Abstract
Parvovirus H1 (H1PV) is an autonomous parvovirus that is transmitted in rodent populations. Its natural host is rats. H1PV infection is nonpathogenic except in rat and hamster fetuses and newborns. H1PV infection of human cancer cells caused strong oncolytic effects in preclinical models. For a clinical trial of H1PV in patients with brain tumors, clinical-grade H1PV was produced according to Good Manufacturing Practices. This report focuses on results obtained after a single high-dose intravenous injection of highly purified H1PV in 30 rats and multiple (n = 17) intravenous injections at 3 dose levels in 223 rats. In both studies, no virus-related mortality or macroscopic organ changes related to H1PV occurred. Histopathology after multiple virus injections revealed minimal diffuse bile duct hyperplasia in livers of animals of the highest dose group and germinal center development in spleens of animals from the high-dose group. Liver changes were reversible within a 2-wk recovery period after the last injection. Hematology, blood chemistry, and coagulation analyses did not reveal significant toxicologic changes due to H1PV. Virus injection stimulated the production of IgG antibodies but did not alter mononuclear cell function or induce cytokine release. PCR analysis showed dose-dependent levels of viral genomes in all organs tested. The virus was excreted primarily through feces. These data provide important information regarding H1PV infection in its natural host. Due to the confirmation of the favorable safety profile of H1PV in a permissive animal model, a phase I/IIa clinical trial of H1PV in brain tumor patients could be initiated.
Abbreviations: H1PV, parvovirus H1; GMP, Good Manufacturing Practice; NS, nonstructural protein; VG, viral genomes
Parvovirus H1 (H1PV) is a small nonenveloped virus which contains a linear single stranded DNA genome of 5.1kb and belongs to the family Parvoviridae. The natural host is the rat, but similar to other related parvoviruses H1PV is able to infect and replicate in cells of other species. This is known for hamsters and humans but H1PV is unable to infect mice. However, no systematic screening of species for susceptibility to H1PV infection has been performed.27 Because H1PV is transmitted in rodent populations, laboratory animals are routinely tested for the presence of antiH1PV antibodies to exclude detrimental effects on research results3,20 Typically only animals without previous parvovirus infection are used in experimental research. In the natural host, rats, as well in hamsters, which can be infected experimentally, H1PV is pathogenic only for newborns and is embryo- and fetotoxic. The effects on progeny depend on the age at infection. Although animals inoculated with H1PV during the ovular preimplantation period do not reveal any harm, infection during midgestation typically results in fetal death. When infected during late gestation or within the first few days after delivery, progeny frequently develop so-called ‘osteolytic syndrome,’ characterized by dwarfism and some mongoloid-like features.10,11,18,19,26
There is no detailed knowledge whether high doses of H1PV or repeated inoculations with the virus lead to pathologic effects that are not observed after normal transmission. H1PV infection in combination with additional factors can lead to aberrant effects, such as hepatocellular necrosis, in infected rats subjected to hepatotoxic chemicals and increased virus proliferation in liver tissue of animals infected with parasites.20 Furthermore, natural infection with a similar virus, Kilham rat parvovirus (KRV), can eventually lead to clinical illness.3
As well as being a naturally occurring virus in the rodent population, H1PV has oncosuppressive properties, which were demonstrated when it inhibited the formation of spontaneous as well as chemically or virally induced tumors in laboratory animals.28,29 More recent investigations demonstrated the direct oncolytic potential of the virus in a number of cancers.7 H1PV infection of tumor cell cultures, including human tumor cells and experimental tumors in animals, led to efficient cell killing and tumor regression.1,2,8,13,14,23,24 The parvoviral cytotoxicity is attributed, at least in part, to the viral regulatory nonstructural protein NS1, and the smaller nonstructural protein NS2 may modulate NS1 cytotoxicity. Tumor entities in which H1PV showed strong oncolytic effects are malignant brain tumors (glioblastomas), lymphoma, pancreatic ductal adenocarcinoma, and others. The encouraging rates of complete remissions after intratumoral or intravenous H1PV injection of rats bearing large intracranial gliomas led to a clinical trial (ParvOryx01) in patients with recurrent glioblastoma multiforme.12,13
For clinical use, H1PV had to be produced according to Good Manufacturing Practice (GMP) standards. To comply with GMP requirements, the purity, infectivity and stability of the virus preparation had to be demonstrated by the manufacturer (IDT Biologica, Dessau, Germany). Prior to use in patients, this medical-grade GMP-produced batch of virus had to undergo extensive toxicology testing in animals. Due to the permissiveness for H1PV infection and replication, rats were defined as the appropriate and sufficient species to investigate the potential toxicity profile of H1PV. This species selection was approved by the regulatory authority (Paul-Ehrlich Institut, Langen, Germany). A minimal regulatory requirement in repeated toxicology testing for clinical trials in humans is a 2-wk treatment period. The duration of the H1PV toxicology study presented here reflects the intended protocol of the clinical trial in brain tumor patients. However, to allow for possible other trials in different tumor entities, the treatment protocol here exceeds the minimal period of 2 wk. An additional reason for the extension of the study beyond the minimal duration was that an immunologic response to the injected virus was not expected to begin before 2 wk after the initial injection. In consequence, additional virus administrations in weeks 3 and 4 were required to investigate potential adverse effects related to the activation of the immune system. These considerations and requirements led to a study protocol that scheduled a total of 17 injections within 28 d (14 injections during the first 14 d, 2 injections in week 3, and one injection in week 4). In light of the absence of pathologic side effects after H1PV injection in preclinical experiments, the investigated doses in this toxicology study were substantially higher than those intended for the initial treatments in tumor patients (1 × 106 pfu).
This report focuses on the biology of H1PV infection after intravenous injection of rats with a single high virus dose at 2 dose levels (8.6 × 107 and 8.6 × 108 pfu per rat) or after a total of 17 administrations at 3 different dose levels (106, 107, 108 pfu per injection). The results of this study were mandatory for the regulatory approval of a clinical trial (ParvOryx01), which started in October 2011. In addition to providing safety information, the data offer insights into the biology of H1PV in the natural host including organ distribution, relative organ concentration, crossing of the blood-brain barrier, virus shedding, and immunologic reactions. Because the data were generated with purified virus under highly regulated test conditions, the results add valuable information to the understanding of H1PV infection in the natural host. In addition, the availability of these results likely will help to reduce the number of future animal experiments in this or related areas of research.
Materials and Methods
H1PV production, virus formulations, and virus administration.
The clinical H1PV stock was manufactured to GMP standards by IDT Biologika (Dessau, Germany). H1PV production was performed according to a modified standard protocol that was adapted to GMP requirements and large-scale production. In brief, H1PV was propagated in human newborn kidney cells (NB-324K) and purified by iodixanol density gradient centrifugation.30 Infective particles were quantified by plaque-forming assay.
The test item formulation for single dose was prepared freshly on the day of administration. All test item formulations for repeated dosage were prepared as stock solutions and stored as aliquots at less than −70 °C until use. Repeated freezing–thawing of the aliquots was avoided.
For the acute intravenous study, H1PV was diluted with 48% iodixanol in Ringer solution (batch no. 09120900079, IDT Biologika, Dessau-Rousslau, Germany). For the repeated-dose study, H1PV was diluted by using 10% iodixanol in Ringer solution (batch no. 290410, 48% Visipaque, IDT Biologika; batch nos. 1004270086, 1006080006, and 1001040005; Ringers solution, IDT Biologika). The iodixanol concentration for multiple dosing was reduced from 48% to 10% to avoid possible nephrotoxic effects.
Stability of virus dilutions stored at −70 °C was proven until day 42. To this end, H1PV formulations were tested weekly for the concentration of viral genomes (VG) by PCR21 and for pfu by plaque assay. All injection doses were stable during that time, confirming full infectivity. The ratio of VG:pfu was approximately 103:1.
All administrations of the test item were performed as slow single or repeated intravenous injections in the tail vein at a volume of 750 μL per injection. All rats were conscious and kept in a restrainer. Animals in the repeated toxicity study received a total of 17 injections. During the first 2 wk of treatment, the rats received daily administrations (days 1 through 14); in the third week of treatment, they received 2 administrations (days 17 and 21); and in the last week, they received only one injection (day 28).
The doses of H1PV for single-dose injection (acute toxicology) were 8.6 × 107 and 8.6 × 108 pfu. For multiple dosing (n = 17), rats received single injections (3 dose levels) of 1× 108 (high dose), 1 × 107 (intermediate dose), and 1 × 106 (low dose) pfu.
Animal experiments.
For all tests, female, nonpregnant, nulliparous and male SPF Wistar rats (Crl: WI[Han]) were obtained from a controlled full-barrier breeding system (Charles River, Sulzfeld, Germany) and tested against viruses, including H1 parvovirus, bacteria and parasites (complete list: http://www.criver.com/HealthData/de/H64W090R.pdf). At the beginning of the study, rats were 8 to 12 wk old. All rats were bred for experimental purposes according to Article 9.2, Number 7 of the German Act on Animal Welfare.6
The studies were conducted by BSL Bioservice (Munich, Germany). All experiments complied with the German Acts on Animal Welfare (Tierschutzgesetz, July 2009), Good Laboratory Practices, and Infection Protection (Infektionsschutzgesetz, January 2001).5,6,9 BSL Bioservice licenses to handle H1PV and conduct acute and repeated-dose toxicity studies were granted by the government of Upper Bavaria, Munich, Germany.
Animals were kept under full-barrier conditions in an air-conditioned room (10 air change hourly) at 22 ± 3 °C, relative humidity of 55% ± 10%, and a 12:12-h light–dark cycle. All rats were kept in individually ventilated cages (type III H, polysulfone cages with wood-fiber bedding and tunnels as enrichment) to avoid cross-infection between animals. Cages were changed weekly. An adequate acclimation period (at least 5 d) was allowed prior to initiating animal experiments. Animals had free access to a maintenance diet for rats and mice (no. 1324, Altromin, Bielefeld, Eastern Westphalia, Germany) and to tap water, which was sulfur-acidified to a pH of approximately 2.8.
Number, sex, and allocation of rats.
Acute toxicity study.
Thirty rats (15 of each sex) were divided in 3 groups of 10 (5 of each sex). The control group received a single intravenous injection of vehicle only (48% iodixanol in Ringer solution), dose group 1 received a single injection of 8.6 × 107 pfu H1PV per animal, and dose group 2 received a single injection of 8.6 × 108 pfu H1PV per animal. The injection volume was 750 µL in all rats.
Repeated toxicity study.
The 223 rats (112 female, 111 male) were allocated into 5 groups of different sizes. All animals received a total of 17 injections. Control group 1 received vehicle only (10% iodixanol in Ringer solution), control group 2 received Ringer solution. Two control groups were included because injection with Ringer solution was unlikely to cause toxic effects, whereas repeated injections of iodixanol could result in toxicity. Rats in groups 3, 4, and 5 each received 17 injections, each of which contained 106 pfu H1PV (low dose, group 3), 107 pfu H1PV (intermediate dose, group 4), or 108 pfu H1PV (high dose, group 5). The allocation of rats and the respective examinations are shown in Table 1. The animals were euthanized either on day 29 (24 h after the last administration) or after a recovery period of 14 d (day 43) after the last administration.
Table 1.
Group definition and allocation of rats
| Euthanized at day 29 |
Euthanized at day 43 |
|||||
| H1PV dose (pfu) | Hematology Clinical biochemistry Urinalysis Histopathology | RT-PCR | Toxicokinetics | Hematology Clinical biochemistry Urinalysis Histopathology | Hematology Clinical biochemistry Urinalysis RT-PCR | |
| Vehiclea | 0 | 10 male | 5 male | 5 male | 7 maleb | 5 male |
| 10 female | 5 female | 5 female | 6 femaleb | 5 female | ||
| Ringer | 0 | 5 male | none | none | 5 male | none |
| 5 female | none | none | 5 female | none | ||
| H1PV | 106 | 10 male | 5 male | 5 male | none | none |
| 10 female | 5 female | 5 female | none | none | ||
| H1PV | 107 | 10 male | 5 male | 5 male | none | none |
| 10 female | 5 female | 5 female | none | none | ||
| H1PV | 108 | 10 male | 5 male | 5 male | 5 male | 5 male |
| 10 female | 5 female | 5 female | 5 female | 5 female | ||
All rats received 750 µL per injection.
The vehicle comprised 10% iodixanol in Ringer solution
2 additional male and one additional female rats were included, because 3 animals had to be euthanized prematurely (2 at day 15 and 1 at day 18) due to deteriorated health status unrelated to the vehicle administration. These additional animals were handled according to the same protocol as for the other rats in this group and received a total of 17 injections over 28 d.
Clinical examinations.
The observation period was 14 d for animals in the acute toxicity study, 28 d for rats in the main repeated-dose study, and 42 d for animals in the recovery group (repeated-dose study only). The rats were checked twice daily for clinical symptoms or death. Any sign of illness before, during, and after administration or reaction to treatment were recorded for each animal. Local tolerance at the injection site was examined. A behavioral examination according to the IRWIN screen and an ophthalmologic examination was performed in rats in the repeated-dose groups.15,22 Rats euthanized on day 29 were examined before virus injection and approximately 24 h after the 16th administration (day 21), and animals euthanized on day 43 were tested before the first and approximately 24 h after the 17th (day 28) administration.
In both studies, body weight was measured before the administration of H1PV and weekly thereafter until completion of the study. Rats in the repeated-dose study were assigned to groups according to their initial body weight to achieve similar body weight distribution. Food consumption was measured weekly by calculating the difference between the weight of input food and leftover food; water consumption was monitored daily by visual inspection.
Laboratory examinations performed after repeated-dose treatment.
After rats had been fasted overnight, blood was sampled at from the abdominal aorta from a subset of animals in each group (Table 1) at euthanasia (day 29 or 43). The rats were anesthetized by using ketamine (100 mg/kg IP) and xylazine (10 mg/kg IP) and euthanized by exsanguination. Blood was collected in uncoated, EDTA-coated, and citrate-coated tubes. The following hematologic parameters were analyzed (ADVIA120, Siemens, Deerfield, IL; ACL 7000 (IL Instrumentation Laboratory, Bedford, MA): Hct, Hgb, RBC, MCV, MCH, MCHC, reticulocytes, platelets, WBC, neutrophils, lymphocytes, monocytes, eosinophils, basophils, prothrombin time, and activated partial thromboplastin time.
To investigate toxic effects in tissues, particularly kidney and liver, the following biochemistry parameters were analyzed (Synchron Cx 5, Beckman Coulter, Pasadena, CA): ALT, AST, ALP, creatinine, total protein, albumin, urea, total bilirubin, total cholesterol, triglycerides, glucose, total globulin (calculated as total protein minus albumin), albumin:globulin ratio, sodium, potassium, calcium.
Urinalysis was performed either on day 29 or 43 in a subset of animals (10 male and 10 female rats of the main and recovery periods, except for the Ringer or vehicle only groups, for which only 5 animals per sex were sampled) by cystocentesis after euthanize. The following parameters were measured by using qualitative indicators of analyte concentration (Henry Schein, Urispec Plus, Melville, NY): specific gravity, nitrite, pH, protein, glucose, ketone bodies, urobilinogen, bilirubin, RBC, and leukocytes.
Measurement of antivirus antibodies by IgG-specific H1PV ELISA.
Preimmune and immune sera were collected for analysis at baseline (before the first administration), after 1 wk of treatment (on day 8), after 2 wk of treatment (on day 15) and at terminal euthanizes. Blood samples of at least 450 μL were sampled from the jugular vein under general anesthesia (either via inhalation of isoflurane (2%) or via intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg)) and immediately transferred into polypropylene tubes. The tubes stayed at room temperature to allow clotting and were then centrifuged for 15 min at 1700 × g. The serum samples were stored at or below −70 °C until analysis. Only baseline and day 29 samples were analyzed in 15 male and 15 female rats per group.
ELISA microplates (C96 Maxisorp, Nalge Nunc, Rochester, NY) were coated with 100 µL positive antigen (dilution, 1: 1000; cell lysate from H1PV-infected NB-324 K cells in VTE buffer with a total protein concentration of 0.45 mg/mL, provided by B Leuchs, German Cancer Research Center, Heidelberg, Germany) and incubated overnight (18 ± 2 h) at 5 ± 3 °C. Microplates were washed 5 times with 300 µL washing buffer (R and D Systems, Minneapolis, MN), blocked with 200 µL of reagent diluent (R and D Systems), and incubated for 1 h at 37 °C. Quality control samples with high, medium, and low concentrations were prepared by spiking with positive rat serum (IgG concentration, 1.09 mg/mL); the final total IgG concentrations were 436, 218, and 109 ng/mL. Serum samples from study animals were thawed and diluted 1:50 (minimal required dilution) in reagent diluent; 100 µL of each sample was incubated for 1 h at 37 °C. All tests were carried out in duplicates. After washing, 100 µL goat–antirat IgG peroxidase conjugate (dilution 1:10; BioCat, Troy, VA) were added to each well, microplates were sealed and incubated for 1 h at 37 °C. After washing 100 µL substrate solution (R and D Systems) were added and plates were incubated for 20 min in the dark at room temperature. The reaction was stopped by adding 75 µL of stop solution per well (R and D Systems). Microplates were sealed, and absorbance was read at 450 nm (measurement wavelength) and 595 nm (reference wavelength) in a microplate reader. For each well, optical density at the reference wavelength was subtracted from that at the measurement wavelength to obtain the difference data. Difference data of the blank sample were subtracted from the difference data of all quality-control and serum samples to obtain blank reduction.
Pathology.
Acute toxicity study.
At the end of the observation period (14 d after virus injection), rats were euthanized by using an overdose of pentobarbital (800 mg/kg IP). All rats underwent a detailed gross necropsy, with careful examination of the external surface of the body; all orifices; and the cranial, thoracic, and abdominal cavities and their contents. All gross pathologic changes were recorded.
Repeated-dose toxicology study.
On study days 29 (main study animals) or 43 (recovery animals), 10 male and 10 female animals per group (except 5 male and 5 female rats that were used for Ringer only control groups) from the main study and 5 male and 5 female rats from the recovery groups underwent a detailed gross necropsy as described earlier. The following organs were weighed: liver, kidneys, thymus, adrenals, spleen, brain, heart, testes, epididymides, uterus with cervix, and ovaries. All rats found moribund or intercurrently deceased underwent a gross necropsy, and the organs were preserved for histopathologic examination.
The pathologic examination was performed without blinding of the pathologist to the treatment groups.
Histopathology (repeated toxicology study).
All rats in the vehicle control, Ringer solution control, and H1PV high-dose groups in the main study (day 29) and recovery animals (day 43) underwent histologic evaluation without blinding of the pathologist to the allocation of animals. The following organs were examined after preparation of paraffin sections and hematoxylin and eosin staining: lung, brain (cerebrum, cerebellum, and pons), trachea, spinal cord, aorta, pituitary gland, uterus with cervix, thyroid–parathyroid glands, ovaries, thymus, vagina, esophagus, testes, salivary glands, epididymides, stomach, prostate, duodenum, jejunum, ileum with Peyer patches, cecum, colon, rectum, skin with mammary gland, liver, urinary bladder, pancreas, lymph nodes (mesenteric and axillary), kidneys, peripheral nerve (for example, sciatic nerve) with skeletal muscle, adrenal glands, sternal bone with bone marrow, spleen, eyes with optic nerves and Harderian glands, heart, seminal vesicles, coagulating glands, ureters, knee joint with femur, larynx, Zymbal glands, and injection site. These examinations were extended to rats in all other dose groups (day 29) that had organs with possible treatment-related changes. Histologic processing of tissues was performed at the histology contract laboratory (Propath UK, Willow Court, Hereford, Great Britain). Histopathologic evaluation was performed at Kaleidis (Saint Louis, France). Blocking, embedding, cutting, staining, and scientific slide evaluation were performed according to the corresponding standard operating procedures of the test sites.
Isolation and mitogenic stimulation of PBMC.
At the time of necropsy on day 29, PBMC were isolated from 5 male and 5 female rats per group and evaluated in vitro. Blood was collected in EDTA tubes. PBMC were isolated by density-gradient centrifugation (Optiprep, Sigma–Aldrich, St Louis, MO) at 700 × g for 20 min at room temperature. The isolated PBMC were frozen (CTL-Cryo ABC Freezing Kit, Cellular Technology Laboratories, Shaker Heights, OH) and stored at or below –70 °C until analysis.
For mitogenic stimulation, all experiments were done in triplicate. After thawing, 220 000 cells were seeded in individual wells of 96-well microplates and stimulated with phorbol myristate acetate (10 ng/mL) and ionomycin (0.4 µg/mL). Proliferation was measured by MTT assay (catalog no. A2231.0001, Thiazolylblau–Tetrazoliumbromid BioChemica, AppliChem, Darmstadt, Germany) at 0 and 48 h after stimulation.
Analysis of cytokine levels.
Blood samples of at least 800 μL were collected for analysis after 1 wk of treatment (day 8, isoflurane anesthesia, jugular vein sampling) and on day 29 at terminal euthanasia. Blood was sampled from all rats assigned to undergo toxicokinetics (5 male and 5 female rats per dose group). After blood collection, the tubes were left at room temperature for 75 min and then centrifuged for 10 min at 1000 × g. Serum samples were stored at or below −70 °C until analysis by ELISA.
Serum samples were analyzed for IL6, TNFα, and IFNβ by ELISA according to the manufacturer's instructions (IL6: Quantikine Rat IL6 Immunoassay, R and D Systems; TNFα: Quantikine Rat TNFα Immunoassay, R and D Systems; IFNβ: ELISA Kit for Rat Interferon, Usen Life Science, Wuhan, Hubai, People's Republic of China). Handling and preparation of all reagents as well as the analysis of cytokine production were performed according to the manufacturer's instructions provided with the commercially available ELISA kits.
Rat IL6, TNFα, and IFNβ ELISA are based on binding of the respective rat cytokine to 2 antibodies. One antibody was immobilized on the microplate. The other antibody was either conjugated directly to an enzyme or conjugated to avidin, which was subsequently bound by streptavidin conjugated to an enzyme. Absorbance was measured at 450 nm by using an ELISA microplate reader (Infinite M200, Tecan Austria, Salzburg, Austria). The concentrations of IL6, TNFα, and IFNβ in samples and positive controls were calculated from a standard curve of the respective cytokine that was generated by using the 4-parameter Marquardt curve-fitting algorithm (Magellan Tracker version 6.4, Tecan Austria).
Due to technical problems, no reliable results for serum concentration of IFNβ, a marker for a specific response to viral infection could be obtained.
Toxicokinetic evaluation.
Toxicokinetic profiles were performed after the first administration (day 1) and after the last of 17 administrations of H1PV on day 28 in 5 female and 5 male rats. Each animal was anesthetized with isoflurane (2%), and approximately 150 μL blood was sampled from the jugular vein at 15 min, 1 h, 2 h, 6 h, 24 h after virus injection (prior to next administration) and collected in heparin–lithium tubes. The samples were stored at or below −70 °C until real-time PCR analysis.
Quantitative real-time PCR analysis of organs and body fluids.
Organs (brain, lung, liver, spleen, kidney, heart, pancreas, prostate, mammary, and bone marrow), urine, saliva, and feces were sampled for real-time PCR evaluation at terminal euthanasia (day 29 or day 43) from 5 male and 5 female rats per group in the repeated-dose toxicity study. Shortly before euthanasia, saliva was collected from anesthetized (ketamine, 100 mg/kg; xylazine, 10 mg/kg) rats by using cotton swabs (Catch-All Sample Collection Swabs, Epicentre, Madison, WI). Blood, urine, feces, and all tissues were stored at or below −70 °C, and saliva swabs were stored at or below −20 °C until processing and analysis.
Blood, urine, and feces samples were thawed; blood and urine samples were resuspended separately after thawing. The required amounts of organs and feces were weighed out and cut into small pieces. Saliva swabs were cut off the applicators.
DNA was extracted by using different kits from Qiagen (Venlo, Limberg, Netherlands) and Quick Extract DNA Extraction Solution (Epicentre) with minor modifications: QIAamp DNA Mini Kit (with Qiagen protease) was used for blood samples (DNA extraction from whole blood) and tissue samples, QIAamp DNA Stool Kit was used for feces samples, QIAamp Viral RNA Mini Kit was used for urine samples (without DNAse treatment), and Quick Extract DNA Extraction Solution was used for saliva swabs. A validation study of the kits was performed. The reference virus suspension was diluted in 10 mM Tris HCl buffer, pH 7.5, before it was used to spike the calibration and quality-control samples, if necessary. Each calibration curve consisted of 2 negative control samples and 7 samples in duplicate corresponding to target concentrations of 5 × 107 (1.25 × 107 for saliva), 5× 106, 5 × 105, 5 × 104, 5× 103, 5 × 102 VG per PCR reaction and 3× 102 to 5× 101 VG per PCR reaction, depending on the validated method for each tissue type.
The master mix per 20-µL reaction (final volume) was prepared as follows: 3.35 µL PCR grade H2O; 10 µL LightCycler 480 Probes Master 2× (Roche Diagnostics, Indianapolis, IN), 0.55 µL FW-H1 (40×, 10 µM; 5′ GCG CGG CAG AAT TCA AAC T 3′), 0.55 µL Rev-H1 (40×, 10 µM; 5′ CCA CCT GGT TGA GCC ATC AT 3′), 0.55 µL Probe H1 [40× 10 µM; 5′-6-FAM-ATG C5*G CC5* G5*C 5*GT TA TAMRA3′ [asterisk indicates locked nucleic acid with a 2′ O, 4′ C methylene bridge]). Each well of a Light Cycler multiwall plate contained 0.15 µL master mix and 5 µL DNA extract. PCR reaction parameters were 95 °C for 10 min; 45 cycles of 95 °C for 15 s and 60 °C for 60 s; followed by 40 °C for 10 min.
Data calculation and statistical analysis.
A statistical assessment of the results of body weight (days 1, 28, and 42) and body weight gain (between days 1 and 28 and days 1 and 42), food consumption (between days 1 and 28 and days 1 and 42); parameters of hematology, blood coagulation, and clinical biochemistry; and absolute and relative organ weights was performed for each sex by using one-way ANOVA and the posthoc Tukey test to compare the values of the H1PV-treated groups with rats that received vehicle or Ringer solution only. Statistics were performed by using Prism software (version 5.01, GraphPad, San Diego, CA). A P value less than 0.05 was considered to be statistically significant.
Results
Toxicology and pathology.
One aim of this study was to assess the possible adverse effects which could arise 1) from single-bolus injection of Wistar rats with a high dose of H1PV or 2) from repeated exposure via intravenous administrations. Repeated injections were performed over 28 d followed by a 14-d recovery period, to investigate the reversibility of possible findings. Furthermore, the 14-d treatment-free period aimed at detecting the occurrence of delayed virus-related effects.
After single intravenous injection of H1PV to the maximal dose of 8.6 × 108 pfu, none of the male and female Wistar rats displayed any clinical signs of toxicity or mortality. All rats of both sexes continued to gain weight throughout the 14-d observation period, and no differences in weight gain between the control and H1PV-treated groups occurred (data not shown). Gross pathology after euthanasia did not reveal any morphologic lesions due to the virus injection. Therefore, the LD50 value exceeded 8.6 × 108 pfu per rat.
Multiple injections of H1PV (17 injections over 28 d) did not result in any overt signs of toxicity or preparation-related mortality to the dose of 108 pfu per rat. The high dose of H1PV led to transient reduced spontaneous physical activity and increased prone position compared with those of control animals in all rats of that dose group, according to cageside clinical evaluation of the rats during the first hour after administration. No H1PV-related behavioral changes were noted in the IRWIN screen that was performed later, at approximately 24 h after the administrations on days 21 or 28.
Compared with the concurrent controls, body weight gain, food consumption, ophthalmologic examinations, gross pathology, and organ weights were similar among all treatment groups of the repeated-dose toxicity study.
Histopathology (repeated-dose toxicology study).
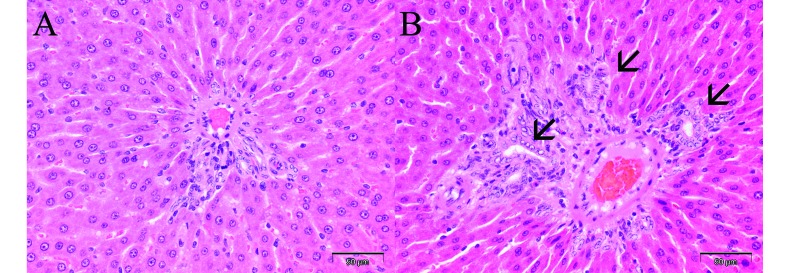
Only minor histopathologic findings attributed to the administration of H1PV occurred. The main treatment-related histopathologic finding at the end of the treatment period (day 29) was minimal diffuse bile duct hyperplasia of the liver (Figure 1) in 1 of 10 female rats after repeated intravenous injection of 107 pfu (intermediate-dose group) and in 1 of 10 male and 6 of 10 female rats repeatedly injected with 108 pfu (high-dose group). This minimal hepatic change was found only in virus-injected rats and therefore is considered to be related to the repeated intravenous treatment with H1PV. The incidence of the liver finding seems to indicate sex-associated dependence, with female rats being more susceptible than male rats. However, considering the H1PV dose on a per-weight basis, relative doses were 30% to 40% higher in female rats than male rats. Therefore, sex-associated dependence of minimal diffuse bile duct hyperplasia is considered to be unlikely. This limited pathologic effect was not noted in any animal of the recovery group, indicating complete reversibility within the 2-wk recovery period.
Figure 1.
Portal triads in liver at the end of treatment. (A) Normal number and epithelial morphology of bile ducts in female rat treated with vehicle only. (B) Minimally increased number and minimally activated epithelial cells of bile ducts (arrows) in female rat treated with 108 pfu H1PV. Magnification, 40×.
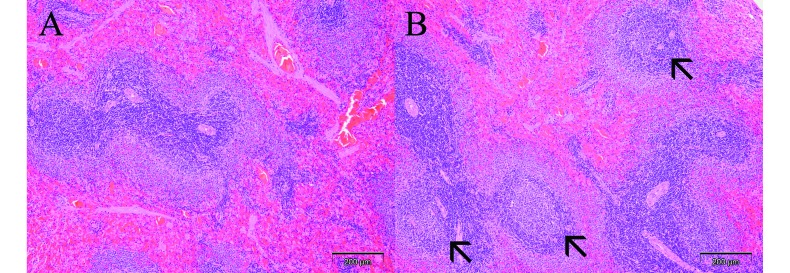
In the spleens of rats repeatedly treated with the high dose (108 pfu), germinal center development was detected only at the end of the recovery period at day 43, possibly as a consequence of an immunologic response after prolonged treatment with H1PV (Figure 2). This feature was present in 3 of 5 male rats and 2 of 5 female rats treated with the high dose, compared with none of the rats in either the vehicle-only or Ringer control groups. Groups treated with the low and intermediate doses of H1PV were not evaluated histologically after the recovery period.
Figure 2.
Germinal centers in spleen at the end of recovery period. (A) Absence of germinal centers in splenic follicles of male rat treated with vehicle only. (B) Mild germinal center development (arrows) in male rat previously treated with 108 pfu H1PV. Magnification, 10×.
Several other minor organ changes, including multifocal minimal vacuolation of corticotubular epithelium in the kidney, were apparent to the same extent in the vehicle- and H1PV-treated groups at the end of the treatment period (data not shown) and therefore are not considered to be caused by virus infection. The changes in kidney remained at the end of the recovery period and therefore are most likely induced by the iodixanol-containing vehicle, because these findings were not noted in the negative control group (Ringer solution).
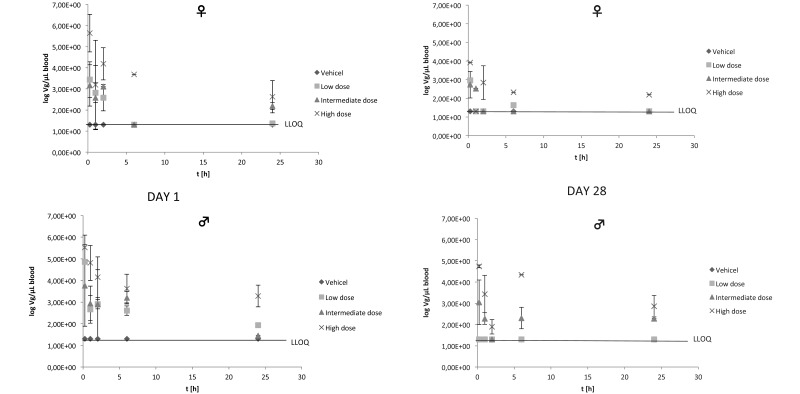
Toxicokinetics after repeated dosing.
Toxicokinetic evaluation of blood was performed on days 1 and 28. Samples were taken at 0.25, 1, 2, 6, and 24 h after virus injection. On both days, VG was highest at the first sampling time point (15 min after injection), as is expected from an intravenous administration. After 24 h, viral DNA could still be detected in blood of some rats of all dose groups, indicating that animals were continuously exposed to H1PV. Dose-dependency could be detected, even though the data were relatively inhomogeneous between different rats of the same group. No significant differences between male and female profiles were seen. The toxicokinetic profile of H1PV in rats on day 28 after repeated administration was generally characterized by lower peak levels and lower overall exposure compared with those after the first administration on day 1. These data indicate an accelerated elimination rate after multiple dosing (Figure 3). We surmise that the altered kinetics most likely were due to the formation of antivirus antibodies. Together, the toxicokinetic data suggest that there is no appreciable sex-associated difference in the plasma kinetics of H1PV in rats.
Figure 3.
Results of toxicokinetics: day 1, left columns; day 28, right columns; upper panels, female rats; lower panels, male rats. Values are given as mean ± 1 SD. Lower limit of quantification (50 VG/PCR reaction) is equivalent to 20 VG/µL blood. Only values above the lower limit of quantification (LLOQ) are included in the calculation of mean values.
Organ distribution and tissue concentration of H1PV after multiple intravenous dosing.
On day 29 and after 2 wk of recovery (day 43), blood and samples of brain, heart, lung, liver, spleen, kidney, pancreas, prostate, mammary gland area, and bone marrow were collected (5 rats of each sex per group). The samples were processed for PCR detection of viral genomes.
After 4 wk of repeated intravenous treatment of rats with 3 different doses (106, 107, and 108 pfu) of H1PV formulated in 10% iodixanol in Ringer solution, viral genomes were detected in all tested organs. In general, the concentrations correlated with the administered dose. The highest concentrations of viral DNA per unit genomic DNA were detected in liver and spleen, whereas the lowest concentrations were in brain, heart, prostate, and mammary gland (Tables 2 and 3). A small number of positive tests in the vehicle control group (11 positive results among 250 analyzed control samples) was not correlated with specific animals and most likely were caused by contamination during DNA sampling or DNA extraction.
Table 2.
Virus distribution (log VG/mg [log VG/µL for blood]; mean ± 1 SD) at terminal euthanasia (day 29 or 43) after multiple dosing in female rats
| Vehicle | Low-dose H1PV | Intermediate-dose H1PV | High-dose H1PV | Recovery vehicle | Recovery high-dose H1PV | ||
| Blood | Mean | LLOQ | 2.68 ± 0.64 | 3.53 ± 1.18 | 4.87± 0.85 | LLOQ | 1.66 ± 0.45 |
| N | 5 | 4 (3) | 5 (4) | 5 | 5 | 4 (2) | |
| Brain | Mean | LLOQ | LLOQ | 3.00 ± 0.24 | 4.04 ± 0.17 | LLOQ | 3.22 ± 0.20 |
| 5 | 5 | 5 | 5 | 5 | 4 | ||
| Lung | Mean | LLOQ | 3.56 ± 0.52 | 5.51 ± 0.35 | 6.84 ± | 2.52 | 4.34 ± 0.27 |
| n | 5 | 5 | 5 | 5 | 5 (4) | 4 (3) | |
| Liver | Mean | LLOQ | 6.28 ± 0.14 | 7.11 ± 0.11 | 7.83 ± 0.15 | 2.44 ± 0.04 | 6.16 ± 0.29 |
| n | 5 | 5 | 5 | 5 (3) | 5 (2) | 4 | |
| Spleen | Mean | LLOQ | 7.11 ± 0.14 | 7.43 ± 0.09 | 8.09 ± 0.16 | LLOQ | 7.04 ± 0.29 |
| n | 5 | 5 (4) | 5 | 5 | 5 | 4 | |
| Kidney | Mean | LLOQ | 3.29 ± 0.06 | 4.73 ± 0.40 | 5.53 ± 0.15 | LLOQ | 4.71 ± 0.40 |
| n | 5 | 5 (4) | 5 | 5 | 5 | 4 | |
| Heart | Mean | LLOQ | LLOQ | 2.95 ± 0.15 | 4.42 ± 0.32 | LLOQ | 2.51 ± 0.03 |
| n | 5 | 5 | 5 | 5 | 5 | 4(2) | |
| Pancreas | Mean | 2.75 | 2.94 ± 0.56 | 3.25 ± 0.76 | 6.54 ± 0.22 | LLOQ | 3.90 ± 0.13 |
| n | 5 (1) | 5 (4) | 5 (4) | 5 | 5 | 4 (2) | |
| Mammary | Mean | 3.32 | 3.35 ± 0.20 | 3.92 ± 0.12 | 5.37 ± 1.03 | 4.04 | 3.54 |
| n | 5 (1) | 5 (2) | 5 | 5 | 5 (1) | 4 (1) | |
| Bone marrow | Mean | 3.23 | 3.80 ± 0.73 | 5.88 ± 0.17 | 6.30 ± 1.16 | LLOQ | 4.76 ± 0.33 |
| n | 5 (1) | 5 (4) | 5 (4) | 5 (4) | 5 | 4 |
The number of analyzed samples is given; the number in parentheses is the number of samples included in the calculation of mean values and standard deviations. Values below the lower limit of quantification (LLOQ) were not included in the calculation.
Table 3.
Virus distribution (log VG/mg [log VG/µL for blood]; mean ± 1 SD) at terminal euthanasia (day 29 or 43) after multiple dosing in male rats
| Vehicle | Low-dose H1PV | Intermediate-dose H1PV | High-dose H1PV | Recovery vehicle | Recovery high-dose H1PV | ||
| Blood | Mean | LLOQ | 2.08 ± 0.33 | 3.73 ± 0.69 | 4.59 ± 0.74 | LLOQ | 2.52 ± 0.60 |
| n | 5 | 5 (4) | 5 (4) | 5 | 5 | 5 (2) | |
| Brain | Mean | LLOQ | LLOQ | 2.94 ± 0.22 | 3.85 ± 0.18 | LLOQ | 3.45 ± 0.20 |
| n | 5 | 5 | 5 | 5 | 5 | 5 | |
| Lung | Mean | 2.50 | 3.68 ± 0.43 | 5.50 ± 0.33 | 5.52 ± 0.17 | LLOQ | 4.95 ± 0.38 |
| n | 5 | 5 | 5 | 5 (4) | 5 | 5 (4) | |
| Liver | Mean | LLOQ | 5.45 ± 0.58 | 6.93 ± 0.20 | 7.71 ± 0.10 | LLOQ | 6.14 ± 0.17 |
| n | 5 | 5 | 5 | 5 | 5 | 5 | |
| Spleen | Mean | 3.00 | 6.78 ± 0.16 | 7.34 ± 0.14 | 7.89 ± 0.09 | LLOQ | 6.84 ± 0.38 |
| n | 5 (1) | 5 | 5 | 5 | 5 | 5 | |
| Kidney | Mean | LLOQ | 3.07 ± 0.18 | 4.56 ± 0.36 | 5.38 ± 0.20 | LLOQ | 4.70 ± 0.08 |
| n | 5 | 5 | 5 | 5 | 5 | 5 | |
| Heart | Mean | LLOQ | LLOQ | 3.12 ± 0.11 | 3.97 ± 0.16 | LLOQ | 2.47 ± 0.05 |
| n | 5 | 5 | 5 | 5 | 5 | 5 (3) | |
| Pancreas | Mean | 2.76 | 3.39 ± 0.33 | 3.21 ± 0.30 | 6.05 ± 0.44 | LLOQ | 4.42 ± 1.09 |
| n | 5 (1) | 5 (2) | 5 (4) | 5 | 5 | 5 | |
| Prostate | Mean | LLOQ | 2.52 | 3.42 ± 0.40 | 5.43 ± 0.84 | LLOQ | 3.92 ± 0.29 |
| n | 5 | 5 (1) | 5 | 5 | 5 | 5 | |
| Mammary | Mean | LLOQ | 3.12 ± 0.05 | 3.97 ± 0.13 | 4.97 ± 0.48 | 3.82 ± 0.22 | 3.22 |
| n | 5 | 5 (2) | 5 (2) | 5 | 5 | 5 (1) | |
| Bone marrow | Mean | LLOQ | 3.65 ± 0.15 | 5.30 ± 0.47 | 6.30 ± 0.58 | LLOQ | 4.59 ± 0.57 |
| n | 5 | 5 (4) | 5 (4) | 5 | 5 | 5 (4) |
The number of analyzed samples is given; the number in parentheses is the number of samples included in the calculation of mean values and standard deviations. Values below the lower limit of quantification (LLOQ) were not included in the calculation.
At the end of the 2-wk treatment-free recovery period, all organs continued to test positive for viral DNA. No increase in the number of VG compared with results of the main study was appreciable in any organ. In contrast, viral DNA concentrations decreased slightly in all tissues compared with the samples collected at the end of the treatment period. No difference between the sexes was detected.
Excretion after multiple intravenous dosing.
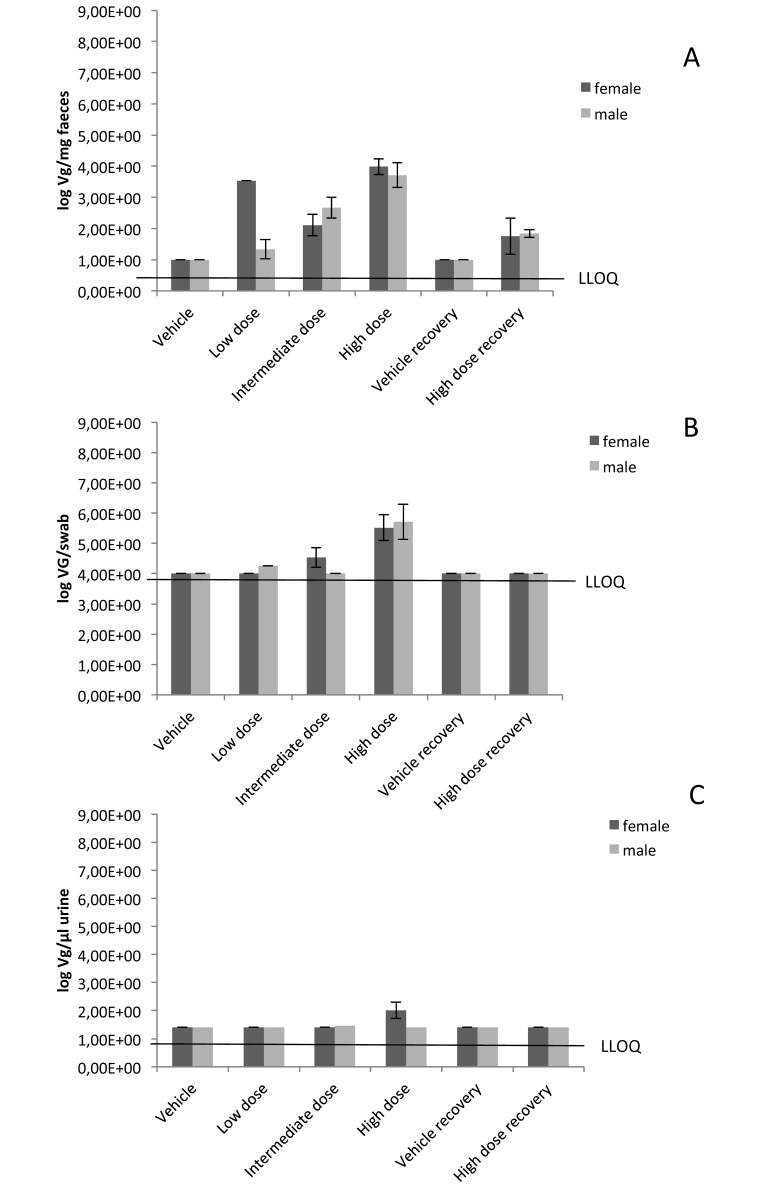
Samples of urine, feces, and saliva were collected on the same days as were the organ samples (days 29 and 43) and were evaluated by using the same PCR protocol. Samples of saliva, urine, and feces from rats treated with 106 to 108 pfu H1PV per dose for 4 wk showed that the highest concentrations of viral DNA were detected in feces (day 29). After the 2-wk treatment-free recovery period (day 43), minor concentrations of VG were still detectable in feces of rats of the high-dose group. At 24 h after the last intravenous treatment with H1PV, viral DNA was detected in the saliva and urine samples of some rats, mainly those in the high-dose (108 pfu per dose) group. However, the amounts of viral DNA were lower in saliva and urine than in feces. This finding points to a substantial contribution of the hepatobiliary system to virus excretion, albeit an H1PV infection of the gastrointestinal tract as a source of fecal VG cannot be excluded. Like blood and tissue samples, all samples of feces, urine, and saliva showed a dose-dependent increase of viral genomes (Figure 4).
Figure 4.
Mean virus excretion. Concentrations below the lower limit of quantification (LLOQ) are presented as the LLOQ. (A) Feces (no. of VG/mg). (B) Saliva (no. of VG/swab). (C) Urine (no. of VG/µL). The standard deviation was calculated (if applicable, more than one value above LLOQ) for the log of single values. Only values above the LLOQ were included in the calculation of mean values, because no definitive values are available for samples with a VG concentration below LLOQ. When all values of a group were lower than the LLOQ, the mean value is presented as the LLOQ.
Because the lower limit of quantification for saliva was higher than those for feces or urine, it cannot be excluded that viral DNA was also present in saliva of rats at the low and intermediate doses of H1PV.
Clinical biochemistry.
At all dose levels on day 29, albumin concentrations (decreased), total globulin concentrations (increased) and albumin:globulin ratios (decreased) were slightly but significantly (P < 0.05) altered in male rats injected with H1PV. At the end of the 2-wk recovery period, the changes for the albumin:globulin ratio and albumin were proven to be reversible in male rats repeatedly treated with 108 pfu H1PV per dose and thus most likely are reversible over the entire dose range. At the end of the recovery period, the serum total globulin level was significantly (P < 0.05) higher in female rats given 108 pfu per dose compared with vehicle or Ringer solution only. These slight changes did not show a dose–response relationship and were present only in one sex. In consequence, these findings are unlikely to be a pathologic effect of H1PV infection.
For the remaining clinical chemical parameters (ALT, AST, ALP, creatinine, total protein, urea, total bilirubin, total cholesterol, triglycerides, glucose, sodium, potassium, and calcium), most mean and individual values were within the biologic ranges (data not shown).
Hematology.
The following hematologic parameters and blood coagulation parameters did not show noteworthy differences between H1PV-injected and control animals: RBC count, MCV, MCH, MCHC, reticulocyte percentage, platelet count, WBC count, neutrophils, lymphocytes, monocytes, prothrombin time, and activated partial thromboplastin time. Some observed changes, such as increased mean Hgb and Hct levels in LD female and HD male rats, decreased mean basophil percentage in vehicle-only animals, or lower mean eosinophil percentages in vehicle-only and H1PV female animals of all dose groups, reached statistical significance. Because the changes were not dose-related, seen only in one sex, or noted only in the vehicle-only control group and were still within the biologic values expected for animals of this strain and age, they were unrelated to H1PV infection. Parameters indicating inflammation were not affected (data not shown).
Urinalysis.
All tested parameters of urinalysis (specific gravity, nitrite, pH, protein, glucose, ketone bodies, urobilinogen, bilirubin, RBC, and leukocytes) were unchanged after H1PV infection when compared with controls (data not shown).
Immunology.
Potential effects of H1PV on the immune system were investigated on days 8 and 29. Blood samples were tested for cytokine release, antiH1PV antibodies, and the function of PBMC (only on day 29). Cytokine measurements showed that serum levels of IL6 (a marker of inflammatory response) and TNFα (indicator for systemic inflammation) were equivalent between virus-treated rats and control groups. All serum concentrations were below the limit of quantitation, irrespective of the virus dose (data not shown). Due to technical problems, no reliable results for serum concentration of IFNβ, a marker for a specific response to viral infection could be obtained.
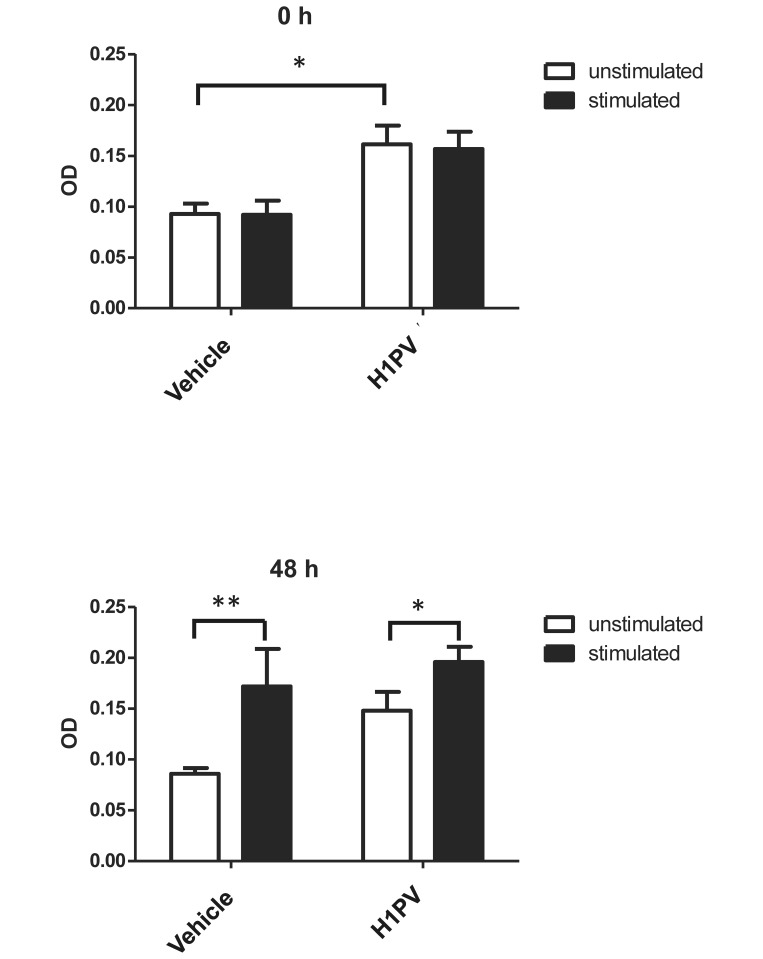
PBMC from rats in the control group and the high-dose were compared for signs of toxicity and for proliferative potential after mitogenic stimulation at 2 different time points (0 and 48 h of stimulation). H1PV had no microscopically visible cytotoxic effect on isolated PBMC from male or female animals (data not shown). At baseline (0 h, unstimulated) after incubation with the solvent DMSO, PBMC from H1PV-treated rats showed significantly (P < 0.05) higher OD values than did vehicle-only controls, indicating a higher percentage of metabolically active PBMC in virus treated animals. After stimulation at 0 h, there was no difference between the 2 animal groups at this time point. Proliferative capacity of PBMC from virus-injected and control rats at 48 h after stimulation was equivalent among male rats tested individually. Stimulation with phorbol myristate acetate and ionomycin had a significant proliferative effect in vehicle-injected rats (P < 0.01) and H1PV-treated animals (P < 0.05; Figure 5). In female rats, PBMC from all control and high-dose groups had to be pooled prior to seeding due to low cell numbers. Pooled samples showed no proliferation difference between H1PV-injected animals and controls, ruling out sex-associated differences.
Figure 5.
Proliferation of PBMC after mitogenic stimulation. Optical density (OD) was measured at 570 nm at 2 time points (0 and 48 h after stimulation). Statistical analysis was performed using a 2-way ANOVA and a Bonferroni post test. *, Value significantly (P < 0.05) different from those for control groups. Results from male rats, which were analyzed individually, are shown. PBMC from female rats had to be pooled due to low cell numbers and showed similar effects to those in male rats (data not shown).
In addition, there was no change in organ weight of thymus, spleen, and lymph nodes or any morphologic changes of lymphatic tissues, such as Peyer patches and bronchus-associated lymphoid tissue, in rats after 17 injections of as much as 108 pfu/injection within 28 d.
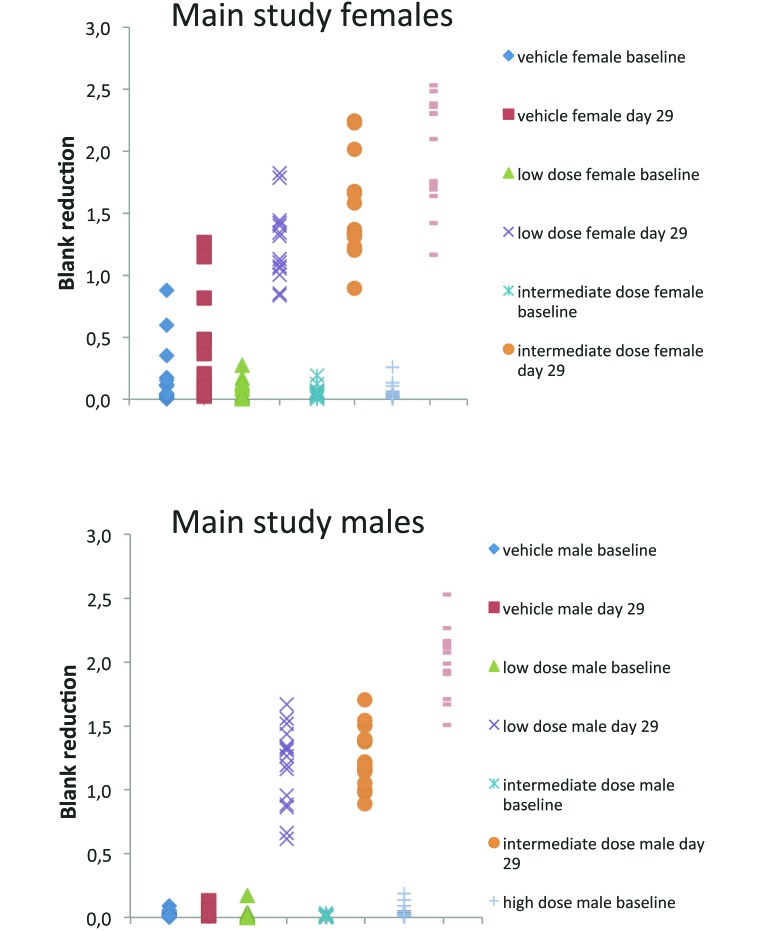
The presence of antivirus antibodies (IgG isotype) was investigated in serum samples from rats of the 4-wk repeated-dose toxicity study. By using a previously characterized direct ELISA format, all sera from virus-treated animals scored positive in the H1PV IgG-specific screening assay in both sexes on day 29 (main study and toxicokinetic animals) and after a 2-wk recovery period on day 43 (recovery animals of the high-dose group). Signal strength was generally dose-dependent with increasing OD values (after blank reduction) from low-dose to high-dose groups (Figure 6).
Figure 6.
Measurement of antiH1PV antibodies (IgG) by specific ELISA. Raw data (blank reductions) are shown groupwise for individual rats; results are shown for female (upper panels) and male (lower panels) rats.
Discussion
The main purpose of the 2 studies was to assess the potential toxicity profile of H1PV in rats as the natural host and thereby representing a model system that is permissive for virus replication, possibly enhancing deleterious effects. In both studies, a total of 253 (126 male and 127 female) rats were investigated. The virus preparation was administered by single intravenous injection at 2 different doses or by repeated (n = 17) intravenous bolus administrations in graduated doses to different groups of male and female test animals, one dose level per group. Doses were 8.6 × 107 and 8.6 × 108 pfu in the acute toxicity study and 106 (low dose), 107 (intermediate dose) and 108 (high dose) pfu per injection per rat in the repeated-dose toxicity study. Control groups received 48% or 10% iodixanol in Ringer solution as vehicle or Ringer solution only. Rats were treated for 4 wk in the repeated-dose toxicity study.
In general, intravenous injections of GMP-grade H1PV at either a single dose of a maximum of 8.6 × 108 pfu/dose or of 17 intravenous doses at 3 dose levels (maximum, 1 × 108 pfu per injection) were well tolerated in rats. There was no virus-related mortality, and no toxicologically relevant general signs of pathology could be demonstrated. We saw no local intolerability at the injection sites, no relevant changes in organ weights, no evidence of late and irreversible toxicities, and no immunotoxicity. In consequence, the intravenous LD50 value of H1PV in rat is higher than is the test dose (8.6 108 pfu H1PV).
Repeated intravenous injections led to virus distribution to all tested body organs at all 3 dose levels. Because H1PV is a replication-competent virus, the organ distribution of H1PV was determined by qPCR, which allowed for quantification of viral genomes and comparison with the input dose. However, positive test results are not equivalent to the presence of active and infectious virus particles. Therefore, one limitation of this study is the inability to assess the possible production of infectious progeny viruses in normal tissue. Because the amount of viral DNA in tested organs did not exceed the inoculum, virus replication at a high rate is unlikely. All organs from rats of the recovery group (high dose only) continued to test positive for viral DNA 14 d after the last administration, and viral DNA remained present in feces at the end of the recovery period. Due to the limitation of the applied methodology of qPCR, whether slow clearance of the inoculum or replication was the reason for the positive test results 2 wk after the last dose cannot be established. The persistence of viral DNA after 2 wk of recovery corroborates previously published results on rat parvoviruses, which showed that infectious parvovirus can be recovered from inoculated rats as late as 6 mo after infection.16
The main excretion route for H1PV was the digestive tract (via feces), whereas the excretion of H1PV via urine was negligible. The saliva data indicate low excretion by this route. However, they probably do not accurately reflect the amount of H1PV excreted via saliva, because contamination of the oral cavity by feces (coprophagy) or bedding (ingestion of bedding) cannot be excluded.
Even though viral DNA was detected in all tested organs after virus infection, there was no indication of functional impairment of infected tissues. Histopathology did not reveal any differences between H1PV-infected and control animals except in 2 organs: liver and spleen. In liver, one female rat in the MD group and one male rat and 6 female rats of the high-dose group had minimal diffuse bile duct hyperplasia; no low-dose or control animals had this alteration. In consequence, this finding was considered to be related to the repeated intravenous treatment with H1PV. This minor effect was completely reversible after the 2-wk recovery period. The dose-dependent changes in the liver further support that the main route of clearance of H1PV is via the reticuloendothelial system and digestive tract. This route of viral excretion is similar to those of other viruses used for oncolytic therapy, such as adenovirus.4
The alterations in liver tissue were not accompanied by pathologic results of blood tests for liver function or by increased serum levels of liver enzymes as sensitive indicators for cytotoxic effects. In conclusion, all test results indicate that repeated injections of high doses of H1PV lead to only minor structural changes in the liver and that these changes are transient and are not accompanied by cellular damage. The clinical relevance of an isolated minimal bile duct hyperplasia that has not been reported as a solitary finding in humans remains unclear. Although unlikely, this feature could be a relevant safety issue in patients who have previously been treated with drugs that affect liver function, given the knowledge that H1PV infection in combination with hepatotoxic chemicals can lead to hepatocellular necrosis in rats.3 Therefore, liver function should be monitored closely during experimental treatment with H1PV. Furthermore, it cannot be ruled out entirely that exposure to even higher doses of H1PV, either by direct injection or by replication in susceptible tissues such as tumors, could lead to more pronounced and possibly pathologic changes of liver tissue or liver function.
In spleen, multiple injections of H1PV led to histologic evidence of prominent germinal center development at the high dose. In contrast to the findings in liver, the alterations in spleen were detected only after the recovery period. Because rats from the low- and intermediate-dose groups were not evaluated at this time point, whether this observation was dose-dependent remains unclear. A likely explanation is the development of a relatively late immunogenic reaction that persisted for more than 2 wk after the last virus injection. Given that the follow-up of rats in this study was restricted to 43 d, the reversibility of the activation in spleen is unknown. However, this histopathologic finding was not associated with clinical abnormalities of the immune system during the test phase. However, long-term effects, even though unlikely, cannot be excluded by the current experimental protocol.
Repeated dosing of H1PV led to transient clinical signs such as reduced spontaneous activity immediately after the last 3 administrations of the highest virus dose. In the absence of other findings, this effect most likely is related to the activation of the immune system by repeated infections. Cytokine levels, which could support this explanation, were tested 1 d after the last virus injection (day 28), when the clinical findings were observed. By this time, the rats had already recovered; therefore the negative cytokine test results do not contradict this most likely explanation. However, there are no specific test results that provide proof for this interpretation. The transient effect of the behavioral changes was further supported by the IRWIN screening, which was done approximately 24 h after the virus injections on day 21 (16th administration) or day 28 (17th administration) in a subset of animals. In this test, the reduced spontaneous activity level which occurred only immediately after virus administration in the high-dose group and lasted for only 1 h, could no longer be observed when the examination was conducted.
The determination of clinical chemical parameters revealed that most mean and individual values were within the biologic ranges. At all dose levels, albumin (decreased) and total globulin (increased) concentrations and albumin:globulin ratio (decreased) were slightly but significantly (P < 0.05) altered in male rats after H1PV treatment. The changes were reversible for the albumin:globulin and albumin in high-dose male rats at the end of the 2-wk recovery period. These slight changes did not show a dose–response relationship and were noted only in one sex. Therefore, the findings are not considered to be toxicologically significant and may in addition reflect the immunogenic potential of H1PV rather than a toxicologic effect.
The assessment of the hematology and coagulation parameters did not reveal any toxicologically relevant changes that could be attributed to H1PV.
The toxicokinetics of H1PV blood levels showed results that could be expected after intravenous injection. The maximal concentrations were measured at the earliest time point after virus administration (0.25 h), and no sex-associated difference could be detected. After multiple injections, peak serum levels were lower compared with those associated with the first dose. This result is most likely due to an increased rate of elimination.
H1PV administration did not impair the activity of the peripheral blood mononuclear cells nor did it lead to systemic release of cytokines (IL6 and TNFα) on the sampling days. Due to technical problems with a test kit leading to erroneous values for negative controls, the measurements for IFNβ could not be used for the study. In consequence it remains unclear whether H1PV infection leads to interferon release as does Kilham rat virus.17 However, given that the other 2 tested cytokine profiles were negative and because H1PV (and other parvoviruses) are known to be inefficient inducers of IFNβ, high serum levels of IFNβ after H1PV infection were considered unlikely, and no further experiments were performed.25
Finally, H1PV consistently induced production of antivirus IgG antibodies in all rats analyzed. The antivirus antibodies were still present at the end of the 2-wk recovery period in all rats previously treated with H1PV. Concurrently viral DNA was still detectable in several tissues and organs in which antivirus antibodies did not lead to viral clearance. This finding is in line with results published for rat parvovirus, which showed that in the presence of antiviral antibodies, active virus could be recovered from 7 wk until 6 mo after infection.16
All investigations were performed in mature rats. Previous evaluations showed that H1PV injection is embryo- and fetotoxic.10,11,18,19,26 This study did not include animals at these young developmental stages. The experiments were not designed to elucidate whether H1PV causes toxicity either by direct injection or by virus replication in noncancerous developing tissue. If H1PV were considered for the treatment of children or adolescents, the toxicologic testing of the virus needs to be extended to young rats at different stages of development.
Acknowledgments
KG and JR have patent rights related to the clinical use of H1PV (US 7,149,456 B2). BH and OK are employed by ORYX. ORYX is the sponsor of the clinical trial in brain tumor patients. All other authors declare no competing interests.
References
- 1.Angelova AL, Aprahamian M, Balboni G, Delecluse HJ, Feederle R, Kiprianova I, Grekova SP, Galabov AS, Witzens-Harig M, Ho AD, Rommelaere J, Raykov Z. 2009. Oncolytic rat parvovirus H1PV, a candidate for the treatment of human lymphoma: in vitro and in vivo studies. Mol Ther 17:1164–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelova AL, Aprahamian M, Grekova SP, Hajri A, Leuchs B, Giese NA, Dinsart C, Herrmann A, Balboni G, Rommelaere J, Raykov Z. 2009. Improvement of gemcitabine-based therapy of pancreatic carcinoma by means of oncolytic parvovirus H1PV. Clin Cancer Res 15:511–519. [DOI] [PubMed] [Google Scholar]
- 3.Besselsen DG, Besch-Williford CL, Pintel DJ, Franklin CL, Hook RR, Jr, Riley LK. 1995. Detection of H1 parvovirus and Kilham rat virus by PCR. J Clin Microbiol 33:1699–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breidenbach M, Rein DT, Wang M, Nettelbeck DM, Hemminki A, Ulasov I, Rivera AR, Everts M, Alvarez RD, Douglas JT, Curiel DT. 2004. Genetic replacement of the adenovirus shaft fiber reduces liver tropism in ovarian cancer gene therapy. Hum Gene Ther 15:509–518. [DOI] [PubMed] [Google Scholar]
- 5.Bundesministeriums der Justiz 2001. Gesetz zur Verhütung und Bekämpfung von Infektionskrankheiten beim Menschen (Infektionsschutzgesetz - IfSG). Available at http://www.gesetze-im-internet.de/bundesrecht/ifsg/gesamt.pdf
- 6.Bundesministeriums der Justiz 2009. Tierschutzgesetz in der Fassung der Bekanntmachung vom 18. Mai 2006 (BGBl. I S. 1206, 1313), das zuletzt durch das Gesetz vom 15. Juli 2009 (BGBl. I S. 1950) geändert worden ist". Available at http://www.gesetze-im-internet.de/tierschg/
- 7.Cornelis JJ, Deleu L, Koch U, Rommelaere J. 2006. Parvovirus oncosuppression, p 365–384. In: Kerr JR, Cotmore SF, Bloom ME, Linden RM. The parvoviruses. London (UK): Hodder Arnold. [Google Scholar]
- 8.Dupressoir T, Vanacker JM, Cornelis JJ, Duponchel N, Rommelaere J. 1989. Inhibition by parvovirus H1 of the formation of tumors in nude mice and colonies in vitro by transformed human mammary epithelial cells. Cancer Res 49:3203–3208. [PubMed] [Google Scholar]
- 9.European Medicines Agency 2009. ICH guideline M3(R2) on non-clinical safety studies for the conduct of human clinical trials and marketing authorisation for pharmaceuticals. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002720.pdf
- 10.Ferm VH, Kilham L. 1963. Rat virus (RV) infection in fetal and pregnant hamsters. Proc Soc Exp Biol Med 112:623–626. [DOI] [PubMed] [Google Scholar]
- 11.Ferm VH, Kilham L. 1964. Congenital anomalies induced in hamster embryos with H1 virus. Science 145:510–511. [DOI] [PubMed] [Google Scholar]
- 12.Geletneky K, Huesing J, Rommelaere J, Schlehofer JR, Leuchs B, Dahm M, Krebs O, von Knebel Doeberitz M, Huber B, Hajda J. 2012. Phase I/IIa study of intratumoral–intracerebral or intravenous–intracerebral administration of parvovirus H1 (ParvOryx) in patients with progressive primary or recurrent glioblastoma multiforme: ParvOryx01 protocol. BMC Cancer 12:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geletneky K, Kiprianova I, Ayache A, Koch R, Herrero YCM, Deleu L, Sommer C, Thomas N, Rommelaere J, Schlehofer JR. 2010. Regression of advanced rat and human gliomas by local or systemic treatment with oncolytic parvovirus H1 in rat models. Neuro-oncol 12:804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrero YCM, Cornelis JJ, Herold-Mende C, Rommelaere J, Schlehofer JR, Geletneky K. 2004. Parvovirus H1 infection of human glioma cells leads to complete viral replication and efficient cell killing. Int J Cancer 109:76–84. [DOI] [PubMed] [Google Scholar]
- 15.Irwin S. 1968. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia 13:222–257. [DOI] [PubMed] [Google Scholar]
- 16.Jacoby RO, Johnson EA, Paturzo FX, Gaertner DJ, Brandsma JL, Smith AL. 1991. Persistent rat parvovirus infection in individually housed rats. Arch Virol 117:193–205. [DOI] [PubMed] [Google Scholar]
- 17.Kilham L, Buckler CE, Ferm VH, Baron S. 1968. Production of interferon during rat virus infection. Proc Soc Exp Biol Med 129:274–278. [DOI] [PubMed] [Google Scholar]
- 18.Kilham L, Ferm VH. 1964. Rat virus (RV) infections of pregnant, fetal, and newborn rats. Proc Soc Exp Biol Med 117:874–879. [DOI] [PubMed] [Google Scholar]
- 19.Kilham L, Margolis G. 1969. Transplacental infection of rats and hamsters induced by oral and parenteral inoculations of Hl and rat (RV) viruses. Teratology 2:111–123. [DOI] [PubMed] [Google Scholar]
- 20.Kilham L, Margolis G, Colby ED. 1970. Enhanced proliferation of H1 virus in livers of rats infected with Cysticercus fasciolaris. J Infect Dis 121:648–652. [DOI] [PubMed] [Google Scholar]
- 21.Lacroix J, Leuchs B, Li J, Hristov G, Deubzer HE, Kulozik AE, Rommelaere J, Schlehofer JR, Witt O. 2010. Parvovirus H1 selectively induces cytotoxic effects on human neuroblastoma cells. Int J Cancer 127:1230–1239. [DOI] [PubMed] [Google Scholar]
- 22.Moscardo E, Maurin A, Dorigatti R, Champeroux P, Richard S. 2007. An optimised methodology for the neurobehavioural assessment in rodents. J Pharmacol Toxicol Methods 56:239–255. [DOI] [PubMed] [Google Scholar]
- 23.Raykov Z, Grekova S, Galabov AS, Balboni G, Koch U, Aprahamian M, Rommelaere J. 2007. Combined oncolytic and vaccination activities of parvovirus H1 in a metastatic tumor model. Oncol Rep 17:1493–1499. [PubMed] [Google Scholar]
- 24.Rommelaere J, Geletneky K, Angelova AL, Daeffler L, Dinsart C, Kiprianova I, Schlehofer JR, Raykov Z. 2010. Oncolytic parvoviruses as cancer therapeutics. Cytokine Growth Factor Rev 21:185–195. [DOI] [PubMed] [Google Scholar]
- 25.Schlehofer JR, Rentrop M, Mannel DN. 1992. Parvoviruses are inefficient in inducing interferon β, tumor necrosis factor α, or interleukin 6 in mammalian cells. Med Microbiol Immunol (Berl) 181:153–164. [DOI] [PubMed] [Google Scholar]
- 26.Soike KF, Iatropoulis M, Siegl G. 1976. Infection of newborn and fetal hamsters induced by inoculation of LuIII parvovirus. Arch Virol 51:235–241. [DOI] [PubMed] [Google Scholar]
- 27.Tattersall P. 2006. The evolution of parvoviral taxonomy, p 5–14. In: Kerr JR, Cotmore SF, Bloom ME, Linden RM, Parrish CR. The parvoviruses. London (UK): Hodder Arnold. [Google Scholar]
- 28.Toolan HW, Ledinko N. 1968. Inhibition by H1 virus of the incidence of tumors produced by adenovirus 12 in hamsters. Virology 35:475–478. [DOI] [PubMed] [Google Scholar]
- 29.Toolan HW, Rhode SL, 3rd, Gierthy JF. 1982. Inhibition of 7,12-dimethylbenz(a)anthracene-induced tumors in Syrian hamsters by prior infection with H1 parvovirus. Cancer Res 42:2552–2555. [PubMed] [Google Scholar]
- 30.Wrzesinski C, Tesfay L, Salome N, Jauniaux JC, Rommelaere J, Cornelis J, Dinsart C. 2003. Chimeric and pseudotyped parvoviruses minimize the contamination of recombinant stocks with replication-competent viruses and identify a DNA sequence that restricts parvovirus H1 in mouse cells. J Virol 77:3851–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]