Abstract
The physiological electric field serves specific biological functions, such as directing cell migration in embryo development, neuronal outgrowth and epithelial wound healing. Applying a direct current electric field to cultured cells in vitro induces directional cell migration, or galvanotaxis. The 2-dimensional galvanotaxis method we demonstrate here is modified with custom-made poly(vinyl chloride) (PVC) chambers, glass surface, platinum electrodes and the use of a motorized stage on which the cells are imaged. The PVC chambers and platinum electrodes exhibit low cytotoxicity and are affordable and re-useable. The glass surface and the motorized microscope stage improve quality of images and allow possible modifications to the glass surface and treatments to the cells. We filmed the galvanotaxis of two non-tumorigenic, SV40-immortalized prostate cell lines, pRNS-1-1 and PNT2. These two cell lines show similar migration speeds and both migrate toward the cathode, but they do show a different degree of directionality in galvanotaxis. The results obtained via this protocol suggest that the pRNS-1-1 and the PNT2 cell lines may have different intrinsic features that govern their directional migratory responses.
Keywords: Cellular Biology, Issue 94, Cell biology, Prostate cells, cell migration, electric field, galvanotaxis, time-lapse imaging
Introduction
Endogenous electric fields are detected in various tissues, such as skin 1, 32, 33 and brain 2. The physiological electric field serves specific biological functions, including directing embryo development 3, 4, guiding the outgrowth of neuronal processes 5, 6 and promoting epithelial and corneal wound closure 1, 7. In vitro, application of a direct current electric field to cultured cells mimics the physiological electric field and induces directional cell migration, or galvanotaxis. Galvanotaxis has been studied in fibroblasts 8, fish keratinocytes 9, human epithelial and corneal keratinocytes 10-12, lymphocytes 13, neuroblasts 2, and neuronal progenitor cells 14. When exposed to the applied field, the majority of studied cells migrate directionally toward the cathodal (-) pole. Yet, several cancer cells, including highly metastatic human breast cancer cells and the human prostate cancer cell line PC-3M, move to the anodal (+) pole 15, 16. Several mechanisms are proposed to mediate galvanotaxis or to explain the ability of the cells to sense the electric field, including activation of EGF receptors 12, the epithelial sodium channel 17, PI3K and PTEN 18, and release of calcium ions 15, 19. The mechanism is not yet fully understood and it is possible that multiple signaling pathways are involved in galvanotaxis.
The 2-dimensional galvanotaxis method we demonstrate here is useful to characterize the directional migration of adherent, motile cells, either to monitor individual cell migration 10, 12, 17 or migration of a sheet of confluent cells 18, 20. This technique is modified from Peng and Jaffe21, and Nishimura et al.10 with custom-made, clear PVC chambers, with removable coverslips allowing for easy cell retrieval after galvanotaxis for secondary analysis, such as immuno-fluorescence imaging. The glass surface of the galvanotaxis chambers is optical-compatible, which allows the filming at high magnification and with fluorescently-labeled cells. It also allows the experimental design with modification of the glass surface, such as changing the surface coating or charges. Spacers made of No. 1 coverglass are used in the chambers to minimize the current flow over the cells; therefore the joule heating, which is proportional to the square of the current flow, would not overheat the cells during the experiment. The connecting agar bridges prevent direct contact of the electrodes with the cells and prevent change of the medium pH or ion concentration during galvanotaxis.
Two non-tumorigenic human prostate cell lines were examined for their galvanotaxis response in this study. The pRNS-1-1 22 and PNT2 23 are both SV40-immortalized, growth factor-dependent cell lines expressing the epithelial markers cytokeratin 5, 8, 18 and 19 with low or no expression of the prostate specific-antigen (PSA). Both cell lines maintain the polygonal morphology of normal epithelial cells, but chromosome abnormality was observed in karyotyping 22, 24. Although pRNS-1-1 and PNT2 share similar behaviors in most experiments, they do show differences in the formation of acinar structure and in galvanotaxis. On a 3-D matrix, Matrigel, the pRNS-1-1 cells form hollow acinar structures with lumens resembling the normal prostate gland tissue 25. However, the PNT2 cells form solid spheroids without a lumen or polarized epithelium 26. The pRNS-1-1 cells also demonstrate a higher galvanotactic response than the PNT2 in the current study. The correlation between the formation of acinar structure and galvanotaxis in pRNS-1-1 suggests that the galvanotactic signals may play a role in organizing the prostate gland tissue movements in response to endogenous electric fields, and provides further characteristics to discriminate between these 2 cell lines.
Protocol
1. Culturing Prostate Cells
Culture the pRNS-1-1 and PNT2 prostate cells on 100 mm culture dishes in RPMI 1640 medium supplemented with 10% FBS and Antibiotic-Antimycotic at 37 °C with 5% CO2. Refresh the culture medium every day until the cells reach 80% confluency for the galvanotaxis experiments.
2. Assembling Galvanotaxis Chambers
- Assembling bottom chambers
- Wipe a plastic galvanotaxis chamber with 2-propanol. Apply the marine grade silicon sealer around the circular openings on the bottom side of a chamber with a syringe and attach a large coverglass (Figure 1). Use the back side of a cotton applicator to push down the coverglass and wipe off the excess glue with cotton swabs. The circular openings allow the medium and electric current to flow over the cells located between the two inner medium reservoirs.
- Cut a small coverglass to make 6 mm x 25 mm spacers with a diamond point marker.
- Flip the chamber. Apply 2 stripes of the silicon glue with a syringe and/or a metal spatula to create a 10 mm x 25 mm channel surface for cell attachment between the 2 circular openings on the bottom glass. Glue 2 pieces of glass spacer between the 2 circular openings. Use the back side of the cotton applicators to push down the spacers and wipe off the excess glue with cotton swabs and/or toothpicks.
- Dry the chamber for 24 hr until the silicon glue is cured. Soak the chamber in distilled water overnight to remove the acetic acid residue from the glue. Dry the chamber for immediate use, or store the chamber for later use in a clean container.
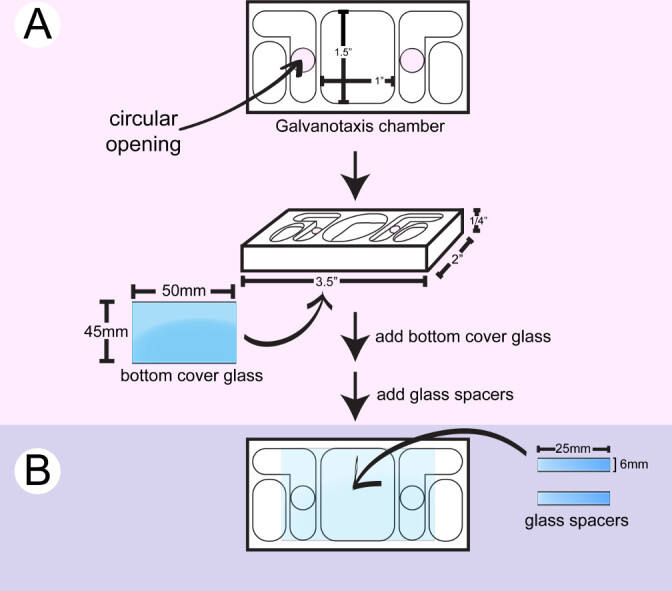 Figure 1: Assembly of the bottom galvanotaxis chamber. A) A large coverglass is attached to the bottom of a clean chamber. B) Two pieces of glass spacers are glued between the 2 circular openings to create a 10 mm x 25 mm channel for cells to attach.
Figure 1: Assembly of the bottom galvanotaxis chamber. A) A large coverglass is attached to the bottom of a clean chamber. B) Two pieces of glass spacers are glued between the 2 circular openings to create a 10 mm x 25 mm channel for cells to attach.
- Seeding cells on the galvanotaxis chambers
- Prepare the require number of galvanotaxis chambers needed for the experiment. Each cell line or treatment requires a single galvanotaxis chamber. Wipe the galvanotaxis chambers with 2-propanol. Wash the chambers with sterile PBS 3 times and check the liquid flow between the 2 circular openings in the chambers.
- Enclose the chambers in sterile cell culture dishes and allow them to equilibrate at 37 °C for 15 min. Detach the prostate cells from their culture dish using 5 ml 0.25% Trypsin-EDTA at 37 °C for 5 min. Neutralize the trypsin-EDTA with 5 ml 10% FBS in PBS.
- Transfer the cells to 15 ml tubes and centrifuge the cells for 5 min at 200 x g, 37 °C. Aspirate the supernatant and re-suspend the cells in 1 ml of culture medium.
- Take 20 µl of cell solution to load to a counting chamber and calculate the cell number. Adjust the cell concentration to 8 x 104 cell/ml with culture medium.
- Take the galvanotaxis chambers out of the 37 °C incubator. Seed 350 µl of the cell suspension onto each chamber.
- Incubate the cells in the chambers overnight in the culture dish with wet Kimwipe or in a humidity chamber in the incubator at 37 °C with 5% CO2.
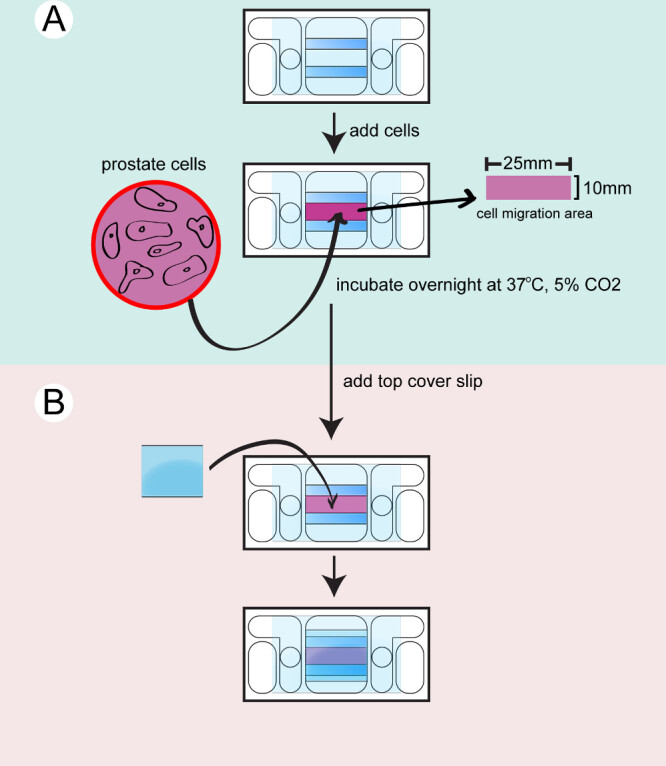 Figure 2: Seeding the cells to the chamber. The bottom chamber is dried and cleaned. A) Prostate cells are trypsinized, counted and transferred to the chamber and incubated overnight. B) A top coverglass is attached to seal the chamber before filming.
Figure 2: Seeding the cells to the chamber. The bottom chamber is dried and cleaned. A) Prostate cells are trypsinized, counted and transferred to the chamber and incubated overnight. B) A top coverglass is attached to seal the chamber before filming.
- Assembling the top chambers
- Assemble the top chamber prior to filming. The microscope can only accommodate imaging a single galvanotaxis chamber at one time. Warm up 10 ml of culture medium at 37 °C for each galvanotaxis chamber. Transfer a galvanotaxis chamber from the incubators to a 37 °C warming plate.
- Rinse the cells with culture medium to remove the unattached cells. Leave 400 µl of medium in the chamber. Use a syringe to apply high vacuum grease on top of both glass spacers.
- Add a small coverglass to seal the chamber. Gently press down the coverglass with the back side of a cotton applicator. Wipe off the excess medium with cotton swabs.
- Dry the glass surface and apply high vacuum grease to seal the gap between the coverglass and the chamber. Use a metal spatula to spread the grease. Add 4 ml of the culture medium to the inner reservoirs and check the liquid flow between the reservoirs.
- Make agar bridges
- Cut a pair of 2 inch long PVC tubing (503/16 ID x 5/16 OD x 1/16 Wall), flip and insert them into a 100 ml beaker.
- Measure 200 mg Bacto-Agar and add it in a 50 ml flask with 10 ml culture medium to make 2% agar gel. Microwave for 30 sec. Load the agar gel to the plastic tubing with a transfer pipet. Leave the agar bridges at room temperature for 10 min to solidify the agar.
- Add 2 ml culture medium to the outer reservoirs of the galvanotaxis chamber. Insert the agar bridges to the inner and outer medium reservoirs to conduct the current.
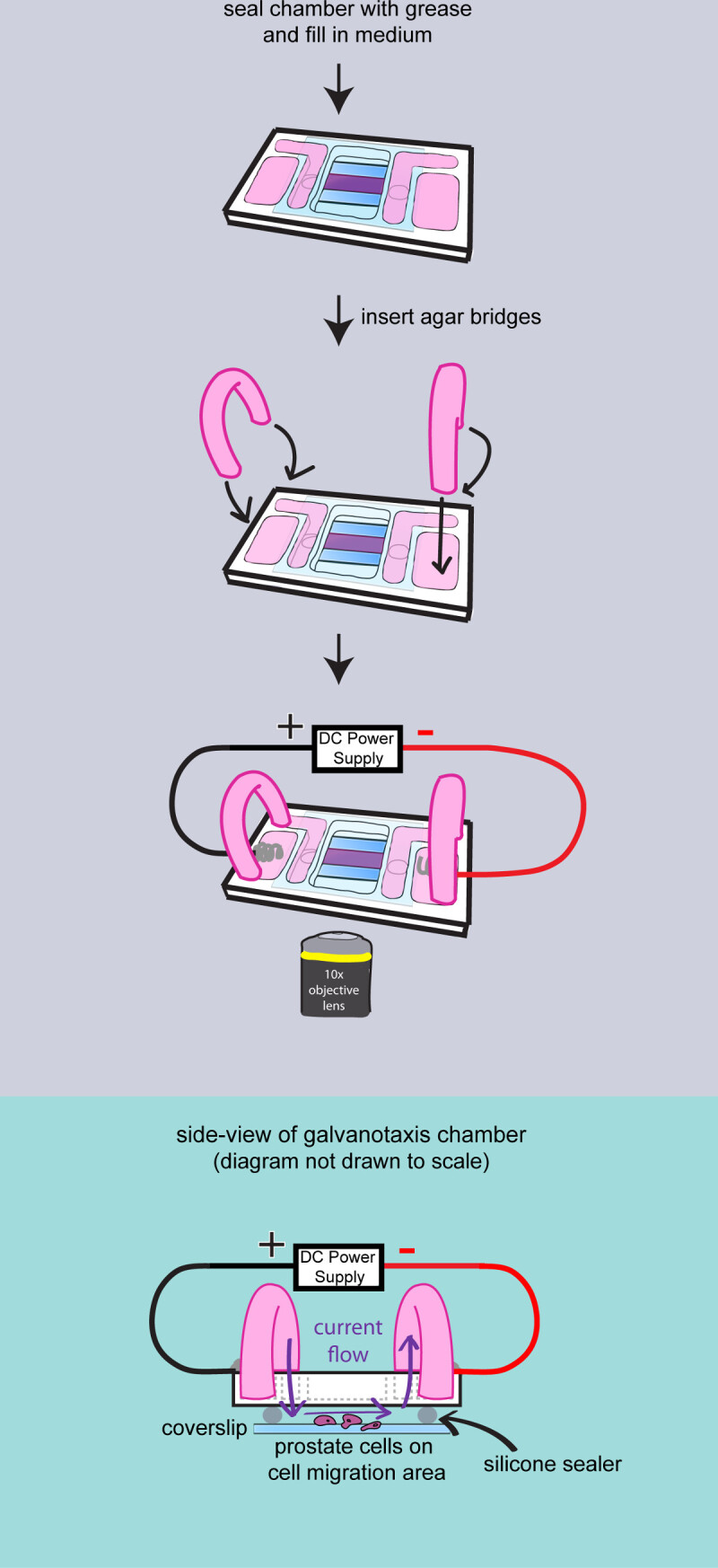 Figure 3: Filming the cells on the chamber. A diagram to demonstrate the final assembly of the galvanotaxis chamber. The chamber is completely sealed with high vacuum grease. Medium is added to fill the reservoirs, and two agar bridges are inserted into the chamber. Then the galvanotaxis chamber is transferred to the microscope stage and the electrodes are attached to apply the electric field. Side-view of the assembled galvanotaxis chamber is shown to demonstrate that the electric current flows over the cells through the agar bridges and the space between the cover glass.
Figure 3: Filming the cells on the chamber. A diagram to demonstrate the final assembly of the galvanotaxis chamber. The chamber is completely sealed with high vacuum grease. Medium is added to fill the reservoirs, and two agar bridges are inserted into the chamber. Then the galvanotaxis chamber is transferred to the microscope stage and the electrodes are attached to apply the electric field. Side-view of the assembled galvanotaxis chamber is shown to demonstrate that the electric current flows over the cells through the agar bridges and the space between the cover glass.
3. Time-lapse Imaging
Switch on the environmental chamber to 37 °C with 5% CO2 in the circulating air 2 hr prior to imaging.
Switch on the microscope and initiate the image-acquiring software. Calibrate the microscope stage and select the 10X objective.
Transfer the galvanotaxis chambers to the microscope stage, secure the chamber with tape and focus on the cells. Insert the galvanotaxis electrodes to the outer reservoirs with cathode on the right side. Secure the wire and the electrodes with tape.
Switch on the power box to apply an electric field to the chamber. Measure the output voltage with the voltmeter across the chamber to reach 2.5 volts for the 25 mm long chamber (100 mV/mm). Maintain the field strength during the experiment by adjusting the output current.
Select 10 points across the chamber to film in the software to generate ten time-lapse movies. Set up the acquisition conditions at 10-min intervals for 2 hr.
Start filming and adjust the output current as needed.
At the end of the experiment, remove the chamber from the stage and fix the cells with 95% alcohol. Break open the chamber with a razor blade to clean and re-use it.
4. Quantification of Galvanotaxis
Rotate the time-lapse movies and re-orient the cathode to the top of the images. Export the movies to the cell tracking software.
Manually track the (x,y) position of 10-20 cells at each time-point from each movie (Figure 4 A, B). The migration distances and the angles relative to the north-south direction, the same orientation of the electric field, will be calculated in the cell tracking software (Figure 4C). NOTE: The average migration speed is converted from the total migration distance and total filming time. The directionality is converted from the angles to the cosine value. If cells migrate with random directionality, the average of net cosine will be close to zero. If the cells migrate directly toward the cathode, the cosine value will be +1. If the cells migrate directly toward the anode, the cosine value will be -1.
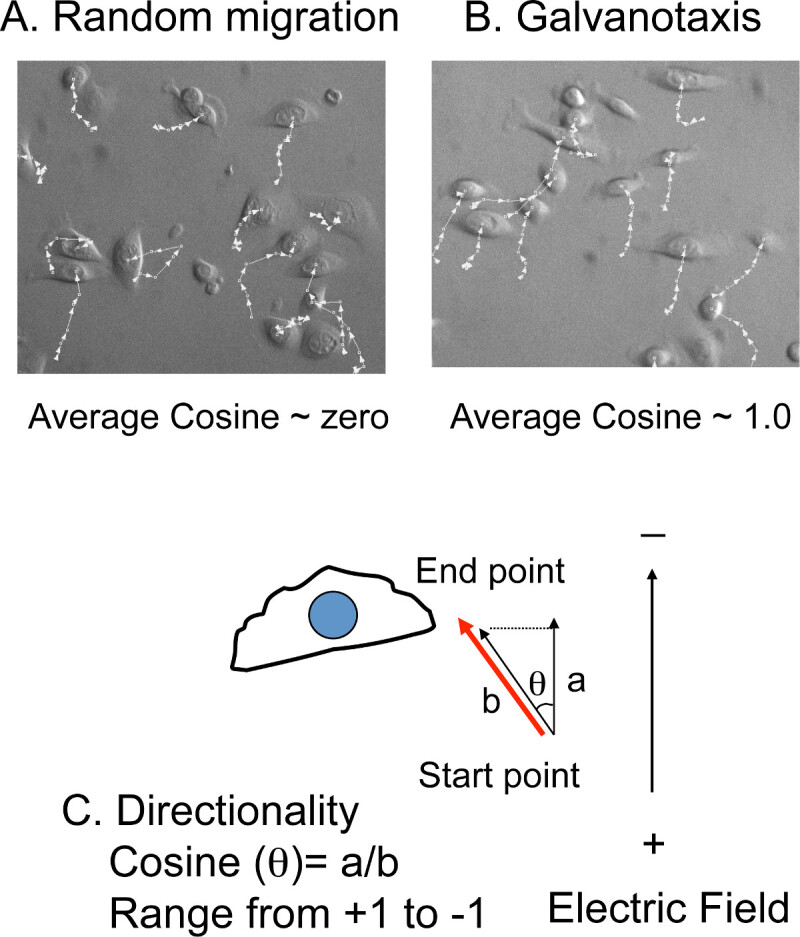 Figure 4: Cell tracking to measure directionality.A) Overlay of the tracking lines with cell images. The (x, y) positions of the cells are manually tracked in the time-lapse movies. If the cells migrate randomly, the average cosine is close to zero. B) However, if the cells migrate toward the cathode or the anode, the value of the average cosine is close to + (cathode) or – (anode) 1.0. C) The directionality is presented by the cosine value, which is converted from the migration angles (θ). The cosine (θ) equals the ratio of the distance a (the migration distance) to distance b (the distance projected to the direction of the electric field).
Figure 4: Cell tracking to measure directionality.A) Overlay of the tracking lines with cell images. The (x, y) positions of the cells are manually tracked in the time-lapse movies. If the cells migrate randomly, the average cosine is close to zero. B) However, if the cells migrate toward the cathode or the anode, the value of the average cosine is close to + (cathode) or – (anode) 1.0. C) The directionality is presented by the cosine value, which is converted from the migration angles (θ). The cosine (θ) equals the ratio of the distance a (the migration distance) to distance b (the distance projected to the direction of the electric field).
Save the measurements. Import the data to a database application to calculate the combined results.
Export the combined data of average migration speed and average cosine value to a spreadsheet to plot the bar graphs (Figure 5).
Representative Results
Two lines of prostate cells (pRNS-1-1 and PNT2) were investigated with this method. Cells in both lines migrate at similar speeds of 1.0 +/- 0.3 microns/min over the course of 2-hours (Figure 5A). However, the directionality to the electric field is 0.7 +/- 0.3 for the pRNS-1-1 line, and 0.2 +/- 0.8 for the PNT2 line (Figure 5B). The results show a significant difference in the galvanotaxis of these two cell lines (p<0.01, 100 cells were tracked), suggesting that they have different cellular signaling mechanisms that result in different directional responses to positional cues.
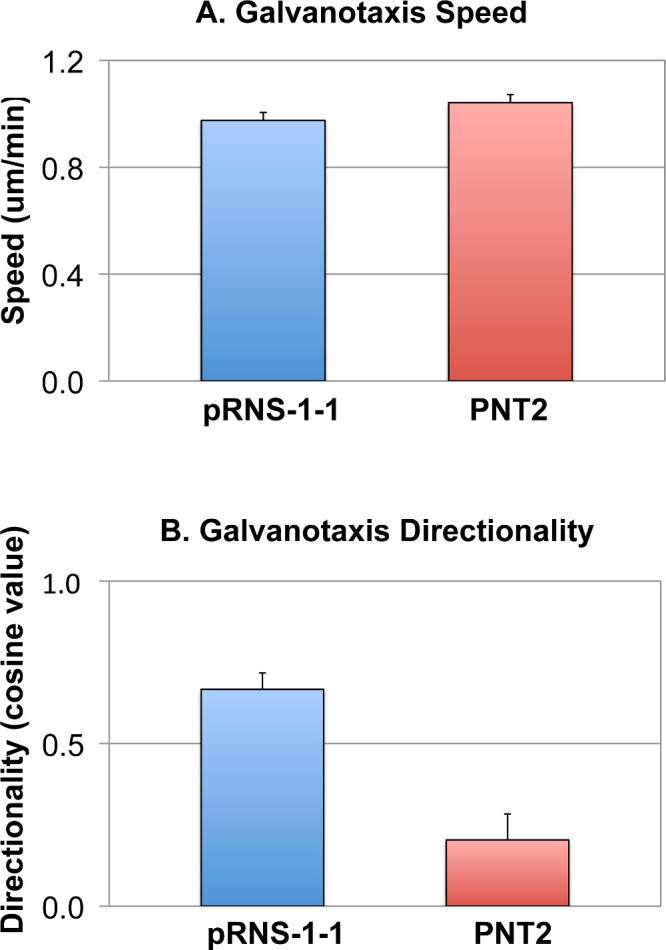 Figure 5: Galvanotaxis of prostate cells. A) The migration speeds of pRNS-1-1 and PNT2 lines are similar when the cells are exposed to the electric field. B) However, the pRNS-1-1 line showed a high directionality toward cathode (cosine = 0.7), while the PNT2 line showed a low directionality (cosine = 0.2) in the electric field.
Figure 5: Galvanotaxis of prostate cells. A) The migration speeds of pRNS-1-1 and PNT2 lines are similar when the cells are exposed to the electric field. B) However, the pRNS-1-1 line showed a high directionality toward cathode (cosine = 0.7), while the PNT2 line showed a low directionality (cosine = 0.2) in the electric field.
Discussion
The analysis of a cell’s galvanotaxis response has been an important functional indicator for many cellular migratory or growth processes 27, 28. Here we use a custom-made chamber with glass surface to film two prostate cell lines. These cell lines demonstrated different degrees of galvanotaxis, and we speculate that the intracellular localization or the activation of the galvanotaxis-mediating proteins might be interfered during the process of generating the immortal cell lines, resulting in the observed difference in the galvanotaxis response.
Compared to other galvanotaxis chamber designs made in culture dishes27, 28, there are several benefits to the current design. First, the glass surface is suitable for imaging and the coverglass with cells can be retrieved after galvanotaxis for immunostaining. In this study we used a low magnification of 10X objective to monitor a group of cells. However, with these improved glass optics, it is possible to film a single cell at higher magnification with a 60X oil objective, for tracking the fast extension and retraction of lamellipodial at the leading edge of the cells 17, or tracking cells labeled with fluorescent proteins. Second, the glass surface could be modified with matrix protein coatings 10, 12 or patterned with micro-channels. Chemical treatments are also possible to pre- treat the cells before filming 29. Matrix coating is not required for the prostate cell lines used here, and the matrix coating is cell type-dependent. For example, collagen I coating is required for the galvanotaxis of human keratinocytes 30, but laminin coating is required for the galvanotaxis of human neural stem cells 31. We tested human keratinocyte galvanotaxis on collagen I, collagen IV, fibronectin, and laminin. Different matrix proteins altered cell migration speed, but did not change the cathodal migration in galvanotaxis 29. Third, the modified platinum electrodes are inert in the electric field. Compared to the silver electrodes as used in other galvanotaxis methods 27, they do not break down or release metal ions to the medium, which reduces the cytotoxicity in galvanotaxis. Fourth, we may change the intervals of frame capturing, which helps us to monitor either fast or slow cell migration. The motorized microscope also provides the opportunity to acquire multiple movies from different areas in one chamber. A control experiment without the applied electric field is important for determining whether the electric field alters the migratory speed of the cells.
However, the current galvanotaxis chambers are limited to filming cell migration for up to 4 hours due to the small volume of medium in the reservoirs. With an enlarged version of the chamber design, it would be possible to film cells for a longer period of time.
Some potential problems in the method include: 1) the cells might die during the chamber assembly if they become desiccated. Since there is a very small volume of medium in the chamber (400 µl), it is important to avoid trapping air bubbles in the chamber and to keep some liquid covering the cells during the cell rinse step. 2) The chamber could leak during imaging, which would decrease the current passing through the chamber. The liquid flow in the chamber should be carefully checked before it is transferred to the microscope stage and more high vacuum grease applied if needed. 3) Smudged high vacuum grease may interfere with filming. The glass surface can be cleaned with 95% alcohol to remove grease. 4) Stage drift or any displacement of the chambers on a motorized microscope stage could bias the measurements of the directional migration. Therefore, it is important to secure the galvanotaxis chambers with adequate stage holder and labeling tape.
Overall, the galvanotaxis method is useful to reveal the intrinsic directionality of motile cells in an applied electric field, which will help us to further understand the directional migration in organisms.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The prostate cell lines are kindly provided by Dr. Ling-Yu Wang and Dr. Hsing-Jien Kung at Cancer Center, UC Davis. This project is supported by NIH galvanotaxis grant 4R33AI080604.
References
- Reid B, Nuccitelli R, Zhao M. Non-invasive measurement of bioelectric currents with a vibrating probe. Nat Protoc. 2007;2(3):661–669. doi: 10.1038/nprot.2007.91. [DOI] [PubMed] [Google Scholar]
- Cao L, et al. Endogenous electric currents might guide rostral migration of neuroblasts. EMBO Rep. 2013;14(2):184–190. doi: 10.1038/embor.2012.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotary KB, Robinson KR. Endogenous electrical currents and voltage gradients in Xenopus embryos and the consequences of their disruption. Dev Biol. 1994;166(2):789–800. doi: 10.1006/dbio.1994.1357. [DOI] [PubMed] [Google Scholar]
- Hotary KB, Robinson KR. Evidence of a role for endogenous electrical fields in chick embryo development. Development. 1992;114(4):985–996. doi: 10.1242/dev.114.4.985. [DOI] [PubMed] [Google Scholar]
- Yamashita M. Electric axon guidance in embryonic retina: galvanotropism revisited. Biochem Biophys Res Commun. 2013;431(2):280–283. doi: 10.1016/j.bbrc.2012.12.115. [DOI] [PubMed] [Google Scholar]
- Wood MD, Willits RK. Applied electric field enhances DRG neurite growth: influence of stimulation media, surface coating and growth supplements. J Neural Eng. 2009;6(4):046003. doi: 10.1088/1741-2560/6/4/046003. [DOI] [PubMed] [Google Scholar]
- Kucerova R, et al. The role of electrical signals in murine corneal wound re-epithelialization. J Cell Physiol. 2011;226(6):1544–1553. doi: 10.1002/jcp.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillman AL, Quang DM, Farboud B, Fang KS, Nuccitelli R, Isseroff RR. Human Dermal fibroblasts do not exhibit directional migration on collagen I in direct-current electric fields of physiological strength. Exp Dermatol. 2003;12(4):396–402. doi: 10.1034/j.1600-0625.2002.120406.x. [DOI] [PubMed] [Google Scholar]
- Allen GM, Mogilner A, Theriot JA. Electrophoresis of cellular membrane components creates the directional cue guiding keratocyte galvanotaxis. Curr Biol. 2013;23(7):560–568. doi: 10.1016/j.cub.2013.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura KY, Isseroff RR, Nuccitelli R. Human keratinocytes migrate to the negative pole in direct current electric fields comparable to those measured in mammalian wounds. J Cell Sci. 1996;109(1):199–207. doi: 10.1242/jcs.109.1.199. [DOI] [PubMed] [Google Scholar]
- Farboud B, Nuccitelli R, Schwab IR, Isseroff RR. DC electric fields induce rapid directional migration in cultured human corneal epithelial cells. Exp Eye Res. 2000;70(5):667–673. doi: 10.1006/exer.2000.0830. [DOI] [PubMed] [Google Scholar]
- Fang KS, Ionides E, Oster G, Nuccitelli R, Isseroff RR. Epidermal growth factor receptor relocalization and kinase activity are necessary for directional migration of keratinocytes in DC electric fields. J Cell Sci. 1999;112(12):1967–1978. doi: 10.1242/jcs.112.12.1967. [DOI] [PubMed] [Google Scholar]
- Li J, et al. Activated T lymphocytes migrate toward the cathode of DC electric fields in microfluidic devices. Lab Chip. 2011;11(7):1298–1304. doi: 10.1039/c0lc00371a. [DOI] [PubMed] [Google Scholar]
- Meng X, Arocena M, Penninger J, Gage FH, Zhao M, Song B. PI3K mediated electrotaxis of embryonic and adult neural progenitor cells in the presence of growth factors. Exp Neurol. 2011;227(1):210–217. doi: 10.1016/j.expneurol.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Ma X, Lin F. DC Electric Fields Direct Breast Cancer Cell Migration, Induce EGFR Polarization, and Increase the Intracellular Level of Calcium Ions. Cell Biochem Biophys. 2013;67(3):1115–1125. doi: 10.1007/s12013-013-9615-7. [DOI] [PubMed] [Google Scholar]
- Martin-Granados C, et al. A role for PP1/NIPP1 in steering migration of human cancer cells. PLoS One. 2012;7(7):40769. doi: 10.1371/journal.pone.0040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HY, Charles RP, Hummler E, Baines DL, Isseroff RR. The epithelial sodium channel mediates the directionality of galvanotaxis in human keratinocytes. J Cell Sci. 2013;126(9):1942–1951. doi: 10.1242/jcs.113225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442(7101):457–460. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
- Shanley LJ, Walczysko P, Bain M, MacEwan DJ, Zhao M. Influx of extracellular Ca2+ is necessary for electrotaxis in Dictyostelium. J Cell Sci. 2006;119(22):4741–4748. doi: 10.1242/jcs.03248. [DOI] [PubMed] [Google Scholar]
- Zhao M, Agius-Fernandez A, Forrester JV, McCaig CD. Directed migration of corneal epithelial sheets in physiological electric fields. Invest Ophthalmol Vis Sci. 1996;37(13):2548–2558. [PubMed] [Google Scholar]
- Peng HB, Jaffe LF. Polarization of fucoid eggs by steady electrical fields. Dev Biol. 1976;53(2):277–284. doi: 10.1016/0012-1606(76)90229-3. [DOI] [PubMed] [Google Scholar]
- Lee M, et al. Characterization of adult human prostatic epithelial-cells immortalized by polybrene-induced DNA transfection with a plasmid containing an origin-defective sv40-genome. Int J Oncol. 1994;4(4):821–830. doi: 10.3892/ijo.4.4.821. [DOI] [PubMed] [Google Scholar]
- Berthon P, Cussenot O, Hopwood L, Leduc A, Maitland N. Functional expression of sv40 in normal human prostatic epithelial and fibroblastic cells - differentiation pattern of non-tumorigenic cell-lines. Int J Oncol. 1995;6(2):333–343. doi: 10.3892/ijo.6.2.333. [DOI] [PubMed] [Google Scholar]
- Aurich-Costa J, Vannier A, Grégoire E, Nowak F, Cherif D. IPM-FISH, a new M-FISH approach using IRS-PCR painting probes: application to the analysis of seven human prostate cell lines. Genes Chromosomes Cancer. 2001;30(2):143–160. [PubMed] [Google Scholar]
- Tyson DR, Inokuchi J, Tsunoda T, Lau A, Ornstein DK. Culture requirements of prostatic epithelial cell lines for acinar morphogenesis and lumen formation in vitro: role of extracellular calcium. Prostate. 2007;67(15):1601–1613. doi: 10.1002/pros.20628. [DOI] [PubMed] [Google Scholar]
- Lang SH, Sharrard RM, Stark M, Villette JM, Maitland NJ. Prostate epithelial cell lines form spheroids with evidence of glandular differentiation in three-dimensional Matrigel cultures. Br J Cancer. 2001;85(4):590–599. doi: 10.1054/bjoc.2001.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babona-Pilipos R, Popovic MR, Morshead CM. A galvanotaxis assay for analysis of neural precursor cell migration kinetics in an externally applied direct current electric field. J Vis Exp. 2012. [DOI] [PMC free article] [PubMed]
- Meng X, et al. Electric field-controlled directed migration of neural progenitor cells in 2D and 3D environments. J Vis Exp. 2012. [DOI] [PMC free article] [PubMed]
- Pullar CE, Isseroff RR. Cyclic AMP mediates keratinocyte directional migration in an electric field. J Cell Sci. 2005;118(9):2023–2034. doi: 10.1242/jcs.02330. [DOI] [PubMed] [Google Scholar]
- Sheridan DM, Isseroff RR, Nuccitelli R. Imposition of a physiologic DC electric field alters the migratory response of human keratinocytes on extracellular matrix molecules. J Invest Dermatol. 1996;106(4):642–646. doi: 10.1111/1523-1747.ep12345456. [DOI] [PubMed] [Google Scholar]
- Feng JF, et al. Guided migration of neural stem cells derived from human embryonic stem cells by an electric field. Stem Cells. 2012;30(2):349–355. doi: 10.1002/stem.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerjee EV, Isseroff RR, Nuccitelli R, Collins SD, Smith RL. Microneedle array for measuring wound generated electric fields. Conf Proc IEEE Eng Med Biol Soc. 2006;1:4326–4328. doi: 10.1109/IEMBS.2006.260205. [DOI] [PubMed] [Google Scholar]
- Nuccitelli R, Nuccitelli P, Li C, Narsing S, Pariser DM, Lui K. The electric field near human skin wounds declines with age and provides a noninvasive indicator of wound healing. Wound Rep. and Reg. 2011;19:645–655. doi: 10.1111/j.1524-475X.2011.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


