Abstract
The autonomous parvovirus H1 (H1PV) is transmitted in rodent populations. The natural host is the rat, in which H1PV infection is pathogenic only in fetuses and newborns. H1PV infection of human cancer cells leads to strong oncolytic effects in preclinical models. In preparation for a clinical trial of H1PV injection in patients with malignant brain tumors, H1PV had to be prepared to Good Manufacturing Practice standards, including extensive toxicology testing in rats. Because the trial involves direct intracerebral injection of H1PV into the tumor and around the resection cavity, possible toxicity to CNS tissue had to be investigated. In addition, quantitative blood levels and the tissue distribution of H1PV after single intracerebral or intravenous injection were measured. Direct injection of H1PV into rat brain at 3 dose levels (maximum, 7.96 × 107 pfu) did not cause any macroscopic or histologic pathology. Furthermore, H1PV infection of the brain did not alter central or autonomous nervous system function. H1PV DNA was detected in almost all organs at 6 h, 48 h, and 14 d after intravenous and intracerebral injection, with the highest levels in liver and spleen. H1PV concentrations in most organs were similar after intravenous and intracerebral injection, indicating high permeability of the blood–brain barrier for this small virus. The current results demonstrate wide organ distribution of H1PV after intravenous or intracerebral injection, confirm that H1PV is nonpathogenic in adult rats even after direct injection into the brain, and form the basis for the ongoing ParvOryx01 clinical trial.
Abbreviations: H1PV, parvovirus H1; GMP, good manufacturing practice; LLOQ, lowest limit of quantification; VG, viral genomes
Parvovirus H1 (H1PV) is a small single-stranded nonenveloped DNA virus of 5.1 kb that belongs to the family Parvoviridae. The natural host is the rat but, similar to other related parvoviruses, H1PV can infect and replicate in cells of various other species, including humans.24 However, a systematic screening of species susceptible to H1PV infection has not yet been performed.24 In its natural host, H1PV is pathogenic only in newborns and is embryo- and fetotoxic. The detrimental effects on progeny depend on the age at infection: whereas rats inoculated with H1PV during the ovular preimplantation period do not reveal any reproductive impairment, infection of dams in the middle of the gestational period typically results in fetal death. When infected during late gestation or within the first few days after birth, the progeny frequently develop the so-called ‘osteolytic syndrome,’ characterized by dwarfism and various mongoloid-like features.10,11,17,18,23
Because H1PV is readily transmitted in rodent populations in the absence of natural immunity, laboratory animals are routinely tested for the presence of antiH1PV antibodies. Antibodies to H1PV were found in 10.4% of rats caught from feral populations.8 To our knowledge, there is currently no detailed information about the bioavailability and tissue distribution of H1PV after natural or experimental infection in vivo, although the route of virus excretion is known to follow fecal and oronasal routes.18
In addition to being a naturally occurring virus in the rodent population, H1PV has oncosuppressive properties, inhibiting the formation of spontaneous as well as chemically or virally induced tumors in laboratory animals.6,25,26 More recent investigations demonstrated the direct oncolytic potential of the virus in a number of cancers.1,2,7,22 H1PV infection of tumor cell cultures, including human tumor cells, and of experimental tumors in vivo led to efficient cell killing and tumor regression.14 The parvoviral cytotoxicity is attributed, at least in part, to the viral regulatory nonstructural protein NS1, and the smaller nonstructural protein NS2 may modulate NS1 cytotoxicity. One promising tumor entity in which H1PV showed strong oncolytic effects is malignant brain tumors (specifically, glioblastoma).13 The encouraging rates of complete remissions after intratumoral or intravenous H1PV injection of rats bearing large intracranial gliomas provided the rationale for a clinical trial in patients with recurrent glioblastoma.12
For clinical use, an H1 virus stock conforming with the standards of Good Manufacturing Practice (GMP) had to be available. To comply with GMP requirements, the purity, infectivity, and stability of the virus preparation had to be demonstrated by the manufacturer. Furthermore, prior to injection in patients, this GMP virus preparation had to undergo extensive nonclinical safety testing. To support the clinical phase I protocol, which includes intravenous infusions as well as direct injection of H1PV into brain tumors, both routes of administration were included in the test protocol. Due to the permissiveness for H1PV infection and replication, rats were defined as the appropriate and sufficient species in which to investigate the potential toxicity profile of H1PV. This species selection was approved by the regulatory authority (Paul-Ehrlich Institut, Langen, Germany).
This report focuses on (1) the tissue availability and biodistribution of H1PV after single intravenous and intracranial injection and (2) the potential CNS toxicity of H1PV after direct injection into the rat brain. The results from these studies, along with those of additional studies after single- and repeated-dose administration (reported elsewhere),15 were mandatory for the regulatory approval of the clinical trial (ParvOryx01) that started in October 2011. In addition to providing safety information of direct clinical relevance, the data offer insights into the biology of H1PV in its natural host (rats), including organ distribution, relative organ concentration, penetration of the blood–brain barrier, viral shedding, and infection-associated behavioral changes. Because the data were generated with purified virus under highly controlled test conditions, the results add valuable information to the understanding of H1PV infection in its natural host. In addition, making these results available may help to reduce the number of future animal experiments in this or related areas of research.
Materials and Methods
Test material.
Replication-competent wild-type H1PV was produced to GMP grade by IDT Biologika (Dessau, Germany, Batch no. 0090810). H1PV production was performed according to a modified standard protocol that was adapted to GMP requirements and large-scale production. In brief, H1PV was propagated in human newborn kidney cells (NB324K) and purified by iodixanol density-gradient centrifugation.27 Infective particles were quantified by plaque-forming assay. The virus titer of H1PV in the test batch used for all animal experiments was 7.96 × 109 pfu/mL.
All test-item formulations were prepared as stock solutions and stored as aliquots at approximately −80 °C until use. Repeated freezing and thawing of the aliquots was avoided. H1PV formulations were tested for the concentration of viral genomes (VG) by PCR and for pfu by plaque assay. All injection doses were fully infective at the time of injection. The ratio of VG to pfu was approximately 1× 103. Test item formulations remaining after individual in vivo administrations were discarded.
Virus formulation for bioavailability testing.
All rats received the same dose of 7.96 × 107 pfu. For intravenous administrations, 650 μL H1PV stock was diluted with 5850 μL 48% iodixanol in Ringer solution (batch 290410; refraction index, 1.41 ± 0.01). For intracerebral administration, aliquots of undiluted virus stock were used.
Virus formulation for investigation of adverse CNS effects.
Intracerebral H1PV injection was performed at 3 dose levels: 7.96 × 107 pfu (high dose), 7.96 × 106 pfu (intermediate dose), and 7.96 × 105 pfu (low dose). For the high-dose group, aliquots of undiluted H1PV were prepared. For intermediate-dose group, 40 μL H1PV was added to 360 μL 48% iodixanol in Ringer solution. For the low-dose group, 40 μL of the intermediate-dose H1PV solution was diluted with 360 μL 48% iodixanol in Ringer solution. The rats in the control group were injected with vehicle only (48% iodixanol in Ringer solution).
Preparation of chlorpromazine and d-amphetamine.
Chlorpromazine (60 mg; batch 097K2503, Sigma, Taufkirchen, Germany) was diluted in a final volume of 30 mL of sterile water (batch 04002D, Delta Select, Munich, Germany). d-Amphetamine (54 mg; batch 058K3350, Sigma) was diluted in a final volume of 27 mL of sterile water (batch 04002D, Delta Select). The final concentration of the active ingredients in the formulation for each substance was 2 mg/mL. These compounds were used as positive controls in the modified IRWIN screening procedure.16,19
Animal experiments.
For tissue-availability testing, male and nonpregnant female nulliparous SPF Wistar rats (Crl:WI[Han]) were used. The safety study on potential CNS effects was performed in accordance with ICH Guidance Document M3(R2)9 by using healthy male Wistar rats. All animals were obtained from a controlled full-barrier breeding system (Charles River, Sulzfeld, Germany) and tested against viruses, including H1 parvovirus, bacteria, and parasites (complete list of tested agents available at http://www.criver.com/HealthData/de/H64W090R.pdf). At the beginning of the studies, rats were 8 to 12 wk old. All animals were bred for experimental purposes according to Article 9.2, Number 7 of the German Act on Animal Welfare.5
The studies were conducted by BSL Bioservice (Munich, Germany). All experiments complied with the German Acts on Animal Welfare (Tierschutzgesetz, July 2009),5 Good Laboratory Practices,20 and Infection Protection (Infektionsschutzgesetz, January 2001).4 Licenses to handle parvovirus H1 and to conduct acute and repeated-dose toxicity studies were granted to BSL Bioservice by the government of Upper Bavaria, Munich, Germany.
Housing and feeding conditions.
Rats were housed under full-barrier conditions in an air-conditioned room (10 air changes hourly) at 22 ± 3 °C, relative humidity of 55% ± 10%, and a 12:12-h photoperiod. Animals had free access to a commercial maintenance diet for rats and mice (lot number 1020, Altromin 1324, Altromin, Lage, Germany) and to tap water that was sulfur-acidified to pH 2.8 (approximately). Rats were housed individually during biodistribution testing but were group-housed for the CNS safety study, unless an animal's health status necessitated individual housing. All rats were kept in individually ventilated cages (type III H polysulfone cages with sawdust bedding and polycarbonate tunnels for enrichment) to avoid cross infection between animals. Cages with fresh bedding were provided every week. Rats were acclimated for at least 5 d before experiments began.
Test groups. Biodistribution study.
This study population comprised 36 female and 36 male Wistar rats, which were divided into 3 groups according to the time point of terminal euthanasia (6 h, 48 h, or 14 d after the single dose). Each of the 3 groups was subdivided in 2 subgroups based on the route of administration (either intracerebral or intravenous). Prior to the first administration, a detailed clinical observation was performed on each animal. The test item was administered either as a single intravenous (volume, 100 μL) or intracerebral (volume, 10 μL) injection. All rats received the same dose of 7.96 × 107 pfu H1PV.
Safety study on potential CNS effects.
The CNS safety study involved 48 male rats, which were divided into 6 groups of 8 animals each. Prior to the first administration, a detailed clinical examination was performed on each animal. One group each received either vehicle only intracerebrally; 7.96 × 107 pfu, 7.96 × 106 pfu; or 7.96 × 105 pfu H1PV intracerebrally; oral chlorpromazine; or oral d-amphetamine. For rats in the H1PV and vehicle groups, the total volume injected was 10 μL. The reference items chlorpromazine and d-amphetamine each were given as a single oral dose of 20 mg/kg in water at an application volume of 10 mL/kg.
Modes of administration.
Intravenous administration was performed in conscious, restrained rats as a slow bolus injection in the tail vein.
Intracerebral administration was performed in isoflurane-anesthetized rats. Animals were placed on a homeothermic blanket, with the head placed in the stereotaxic frame (DKI 900, David Kopf Instruments, Tujunga, CA). The head was shaved and disinfected with iodine solution. After midline skin incision, the skull was punctured with a 20-gauge needle 1 mm anterior to the junction of the sutura coronalis and the sutura sagittalis and 2 mm lateral to the right. A 10-μL Hamilton syringe was mounted to the stereotactic frame and inserted 6 mm below bone level. Prior to injection, the needle was retracted by 1 mm. The test item was injected slowly, at a rate of approximately 1 μL/min, for a total volume of 10 μL. After administration, the needle was left in place for approximately 2 min to allow for further diffusion of the test item from the application site. The syringe then was removed slowly, at approximately 1 mm/min. The incision site was sutured, and the rat returned to its cage for recovery. No postoperative analgesics were administered.
Mortality check and clinical observation.
Rats were monitored for 6 h, 48 h, or 14 d after dosing in the biodistribution study and for 48 h in the CNS toxicity study. The animals were checked twice daily for clinical symptoms or death. Any signs of illness before, during, or after administration or reaction to treatment were recorded.
Body weight.
In both studies, body weight was measured before administration and at time of euthanasia for the H1PV and vehicle-only groups. Rats in the chlorpromazine and d-amphetamine groups were weighed before administration only.
Sampling for qPCR evaluation.
For the biodistribution study, 24 rats were placed in metabolic cages the day before H1PV administration to enable the collection of urine, feces, and saliva. From 72 rats (3 groups of 24 rats; 12 per route of administration, serial interim blood samples were obtained from subgroups of 3 male and 3 female rats at each time point for each route of administration (intravenous and intratumor). Rats in the first group were sampled at 0 min, 15 min, and 2 h, with all 24 rats sampled at 6 h (at euthanasia); rats in the second group were sampled at 0 min, 30 min, 12 h, and 24 h (6 rats each time point), with all sampled before euthanasia at 48 h; and rats in the final group were sampled at 0 min and days 4, 7, 10, and 14 (at euthanasia).
All blood samples (400 μL) were obtained from the jugular vein of isoflurane anesthetized rats. Blood was collected in heparin lithium tubes. In addition, feces, saliva, and urine were collected on the same day as blood-sampling from the rats sampled on days 4, 7, and 10.
For qPCR quantification of H1PV viral genomes, samples of blood (treated with heparin), brain, heart, ovaries, lung, liver, spleen, kidney, mandibular lymph nodes, mesenteric lymph nodes, pancreas, prostate, mammary, bone marrow, feces, and urine were collected at euthanasia. Feces were collected by sampling from the colon, and urine was obtained by cystocentesis. Saliva was collected by using cotton swabs (Catch-All Sample Collection Swabs, Epicentre, Madison, WI). All samples were frozen in liquid nitrogen and stored at −80 °C (saliva swabs were stored below −20 °C) until processing and analysis.
qPCR procedure.
After thawing, the required amounts of organs and feces were weighed and cut into small pieces. Saliva swabs were cut off the applicators. Blood samples were analyzed as whole blood, without separation of serum and buffy coat.
DNA was extracted by using various kits (Qiagen, Venlo, Limburg, The Netherlands) and Quick Extract DNA Extraction Solution (Epicentre), with minor modifications; a validation study was performed.
The reference virus suspension was diluted in 10 mM Tris HCl pH 7.5 before spiking with virus of the calibration and quality-control samples as necessary. Each calibration curve consisted of 2 negative-control samples and 7 duplicate samples corresponding to target concentrations of 5 × 107 (1.25 × 107 for saliva), 5 × 106, 5 × 105, 5 × 104, 5 × 103, 5 × 102, and 3 × 102 to 5 × 101 VG per PCR reaction, depending on the validated method for each sample type.
The master mix per 20-µL reaction (final volume) contained 3.35 µL PCR-grade H2O; 10 µL LightCycler 480 Probes Master (Roche, Mannheim, Germany) 2×, 0.55 µL FW-H1 (40×, 10 µM; 5′ GCG CGG CAG AAT TCA AAC T 3′), 0.55 µL Rev-H1 (40×, 10 µM; 5′-CCA CCT GGT TGA GCC ATC AT 3′); and 0.55 µL probe H1 (40× 10 µM; 5′-6-FAM-ATG C5*G CC5* G5*C 5*GT TA TAMRA 3′ [* indicates a ‘locked nucleic acid’]). Aliquots (0.15 µL) of the master mix were added to each well of a LightCycler multiwall plate, after which 5 µL of the DNA extract was added. Cycling parameters were 95 °C for 10 min; followed by 45 cycles of 95 °C for 15 s, 60 °C for 60 s; and concluding with 40 °C for 10 min.
Assessment of central and autonomic nervous system function (CNS toxicity study only).
In the safety study on potential CNS effects, neurologic examination according to a modified IRWIN screening procedure and observation of spontaneous activity in the open field was performed before and at and 48 h after single intracerebral administration of H1PV or vehicle.16,19 Rats receiving positive-control compounds (chlorpromazine and D-amphetamine) were observed before and at 1 h (except ophthalmoscopy), 2 h, and 24 h after administration.
The examination included a brief homecage observation, rating of the reactivity to removal from the cage and handling, and assessment of salivation, lacrimation, exophthalmos, respiration, piloerection, and changes in skin or mucous membranes.
After a rat acclimated for 2 min in the open field, the arousal level (alertness), behavior, locomotor activity and gait characteristics were recorded. The numbers of supported (using the borders of the open field as support) and unsupported (unassisted) rears were counted separately. In addition, fecal boluses and pools of urine were counted. Any twitches, tremors, seizures, and unusual behavior or stereotypies were noted, if present. Reactions to a touch on the head and tail and toe pinches were noted. Extensor thrust, flexion reflex, equilibrium, positional passivity, visual placing, grasping reflex (together with grip strength), righting reflexes, and auditory startle response were tested. Pupil response to light was tested, and the anterior eye chamber as well as the fundus of the eye were examined after topical use of a mydriatic (batch 240051, Atropin-Pos, Ursapharm, Saarbrücken, Germany). Body temperature was measured after all examinations had been done.
Pathology (CNS toxicity study only).
In the safety study on potential CNS effects, all rats were euthanized by pentobarbital overdose (Narcoren, Merial, Hallbergmoos, Germany) at the end of the observation period (48 h). Rats in the H1PV and vehicle groups underwent gross necropsy, including examination of the brain. Subsequently, the brain was preserved in 10% neutral buffered formalin for histopathologic evaluation. The pathologic examination was performed without blinding of the pathologist to the treatment groups.
Histopathology (CNS toxicity study only).
Histopathologic evaluation of all euthanized rats in the H1PV and vehicle groups was done without blinding of the pathologist to the allocation of animals.
Brains fixed in 10% neutral buffered formalin were sent from the test facility to Propath UK (Hereford, England, UK) for histologic processing. Each brain was dissected by using the RBM-4000C coronal brain matrix (Activational Systems, Mableton, GA). This matrix has 24 divisions (1 mm each), and each brain was cut at divisions 3, 6, 9, 12, 15, 18, 21, and 24 to provide 8 coronal sections. After paraffin embedding, sections of approximately 4 to 5 μm thickness were stained with hematoxylin and eosin. Injection-site–associated lesions were graded by a board-certified pathologist (GPE).
Results
Bioavailability of H1PV after intravenous or intracerebral injection.
Bioavailability data were obtained until 14 d after single intracerebral or intravenous administration of 7.96 × 107 pfu H1PV to Wistar rats. Three groups, each consisting of 12 adult male and 12 adult female Wistar rats and differing according to the time of euthanasia after injection (6 h, 48 h, or 14 d), were evaluated; each of these groups was subdivided according to the route of administration.
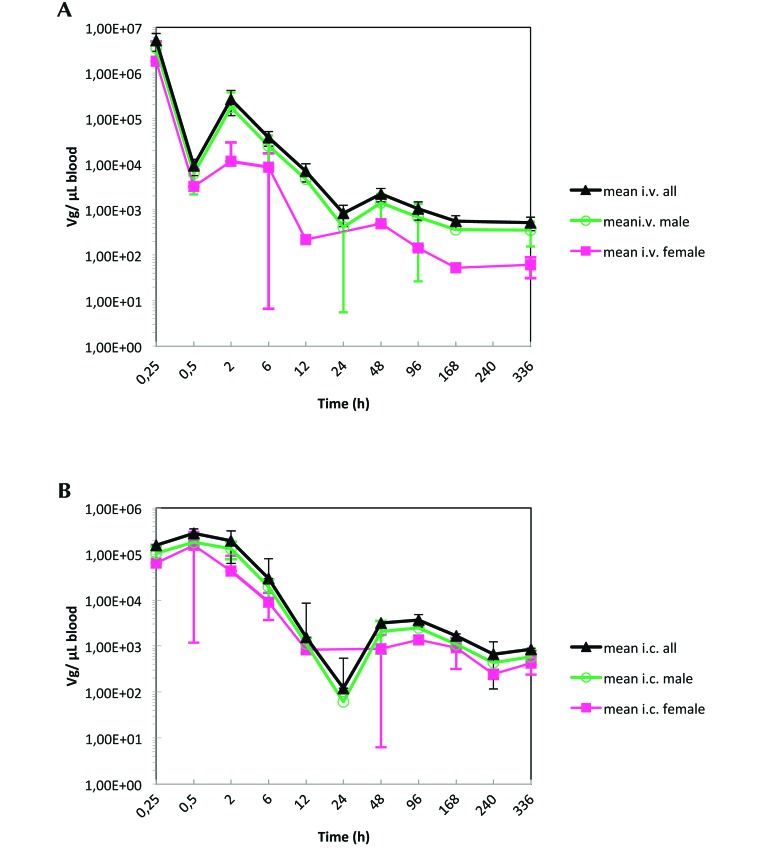
The highest concentrations of H1PV in blood were observed at the first sampling time point (that is, 15 min), regardless of route of administration. The pharmacokinetic profiles for both routes (intravenous and intracerebral) followed a biphasic decline, with rapid elimination and distribution phases and a flattened terminal phase (Figures 1 and 2). At 15 min after administration, blood concentrations of H1PV (in no. of VG/µL) appeared to be higher after intravenous compared with intracerebral injection. At all later time points, blood concentrations did not differ between administration routes. Quantifiable levels of viral DNA were still detectable in blood at the last sampling time point (14 d after dosing) in 9 of the 12 rats given intracerebral injections and in 7 of the 12 animals dosed intravenously.
Figure 1.
(A) H1PV concentration in blood after a single intravenous administration of 7.96 × 107 pfu to male and female Wistar rats (n = 3 at each time point, except n = 6 rats of each sex at 6, 48, and 336 h). (B) H1PV concentration in blood after single intracerebral administration of 7.96 × 107 pfu to male and female Wistar rats (n = 3 at each time point, except n = 6 rats of each sex at 6, 48, and 336 h).
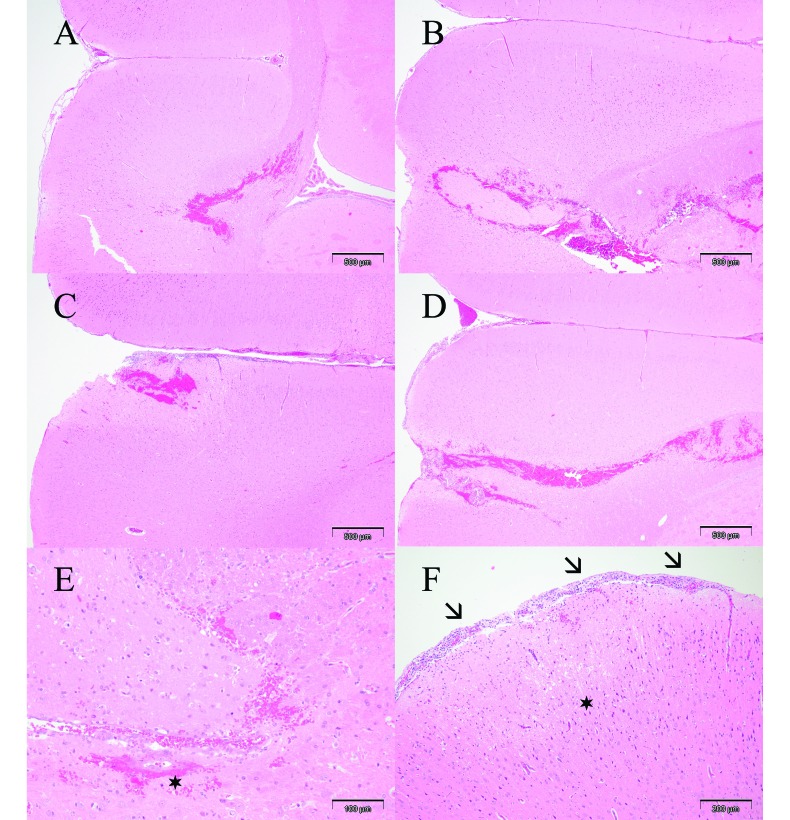
Figure 2.
Coronal brain sections (magnification, 4×) showing intracerebral injection channel in rats treated (A) with vehicle only or (B) 7.96 × 105 pfu, (C) 7.96 × 106 pfu, or (D) 7.96 × 107 pfu H1PV. (E) Parenchymal hemorrhages and focal minimal vasculitis (asterisk) in a rat treated with vehicle only (magnification, 20×). (F) Mild meningeal inflammatory cell infiltrates (arrows) and a small area of encephalomalacia (asterisk) near the injection channel in a rat treated with 7.96 × 106 pfu H1PV (magnification, 10×).
The calculation of bioavailability after intracerebral administration of 7.96 × 107 pfu H1PV yielded the values of 42% for male rats and 97% for female rats (Table 1). In general, there was high interindividual variability in H1PV blood concentration, regardless of mode of administration. Maximal concentrations were detected at 0.5 to 2 h after dosing, indicating that H1PV distributed to the blood compartment within a relatively short time after administration into the brain.
Table 1.
Mean blood pharmacokinetics of H1PV in Wistar rats after a single intracerebral or intravenous administration of 7.96× 107 pfu
| Route | Maximal concentration (no. VG/µL) | Time at maximal concentration (h) | AUC0–t (no. VG × h/µL) | Absolute bioavailability (%) | |
| Male | IC | 8.78 × 104 | 2.00 | 4.77 × 105 | 42 |
| IV | 1.64 × 106 | 0.25 | 1.13 × 106 | not done | |
| Female | IC | 1.53 × 105 | 0.50 | 5.55 × 105 | 97 |
The peak blood levels were slightly higher in female rats, but overall exposure was comparable between sexes. Given that the overall blood exposure after intracerebral dosing was similar between male and female rats, the calculated difference in the mean bioavailability between both sexes appears to be related to the determination of the blood concentrations after intravenous administration and thus most probably owing to the high variability of the individual values. Therefore, despite the calculated difference, similar intracerebral bioavailablity in both sexes can reasonably be assumed (Table 1).
Biodistribution of H1PV after a single intracerebral or intravenous dose.
Samples of brain, lung, liver, spleen, kidney, mandibular and mesenteric lymph nodes, pancreas, prostate, mammary gland area, bone marrow, and myocardial tissue were collected at 6 h, 48 h, and 14 d after injection from male and female rats after intracerebral or intravenous administration of 7.96× 107 pfu H1PV. Viral DNA was detected by qPCR in all tested organs and tissues analyzed (Table 2). The highest concentrations of viral DNA were found in liver and spleen at 6 h after H1PV administration. The lowest concentrations were in lung, mammary gland, and prostate samples. In most tissue types, viral DNA (no. of VG) decreased slightly over time. No decrease over time was detected in lung samples. In some animals, viral DNA was still detectable in the entire panel of organs tested 14 d after injection.
Table 2.
Concentration of H1PV ((log mean ± 1 SD; no. of VG/µg genomic DNA) in tissues after single intravenous or intracerebral administration of Wistar rats with 7.96 × 107pfu
| IC 6 h | IC 48 h | IC 14 d | IV 6 h | IV 48 h | IV 14 d | ||
| Male | Brain | 6.64 ± 0.58 | 6.26 ± 1.10 | 4.99 ± 1.13 | 4.50 ± 0.67 | 3.67 ± 0.31 | 3.05 ± 0.56 |
| 6 (5)a | 6 (5) | 6 | 6 | 6 | 6 (5) | ||
| Heart | 4.99 ± 0.62 | 4.17 ± 8.00 | 3.29 | 4.99 ± 0.91 | 4.29 ± 0.77 | 3.62 ± 0.11 | |
| 6 | 6 (5) | 6 (1) | 6 | 6 (5) | 6 (3) | ||
| Lung | 5.49 ± 0.86 | 5.88 ± 1.05 | 5.71 ± 1.15 | 6.16 ± 1.30 | 6.36 ± 1.51 | 5.88 ± 0.86 | |
| 6 (3) | 6 (5) | 6 | 6 (3) | 6 | 6 (5) | ||
| Liver | 6.90 ± 0.62 | 6.25 ± 1.04 | 4.83 ± 0.53 | 7.21 ± 0.45 | 6.50 ± 0.85 | 5.09 ± 0.99 | |
| 6 | 6 | 6 | 6 | 6 | 6 | ||
| Spleen | 6.79 ± 0.45 | 6.31 ± 1.02 | 5.62 ± 0.70 | 7.07 ± 0.46 | 6.78 ± 0.65 | 5.66 ± 0.87 | |
| 6 | 6 | 6 | 6 | 6 | 6 | ||
| Kidney | 5.80 ± 0.53 | 5.01 ± 0.97 | 4.44 ± 0.22 | 6.06 ± 0.66 | 5.58 ± 0.64 | 4.48 ± 0.94 | |
| 6 | 6 | 6 (5) | 6 | 6 | 6 (5) | ||
| Mandibular lymph node | 5.82 ± 1.03 | 5.16 ± 1.36 | 3.42 ± 1.34 | 3.96 ± 0.46 | 4.26 ± 0.96 | 2.66 ± 1.01 | |
| 6 | 6 (5) | 6 | 6 | 6 | 6 (4) | ||
| Mesenteric lymph node | 4.49 ± 0.95 | 4.13 ± 1.24 | 3.74 ± 1.17 | 5.41 ± 0.39 | 5.12 ± 0.87 | 3.79 ± 0.81 | |
| 6 | 6 | 6 | 6 | 6 | 6 | ||
| Pancreas | 5.30 ± 0.53 | 4.65 ± 0.45 | 4.88 ± 0.65 | 5.38 ± 0.72 | 4.71 ± 0.54 | 3.77 ± 0.71 | |
| 6 | 6 | 6 | 6 | 6 | 6 | ||
| Prostate | 4.39 ± 0.52 | 3.81 ± 0.76 | 4.05 ± 0.85 | 4.47 ± 0.43 | 4.42 ± 0.32 | 3.43 ± 0.42 | |
| 6 | 6 (4) | 6 (5) | 6 | 6 (4) | 6 (3) | ||
| Mammary | 5.1 ± 0.15 | 4.27 ± 0.58 | 5.54 | 4.97 ± 0.45 | 5.92 ± 0.78 | 3.83 | |
| 4 (3) | 6 (4) | 6 (1) | 5 | 6 (2) | 5 (1) | ||
| Bone marrow | 4.95 ± 0.56 | 4.49 ± 0.92 | 2.97 ± 0.69 | 5.05 ± 0.74 | 4.61 ± 0.71 | 3.10 ± 0.68 | |
| 6 | 6 | 6 | 6 | 6 | 6 | ||
| Female | Brain | 6.78 ± 0.61 | 5.92 ± 1.12 | 6.26 ± 0.48 | 4.47 ± 0.33 | 3.48 ± 0.35 | 2.55 ± 0.79 |
| 6 (5) | 6 (4) | 6 | 6 | 6 | 6 (4) | ||
| Heart | 4.97 ± 0.60 | 4.13 ± 0.54 | 3.12 ± 0.19 | 4.48 ± 0.76 | 3.61 ± 0.44 | LLOQ | |
| 6 | 6 (4) | 6 (4) | 6 | 6 | 6 | ||
| Lung | 4.82 ± 0.87 | 4.91 ± 1.35 | 4.93 ± 0.64 | 4.20 ± 0.91 | 4.71 ± 0.23 | 4.10 ± 0.64 | |
| 6 (5) | 6 (5) | 6 (5) | 6 (4) | 6 | 6 | ||
| Liver | 7.35 ± 0.49 | 6.10 ± 1.58 | 5.70 ± 0.37 | 7.14 ± 0.71 | 6.20 ± 0.25 | 4.38 ± 0.22 | |
| 6 | 6 | 6 | 6 | 6 | 6 | ||
| Spleen | 7.14 ± 0.60 | 6.01 ± 1.56 | 6.43 ± 0.26 | 6.76 ± 0.63 | 6.20 ± 0.27 | 5.84 ± 0.55 | |
| 6 | 6 | 6 | 6 | 6 | 6 | ||
| Kidney | 6.19 ± 0.74 | 5.75 ± 0.39 | 4.89 ± 0.16 | 6.18 ± 0.74 | 5.04 ± 0.28 | 3.67 ± 0.28 | |
| 6 (5) | 6 (4) | 6 | 6 | 6 | 6 (4) | ||
| Mandibular lymph node | 6.30 ± 0.92 | 4.61 ± 1.57 | 3.93 ± 1.73 | 4.26 ± 0.74 | 3.56 ± 0.92 | 4.36 ± 0.45 | |
| 6 | 6 | 6 | 6 | 6 | 6 (2) | ||
| Mesenteric lymph node | 4.99 ± 0.64 | 3.73 ± 1.30 | 4.66 ± 0.80 | 4.41 ± 0.78 | 3.75 ± 0.76 | 4.20 ± 0.80 | |
| 6 | 6 | 6 | 6 | 6 | 5 | ||
| Pancreas | 5.39 ± 0.56 | 4.80 ± 0.46 | 4.51 ± 0.33 | 5.26 ± 0.71 | 5.03 ± 0.93 | 4.17 ± 1.12 | |
| 6 | 6 | 6 | 6 | 6 | 5 | ||
| Mammary | 4.81 ± 0.66 | 4.53 ± 0.73 | 4.46 | 5.33 ± 0.40 | 4.12 ± 0.70 | LLOQ | |
| 6 | 6 (2) | 6 (1) | 6 (5) | 6 (2) | 6 | ||
| Bone marrow | 5.75 ± 0.41 | 4.40 ± 1.24 | 3.91 ± 0.55 | 5.28 ± 0.76 | 4.53 ± 0.42 | 2.90 ± 0.50 | |
| 6 | 6 | 6 | 6 | 6 | 6 |
LLOQ, lower limit of quantification; VG, viral genomes
No, of samples analyzed (no. of results included in calculation of mean values and standard deviations); values below the LLOQ or above the upper limit of quantification were not included in the calculation.
The pattern of viral concentration over time depended on the route of administration in 2 tissues only. As expected, higher concentrations were present in the brains of rats that received intracerebral injections of H1PV compared with those exposed by intravenous administration. This difference was still apparent even at 14 d after treatment. Moreover, viral DNA concentrations in mandibular lymph nodes at 6 h were higher after intracerebral compared with intravenous injection. This difference was not apparent in mesenteric lymph nodes. There was no overt sex-associated difference in the organ distribution of H1PV in rats.
Excretion of H1PV after a single intracerebral or intravenous dose.
A single intracerebral or intravenous administration of H1PV (7.96 × 107 pfu) yielded at most trace amounts of viral DNA in urine and saliva when evaluated as long as 14 d after dosing. Moderate concentrations of viral DNA were found in feces within 14 d after injection of H1PV. Neither the route of administration nor sex of the rats seemed to affect the amount of H1PV that was shed (Table 3).
Table 3.
Excretion (log mean ± 1 SD) of H1PV in feces, urine, and saliva of rats after single administration of 7.96 × 107pfu
| Time | Male |
Female |
|||||
| Feces (VG/mg) | Urine (VG/µL) | Saliva (VG/swab) | Feces (VG/mg) | Urine (VG/µL) | Saliva (VG/swab) | ||
| Intracerebral | 0 h | LLOQ | LLOQ | 5.14 ± 0.60 | 1.66 ± 0.68 | LLOQ | 4.28 |
| 6 h | 3.11 ± 1.69 | 1.89 ± 0.24 | 4.32 ± 0.13 | 2.42 ± 1.07 | 2.10 ± 0.14 | 4.48 | |
| 48 h | 3.38 ± 0.56 | 2.02 ± 0.80 | LLOQ | 3.95 ± 0.38 | 2.10 ± 0.85 | LLOQ | |
| 4 d | 2.84 ± 0.35 | 2.23 ± 0.89 | 4.59 ± 0.61 | 2.80 ± 0.35 | 1.98 ± 0.79 | 5.48 ± 0.49 | |
| 7 d | 2.57 ± 0.26 | 2.19 ± 0.86 | LLOQ | 2.78 ± 0.05 | 1.93 ± 0.74 | LLOQ | |
| 10 d | 2.22 ± 0.57 | 2.01 | 4.10 | 2.75 ± 0.42 | 2.27 ± 0.66 | 4.27 | |
| 14 d | 2.38 ± 0.48 | LLOQ | 4.18 | 2.11 ± 0.22 | LLOQ | LLOQ | |
| Intravenous | 0 h | LLOQ | LLOQ | 4.46 ± 0.35 | LLOQ | LLOQ | LLOQ |
| 6 h | 3.03 ± 1.24 | 1.43 | LLOQ | 1.50 ± 0.49 | 2.01 ± 0.55 | 4.41 ± 0.24 | |
| 48 h | 3.26 ± 0.80 | 2.46 ± 1.34 | LLOQ | 2.81 ± 0.29 | 2.04 ± 0.19 | LLOQ | |
| 4 d | 3.00 ± 0.95 | 1.62 ± 0.18 | 5.05 | 2.21 ± 0.27 | 2.28 ± 0.65 | LLOQ | |
| 7 d | 2.96 ± 1.06 | LLOQ | LLOQ | 2.59 ± 0.26 | 1.93 | LLOQ | |
| 10 d | 2.50 ± 1.00 | LLOQ | LLOQ | 2.07 ± 0.54 | LLOQ | 5.05 | |
| 14 d | 2.51 ± 0.75 | LLOQ | LLOQ | 2.11 ± 0.36 | LLOQ | LLOQ | |
LLOQ, lower limit of quantification; VG, viral genomes
Mortality and clinical observations.
None of the H1PV-treated rats died during the observation period (6 h to 14 d after administration), nor did any demonstrate any clinical findings associated with the viral preparation.
Body weight.
Minimal weight loss (6 g or less) was apparent without any bias regarding sex, route of administration, or treatment group at the 48-h time point in some rats. At 14 d after intracerebral or intravenous administration, all male and female rats had gained weight compared with baseline. No relevant differences in the increase in body weight were seen between rats treated via the intravenous compared with the intracerebral route (data not shown).
Potential adverse effects on CNS after intracerebral injection of H1PV. Mortality and clinical signs.
No mortality was recorded after intracerebral injection of 10 µL H1PV in vehicle (48% iodixanol in Ringer solution) containing doses of 0, 7.96 × 107, 7.96 × 106, or 7.96 × 105 pfu H1PV. During the 48-h observation period, no clinical findings specifically related to either the vehicle or H1PV were noted at any of the 3 dose levels.
CNS functionality.
After single intracerebral injection, no relevant differences in functional or behavioral parameters were apparent in any of the H1PV-treated rats (all dose groups) compared with vehicle-injected control animals. Observation included posture, gait, palpebral closure, lacrimation, piloerection, arousal, and vocalization. No convulsions, tremors, stereotypy, or bizarre behavior were observed in any of the control or and H1PV-treated groups. No abnormalities in the numbers of supported and unsupported rears were detected. Delayed motor responses to pinching of the tail were recorded in 4 rats of the vehicle-only group. None of the responses to reflexes were altered compared with pretreatment evaluations in H1PV-treated rats. Pupillary responses and ophthalmologic examinations were normal in all animals in both the vehicle and H1PV rats.
Body temperature and frequency and amount of urination and defecation were similar between H1PV- and vehicle-injected animals and did not differ from values before treatment.
Positive control animals behaved as anticipated. Control rats showed reduced locomotor activity and attention after treatment with chlorpromazine as compared with prior to treatment. After treatment with d-amphetamine, most rats showed expected symptoms such as excessive salivation and increased response to handling, including signs of aggressiveness or vocalization.
Macroscopic findings.
In the vehicle- and H1PV-injected rats that underwent necropsy 48 h after administration, gross pathology findings in the brain showed evidence of the surgical approach used for the administration of the virus preparation or diluent. The application site on the right frontal lobe of the brain could be identified in all rats except 3 in the vehicle-only group.
Macroscopic organ changes were very few and were unrelated to the test item. In all rats (H1PV and control groups), abdominal blood vessels were injected with blood, most likely as a result of the intraperitoneal injection needed during euthanasia and the lack of exsanguination at necropsy.
Histopathology. Injection site.
In most rats, histologic examination of the intracerebral injection site showed minimal to marked hemorrhage, minimal to marked necrosis or malacia, minimal or mild vasculitis and perivasculitis (Figure 2 E), and minimal to marked meningeal infiltrates (Figure 2 F). Injection-site–associated histologic lesions were classified by severity and number of affected animals (Table 4). Comparison of the group incidences and severity grades revealed that the average severity of injection-site–associated vasculitis–perivasculitis and meningeal infiltrates appeared to be slightly higher in rats treated with high-dose H1PV. The difference was minimal only, and, in view of the interindividual variation, was not considered to be clear evidence of a test-item–related adverse effect (Figure 2 A through D). However, relationship to the injected virus preparation could not be excluded definitively.
Table 4.
No. of injection-site–associated histologic lesions after single intracerebral injection of 8 rats with H1PV at 3 dose levels
| Vehicle (n = 5)a | 7.96 × 105 pfu (n = 8)a | 7.96 × 106 pfu (n = 7)a | 7.96 × 107 pfu (n = 7)a | |
| Haemorrhage | ||||
| minimal | 2 | 1 | 3 | 2 |
| mild | 0 | 3 | 3 | 4 |
| moderate | 2 | 3 | 0 | 1 |
| marked | 0 | 1 | 1 | 0 |
| total | 4 | 8 | 7 | 7 |
| Necrosis or malacia | ||||
| minimal | 3 | 3 | 2 | 4 |
| mild | 1 | 4 | 4 | 2 |
| moderate | 1 | 0 | 1 | 1 |
| marked | 0 | 1 | 0 | 0 |
| total | 5 | 8 | 7 | 7 |
| Vasculitis or perivasculitis or both | ||||
| minimal | 2 | 6 | 5 | 1 |
| mild | 1 | 1 | 2 | 6 |
| total | 3 | 7 | 7 | 7 |
| Meningeal inflammatory infiltrates | ||||
| minimal | 2 | 1 | 4 | 3 |
| mild | 1 | 1 | 3 | 2 |
| moderate | 0 | 1 | 0 | 1 |
| marked | 0 | 0 | 0 | 1 |
| total | 3 | 3 | 7 | 7 |
No. of samples with histologically discernable injection site
Remote brain parenchyma and meninges.
Other histologic brain findings were diffuse meningeal or parenchymal congestion or hemorrhage, which was present in most brain sections. No relationship to the test item could be demonstrated. These changes therefore were considered to result from the technical procedure of euthanasia and necropsy without exsanguination.
Discussion
These studies were conducted to address the bioavailability, biodistribution, and potential CNS toxicity of H1PV as part of the required toxicology testing during preparation for a clinical trial in brain tumor patients. All tests were performed in rats, the natural host of the virus, because this model system is permissive for virus replication, possibly enhancing relevant side effects of H1PV infection. All injections were performed with highly purified H1PV that was produced to GMP standards, as is mandatory for clinical use in patients, thus guaranteeing virus infectivity and purity. Bioavailability and biodistribution were assessed by qPCR quantification of the number of viral genomes present. Because H1PV is replication-competent and therefore has the potential to infect and replicate in normal tissues, viral nucleic acid levels were quantified to be able to detect relevant changes. However, because the presence of viral genomes is not equivalent to the presence of infectious virus particles, these studies are not suited to assess the infectivity of H1PV-injected animals.
The investigations revealed valuable information on viral safety after different routes of administration. In addition, these studies confirmed that H1PV is nonpathogenic in the natural host even upon direct intracerebral injection, at least over the time course and within the limitations of the experimental conditions. Furthermore, the investigations provide new information on the biologic properties of H1PV in rats, including the passage of the virus through the blood–brain barrier, main routes of viral elimination, and viral shedding. However, because rats are the natural host of H1PV, virus distribution and elimination in humans will most likely differ. Regardless, the information obtained in this investigation yielded crucial direction for appropriate planning of the first clinical trial in humans.
Systemic exposure to H1PV induced only minor differences between both routes of administration, suggesting high systemic availability after intracerebral administration. This finding may have important implications for the clinical use of H1PV with regard to both efficacy and safety. H1PV is one of the smallest viruses and can readily pass across the blood–brain barrier, even in the absence of experimental brain tumors, which are known to have the ability to compromise the functionality of this system.3 Because the substantial differences in viral blood concentrations between both routes of administration occurred only within the first hour after injection and because the time to achieve the maximal blood concentration after intracerebral administration was in the range of 0.5 to 2 h, a high rate of viral passage through the blood–brain barrier is likely. This attribute is particularly demonstrated by the presence of H1PV DNA in brain tissue after intravenous injection. Because the rats were not perfused after euthanasia, minor contamination of brain tissue samples with blood containing viral DNA is likely. However, given that the proportion of blood-containing vessels per volume of cortical brain tissue is calculated to be less than 2% and that the concentration of viral genomes was higher in brain tissue than in blood, the conclusion of virus penetration across the blood–brain barrier can be made.21 Based on these findings in conjunction with a range of additional preclinical data, a proportion of patients in the ongoing clinical trial will be treated by intravenous injection prior to tumor resection. Resected tumor tissue will be tested for the presence of viral genomes, as an indicator of virus distribution across the human blood–brain barrier.
Organ distribution showed no substantial differences after intravenous compared with intracerebral administration of H1PV. Only after intracerebral injection did brain tissue and draining mandibular lymph nodes contain higher levels of viral genomes than those achieved after intravenous administration. For brain tissue, this difference could be detected throughout the 14-d observational period. This result was most likely due to the initial distribution of virus to all body organs after intravenous injection, thereby substantially reducing the amount available for passage through the blood–brain barrier. In contrast, after intracerebral administration, a higher proportion of the inoculum remained within the brain tissue despite demonstrated virus distribution from the brain into extraCNS compartments. In mandibular lymph nodes, the number of viral genomes, which was higher at the early time points after intracerebral injection compared with intravenous injection, had nearly equalized by day 14. Because the lymph of the brain drains to the cervical (mainly) and adjacent (mandibular) lymph nodes, the temporal difference in the amount of virus at these locations is probably related to lymphatic clearance of the test item from the brain.
Furthermore, a single intravenous or intracerebral injection of 7.96 × 107 pfu H1PV was sufficient to maintain H1PV genomes in blood even 14 d after administration. H1PV distribution was widespread, with positive virus signals obtained in all tested organs, and no relevant sex-specific findings were obtained in regard to tissue distribution. Virus shedding and excretion occurred mainly via feces, which continued to test positive for viral genomes 14 d after injection. Urine samples and mouth swabs showed virus-positive signals primarily during the first 7 d, and the concentration of viral genomes was lower in urine and saliva than in feces.
Virus levels declined over time in all tested organs and blood, so there is no evidence for virus replication, at least at a higher level under the conditions of this investigation. Because testing for active infectious virus particles, viral RNA, or viral proteins was beyond the scope of information required for approval of clinical use of the viral preparations investigated in this study, the possibility of low-level virus replication in normal tissue cannot be fully excluded on the basis of our results. Furthermore, it was not possible to identify possible organ sites where virus persistence might occur. The current findings indicate that additional detailed examinations are required to further elucidate how H1PV persists in rodent populations.
To assess cerebral toxicity, H1PV was injected into the brain at 3 dose levels. Because the volume of injection in the brain was limited to 10 µL, the highest dose was 7.96× 107 pfu, the maximal amount of virus particles in the undiluted stock. Histopathologic investigations showed pronounced changes indicative of inflammation at the injection site as well as at distant sites in the intermediate- and high-dose group. Because the injected material contained viral particles, these findings were expected and were not considered to be severe enough to be clear evidence for virus-related, significant pathology after direct injection of H1PV into the brain. However, it cannot be entirely ruled out that H1PV might cause local toxicity when given at higher dose levels or after a longer observation period than 48 h. No treatment-related macroscopic organ pathology associated with the administration of either the vehicle or H1PV preparation was seen in this study, but because histopathology was performed only on brain tissue, microscopic changes in other tissues cannot be excluded. To answer this question, a detailed histologic examination of all sampled organs was performed during a 28-d intravenous repeated-dose toxicology study in rats, for which the results are reported elsewhere.15
The influence of a single intracerebral administration of H1PV on central and autonomic nervous system functions was assessed according to a modified IRWIN screening procedure, which included observation of the spontaneous activity in the open field and a detailed neurologic examination. This extensive testing revealed no clinical or behavioral abnormalities, in line with the lack of significant pathologic findings in the brain. Because aberrant or pathologic behavior can occur in the absence of related structural changes of the brain architecture, this in-depth analysis adds valuable functional information to supplement the histologic evidence.
In conclusion, H1PV shows advantageous kinetic characteristics for possible therapeutic use. Specifically, the virus can pass rapidly through the blood–brain barrier and spread widely even after local injection. These features support a favorable rationale for the treatment of brain tumors by local as well as systemic administration of this agent. Moreover, no signs of relevant morphologic or functional pathologies were observed after cerebral administration of H1PV to Wistar rats. The data obtained on the tissue distribution and CNS safety of H1PV in a relevant animal species (that is, rats) support the assumption of a favorable tissue distribution and good tolerability in humans.
Acknowledgments
KG and JR have patent rights related to the clinical use of H1PV (US 7,149,456 B2). BH, MD, and OK are employed by ORYX. ORYX is the sponsor of the clinical trial in brain-tumor patients. All other authors declare no competing interests.
References
- 1.Angelova AL, Aprahamian M, Balboni G, Delecluse HJ, Feederle R, Kiprianova I, Grekova SP, Galabov AS, Witzens-Harig M, Ho AD, Rommelaere J, Raykov Z. 2009. Oncolytic rat parvovirus H1PV, a candidate for the treatment of human lymphoma: in vitro and in vivo studies. Mol Ther 17:1164–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelova AL, Aprahamian M, Grekova SP, Hajri A, Leuchs B, Giese NA, Dinsart C, Herrmann A, Balboni G, Rommelaere J, Raykov Z. 2009. Improvement of gemcitabine-based therapy of pancreatic carcinoma by means of oncolytic parvovirus H1PV. Clin Cancer Res 15:511–519. [DOI] [PubMed] [Google Scholar]
- 3.Black KL, Ningaraj NS. 2004. Modulation of brain tumor capillaries for enhanced drug delivery selectively to brain tumor. Cancer Control 11:165–73. [DOI] [PubMed] [Google Scholar]
- 4.Bundesministeriums der Justiz. [Internet] 2001. Gesetz zur Verhütung und Bekämpfung von Infektionskrankheiten beim Menschen (Infektionsschutzgesetz - IfSG). [Cited November 2014]. Available at http://www.gesetze-im-internet.de/bundesrecht/ifsg/gesamt.pdf
- 5.Bundesministeriums der Justiz. [Internet] 2009. Tierschutzgesetz in der Fassung der Bekanntmachung vom 18. Mai 2006 (BGBl. I S. 1206,1313), das zuletzt durch das Gesetz vom 15. Juli 2009 (BGBl. I S. 1950) geändert worden ist". [Cited November 2014]. Available at http://www.gesetze-im-internet.de/tierschg/
- 6.Cornelis JJ, Deleu L, Koch U, Rommelaere J. 2006. Parvovirus oncosuppression, p 365–384. In: Kerr JR, Cotmore SF, Bloom ME, Linden RM. The parvoviruses. London (UK): Hodder Arnold. [Google Scholar]
- 7.Dupressoir T, Vanacker JM, Cornelis JJ, Duponchel N, Rommelaere J. 1989. Inhibition by parvovirus H1 of the formation of tumors in nude mice and colonies in vitro by transformed human mammary epithelial cells. Cancer Res 49:3203–3208. [PubMed] [Google Scholar]
- 8.Easterbrook JD, Kaplan JB, Glass GE, Watson J, Klein SL. 2008. A survey of rodent-borne pathogens carried by wild-caught Norway rats: a potential threat to laboratory rodent colonies. Lab Anim 42:92–98. [DOI] [PubMed] [Google Scholar]
- 9.European Medicines Agency. [Internet] 2009. ICH guideline M3(R2) on non-clinical safety studies for the conduct of human clinical trials and marketing authorisation for pharmaceuticals. [Cited November 2014]. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002720.pdf
- 10.Ferm VH, Kilham L. 1963. Rat virus (RV) infection in fetal and pregnant hamsters. Proc Soc Exp Biol Med 112:623–626. [DOI] [PubMed] [Google Scholar]
- 11.Ferm VH, Kilham L. 1964. Congenital anomalies induced in hamster embryos with H1 virus. Science 145:510–511. [DOI] [PubMed] [Google Scholar]
- 12.Geletneky K, Huesing J, Rommelaere J, Schlehofer JR, Leuchs B, Dahm M, Krebs O, von Knebel Doeberitz M, Huber B, Hajda J. 2012. Phase I/IIa study of intratumoral/intracerebral or intravenous/intracerebral administration of parvovirus H1 (ParvOryx) in patients with progressive primary or recurrent glioblastoma multiforme: ParvOryx01 protocol. BMC Cancer 12:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geletneky K, Kiprianova I, Ayache A, Koch R, Herrero YCM, Deleu L, Sommer C, Thomas N, Rommelaere J, Schlehofer JR. 2010. Regression of advanced rat and human gliomas by local or systemic treatment with oncolytic parvovirus H1 in rat models. Neuro-oncol 12:804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrero YCM, Cornelis JJ, Herold-Mende C, Rommelaere J, Schlehofer JR, Geletneky K. 2004. Parvovirus H1 infection of human glioma cells leads to complete viral replication and efficient cell killing. Int J Cancer 109:76–84. [DOI] [PubMed] [Google Scholar]
- 15.Geletneky K, Leoni A, Pohlmeyer-Esch G, Loebhard S, Baetz A, Leuchs B, Roscher B, Hoefer C, Jochims K, Dahm M, Huber B, Rommelaere J, Krebs O, Hajda J.2014. Pathology, Organ Distribution, and Immune Response after Single and Repeated Intravenous Injection of Rats with Clinical-Grade Parvovirus H1 Comp Med in press.
- 16.Irwin S. 1968. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia 13:222–257. [DOI] [PubMed] [Google Scholar]
- 17.Kilham L, Ferm VH. 1964. Rat virus (RV) infections of pregnant, fetal, and newborn rats. Proc Soc Exp Biol Med 117:874–879. [DOI] [PubMed] [Google Scholar]
- 18.Kilham L, Margolis G. 1969. Transplacental infection of rats and hamsters induced by oral and parenteral inoculations of H1 and rat viruses (RV). Teratology 2:111–123. [DOI] [PubMed] [Google Scholar]
- 19.Moscardo E, Maurin A, Dorigatti R, Champeroux P, Richard S. 2007. An optimised methodology for the neurobehavioural assessment in rodents. J Pharmacol Toxicol Methods 56:239–255. [DOI] [PubMed] [Google Scholar]
- 20.Organisation for Economic Co-operation and Development. [Internet] 1997. OECD Series on Principles of Good Laboratory Practice (GLP) and Compliance Monitoring. [Cited November 2014]. Available at http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/mc/chem(98)17&doclanguage=en.
- 21.Patt S, Sampaolo S, Theallier-Janko A, Tschairkin I, Cervos-Navarro J. 1997. Cerebral angiogenesis triggered by severe chronic hypoxia displays regional differences. J Cereb Blood Flow Metab 17:801–806. [DOI] [PubMed] [Google Scholar]
- 22.Rommelaere J, Geletneky K, Angelova AL, Daeffler L, Dinsart C, Kiprianova I, Schlehofer JR, Raykov Z. 2010. Oncolytic parvoviruses as cancer therapeutics. Cytokine Growth Factor Rev 21:185–195. [DOI] [PubMed] [Google Scholar]
- 23.Soike KF, Iatropoulis M, Siegl G. 1976. Infection of newborn and fetal hamsters induced by inoculation of LuIII parvovirus. Arch Virol 51:235–241. [DOI] [PubMed] [Google Scholar]
- 24.Tattersall P. 2006. The evolution of parvoviral taxonomy, p 5–14. In: Kerr JR, Cotmore SF, Bloom ME, Linden RM, Parrish CR. The parvoviruses. London (UK): Hodder Arnold. [Google Scholar]
- 25.Toolan HW, Ledinko N. 1968. Inhibition by H1 virus of the incidence of tumors produced by adenovirus 12 in hamsters. Virology 35:475–478. [DOI] [PubMed] [Google Scholar]
- 26.Toolan HW, Rhode SL, 3rd, Gierthy JF. 1982. Inhibition of 7,12-dimethylbenz(a)anthracene-induced tumors in Syrian hamsters by prior infection with H1 parvovirus. Cancer Res 42:2552–2555. [PubMed] [Google Scholar]
- 27.Wrzesinski C, Tesfay L, Salome N, Jauniaux JC, Rommelaere J, Cornelis J, Dinsart C. 2003. Chimeric and pseudotyped parvoviruses minimize the contamination of recombinant stocks with replication-competent viruses and identify a DNA sequence that restricts parvovirus H1 in mouse cells. J Virol 77:3851–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]