Abstract
Pulmonary embolism (PE) is a leading cause of sudden cardiac death, and a model is needed for testing potential treatments. In developing a model, we compared the hemodynamic effects of isoflurane and α-chloralose in an acute swine model of PE because the choice of anesthesia will likely affect the cardiovascular responses of an animal to PE. At baseline, swine that received α-chloralose (n = 6) had a lower heart rate and cardiac output and higher SpO2, end-tidal CO2, and mean arterial pressure than did those given isoflurane (n = 9). After PE induction, swine given α-chloralose compared with isoflurane exhibited a lower heart rate (63 ± 10 compared with 116 ± 15 bpm) and peripheral arterial pressure (52 ± 12 compared with 61 ± 12 mm Hg); higher SpO2 (98% ± 3% compared with 95% ± 1%), end-tidal CO2 (35 ± 4 compared with 32 ± 5), and systolic blood pressure (121 ± 8 compared with 104 ± 20 mm Hg); and equivalent right ventricular:left ventricular ratios (1.32 ± 0.50 compared with 1.23 ± 0.19) and troponin I mean values (0.09 ± 0.07 ng/mL compared with 0.09 ± 0.06 ng/mL). Isoflurane was associated with widely variable fibrinogen and activated partial thromboplastin time. Intraexperiment mortality was 0 of 6 animals for α-chloralose and 2 of 9 swine for isoflurane. All swine anesthetized with α-chloralose survived with sustained pulmonary hypertension, RV-dilation-associated cardiac injury without the confounding vasodilatory or coagulatory effects of isoflurane. These data demonstrate the physiologic advantages of α-chloralose over isoflurane for anesthesia in a swine model of severe submassive PE.
Abbreviations: LV, left ventricle; PAP, pulmonary arterial pressure; PE, pulmonary embolism; RV, right ventricle
Pulmonary embolism (PE) is one of the leading causes of noncardiac sudden death in Western nations and is the third most common cause of cardiovascular morbidity.4,6,7,18 In survivors, severe PE damages the right heart, leading to a clinical course complicated by hypotension and circulatory shock, suggesting acute right heart failure in about 10% of patients and followed by persistent pulmonary hypertension or right ventricular dysfunction and dyspnea in at least 15% of patients.9,15,16,23,29 To test treatments to reduce right heart failure, a standardized model that is repeatable, accurate, and precise and that mimics the gross pathologic, cardiovascular, pulmonary, autonomic, hematologic, biochemical, and cellular characteristics of PE in humans with disease is needed.8
Three lines of rationale favor domestic pigs as a model for PE. Several publications, using different methods of anesthesia, have found that swine manifest hemodynamic responses similar to those of humans in the presence of autologous PE, including elevated heart rate, decreased cardiac output, and reduced oxygen saturation.2,12,30 Swine have similar platelet concentrations, and their coagulation profile on thromboelastography has been shown to be similar to humans, with the exception of higher fibrin crosslinking but less fibrin, leading to resistance to plasmin.5,11,19,34 Market swine, which would otherwise be destined for slaughter, are relatively cost effective compared with other large animals and are of sufficient size for placement of an adult pulmonary arterial catheter for measurement of pulmonary vascular resistance in a closed-chest preparation.
In view of the differences in the hemodynamic effects of different anesthetic agents, the choice of anesthesia will likely affect the cardiovascular responses of an animal to PE. However, current literature lacks a methodologic publication that compares the cardiovascular, right ventricular, pulmonary, and hematologic responses to PE in closed-chest swine models incorporating different anesthetic regimens.
Figure 1 presents features of an ideal animal model for the purpose of testing treatments for PE. To develop a swine model of PE that closely resembles this physiologic ideal model, we induced PE in swine maintained in a surgical plane of anesthesia with either isoflurane or α-chloralose. Each of these agents has potential advantages and disadvantages. Isoflurane can be titrated minute by minute but causes undesirable vasodilation, whereas α-chloralose is believed to preserve cardiovascular reflexes but requires heating to dissolve and continuous infusion or repeated boluses.26,35 We hypothesized that, compared with isoflurane, α-chloralose would meet more of the features described in Figure 1.
Figure 1.
Desirable features of large animal model of severe submassive PE designed for translational research.
Materials and Methods
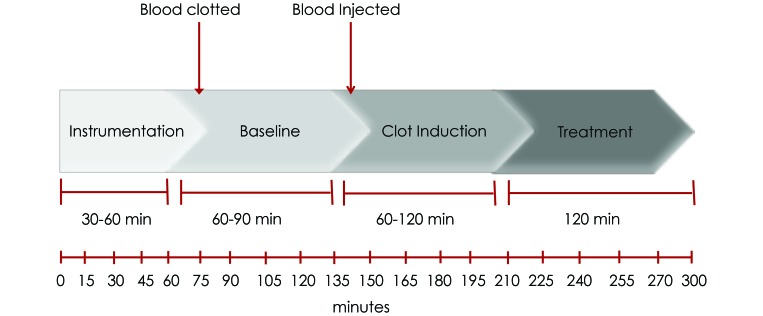
This study was approved by the IACUC at the Indiana School of Medicine. Domestic Yorkshire swine (Sus scrofa) of either sex were obtained from a local distributor (John Hardin Farms, Danville, IN) and were allowed to acclimate a minimum of 3 d prior to experimentation. Swine were monitored to confirm normal health status. Figure 2 shows the experimental overview, which had 4 phases: surgical preparation, equilibration (baseline), clot induction, and treatment (saline only).
Figure 2.
Time line and overview of experiment.
Initiation of surgery and monitoring.
Anesthesia was induced with a combination of tiletamine, ketamine, and xylazine (500, 250, and 250 mg/mL, respectively; dose, 1 mL/22.7 kg IM). A single dose of morphine (3 mg/kg IM) was used for analgesia. After induction, swine were intubated by using a Miller 7 laryngoscope blade, and an appropriately sized cuffed endotracheal tube was placed, with confirmation of correct placement by monitoring of end-tidal CO2. In one group of swine (n = 9; 4 male, 5 female), isoflurane in conjunction with 100% oxygen was titrated to an inspired concentration of 1% to 2% to maintain anesthesia. In the other group (n = 6; 3 male, 3 female), swine received 1000 mL of 0.9% normal saline infused over 3 h to balance fluid volume required to deliver α-chloralose. Prior to injection, 1% α-chloralose (w/w) was dissolved in 0.9% normal saline heated to 50 °C and allowed to cool to 30 to 35 °C. For induction, swine received an intravenous bolus of 70 mg/kg α-chloralose, followed by maintenance of anesthesia with 10 mg/kg IV every 2 h. In both groups, the appropriate level of anesthesia was maintained, defined as muscle relaxation and absent reflexes (corneal, hoof pinch). Animals were monitored throughout the experiment for signs and symptoms of inadequate sedation (eye blink, jaw, papillary or limb withdrawal). Swine were placed on a mechanical ventilator, with a respiratory rate of 12 to 17 breaths per minute to normalize end-tidal CO2 prior to introduction of PE. Heart rate and SpO2 were monitored continuously.
Vascular access was obtained via cut downs (4 to 5 cm) to expose both external jugular veins, one femoral artery, and one femoral vein for line placement. Blood (2 mL/kg) was drawn from the first available accessed vein, and 4-mL aliquots were placed into glass tubes (0.5 × 6 cm) and clotted at 37 °C for 1 h. The blood volume removed was replaced 2:1 with 0.9% normal saline. During this time, a 7-French Swan–Ganz (Edwards Lifescience, Irvine, CA) catheter was placed into the right external jugular vein, and a silastic tube (inner diameter, 0.25 in.; outer diameter, 0.375 in.; width, 0.0625 in.) was placed into the left external jugular vein to monitor pulmonary arterial pressure (PAP), cardiac output, and pulmonary capillary wedge pressure. Cardiac output was measured using thermodilution technique and the pulmonary capillary wedge pressure was measured with ventilations temporarily interrupted; measurements were made in triplicate at each time point. Output data from the Swan–Ganz catheter were analyzed automatically (model SC7000 Hemopod, Siemens, Deerfield, IL) with manual recording of results. Tygon tubing (inner diameter, 4 mm; Saint Gobain, Courbevoie, Ile-de-France, France) was placed in the femoral artery in fluid connection with a transducer to monitor arterial pressures.
Echocardiographic measurements.
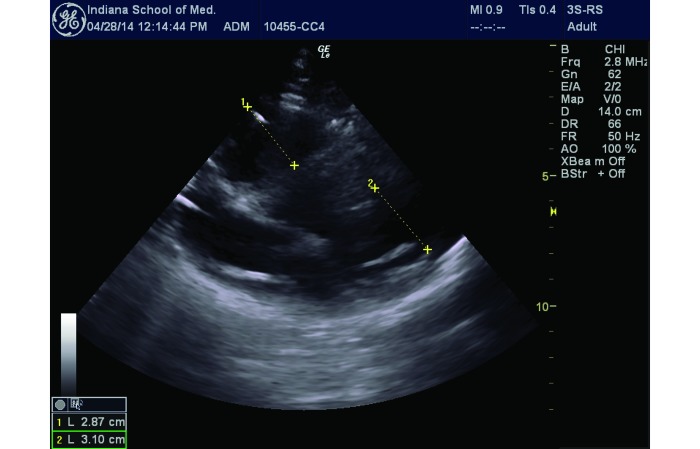
Real-time, transthoracic B-mode ultrasonic images were obtained (Logiq e BT12, GE Healthcare, Little Chalfont, United Kingdom) by using a 2.0-MHz probe. We used the ratio of the chamber diameter of the right ventricle to that of the left ventricle (RV:LV ratio) as our main index of right ventricular enlargement during clot induction. Because of the anatomy of swine, we measured the RV:LV ratio from a modified parasternal long-axis view (Figure 3).
Figure 3.
Modified parasternal long-axis view of the heart obtained by cardiac ultrasonography for the RV:LV measurement. The ratio of distance A to distance B represents the RV:LV ratio.
Blood sampling.
Blood samples were obtained in tubes containing 0.11 mM (3.2%) sodium citrate. Coagulation studies were performed by using an automated analyzer (STAcompact, Diagnostica Stago, Parsippany, NJ), and troponin measurements were performed by using a handheld hematology analyzer (iSTAT System, Abbot Point of Care, Princeton, NJ).
Induction of severe submassive PE.
After 60 to 90 min incubation, clotted blood was removed as well-formed cylindrical casts (0.5 cm × 6.0 cm) of the glass tubes. Several (5 or 6) of the cylinders were cut into 0.5 × 0.5-cm cubes, and the remainder were retained as cylinders. Clots were placed on cotton fabric to remove excess serum, weighed, and washed 3 times in warm 0.9% NaCl. Clots then were delivered via the external jugular silastic catheter. Confirmation of clot transit through the right ventricle was shown visually via real-time echocardiography. Approximately 15 cubes were delivered first, followed by 15 to 20 cylindrical clots. Clots were injected over a period of approximately 90 min or until one of the following parameters occurred: 1) the systolic PAP reached or exceeded 60 mm Hg; 2) the measured systemic arterial pressure decreased below 90 mm Hg; 3) the end-tidal CO2 decreased by more than 10 torr; and 4) echocardiography revealed global RV hypokinesis. When any one of these parameters manifested, no additional clot was given for 10 min. After 10 min, if the systolic PAP was below 50 mm Hg, additional clot (cubes, cylinders, or both) was instilled as needed to increase and sustain the systolic PAP above 60 mm Hg, providing that the remaining 3 parameters did not occur. Sustained severe submassive PE was considered to be present when the systolic PAP remained above 60 mm Hg for more than 15 min.
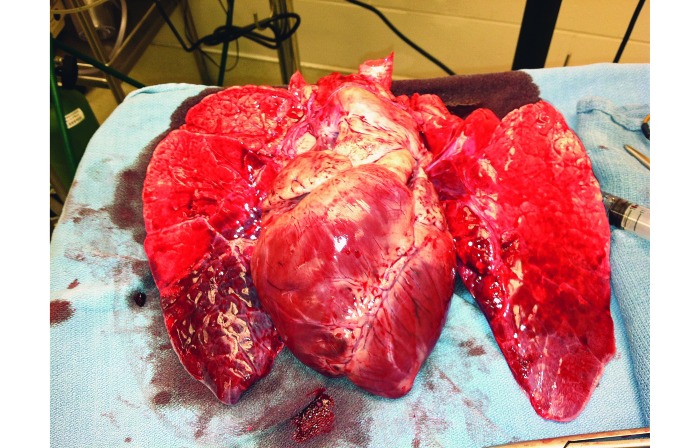
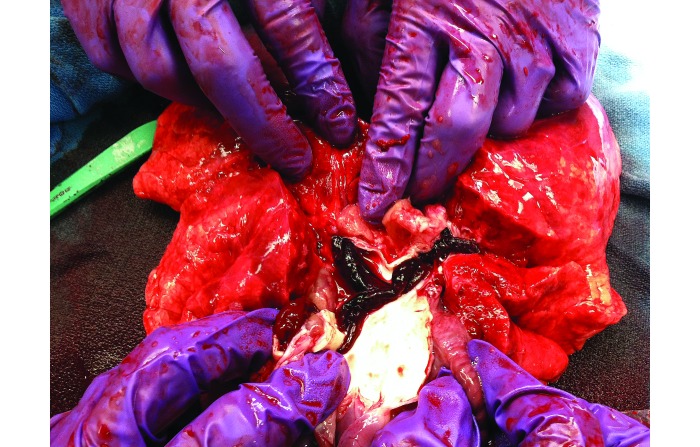
After embolization, swine received 500 mL of 0.9% normal saline (250 mL) over 120 min through the proximal port of the Swan–Ganz catheter as a diluent control for future treatments. At the completion of the experiment, experimental variables were recorded as done prior to and after embolization. All swine were euthanized with accordance of our local IACUC by using an appropriate commercial euthanasia solution. During necropsy, the pulmonary vasculature was dissected to the segmental arterial branches to determine location of clot and to visibly inspect lungs for infarction and hemorrhage (Figures 4 and 5).
Figure 4.
Dilated RV and pulmonary infarction at the bases.
Figure 5.
Pulmonary artery outflow tract with a saddle pulmonary embolism.
Data analysis.
All variables recorded were categorized into the 3 phases of the experiment: baseline, clot induction, and treatment. Baseline measurements were recorded after the pig was instrumented but prior to clot embolization. The clot induction phase was defined as the time from when clotted blood began to be introduced until 20 min after the last clot given to achieve the predefined goals of sustained pulmonary hypertension or systemic hypotension. The treatment phase lasted from the end of clot induction until the conclusion of the experiment. Cardiac output and pulmonary capillary wedge pressure data represent the means of 3 measurements at each time point. Data are presented as means ± SEM. Within-group comparisons of normally distributed, parametric data (that is, serial measurements in either group) were compared by using paired t tests. Between-group comparisons (isoflurane compared with α-chloralose) were made at the 3 main time points (baseline, after clot induction, and after treatment). These data were tested for normality, and normally distributed data were compared by using an unpaired Student t test. To control for a multiple comparisons, Bonferroni correction was used such that a P value less than 0.004 was significant.1
Results
Of the 9 swine anesthetized with isoflurane, 7 pigs (4 female, 3 male) survived and provided complete data sets. All 6 swine (3 female, 3 male) anesthetized with α-chloralose survived until the end of the study. Clots were confirmed with real-time echocardiography (data not shown) and on postmortem necropsy (Figures 4 and 5). In all groups the RV:LV ratio (mean ± SEM) increased from 0.82 ± 0.23 to 1.27 ± 0.31 (P = 0.002). Necropsy revealed RV dilation and pulmonary infarction in all animals (Figure 4). The clot burden was 21.8 ± 6.7 g for both groups combined, and this measure did not differ significantly between the 2 anesthesia groups. For all swine combined, serum troponin I concentration increased throughout the experiment from 0.02 ± 0.03 ng/mL at baseline to 0.09 ± 0.07ng/mL at the conclusion of the experiment (P < 0.01, paired t test). Peak troponin I concentration did not differ between the isoflurane and α-chloralose groups.
Animal monitoring and anesthesia.
The total experimental time (mean ± SEM) was 319 ± 98 min and ranged from 157 to 430 min. On average, the total preparation time for pigs anesthetized with α-chloralose was about 10 min longer than that for the isoflurane group because of the time required to infused the drug. The largest source of variation was instrumentation time. Adequate anesthesia depth was maintained with a mean isoflurane concentration of 1.30% ± 0.37% (range, 0.75% to 2.0%). In the isoflurane group, 2 pigs (the 3rd and 5th in the sequence of 9) died during PE induction. Despite receiving an inhaled dose of greater than 1% isoflurane that produced marginally low blood pressure in all 7 of the animals remaining, 4 of these pigs showed signs of insufficient anesthesia, including large muscle movement that required rapid action by the research team, including the administration of supplemental morphine.
The mean volume of α-chloralose administered was 633 ± 140 mL (equivalent to 6.33 g α-chloralose per swine or 0.12 g/kg). None of the swine in the α-chloralose group died, and none showed signs of emergence from anesthesia. Each pig in both groups received a total volume of 1500 mL intravenous fluids, inclusive of 0.9% NaCl used for blood replacement, clot delivery, and fluid balancing or α-chloralose administration.
Hemodynamic measurements.
All hemodynamic measurements from each phase of the experiment are shown in Tables 1 and 2. During the baseline phase, isoflurane-anesthetized swine demonstrated higher heart rate, lower oxygen saturation, lower end-tidal CO2, and lower systolic blood pressure when compared with pigs given α-chloralose. During clot induction, animals in the isoflurane group showed lower oxygen saturation and systolic blood pressure but higher heart rate and systolic pulmonary arterial pressure than measures in the α-chloralose group. At baseline and after PE induction, isoflurane-treated swine demonstrated a trend toward lower pulmonary vascular resistance compared with the α-chloralose group. Systemic vascular resistance was consistently lower in the isoflurane group than in the α-chloralose group.
Table 1.
Hemodynamic measurements
| Isoflurane | α-Chloralose | P | |
| Baseline | |||
| Heart rate (bpm) | 103 ± 4.0 | 60 ± 0.8a | <0.004 |
| SpO2 (%) | 95 ± 0.3 | 98 ± 0.2a | <0.004 |
| Respiratory rate (breaths/min) | 18 ± 0.5 | 16 ± 0.2a | <0.004 |
| End-tidal CO2 | 39 ± 0.6 | 42 ± 0.4a | <0.004 |
| Systolic blood pressure (mm Hg) | 107 ± 2.0 | 126 ± 1.0a | <0.004 |
| Diastolic blood pressure (mm Hg) | 70 ± 1.5 | 75 ± 1.0 | 0.004 |
| Mean arterial pressure (mm Hg) | 83 ± 1.7 | 96 ± 1.1a | <0.004 |
| Systolic pulmonary arterial pressure (mm Hg) | 33 ± 2.4 | 32 ± 0.9 | 0.704 |
| Diastolic pulmonary arterial pressure (mm Hg) | 18 ± 2.2 | 14 ± 0.7 | 0.011 |
| Clot induction | |||
| Heart rate (bpm) | 116 ± 3.0 | 63 ± 1.1a | <0.004 |
| SpO2 (%) | 95 ± 0.2 | 98 ± 0.3a | <0.004 |
| Respiratory rate (breaths/min) | 16 ± 0.6 | 16 ± 0.3 | 0.547 |
| End-tidal CO2 (mm Hg) | 32 ± 0.7 | 35 ± 0.6a | <0.004 |
| Systolic blood pressure (mm Hg) | 104 ± 1.4 | 121 ± 2.1a | <0.004 |
| Diastolic blood pressure (mm Hg) | 72 ± 1.0 | 73 ± 1.9 | 0.816 |
| Mean arterial pressure (mm Hg) | 84 ± 1.0 | 92 ± 2.1 | 0.020 |
| Systolic pulmonary arterial pressure (mm Hg) | 61 ± 1.9 | 53 ± 1.3a | <0.004 |
| Diastolic pulmonary arterial pressure (mm Hg) | 25 ± 3.0 | 27 ± 0.7 | 0.503 |
| Treatment | |||
| Heart rate (bpm) | 106 ± 3.0 | 61 ± 1.3a | <0.004 |
| SpO2 (%) | 95 ± 0.2 | 97 ± 1.0 | 0.158 |
| Respiratory rate (breaths/min) | 17 ± 0.4 | 16 ± 0.3 | 0.083 |
| End-tidal CO2 (mm Hg) | 34 ± 0.7 | 38 ± 0.6a | <0.004 |
| Systolic blood pressure (mm Hg) | 100 ± 1.0 | 131 ± 1.4a | <0.004 |
| Diastolic blood pressure (mm Hg) | 71 ± 1.3 | 78 ± 1.8 | 0.005 |
| Mean arterial pressure (mm Hg) | 82 ± 1.0 | 99 ± 1.8a | <0.004 |
| Systolic pulmonary arterial pressure (mm Hg) | 51 ± 0.8 | 51 ± 0.6 | 0.932 |
| Diastolic pulmonary arterial pressure (mm Hg) | 31 ± 1.1 | 25 ± 0.6a | <0.004 |
Value significantly different from that for isoflurane group.
Table 2.
Calculated measurements (mean ± SEM) of hemodynamics
| Isoflurane | α-Chloralose | P | |
| Baseline | |||
| Cardiac output (L/min) | 2.07 ± 0.13 | 1.66 ± 0.06a | <0.004 |
| Systemic vascular resistance (dyn × s/cm5) | 2348 ± 162 | 3690 ± 158a | <0.004 |
| Pulmonary vascular resistance (dyn × s/cm5) | 185 ± 43 | 450 ± 101 | 0.078 |
| RV:LV ratio | 0.80 ± 0.26 | 0.83 ± 0.03 | 0.843 |
| Clot induction | |||
| Cardiac output (L/min) | 1.70 ± 0.09 | 1.49 ± 0.04 | 0.020 |
| Systemic vascular resistance (dyn × s/cm5) | 2366 ± 204 | 3256 ± 160a | <0.004 |
| Pulmonary vascular resistance (dyn × s/cm5) | 907 ± 58 | 1617 ± 192 | 0.020 |
| RV:LV ratio | 1.32 ± 0.25 | 1.23 ± 0.07 | 0.669 |
| Treatment | |||
| Cardiac output (L/min) | 1.48 ± 0.09 | 1.75 ± 0.09 | 0.030 |
| Systemic vascular resistance (dyn × s/cm5) | 2334 ± 263 | 3096 ± 123 | 0.008 |
| Pulmonary vascular resistance (dyn × s/cm5) | 954 ± 160 | 1184 ± 133 | 0.305 |
| RV:LV ratio | 1.12 ± 0.14 | 1.02 ± 0.02 | 0.393 |
Value significantly different from that for isoflurane group.
After the sham treatment phase, the intergroup difference in oxygen saturation was no longer significant, but heart rate and diastolic pulmonary arterial pressure were still significantly higher and end-tidal CO2, systolic blood pressure, and mean arterial pressure were lower in swine anesthetized with isoflurane compared with α-chloralose.
Measurements of coagulation.
Coagulation parameters are recorded in Table 3. No meaningful intergroup difference in prothrombin time or fibrinogen level was present, but the activated partial thromboplastin time and fibrinogen concentration varied more widely in swine anesthetized with isoflurane than α-chloralose. In particular, 30% of activated partial thromboplastin times from isoflurane-treated swine were below the lower limit of the range in healthy awake swine.34
Table 3.
Measurements (mean ± SEM) of coagulation
| Phase | Group | Prothrombin time (s) | Activated partial thromboplastin time (s) | Fibrinogen (mg/dL) |
| Baseline | Isoflurane | 13.3 ± 0.8 | 30.5 ± 11.7 | 208 ± 44 |
| α-Chloralose | 13.2 ± 0.5 | 28.3 ± 0.3 | 147 ± 6 | |
| Clot induction | Isoflurane | 13.0 ± 0.6 | 29.9 ± 9.8 | 182 ± 35 |
| α-Chloralose | 13.4 ± 0.2 | 33.6 ± 3.1 | 161 ± 10 | |
| Treatment | Isoflurane | 13.4 ± 0.5 | 28.0 ± 8.6 | 167 ± 58 |
| α-Chloralose | 13.6 ± 0.4 | 35.0 ± 1.5 | 163 ± 10 |
The effects of each method of anesthesia on key physiologic measurements in the swine PE model are compared in Table 4.
Table 4.
Comparison of methods of anesthesia for a swine model of PE
| Isoflurane | Chloralose | |
| Normal baseline hemodynamics | No | Yes |
| Tachycardia in response to PE | Yes | No |
| Nonhypotensive systolic blood pressure | Yes | Yes |
| Sustained pulmonary hypertension | No | Yes |
| Increased RV:LV ratio | Yes | Yes |
| Persistent intrapulmonary clot on necropsy | Yes | Yes |
| Isolated RV dilation on necropsy | Yes | Yes |
| <10% loss of animals | No | Yes |
Discussion
We here describe a model of submassive PE in swine and compare 2 methods of anesthesia to determine which agent best mimics the expected hemodynamic consequences of PE in humans. Our model and method of PE induction provide reproducible, clinically relevant patterns of hemodynamic change, including sustained pulmonary hypertension, RV dilation, lowered end-tidal CO2, and persistent clots in the pulmonary arterial tree. The data in Table 4 show several benefits of α-chloralose over isoflurane. First, the baseline systolic arterial blood pressure was normal with α-chloralose, whereas isoflurane-anesthetized swine were marginally hypotensive prior to clot induction, with lower systemic vascular resistance. Although no statistical inference can be made, this baseline hypotension suggests excessive vasodilation with isoflurane but not α-chloralose, a difference that explain why all pigs provided α-chloralose survived, compared with 7 of the 9 given isoflurane. Second, α-chloralose-anesthetized swine had less instability in systolic PAP during clot induction and showed an appropriate baseline pulmonary vascular resistance and increase with clot induction. At baseline, isoflurane anesthetized animals often had abnormally low values, suggesting pulmonary vasodilation induced by isoflurane.32 Third, although α-chloralose- and isoflurane-anesthetized pigs both manifested a drop in mean end-tidal CO2 at the prespecified time points, we found that the end-tidal CO2 at the end of surgery was a much more reliable index to guide the PE titration process in the α-chloralose compared with the isoflurane group.13 This observed loss of end-tidal CO2 variability may result from isoflurane-associated blunted pulmonary vasoconstriction that often accompanies PE.32,37,42 Isoflurane's blunting effect on end-tidal CO2 variability hampered the delicate process of clot titration during induction.13
Fourth, the depth of anesthesia was more reliable with α-chloralose, and our experience with isoflurane-treated swine that showed signs of emergence during PE induction raises the possibility that ventilation–perfusion mismatch due to PE leads to erratic pulmonary uptake of isoflurane.32,37,42 Moreover, we emphasize that isoflurane concentrations above 1.4% consistently produced systolic blood pressures below 100 mm Hg prior to PE induction. The isoflurane-associated narrow margin between hypotension and animal emergence required intense attention from the investigators in the midst of a complicated experimental protocol. In addition, the activated partial thrombin times and fibrinogen concentrations tended to vary more with isoflurane, according to the SEM values. The fibrinogen concentrations in our α-chloralose-treated swine were consistent with those in pigs reported previously.34 Most isoflurane-anesthetized animals displayed activated partial thrombin times that were below the range of those of awake, healthy swine and higher fibrinogen concentrations.34 These features increase endogenous clot-generating potential in a degree-dependent fashion, especially in the setting of indwelling catheters, introducing a possible confounding variable.33,39 Finally, 2 of the 9 isoflurane animals but none of the 6 α-chloralose animals died during PE induction (P = 0.34, Fisher exact test). The major cardiovascular response that α-chloralose-anesthetized pigs lacked was an increase in heart rate, suggesting either blunting of the baroreceptor reflex or induction of another mechanism to increase stroke volume. Taken together, our data and observations suggest an advantage of α-chloralose over isoflurane. To our knowledge, this study is the first to compare 2 anesthetic regimens in a large animal model of submassive PE.
We propose that α-chloralose may not be well suited for chronic survival surgeries secondary to its problems with adynamic ileus.35 However, in acute, terminal experiments, its advantages of protection of the sympathetic drive and cardiac output show the usefulness of α-chloralose compared with isoflurane. The primary drawback to isoflurane appears to be its vasodilatory properties, which we believe explain the approximately 30% higher baseline heart rate and sustained low systolic arterial blood pressure measured at the end of the treatment (100 ± 8 compared with 131 ± 15 mm Hg) phase in the isoflurane compared with α-chloralose animals.
We also here provide the detailed methodology for a model of submassive PE in swine that used a large-bore catheter to embolize clots of 2 sizes, thus mimicking the pathophysiology of severe, submassive PE, including sustained pulmonary hypertension, in humans. In pilot work, we found that embolizing only large clots did not produce sustained pulmonary hypertension and was more likely to cause animal loss compared with embolizing clots of 2 sizes. The concept of using large and small clots is supported by human autopsies, which found most that patients with PE had evidence of numerous sublobar, organizing emboli, with or without lung infarction, that occurred prior to embolization of a larger thrombus.27,28,31 One author has referred to this finding as the “heterochronic” quality of PE.28 In addition, the smaller clot was intended to completely occlude the segmental and subsegmental pulmonary arteries to augment the vasoconstrictive and inflammatory effects of PE. Distal pulmonary vascular occlusion stimulates the production of vasoconstrictor molecules and inflammatory chemokines, which may help to sustain pulmonary hypertension.10,46,47 The large clots in our swine model then rapidly augment pulmonary arterial pressures, with resultant RV dilation and heart muscle injury that causes troponin release, as is observed in humans with PE.3
The current study has several limitations. First, the model we used is only relevant to an acute, nonsurvival model to test treatments that mitigate right heart damage in the first hours after embolization. Second, we did not compare α-chloralose with an infusion of ketamine and midazolam, which may be better suited for survival surgery. We hypothesize that ketamine would have blunted the LV function, as seen in other acute cardiac swine models.45 Midazolam likely would have an acute negative cardiac inotropic effect and contribute to a decreased respiratory rate, as has been demonstrated previously in swine.36 Third, according to local IACUC guidelines, all pigs in our study received a single dose of tiletamine–ketamine–xylazine; several case studies suggest the cardiodepressant effects of this combination.20 All swine in both groups received the same weight-based dose of tiletamine–ketamine–xylazine for induction at the beginning of the experiment. In addition, the animals were allowed to acclimate during the instrumentation portion of the procedure, which required on average 90 min and after which the cardiodepressant effect of the TKX likely had dissipated.
In conclusion, we here present a new swine model for submassive PE in which using α-chloralose for anesthesia was minimally invasive and advantageous over using isoflurane. The pigs anesthetized with α-chloralose showed similar PE-associated cardiovascular effects to those in humans. This model may be suitable for testing treatments for PE.
Acknowledgments
Funding support included Lilly Physician Scientist Award and Emergency Medicine Foundation Research Fellowship.
References
- 1.Aickin M, Gensler H. 1996. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health 86:726–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbash IM, Schenke WH, Halabi M, Ratnayaka K, Faranesh AZ, Kocaturk O, Lederman RJ. 2011. Experimental model of large pulmonary embolism employing controlled release of subacute caval thrombus in swine. J Vasc Interv Radiol 22:1471–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becattini C, Vedovati MC, Agnelli G. 2007. Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation 116:427–433. [DOI] [PubMed] [Google Scholar]
- 4.Courtney DM, Kline JA. 2001. Identification of prearrest clinical factors associated with outpatient fatal pulmonary embolism. Acad Emerg Med 8:1136–1142. [DOI] [PubMed] [Google Scholar]
- 5.Flight SM, Masci PP, Lavin MF, Gaffney PJ. 2006. Resistance of porcine blood clots to lysis relates to poor activation of porcine plasminogen by tissue plasminogen activator. Blood Coagul Fibrinolysis 17:417–420. [DOI] [PubMed] [Google Scholar]
- 6.Goldhaber SZ. 2012. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haematol 25:235–242. [DOI] [PubMed] [Google Scholar]
- 7.Hess EP, Campbell RL, White RD. 2007. Epidemiology, trends, and outcome of out-of-hospital cardiac arrest of noncardiac origin. Resuscitation 72:200–206. [DOI] [PubMed] [Google Scholar]
- 8.Houser SR, Margulies KB, Murphy AM, Spinale FG, Francis GS, Prabhu SD, Rockman HA, Kass DA, Molkentin JD, Sussman MA, Koch WJ, Koch W. 2012. Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res 111:131–150. [DOI] [PubMed] [Google Scholar]
- 9.Jimenez D, Kopecna D, Tapson V, Briese B, Schreiber D, Lobo JL, Monreal M, Aujesky D, Sanchez O, Meyer G, Konstantinides S, Yusen RD, On Behalf Of The Protect Investigators 2014. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med 189:718–726. [DOI] [PubMed] [Google Scholar]
- 10.Jones AE, Watts JA, Debelak JP, Thornton LR, Younger JG, Kline JA. 2003. Inhibition of prostaglandin synthesis during polystyrene microsphere-induced pulmonary embolism in the rat. Am J Physiol Lung Cell Mol Physiol 284:L1072–L1081. [DOI] [PubMed] [Google Scholar]
- 11.Kessler U, Grau T, Gronchi F, Berger S, Brandt S, Bracht H, Marcucci C, Zachariou Z, Jakob SM. 2011. Comparison of porcine and human coagulation by thrombelastometry. Thromb Res 128:477–482. [DOI] [PubMed] [Google Scholar]
- 12.Kjærgaard B, Rasmussen BS, de Neergaard S, Rasmussen LH, Kristensen SR. 2012. Extracorporeal cardiopulmonary support may be an efficient rescue of patients after massive pulmonary embolism. An experimental porcine study. Thromb Res 129:e147–e151. [DOI] [PubMed] [Google Scholar]
- 13.Kline JA, Israel EG, Michelson EA, O'Neil BJ, Plewa MC, Portelli DC. 2001. Diagnostic accuracy of a bedside d-dimer assay and alveolar dead-space measurement for rapid exclusion of pulmonary embolism: a multicenter study. JAMA 285:761–768. [DOI] [PubMed] [Google Scholar]
- 14.Kline JA, Marchick MR, Hogg MM. 2009. Reduction in plasma haptoglobin in humans with acute pulmonary embolism causing tricuspid regurgitation. J Thromb Haemost 7:1597–1599. [DOI] [PubMed] [Google Scholar]
- 15.Kline JA, Nordenholz KE, Courtney DM, Kabrhel C, Jones AE, Rondina MT, Diercks DB, Klinger JR, Hernandez J. 2014. Treatment of submassive pulmonary embolism with tenecteplase or placebo. Cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost 12:459–468. [DOI] [PubMed] [Google Scholar]
- 16.Kline JA, Steuerwald MT, Marchick MR, Hernandez-Nino J, Rose GA. 2009. Prospective evaluation of right ventricular function and functional status 6 months after acute submassive pulmonary embolism: frequency of persistent or subsequent elevation in estimated pulmonary artery pressure. Chest 136:1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kline JA, Watts J, Courtney D, Lee YY, Hwang S. 2014. Severe pulmonary embolism decreases plasma l-arginine. Eur Respir J 43:906–909. [DOI] [PubMed] [Google Scholar]
- 18.Kurkciyan I, Meron G, Behringer W, Sterz F, Berzlanovich A, Domanovits H, Mullner M, Bankl HC, Laggner AN. 1998. Accuracy and impact of presumed cause in patients with cardiac arrest. Circulation 98:766–771. [DOI] [PubMed] [Google Scholar]
- 19.Landskroner K, Olson N, Jesmok G. 2005. Cross-species pharmacologic evaluation of plasmin as a direct-acting thrombolytic agent: ex vivo evaluation for large animal model development. J Vasc Interv Radiol 16:369–377. [DOI] [PubMed] [Google Scholar]
- 20.Lefkov SH, Mussig D. 2007. Tiletamine–zolazepam and xylazine is a potent cardiodepressive combination: a case report. J Am Assoc Lab Anim Sci 46:63–64. [PubMed] [Google Scholar]
- 21.Leitner JM, Jilma B, Spiel AO, Sterz F, Laggner AN, Janata KM. 2010. Massive pulmonary embolism leading to cardiac arrest is associated with consumptive coagulopathy presenting as disseminated intravascular coagulation. J Thromb Haemost 8:1477–1482. [DOI] [PubMed] [Google Scholar]
- 22.Levi M. 2010. Disseminated intravascular coagulation or extended intravascular coagulation in massive pulmonary embolism. J Thromb Haemost 8:1475–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin BW, Schreiber DH, Liu G, Briese B, Hiestand B, Slattery D, Kline JA, Goldhaber SZ, Pollack CV. 2012. Therapy and outcomes in massive pulmonary embolism from the Emergency Medicine Pulmonary Embolism in the Real World Registry. Am J Emerg Med 30:1774–1781. [DOI] [PubMed] [Google Scholar]
- 24.Manara A, D'Hoore W, Thys F. 2013. Capnography as a diagnostic tool for pulmonary embolism: a meta-analysis. Ann Emerg Med 62:584–591. [DOI] [PubMed] [Google Scholar]
- 25.McIntyre KM, Sasahara AA. 1971. The hemodynamic response to pulmonary embolism in patients without prior cardiopulmonary disease. Am J Cardiol 28:288–294. [DOI] [PubMed] [Google Scholar]
- 26.Meyer RE, Fish RE. 2008. Pharmacology of injectable anesthetics, sedatives, and tranquilizers, p 27–82. In: Fish RE, Brown MJ, Danneman PJ, Karas AZ. Anesthesia and analgesia in laboratory animals, 2nd ed. San Diego (CA): Academic Press. [Google Scholar]
- 27.Morgenthaler TI, Ryu JH. 1995. Clinical characteristics of fatal pulmonary embolism in a referral hospital. Mayo Clin Proc 70:417–424. [DOI] [PubMed] [Google Scholar]
- 28.Morpurgo M, Schmid C. 1995. The spectrum of pulmonary embolism. Clinicopathologic correlations. Chest 107:18S–20S. [DOI] [PubMed] [Google Scholar]
- 29.Otero R, Oribe M, Ballaz A, Jimenez D, Uresandi F, Nauffal D, Conget F, Rodriguez C, Elias T, Jara L, Cayuela A, Blanco I, Barbera J. 2011. Echocardiographic assessment of pulmonary arterial pressure in the follow-up of patients with pulmonary embolism. Thromb Res 127:303–308. [DOI] [PubMed] [Google Scholar]
- 30.Pereira DJ, Moreira MM, Paschoal IA, Martins LC, Metze K, Moreno Junior H. 2011. Near-fatal pulmonary embolism in an experimental model: hemodynamic, gasometric, and capnographic variables. Rev Bras Cir Cardiovasc 26:462–468. [DOI] [PubMed] [Google Scholar]
- 31.Pineda LA, Hathwar VS, Grant BJ. 2001. Clinical suspicion of fatal pulmonary embolism. Chest 120:791–795. [DOI] [PubMed] [Google Scholar]
- 32.Roehl AB, Steendijk P, Rossaint R, Bleilevens C, Goetzenich A, Hein M. 2012. Xenon is not superior to isoflurane on cardiovascular function during experimental acute pulmonary hypertension. Acta Anaesthesiol Scand 56:449–458. [DOI] [PubMed] [Google Scholar]
- 33.Senthil M, Chaudhary P, Smith DD, Ventura PE, Frankel PH, Pullarkat V, Trisal V. 2014. A shortened activated partial thromboplastin time predicts the risk of catheter-associated venous thrombosis in cancer patients. Thromb Res 134:165–168. [DOI] [PubMed] [Google Scholar]
- 34.Siller-Matula JM, Plasenzotti R, Spiel A, Quehenberger P, Jilma B. 2008. Interspecies differences in coagulation profile. Thromb Haemost 100:397–404. [PubMed] [Google Scholar]
- 35.Silverman J, Muir WW., 3rd 1993. A review of laboratory animal anesthesia with chloral hydrate and chloralose. Lab Anim Sci 43:210–216. [PubMed] [Google Scholar]
- 36.Smith AC, Zellner JL, Spinale FG, Swindle MM. 1991. Sedative and cardiovascular effects of midazolam in swine. Lab Anim Sci 41:157–161. [PubMed] [Google Scholar]
- 37.Smulders YM. 2000. Pathophysiology and treatment of haemodynamic instability in acute pulmonary embolism: the pivotal role of pulmonary vasoconstriction. Cardiovasc Res 48:23–33. [DOI] [PubMed] [Google Scholar]
- 38.ten Wolde M, Sohne M, Quak E, Mac Gillavry MR, Buller HR. 2004. Prognostic value of echocardiographically assessed right ventricular dysfunction in patients with pulmonary embolism. Arch Intern Med 164:1685–1689. [DOI] [PubMed] [Google Scholar]
- 39.Tripodi A, Chantarangkul V, Martinelli I, Bucciarelli P, Mannucci PM. 2004. A shortened activated partial thromboplastin time is associated with the risk of venous thromboembolism. Blood 104:3631–3634. [DOI] [PubMed] [Google Scholar]
- 40.Vanni S, Viviani G, Baioni M, Pepe G, Nazerian P, Socci F, Bartolucci M, Bartolini M, Grifoni S. 2013. Prognostic value of plasma lactate levels among patients with acute pulmonary embolism: the thromboembolism lactate outcome study. Ann Emerg Med 61:330–338. [DOI] [PubMed] [Google Scholar]
- 41.Vedovati MC, Becattini C, Agnelli G, Kamphuisen PW, Masotti L, Pruszczyk P, Casazza F, Salvi A, Grifoni S, Carugati A, Konstantinides S, Schreuder M, Golebiowski M, Duranti M. 2012. Multidetector CT scan for acute pulmonary embolism: embolic burden and clinical outcome. Chest 142:1417–1424. [DOI] [PubMed] [Google Scholar]
- 42.Watts JA, Gellar MA, Fulkerson M-BK, Kline JA. 2011. Pulmonary vascular reserve during experimental pulmonary embolism: effects of a soluble guanylate cyclase stimulator, BAY 41-8543. Crit Care Med 39:2700–2704. [DOI] [PubMed] [Google Scholar]
- 43.Watts JA, Marchick MR, Kline JA. 2010. Right ventricular heart failure from pulmonary embolism: key distinctions from chronic pulmonary hypertension. J Card Fail 16:250–259. [DOI] [PubMed] [Google Scholar]
- 44.Watts JA, Zagorski J, Gellar MA, Stevinson BG, Kline JA. 2006. Cardiac inflammation contributes to right ventricular dysfunction following experimental pulmonary embolism in rats. J Mol Cell Cardiol 41:296–307. [DOI] [PubMed] [Google Scholar]
- 45.Wessler B, Madias C, Pandian N, Link MS. 2011. Short-term effects of ketamine and isoflurane on left ventricular ejection fraction in an experimental swine model. ISRN Cardiol 2011:582658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zagorski J, Debelak J, Gellar M, Watts JA, Kline JA. 2003. Chemokines accumulate in the lungs of rats with severe pulmonary embolism induced by polystyrene microspheres. J Immunol 171:5529–5536. [DOI] [PubMed] [Google Scholar]
- 47.Zagorski J, Marchick MR, Kline JA. 2010. Rapid clearance of circulating haptoglobin from plasma during acute pulmonary embolism in rats results in HMOX1 upregulation in peripheral blood leukocytes. J Thromb Haemost 8:389–396. [DOI] [PubMed] [Google Scholar]