sprouting across the midline into the deprived areas. It is challenging to obtain >90% complete lesions consistently due to the proximity to the basilar artery in the medulla oblongata and survival rates can be low. Alternative surgical approaches and behavioural testing are described in this protocol. The pyramidotomy model is a good tool for assessing neuroplasticity-inducing treatments, which increase sprouting of intact fibers after injury.
Keywords: Neuroscience, Issue 94, central nervous system lesions, pyramids, unilateral spinal cord injury, in vivo, corticospinal tract lesion, forelimb function,
Introduction
The corticospinal tract (CST) is one major motor tract in the human spinal cord. Damage to this tract after spinal cord injury results in considerable loss of dexterity. The CST is especially important for fine motor movement in humans, such as digit movement and distal muscle control. It is also found in rodents although its anatomical location within the spinal cord is different to that of humans and CST damage is less disabling 1-4.
One in vivo model of CST injury involves unilateral pyramidotomy, where one pyramid in the ventral brainstem is cut rostral to the decussation (Figure 1). The pyramids are a clear anatomical feature on the ventral side of the medulla oblongata through which the CST runs before it decussates. In rats, the majority of fibers (around 95%) decussate at the caudal end of the medulla and are thereafter found in the dorsal medial part of the spinal cord. However, approximately 10% of the decussated fibers run in the dorsolateral columns. The remaining ~5% of fibers stay uncrossed on the ipsilateral side and travel in the ventral medial part 5,6. After pyramidotomy the spinal cord loses its direct input from the contralateral CST (Figure 1).
The pyramidotomy model is particularly good for testing the responses of the intact fibers of the unlesioned CST to a treatment. It allows assessment of plasticity and sprouting of the intact fibers across the midline in the spinal cord, which innervate the denervated areas. The corticospinal tract has been observed to sprout considerably in spinal injury models and mediates especially forelimb recovery 7-10. A treatment might aim to enhance this forelimb recovery. The efficacy of a therapy can be tested with behavioural assessments, anatomical tracing techniques of the unlesioned tract and terminal electrophysiological experiments 3,11,12.
The advantage in comparison to most other preclinical models of spinal injuries is that it specifically affects one tract compared to other spinal injuries often affecting multiple tracts. Only one tract in a clear, superficial anatomical location is lesioned. Another advantage is that the pyramidotomy is a reproducible injury model. It is a suitable in vivo resource for researchers to test the effect of potential neuroplasticity-inducing therapies, which aim at recovery of motor function after injuries or disorders affecting the motor system 13,14.
Protocol
Ethics statement: All procedures were in accordance with guidelines from the UK Home Office and Animals (Scientific Procedures) Act of 1986.
1. Surgery and Injury
NOTE: Using rats heavier than 220 g can help with postoperative recovery if the surgeon finds animals often lose more than 10% of their preoperative body weight.
Before surgery, test the animals for their forepaw preference with the cylinder rearing test 15 or the Montoya staicase test 24. Preferably, cut the pyramid/ the CST corresponding to the dominant forepaw. NOTE: For two days prior to surgery, place the following items into the cages, so that the rats become familiar with food items that will be beneficial during recovery from surgery: hydrogel packs, wet mash, and high caloric food supplements. Sterile versions of these food supplements are available from various vendors.
Anesthetize female Lister hooded rats (weighing between 200 and 250 g) with isoflurane (5% for induction and 1 to 2% for maintenance) in oxygen (flow-rate: 1.5 L/min). NOTE: The choice of anesthetic is important. Inhalation anesthetic allows for faster regulation of the depth of anesthesia than ketamine, which is also commonly used. Isoflurane allows for better control of the breathing frequency and depth. The choice of anesthetic is isoflurane. NOTE: Using rats heavier than 220 g can help with postoperative recovery if the surgeon finds animals often lose more than 10% of their preoperative body weight.
Administer Carprofen subcutaneously as an analgesic when the animals are anaesthetized to reduce inflammation and to provide analgesia. Note: Carprofen is given during pyramidotomy surgery in our laboratory according to our veterinarian's advice. Opioids might cause further respiratory depression during surgery.
Maintain the animals at 37 °C during surgery using a homeothermic blanket system and a rectal temperature probe.
Confirm full anesthesia by checking for the paw pinch withdrawal reflex and the blink reflex. Note: Optional: To counter respiratory problems during surgery, intubation and ventilation might be feasible. However, bear in mind that the trachea needs to be displaced during surgery.
Once anesthetized, place the rat in a supine position, shave the ventral neck and sterilize it with 1% chlorhexidine wipes and/or alcohol wipes. Apply a sterile surgical blanket over the animal and keep the area sterile at all times.
Once anesthetized, place the rat in a supine position, shave the ventral neck and sterilize it with 1% chlorhexidine and alcohol wipes. Apply a sterile surgical blanket over the animal and keep the area sterile at all times. NOTE: Optionally, the rat’s head can be fixed in a stereotaxic frame in the supine position during surgery to disable mobility of the head, i.e. the occipital bone.
Make a 2 to 3 cm long midline incision from the chin to almost the rostral end of the sternum using a sterile scalpel (#10). Optionally, apply small bulldog clamps to retract the skin.
Blunt dissect the upper layers of tissue such as glands (e.g. submaxillary gland and parotid gland) and muscles covering the trachea using reverse action with blunted scissors and toothed forceps. Always stay on the midline. The tissues should separate easily.
Once the trachea is exposed, displace it to one side. The midline is visible underneath it with two white fat pads, marking the midline at the rostral end.
Rostral to the white fat pads, blunt dissect tissue until the ventral surface of the skull (the basioccipital bone) is reached.
Insert long-toothed retractors (tooth length 5.5 mm or 16 mm) to keep the trachea displaced to one side (maximum 1 cm) and to expose the base of the skull. Displacement of the trachea can obstruct breathing. NOTE: For some animals, loosen the retractors at multiple times during the surgery to remove the strain on the trachea, e.g. every 10 min for 2 min. Hook retractors can be used instead of long-toothed retractors to reduce the strain of displacement.
Carefully cauterize small visible blood vessels at the rostral end going towards the larynx to avoid bleeding. NOTE: In order to prevent cauterizing the recurrent laryngeal nerve blood vessels are carefully pulled away from the tissue wall before they are cauterized.
Adjust the microscope for the remainder of the surgery.
Remove the periosteum covering the base of the skull with fine forceps. Adjust the retractors to achieve the best possible view for drilling.
Feel the uneven surface of the basioccipital bone with fine forceps, noting an elevation at the midline, which covers the basilar artery. Rostrally, the skull becomes raised as a slight convexity.
Depending on which side the lesion is desired, drill a hole roughly 1 mm lateral to the midline in mediolateral movements. NOTE: Remember that the rat is in the supine position, and its left side may be on the investigators right hand side!
Once a hole is made, enlarge towards the midline with small vertical up-and-downwards-movements with the drill until the basilar artery is clearly visible and enlarge laterally for at least 2 mm.
Make sure the basilar artery is clearly visible. The pyramid can be identified by its slightly bulging shape (Figure 2) and by bordering blood vessels.
Remove any remaining bone fragments with fine forceps.
Apply one drop of Doxapram hydrochloride, a respiratory stimulant, on the tongue.
Open the dura longitudinally with a 26 gauge needle and fine forceps lateral to the basilar artery. Soak up the cerebrospinal fluid (CSF) and any bleeding with cotton buds.
Avoiding injury to the basilar artery, make a cut approximately 1.5 mm wide spanning the width of the pyramid and 0.5 mm deep with Vannas Spring Scissors perpendicular to the basilar artery (Figure 2) to interrupt the CST fibers. NOTE: Mark the microscissors beforehand at 0.5 mm from the tips. Once the dura is opened the basilar artery can be moved slightly to one side allowing better access to the pyramid.
Repeat the cut using the tip of a 26 gauge needle to ensure the lesion includes the fibers close to the basilar artery.
Stop the bleeding with light pressure applied by cotton buds. Optionally, the cut can be covered with gelfoam.
Remove the retractors, replace the tissues and suture only the skin with 3-0 Vicryl sutures.
After the surgery, keep the animal in an incubator at 32 °C until fully awake and administer 5 ml saline subcutaneously, if required. Do not leave the animal unattended.
Give Carporfen subcutaneously as an antiinflammatory and as an analgesic for the post-surgical pain one day postsurgery.
Monitor animals closely for one week after surgery and at least once a week thereafter.
As required after surgery, place hydration gel packs, pureed baby food, wet mash and dry chow on cage floor. Provide water bottles with long spouts (lacking ball bearing valves).
Representative Results
Survival rates.
In a representative study conducted by us we had a survival rate of 16 out of 20 female Lister hooded rats (200 to 250 g). The most common complication is breathing difficulties because of the trachea displacement or damage to the respiratory centers in the brainstem just dorsal to the pyramids (nucleus solitarii, ambiguous and parabrachilis). Similar results have been reported in the literature 16.
Behavioural outcomes.
A range of behavioural tests have been used to assess sensorimotor outcome after pyramidotomy in rodents: the horizontal ladder test, the single pellet reaching test, the cylinder rearing test and Montoya staircase test are the most commonly used behavioural assessments. Other tests described in the literature are the pellet reaching test, rope climbing test, gait analysis and the sticky tape test 3,17-19. Most of these show a functional motor deficit 1 week after injury. Currently, most studies described in the literature only perform behavioural testing up to 42 days 4,11,13,20,21, or for a shorter time 3,18,22. We have performed behavioural testing for a prolonged period of time and have seen essentially full recovery of motor function 8 weeks post pyramidotomy for some of the behavioural tests, except the Montoya staircase test and the horizontal ladder test. This is probably for several reasons. Firstly, the injured CST rostral to the lesion may form synapses on neurons in the brainstem that act as indirect relays to motor neurons in the spinal cord. Secondly, the uninjured (contralateral) CST may sprout and forms synapses on neurons in the brainstem or spinal cord that act as relays to motor neurons. Thirdly, other spared motor tracts such as the reticulospinal, vestibulospinal or rubrospinal tracts may take over some function. Thus, a lasting treatment effect is difficult to detect with many behavioural tests in the pyramidotomy model. Some behavioural testing in this model however could indicate accelerated recovery early after the injury, which is related to a treatment.
Cylinder Rearing test 3,4,23
Based on the cylinder rearing test the CST corresponding to the dominant forepaw was injured. Rats are placed in a plexiglass cylinder and rearing behaviour is observed for a period of 3 min. Preinjury there was a slight preference for the use of one forepaw, the dominant forepaw, during vertical exploration (Figure 3A). The injury affects the dominant side and the use of the contralesional forepaw decreased to 28% at 1 week postinjury (Repeated Measures ANOVA p <0.05; post-hoc analysis revealed animals have significant deficits at Week 1, 2 and 3, p <0.05, Fisher’s LSD). 4 weeks postinjury the animals recovered and used the affected forelimb for 45% of rears, and after 6 weeks this was 57% (n = 16, mean ± standard error is shown). Similarly, Starkey et al. (4) have assessed mice using the cylinder test for up to 42 days after pyramidotomy and reported a significant decrease in the use of the affected forepaw throughout the whole testing period.
Montoya Staircase test 24
Rats require pretraining for this test to acclimatize prior to injury. To increase motivation during the testing session animals are food restricted to 15 g food per rat the night before. The food deprived rats are placed in the Montoya staircase for 15 min. On each side, there is a staircase of 7 steps with wells, each containing 3 pellets. The total number of retrieved pellets on each side are recorded. The number of retrieved pellets with the contralesional/affected forepaw decreased significantly from 61% preinjury to 3% 3 days postinjury (Figure 3B). The ability to retrieve pellets was still significantly impaired at 4 weeks postinjury with 20% of pellets retrieved. Rats show a significant deficit up to week 8 with only 29% of the pellets eaten at that time point (Repeated Measures ANOVA p <0.05, post hoc analysis revealed animals have significant deficits on the contralesional side at all weeks, p <0.05, Fisher’s LSD). The ipsilesional forepaw is affected up to 1 week postinjury; however this recovers by the second week (Repeated Measures ANOVA p <0.05, post hoc analysis revealed animals have significant deficits at Day 3 and Week 1 on the ipsilesional forepaw, p <0.05, Fisher’s LSD). The initial deficits of the ipsilesional forepaw might be explained by the lesioning of the ipsilateral portion of corticospinal tract running through the cut pyramid, with subsequent sprouting and recovery.
Horizontal Ladder test 25
Rats require pretraining for this test. Rats walk three times over a 1 m long ladder with irregular rung spacing. Videos are analysed later in slow motion and the total number of foot slips and misses for each paw is quantified. The graph in Figure 3C represents the total number of misses and slips of the affected and less affected side (forepaw + hindpaw) as a percentage to the total number of steps taken. Rats have a significantly increased number of errors 1 week postinjury with 10% or 11% on the affected side and less affected side respectively. This deficit is persistent on the contralesional/affected side up to week 8 with 10% errors made, however, resolves on the ipsilesional/less affected side by 2 weeks (Repeated Measures ANOVA, post hoc analysis revealed animals have significant deficits at all weeks on the contralesional side and significant deficits at Week 1 and 3 on the ipsilesional side, p <0.05, Fisher’s LSD).
Electrophysiological testing
In a terminal electrophysiological set-up the animal was anaesthetized intraperitoneally with urethane. Throughout the procedure, it was kept at 37 °C ± 1 °C. Its ventral neck region, its forelimbs and chest region were shaved and disinfected with iodine scrub. The pyramids were exposed as previously described and the radial nerve was exposed in the upper arm by a ventral approach incising the skin and the pectoralis major. The distal end of the radial nerve was cut and the nerve was laid over two silver-wire hook-electrodes in a mineral oil bath. Either pyramid was stimulated with concentric bipolar electrodes at different depths with 5 pulses at 300 Hz and increasing stimulation amplitude. Recordings were always made from the radial nerve of the disabled arm (Figure 4).
Uninjured animals had a strong response at latencies between 12 to 20 msec in the radial nerve when the contralateral pyramid was stimulated (Figure 4B). Ipsilateral stimulation only results in occasional activation (Figure 4C). Animals, which have previously received pyramidotomies, on the contralateral side to the recording arm show no response in the radial nerve when the lesioned tract is stimulated above the lesion (Figure 4B’). However, stimulation of the CST fibers in the pyramids ipsilateral to the recording electrodes resulted in a stronger activation in the radial nerve compared to uninjured animals at 12 weeks postinjury (Figure 4C’).
Anatomical outcomes.
Animals were euthanized 10 weeks postsurgery according to Schedule 1 of the Animals (Scientific Procedures) Act 1986. Two weeks previously, the uninjured CST was traced with biotinylated dextran amine (BDA) injections into the motor cortex 26 ipsilateral to the disabled arm (Figure 1). This allows quantification of uninjured fibers, which cross the midline and supply the contralesional spinal cord (Figure 5C, D). Uninjured animals only have a few fibers crossing the midline, whereas this increases in response to injury. Transverse cervical spinal cord sections were stained with protein kinase C (PKC) gamma to assess the proportion of denervation in the lesioned CST (Figure 5E)16. Alternatively, the completeness of the lesion can be assessed by injecting BDA tracer into the motor cortex on the ipsilesional side of the pyramidotomy injury. The absence of labeling in the contralateral dorsomedial CST and the ipsilateral ventromedial CST provides evidence of complete unilateral transection.
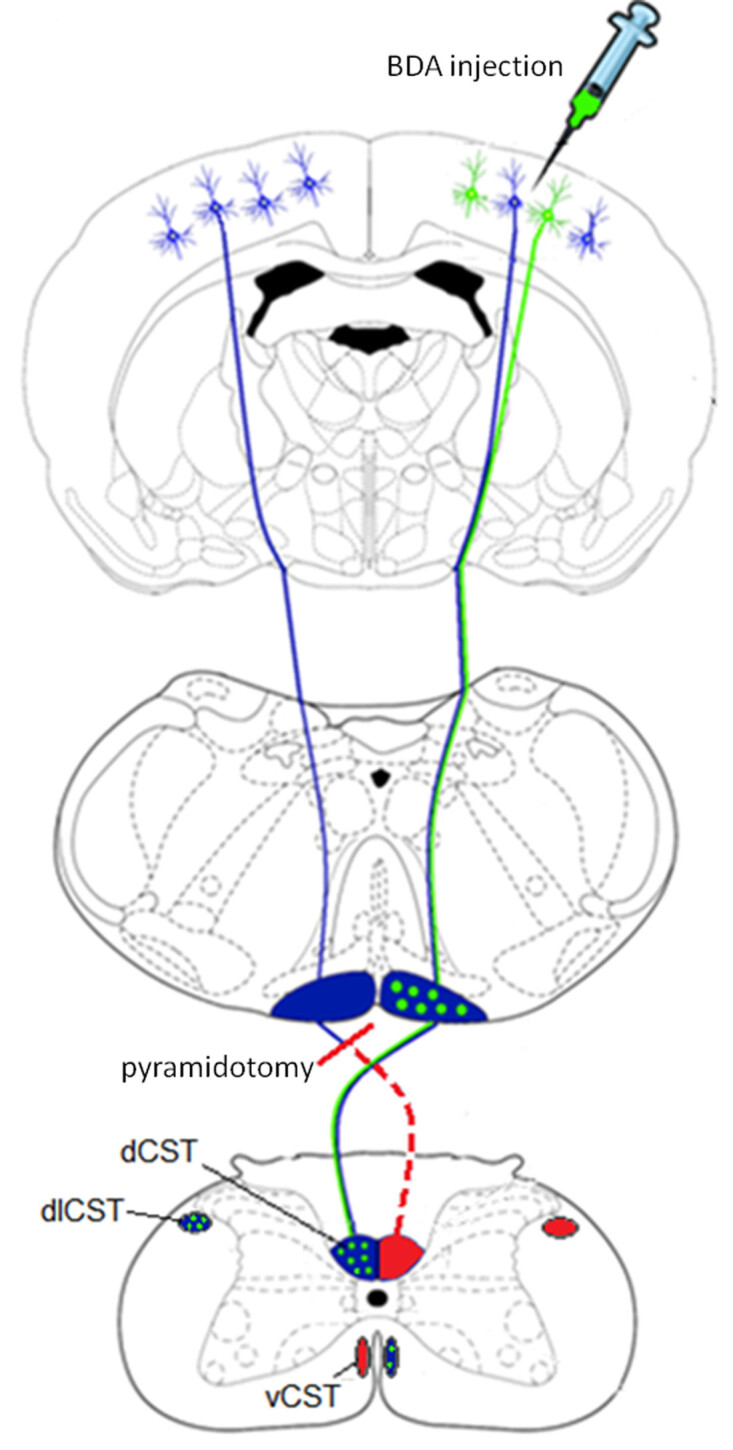 Figure 1: Schematic showing the corticospinal tracts in the rat and unilateral axotomy of the CST in the pyramids. Top image: Corticospinal neurons originate in the cortical layer 5 pyramidal cells. Biotinylated dextran amine (depicted in green) is injected into the contralesional cortex two weeks prior to the end of the experiment. Middle image: The tracts form the pyramids on the ventral surface of the medulla and at the spinomedullary junction the majority of axons decussate. Unilateral pyramidotomy is performed in the caudal medulla (shown in red). Lower image: The majority of the corticospinal tracts run down in the dorsomedial and dorsolateral parts of the spinal cord. A minority stays ipsilaterally and runs ventromedially.
Figure 1: Schematic showing the corticospinal tracts in the rat and unilateral axotomy of the CST in the pyramids. Top image: Corticospinal neurons originate in the cortical layer 5 pyramidal cells. Biotinylated dextran amine (depicted in green) is injected into the contralesional cortex two weeks prior to the end of the experiment. Middle image: The tracts form the pyramids on the ventral surface of the medulla and at the spinomedullary junction the majority of axons decussate. Unilateral pyramidotomy is performed in the caudal medulla (shown in red). Lower image: The majority of the corticospinal tracts run down in the dorsomedial and dorsolateral parts of the spinal cord. A minority stays ipsilaterally and runs ventromedially.
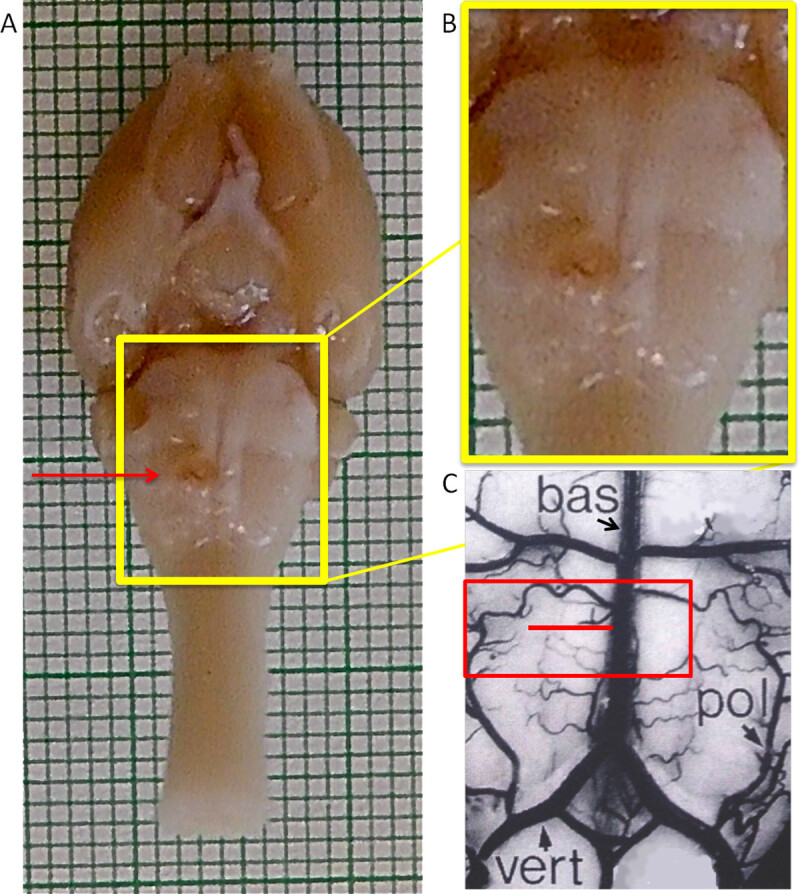 Figure 2: Ventral view of the rat brain. The pyramids are distinctive because they are raised, parallel convexities (yellow box). The borders of the pyramids are defined medially by the basilar artery (bas) over the midline and laterally at the base of the convexity halfway between the basilar artery and the paraolivary artery (pol) as seen in (C). Craniotomy is performed over the basilar artery and lateral extending to the paraolivary artery (red box in C). One pyramid is cut perpendicularly to the basilar artery (red line in C, cut is shown on specimen in A and B). Lesions placed too caudally near the vertebral arteries (vert) will be incomplete because the CST starts to decussate and because the vertebral artery is set off the midline. (C) Image adapted from “The Rat Nervous System” by George Paxinos27. NOTE: This Figure shows a lesion on the left hand side of the images: this is the animal’s right pyramidal tract because it is lying supine. Please note however that the video shows a lesion to the animal’s left pyramidal tract.
Figure 2: Ventral view of the rat brain. The pyramids are distinctive because they are raised, parallel convexities (yellow box). The borders of the pyramids are defined medially by the basilar artery (bas) over the midline and laterally at the base of the convexity halfway between the basilar artery and the paraolivary artery (pol) as seen in (C). Craniotomy is performed over the basilar artery and lateral extending to the paraolivary artery (red box in C). One pyramid is cut perpendicularly to the basilar artery (red line in C, cut is shown on specimen in A and B). Lesions placed too caudally near the vertebral arteries (vert) will be incomplete because the CST starts to decussate and because the vertebral artery is set off the midline. (C) Image adapted from “The Rat Nervous System” by George Paxinos27. NOTE: This Figure shows a lesion on the left hand side of the images: this is the animal’s right pyramidal tract because it is lying supine. Please note however that the video shows a lesion to the animal’s left pyramidal tract.
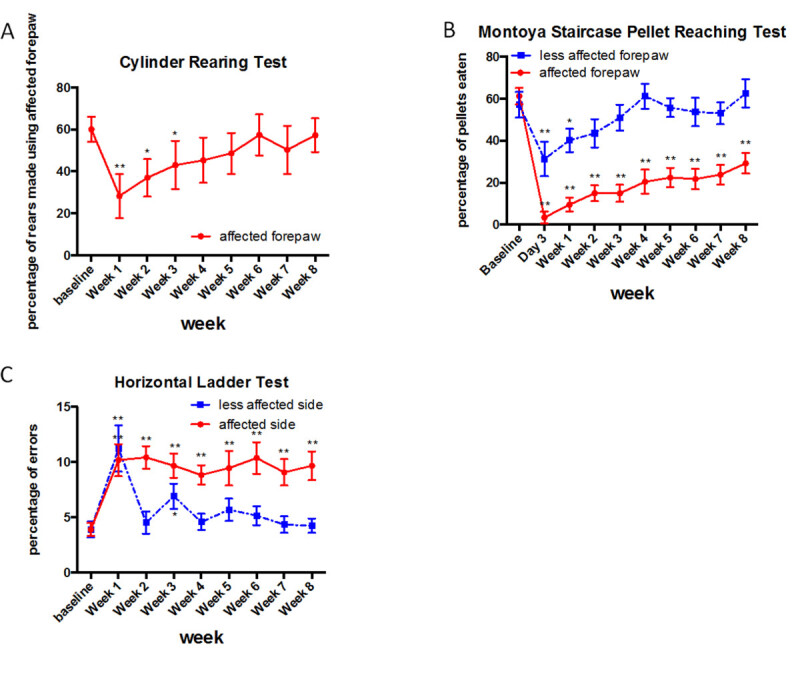 Figure 3: Representative results for the cylinder rearing test, Montoya staircase pellet reaching test and Horizontal ladder test. There is a motor deficit detected by all tests after injury. However, no deficit can be detected with the cylinder rearing test at 4 weeks postinjury, whereas sustained deficits can be detected with the Montoya staircase test and the horizontal ladder test up to 8 weeks postinjury (n = 16 per group, means and standard error are shown). Asterisk indicates p <0.05 relative to preinjury baseline (Fisher’s LSD test).Please click here to view a larger version of this figure.
Figure 3: Representative results for the cylinder rearing test, Montoya staircase pellet reaching test and Horizontal ladder test. There is a motor deficit detected by all tests after injury. However, no deficit can be detected with the cylinder rearing test at 4 weeks postinjury, whereas sustained deficits can be detected with the Montoya staircase test and the horizontal ladder test up to 8 weeks postinjury (n = 16 per group, means and standard error are shown). Asterisk indicates p <0.05 relative to preinjury baseline (Fisher’s LSD test).Please click here to view a larger version of this figure.
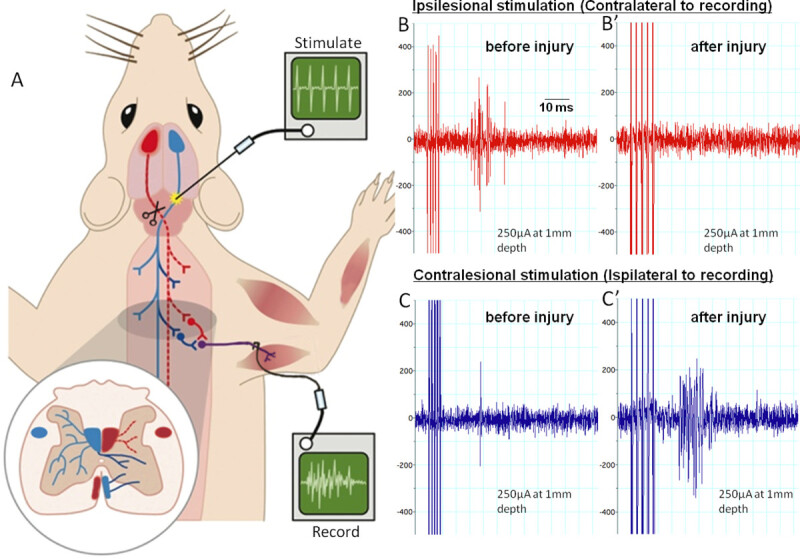 Figure 4: Electrophysiology assessing the corticospinal tract before and after pyramidotomy.(A) shows the set-up for the terminal experiment. We stimulate the pyramids with a train of 5 pulses at various depths rostral to the site of lesioning and decussation. The red tract is the lesioned tract. We record from the radial nerve on the contralesional side. (B) and (C) show example recordings with ipsilesional stimulation or contralesional stimulation before or 12 weeks after pyramidotomy. Ipsilesional stimulations evoke multiple compound action potentials (spikes in the trace) in the radial nerve before injury (B). These are abolished by the injury and activity does not return within 12 weeks (B’). Contralesional stimulation evokes rare single compound action potentials preinjury (C). 12 weeks after injury activity considerably is increased (C’), possibly due to intact axons sprouting across the midline and plastic changes within the spinal cord. Please click here to view a larger version of this figure.
Figure 4: Electrophysiology assessing the corticospinal tract before and after pyramidotomy.(A) shows the set-up for the terminal experiment. We stimulate the pyramids with a train of 5 pulses at various depths rostral to the site of lesioning and decussation. The red tract is the lesioned tract. We record from the radial nerve on the contralesional side. (B) and (C) show example recordings with ipsilesional stimulation or contralesional stimulation before or 12 weeks after pyramidotomy. Ipsilesional stimulations evoke multiple compound action potentials (spikes in the trace) in the radial nerve before injury (B). These are abolished by the injury and activity does not return within 12 weeks (B’). Contralesional stimulation evokes rare single compound action potentials preinjury (C). 12 weeks after injury activity considerably is increased (C’), possibly due to intact axons sprouting across the midline and plastic changes within the spinal cord. Please click here to view a larger version of this figure.
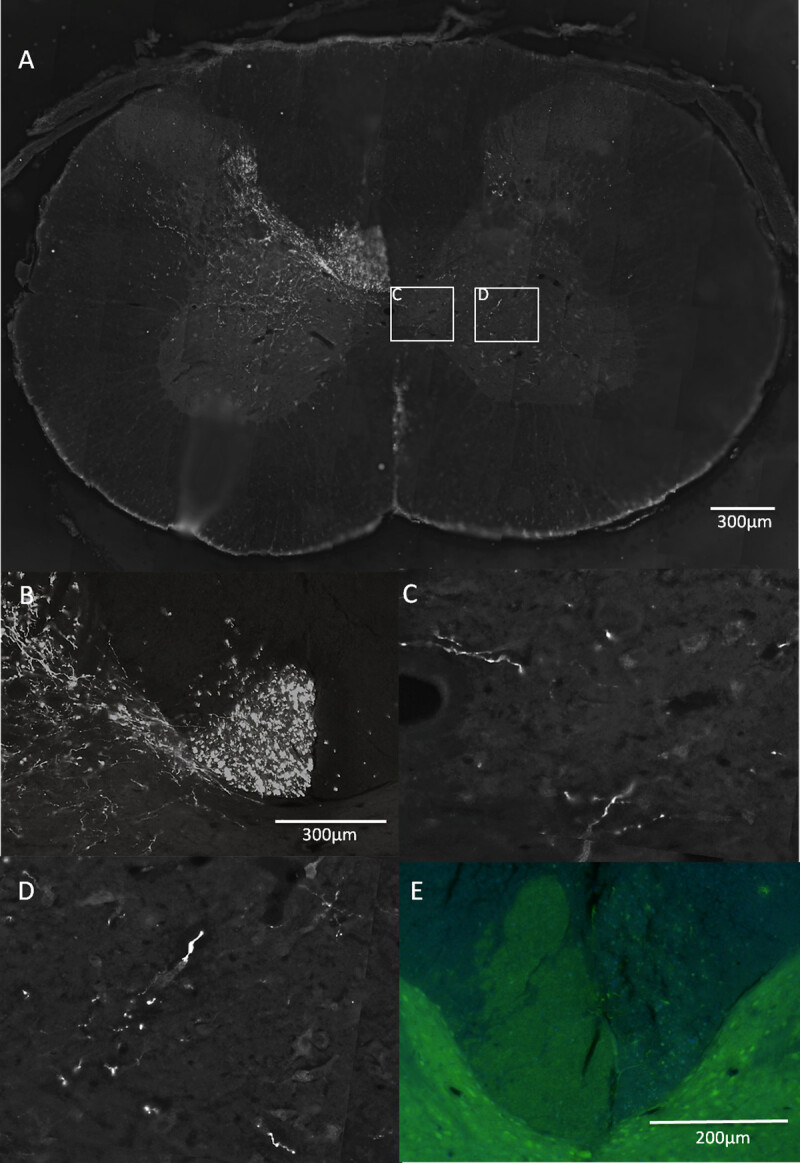 Figure 5: Anatomical evaluation after pyramidotomy.(A, B, C, D) Biotinylated dextran amine was used to trace the corticospinal tract by injecting it into the motor cortex corresponding to the unlesioned CST (see green in Figure 1). The dorsal medial, the ventral medial components of the CST are clearly visible (A, dorsal medial part magnified in B). (C) and (D) are magnifications from (A) of fibers sprouting across the midline into the grey matter of the denervated side in response to a treatment. To assess the completeness of the lesion transverse sections of the cervical level 4 (12 weeks postinjury tissue) were cut and stained with antibodies against PKC gamma (E). The CST on the left hand side of the image is intact and can be seen bulging across the midline into the denervated side, presumably because of the structural disintegration of the injured dorsal CST.
Figure 5: Anatomical evaluation after pyramidotomy.(A, B, C, D) Biotinylated dextran amine was used to trace the corticospinal tract by injecting it into the motor cortex corresponding to the unlesioned CST (see green in Figure 1). The dorsal medial, the ventral medial components of the CST are clearly visible (A, dorsal medial part magnified in B). (C) and (D) are magnifications from (A) of fibers sprouting across the midline into the grey matter of the denervated side in response to a treatment. To assess the completeness of the lesion transverse sections of the cervical level 4 (12 weeks postinjury tissue) were cut and stained with antibodies against PKC gamma (E). The CST on the left hand side of the image is intact and can be seen bulging across the midline into the denervated side, presumably because of the structural disintegration of the injured dorsal CST.
Discussion
We have described the pyramidotomy injury model. The corticospinal tract is cut at the level of the brainstem. From a technical point of view this surgery requires a lot of cautiousness and accuracy. The brainstem accommodates the breathing centers and the cardiovascular centers in multiple nuclei such as the nucleus ambiguous and solitarii embedded in the reticular formation underneath the pyramids. If the cut is made too deep this can lead to sudden breathing difficulties, which may be fatal. We give Dopram just before the cut to increase the breathing activity to prevent a sudden breathing cessation. If the breathing stops it is worth ventilating the animal for the following five minutes with oxygen as it may recover. Additionally, any damage to the basilar artery should absolutely be avoided. Complications during the first few days postinjury are: another hemorrhage in the brainstem affecting vital functions, considerable weight loss, severe breathing difficulties due to trachea displacement and severe orientation and balance problems of the animal due to extensive injury including the olives just lateral to the pyramids. These complications can be treated as end-points and animals may be humanely killed. Close monitoring during the first week after surgery is essential. Supplementary food can be given in order to counterbalance weight loss. Airway inflammation (raspy breathing) normally resolves within one to two days. Generally, younger animals are easier to operate on as their neck is less deep. Furthermore, they are more resilient and have less breathing complications.
It requires some practice to achieve a complete lesion of the CST with a pyramidotomy. Similarly, in two studies by Steward et al. 16, which set out to replicate a former experiment by Benowitz et al. 28, reported initially high mortality rates and that some of their lesions were incomplete. Behavioural deficits will also be variable. We recommend that a new surgeon performs many practice pyramidotomy surgeries before any full experiment to test a candidate therapy is performed. Ways to prevent incomplete lesions are retracing the cut with a hypodermic needle making sure the corticospinal fibers close to the basilar artery are severed as described in this protocol. Other options are to aspirate the lesion site with a tipped glass suction pipette as described in Zhou et al. 2003 29. A fine tungsten wire can also be used to cut the tract 22. An alternative to pyramidotomy is to cut the cervical C3 dorsal CST unilaterally and then to cut with a ventral approach the ipsilesional C2 ventral part of the CST 18. However arguably, a complete lesion is not necessary if the goal is to study sprouting of the contralesional CST; if the majority of the pyramid is lesioned and a sprouting response from the unlesioned side occurs this can be sufficient.
One aim of the pyramidotomy lesion model is to ablate one side of the corticospinal tract and use neuroplasticity-inducing therapies to stimulate the plasticity and sprouting of the intact non injured fibers. To test neuro-protective therapies this model is unsuitable and other injury models such as contusion injuries should be used. Outcomes of pyramidotomies are assessed most often with behavioural testing and anatomy. The horizontal ladder test, the cylinder rearing test and Montoya staircase test are the most commonly used behavioural assessments. Other tests described in the literature are the pellet reaching test, rope climbing test, gait analysis and the sticky tape test 3,17-19. However, the outcomes of most behavioural test are often only monitored up to a maximum of 42 days postlesion 3,4,11,17,18,20-22. From past experiences, rodents recover most function at later time points without any treatment intervention. We have shown that the horizontal ladder test and the Montoya staircase pellet reaching test detect disabilities up to a later time point. They can be used to measure functional recovery due to a treatment. The lesion completeness is judged by PKC gamma staining (which labels CST fibers in the dorsal columns) of the spinal cord at rostral cervical level or eriochrome cyanine staining of transverse sections of the lesion site. Biotinylated dextran amine can be injected into the contralesional cortex to assess the sprouting of the intact fibers across the midline into the denervated spinal cord area.
In conclusion, the pyramidotomy model is good for assessing neuroplasticity-inducing abilities of a treatment drug for central nervous system injuries if the surgery is successfully mastered. It assesses plasticity of intact fibers. The next step would be to use a more clinically relevant model such as contusion injuries to assess the drug’s ability to overcome larger injuries affecting multiple tracts.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by the International Spinal Research Trust and the Rosetrees Trust.
References
- Lemon RN. Descending pathways in motor control. Annual review of neuroscience. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Griffiths J. Comparing the function of the corticospinal system in different species: organizational differences for motor specialization. Muscle & nerve. 2005;32:261–279. doi: 10.1002/mus.20333. [DOI] [PubMed] [Google Scholar]
- Starkey ML, et al. Assessing behavioural function following a pyramidotomy lesion of the corticospinal tract in adult mice. Experimental neurology. 2005;195:524–539. doi: 10.1016/j.expneurol.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Starkey ML, Bartus K, Barritt AW, Bradbury EJ. Chondroitinase ABC promotes compensatory sprouting of the intact corticospinal tract and recovery of forelimb function following unilateral pyramidotomy in adult mice. Eur J Neurosci. 2012;36:3665–3678. doi: 10.1111/ejn.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosamle C, Schwab ME. Cells of origin, course, and termination patterns of the ventral, uncrossed component of the mature rat corticospinal tract. The Journal of comparative neurology. 1997;386:293–303. doi: 10.1002/(sici)1096-9861(19970922)386:2<293::aid-cne9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Brosamle C, Schwab ME. Ipsilateral, ventral corticospinal tract of the adult rat: ultrastructure, myelination and synaptic connections. Journal of neuroctology. 2000;29:499–507. doi: 10.1023/a:1007297712821. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Chen Q, Smith GM, Shine HD. Immune activation is required for NT-3-induced axonal plasticity in chronic spinal cord injury. Exp Neurol. 2008;209:497–509. doi: 10.1016/j.expneurol.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Z'Graggen WJ, et al. Compensatory sprouting and impulse rerouting after unilateral pyramidal tract lesion in neonatal rats. J Neurosci. 2000;20:6561–6569. doi: 10.1523/JNEUROSCI.20-17-06561.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig ES, et al. Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nat Neurosci. 2010;13:1505–1510. doi: 10.1038/nn.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Z'Graggen WJ, Metz GA, Kartje GL, Thallmair M, Schwab ME. Functional recovery and enhanced corticofugal plasticity after unilateral pyramidal tract lesion and blockade of myelin-associated neurite growth inhibitors in adult rats. J Neurosci. 1998;18:4744–4757. doi: 10.1523/JNEUROSCI.18-12-04744.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartje-Tillotson G, O'Donoghue DL, Dauzvardis MF, Castro AJ. Pyramidotomy abolishes the abnormal movements evoked by intracortical microstimulation in adult rats that sustained neonatal cortical lesions. Brain Res. 1987;415:172–177. doi: 10.1016/0006-8993(87)90283-6. [DOI] [PubMed] [Google Scholar]
- Thallmair M, et al. Neurite growth inhibitors restrict plasticity and functional recovery following corticospinal tract lesions. Nature Neuroscience. 1998;1:124–131. doi: 10.1038/373. [DOI] [PubMed] [Google Scholar]
- Akbik FV, Bhagat SM, Patel PR, Cafferty WB, Strittmatter SM. Anatomical plasticity of adult brain is titrated by Nogo Receptor 1. Neuron. 2013;77:859–866. doi: 10.1016/j.neuron.2012.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacol. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Steward O, Sharp K, Yee KM. A re-assessment of the effects of intracortical delivery of inosine on transmidline growth of corticospinal tract axons after unilateral lesions of the medullary pyramid. Experimental neurology. 2012;233:662–673. doi: 10.1016/j.expneurol.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thallmair M, et al. Neurite growth inhibitors restrict plasticity and functional recovery following corticospinal tract lesions. Nature neuroscience. 1998;1:124–131. doi: 10.1038/373. [DOI] [PubMed] [Google Scholar]
- Weidner N, Ner A, Salimi N, Tuszynski MH. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl Acad Sci U S A. 2001;98:3513–3518. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell L, Schneider R, Kolbeck R, Barde YA, Schwab ME. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature. 1994;367:170–173. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- Starkey ML, Bleul C, Maier IC, Schwab ME. Rehabilitative training following unilateral pyramidotomy in adult rats improves forelimb function in a non-task-specific way. Experimental neurology. 2011;232:81–89. doi: 10.1016/j.expneurol.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Ueno M, Hayano Y, Nakagawa H, Yamashita T. Intraspinal rewiring of the corticospinal tract requires target-derived brain-derived neurotrophic factor and compensates lost function after brain injury. Brain : a journal of neurology. 2012;135:1253–1267. doi: 10.1093/brain/aws053. [DOI] [PubMed] [Google Scholar]
- Maier IC, et al. Constraint-induced movement therapy in the adult rat after unilateral corticospinal tract injury. J Neurosci. 2008;28:9386–9403. doi: 10.1523/JNEUROSCI.1697-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Montoya CP, Campbell-Hope LJ, Pemberton KD, Dunnett SB. The "staircase test": a measure of independent forelimb reaching and grasping abilities in rats. Journal of neuroscience methods. 1991;36:219–228. doi: 10.1016/0165-0270(91)90048-5. [DOI] [PubMed] [Google Scholar]
- Soblosky JS, Song JH, Dinh DH. Graded unilateral cervical spinal cord injury in the rat: evaluation of forelimb recovery and histological effects. Behavioural brain research. 2001;119:1–13. doi: 10.1016/s0166-4328(00)00328-4. [DOI] [PubMed] [Google Scholar]
- Soleman S, Yip P, Leasure JL, Moon L. Sustained sensorimotor impairments after endothelin-1 induced focal cerebral ischemia (stroke) in aged rats. Experimental neurology. 2010;222:13–24. doi: 10.1016/j.expneurol.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G. The Rat Nervous System. Academic Press, Inc; 1995. [Google Scholar]
- Benowitz LI, Goldberg DE, Madsen JR, Soni D, Irwin N. Inosine stimulates extensive axon collateral growth in the rat corticospinal tract after injury. Proc Natl Acad Sci U S A. 1999;96:13486–13490. doi: 10.1073/pnas.96.23.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Baumgartner BJ, Hill-Felberg SJ, McGowen LR, Shine HD. Neurotrophin-3 expressed in situ induces axonal plasticity in the adult injured spinal cord. J Neurosci. 2003;23:1424–1431. doi: 10.1523/JNEUROSCI.23-04-01424.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


