Abstract
Recent significant advances in stem cell research and bioengineering techniques have made great progress in utilizing biomaterials to regenerate and repair damage in simple tissues in the orthopedic and periodontal fields. However, attempts to regenerate the structures and functions of more complex three-dimensional (3D) organs such as lungs have not been very successful because the biological processes of organ regeneration have not been well explored. It is becoming clear that angiogenesis, the formation of new blood vessels, plays key roles in organ regeneration. Newly formed vasculatures not only deliver oxygen, nutrients and various cell components that are required for organ regeneration but also provide instructive signals to the regenerating local tissues. Therefore, to successfully regenerate lungs in an adult, it is necessary to recapitulate the lung-specific microenvironments in which angiogenesis drives regeneration of local lung tissues. Although conventional in vivo angiogenesis assays, such as subcutaneous implantation of extracellular matrix (ECM)-rich hydrogels (e.g., fibrin or collagen gels or Matrigel - ECM protein mixture secreted by Engelbreth-Holm-Swarm mouse sarcoma cells), are extensively utilized to explore the general mechanisms of angiogenesis, lung-specific angiogenesis has not been well characterized because methods for orthotopic implantation of biomaterials in the lung have not been well established. The goal of this protocol is to introduce a unique method to implant fibrin gel on the lung surface of living adult mouse, allowing for the successful recapitulation of host lung-derived angiogenesis inside the gel. This approach enables researchers to explore the mechanisms by which the lung-specific microenvironment controls angiogenesis and alveolar regeneration in both normal and pathological conditions. Since implanted biomaterials release and supply physical and chemical signals to adjacent lung tissues, implantation of these biomaterials on diseased lung can potentially normalize the adjacent diseased tissues, enabling researchers to develop new therapeutic approaches for various types of lung diseases.
Keywords: Basic Protocol, Issue 94, lung, angiogenesis, regeneration, fibrin, gel implantation, microenvironment
Introduction
The overall goal of this protocol is to introduce a method to implant fibrin gel on the lung surface of adult mouse, which allows researchers to characterize the molecular mechanisms of lung vascular and alveolar development, and to leverage this knowledge in order to develop biomimetic materials capable of recapitulating physiological lung vascular and alveolar formation to treat various lung diseases.
More than 35 million Americans suffer from chronic lung diseases including chronic obstructive pulmonary disease and pulmonary fibrosis. These patients have long-lasting chronic respiratory symptoms such as shortness of breath, chest tightness, nagging cough, and tiredness, which significantly impair their daily life 1-3. Despite a great amount of effort to develop effective therapies for these lung diseases, currently there is no cure; therefore, quality of life for these patients is poor and economic and human costs are high 4-7. Currently, lung transplantation is the only way to save patients with end-stage chronic lung diseases. However, because of the shortage of transplant donors, high cost, serious complications, and low survival rate 8-11, transplantation is not an optimal approach. Recent rapid progress in tissue engineering techniques has enabled researchers to bioengineer implantable lung by repopulating decellularized whole lung with various types of progenitor cells or induced pluripotent stem (iPS) cells 12,13. However, these bioengineered lungs are functional in host animals only for several hours after implantation 12,14,15. Utilizing biomaterials to regenerate the complex structures and functions of lungs has also been fairly unsuccessful. This may be because key biological processes that govern adult lung regeneration have not been well explored. In the lung, formation of the vascular system is one of the earliest and most important events during development and regeneration 16-21. Newly formed vasculatures in the lung not only deliver oxygen, nutrients and various cell components required for organ formation, but also provide instructive regulatory signals to surrounding cells 22-25. Thus, angiogenesis plays key roles in regenerative alveolarization in adult lungs 24,26,27. In addition, deregulated angiogenesis contributes to chronic lung diseases such as chronic obstructive pulmonary disease (COPD) 28, bronchopulmonary dysplasia (BPD) 21-23, and pulmonary fibrosis 29. Thus, to develop more efficient strategies for engineering lungs or treating chronic lung diseases, it is necessary to understand the fundamental mechanisms of lung-specific angiogenesis.
Each organ displays unique mechanical and chemical properties, which may differ between physiological and pathological conditions 30-33. These organ-specific microenvironments regulate endothelial cell behaviors and orchestrate vascular network formation in an organ-specific manner 24,34-36. Thus, to develop more efficient strategies for lung regeneration, the mechanism underlying lung-specific angiogenesis needs to be understood. While conventional in vivo angiogenesis assays such as subcutaneous hydrogel implantation have been used extensively for angiogenesis research 37-39, those methods do not recapitulate organ-specific angiogenesis. Recently, a novel method to implant Matrigel in an elastic mold on the mouse lung has been developed and shown to successfully recruit blood vessels and lung epithelial cells into the gels 22. This unique approach will allow researchers to explore the mechanism of lung-specific angiogenesis as well as interactions between blood vessels and non-vascular lung cells in physiological and pathological conditions. Since 1) Matrigel is not suitable for clinical application; 2) the elastic mold used to cast the gel may affect interactions between hydrogels and host lung tissue and 3) the elastic mold on the lung potentially causes impairment of lung function and pain during respiration, as a more clinically relevant approach, a 3D fibrin matrix containing angiogenic factors (vascular endothelial growth factor (VEGF)/ basic fibroblast growth factor (bFGF)) has been implanted on the mouse lung without casting in the elastic mold, and has successfully recapitulated host lung-derived angiogenesis. Fibrin gel, polymer fibrils generated from thrombin-cleaved fibrinogen, is known to trap a variety of angiogenic factors such as bFGF and VEGF to accelerate angiogenesis in vivo 40,41. Because of its regenerative ability and biodegradable nature 42, fibrin gel is widely used in the field of tissue engineering.
This article introduces a novel and unique approach to implant fibrin gel on the lung surface of living adult mouse and demonstrates that host lung-derived angiogenesis is recapitulated inside the gels in vivo. This method, which enables researchers to study lung-specific angiogenesis, will likely lead to the development of new therapeutic approaches for various types of lung diseases and significantly advance efforts to successfully regenerate adult lung.
Protocol
NOTE: The in vivo animal study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was reviewed and approved by the Animal Care and Use Committee of Boston Children’s Hospital (Protocol Numbers: 13-10-2526R,14-02-2568R). All drugs used in this protocol are pharmaceutical grade and these drugs are prepared under sterile conditions.
1. Fibrin Gel Preparation
- Prepare fibrin gel that contains VEGF and bFGF.
- Thaw stock solutions of fibrinogen and thrombin that are stored at -80 °C to room temperature (25 °C).
- Add thrombin (final concentration: 2.5 U/ml), CaCl2 (final concentration: 45 mM), VEGF (final concentration: 0-100 ng/ml) and bFGF (final concentration: 0-100 ng/ml) to the fibrinogen solution (final concentration: 12.5 mg/ml in 0.9% sodium chloride solution 43-45) in a 1.5 ml tube.
- Mix gently by pipetting.
- Gently pipette 200 µl of the mixture onto a sterile plastic dish in a drop-wise fashion using p200 pipette tip.
- Incubate the drops at 37 °C for 30-60 min until they solidify. NOTE: The solidified gel can be kept in the sealed plastic dish at room temperature (25 °C) for several hours before implantation (Figure 1a).
Trim the fibrin gel into approximately 3 x 3 x 3 mm cubes using small surgical scissors before implantation.
2. Mouse Preparation
- Anesthetize adult mouse (8-12 weeks) by intraperitoneal (IP) injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) and confirm that the mouse is adequately anesthetized by pinching toe of the mouse.
- Use vet ointment on eyes of the mouse to prevent dryness during the experiment.
Shave fur over left side of the rib cage of the mouse.
Perform endotracheal intubation of the mouse.
Place the mouse on the intubation stand angled at 70° and hold mouse in place by hooking its upper incisors over a small rubber band located at the top of the stand.
Gently retract the tongue to one side using blunt forceps.
Visualize the larynx with the aid of a fiber-optic gooseneck microscope illuminator.
Insert endotracheal elastic catheter (21 G) into the trachea.
Confirm that the mouse is spontaneously breathing in a smooth way (regular 100-150 breaths/min, no paradoxical or shallow respiration).
Place the mouse in prone position under the dissection microscope.
Mechanically ventilate the mouse using a rodent ventilator (150 breaths/min and 7 ml/kg tidal volume).
Count ribs to locate intercostal space between 4th and 5th rib.
Create a sterile field over the area by thoroughly wiping down with alcohol and Povidone-Iodine. Cover the surgical field adequately with a sterile surgical drape.
3. Mouse Surgery
After local injection of 0.25% bupivacaine (200 μl) in the skin, make a transverse skin incision (approximately 1 cm length) over the intercostal space using dissecting scissors.
After injection of 0.25% bupivacaine (200 μl) into the intercostal muscle, make a muscle incision between the 4th and 5th rib using fine small scissors.
- Insert a dissecting retractor between the ribs to fully visualize the left lung.
- Scrape a small area (1 x 1 mm square) of visceral pleura of the center of left lung using fine forceps.
- Apply gentle pressure on the area using a sterile cotton swab until bleeding and air leaks are completely controlled.
- Put small amount of fresh mixture of fibrinogen/thrombin (fibrin glue) (step 1.1.2. 20 µl) over the area using p200 pipette tip.
- Gently place one fibrin gel (step 1.2) using small forceps over the area (Figure 1b).
- Confirm that the gel is well fixed on the area during respiratory movements of the lung.
Make sure that there is neither massive air leaking nor bleeding from the lung.
Close incisions (muscle and skin layers) with absorbable suture, which does not have to be removed.
Aspirate the thoracic cavity using 27 G needle and 1 ml syringe to prevent pneumothorax.
Terminate mechanical ventilation.
4. Mouse Recovery
Make sure the mouse is spontaneously breathing in a smooth way (regular 100-150 breaths/min, no paradoxical or shallow respiration).
IP inject 1 ml of pre-warmed 0.9% NaCl to prevent dehydration.
Allow mouse to recover on the circulating warm water pad.
Remove the endotracheal tube after confirming that the mouse has stable breathing.
Inject Meloxicam (5 mg/kg, subcutaneous injection (SC), for 3 days as postoperative analgesic.
Monitor the movements of the mouse carefully at a minimum of 15 min intervals until it is sternal (able to roll onto its stomach and remain upright) and conscious.
After recovery, return the mouse to a new cage isolated from mice without surgery.
Monitor the surgical site for signs of infection (redness, swelling, discharge), animal’s basic biologic functions (food and water intake, urination, defecation, body weight gain) as well as clinical signs of distress (piloerection, reduced locomotion) daily following the surgical procedure.
5. Harvesting the Lung
7 to 30 days after implantation, euthanize the mouse using CO2 via source of compressed gas.
Make an incision between the tip of xyphoid process and the sternal notch (median sternotomy) and harvest whole lung with the implanted gel for histological and biochemical analysis by cutting the trachea and dissecting all connections to the heart, lungs, and trachea.
Fix implanted gel with lung with 4% paraformaldehyde solution overnight at 4 ºC, embed in OCT compound, and take serial step sections of 30 µm thickness.
Perform histological (hematoxylin and eosin staining) and immunohistochemical analyses (endothelial marker: CD31, epithelial marker: aquaporin (AQP)5 and surfactant protein (SP)-B) using confocal microscope 22,37,40.
Compile stacks of optical sections (30 µm thick) to form three-dimensional images of lung endothelial and epithelial cells using 3D image analysis software 37.
Quantify projected areas of newly formed blood vessels using image analysis software 46.
Representative Results
To examine whether host lung-derived vascular formation is recapitulated inside the biomaterials implanted on the lung, fibrin gels supplemented with major angiogenic factors VEGF and bFGF (0, 10 and 100 ng/ml each) were implanted on the surface of living mouse lungs as reported using Matrigel 22. Fibrin gels 47 that contain these angiogenic growth factors were fabricated as shown in Figure 1a. After thoracotomy, a small area of the left lung surface was scraped using forceps and the fabricated fibrin gel was implanted on the lung of the adult mouse using a small amount of fibrin glue, which is FDA approved and widely used as an effective sealant to stop air leaks and reduce bleeding in lung surgery 48,49 (Figure 1b). Most mice recovered without severe respiratory symptoms (e.g., pneumothorax, respiratory distress). Seven days after implantation, mice were euthanized and lungs were harvested. Implanted fibrin gel was incorporated into the host lung 7 days after implantation (Figure 1c). 3D reconstruction of confocal fluorescence images has shown that host-derived CD31-positive endothelial cells formed vascular networks inside the gels 7 days after implantation in a VEGF/bFGF dose-dependent way (Figure 2a, c). Type I (AQP5 positive) and type II (SP-B positive) lung epithelial cells were also recruited along newly formed blood vessels inside the gels that were supplemented with higher concentrations of VEGF and bFGF (each 100 ng/ml) (Figure 2a). H&E staining of histological sections revealed that other types of host cells also migrated into the gel 7 days after implantation (Figure 2b). These findings suggest that host lung-derived regenerative vascular networks are successfully constructed inside the fibrin gels that are supplemented with angiogenic factors and implanted on the surface of adult mouse lung.
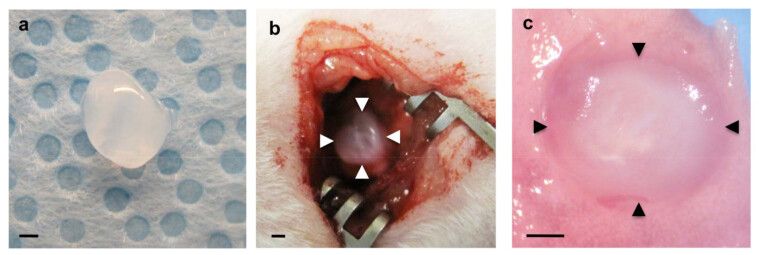 Figure 1: (a) Fibrin gel prepared before implantation. (b) Fibrin gel implanted over the scraped visceral pleura of the left lung (arrowheads). (c) Implanted fibrin gel (arrowheads) incorporated into the host lung 7 days after implantation. Scale bars 1 mm.
Figure 1: (a) Fibrin gel prepared before implantation. (b) Fibrin gel implanted over the scraped visceral pleura of the left lung (arrowheads). (c) Implanted fibrin gel (arrowheads) incorporated into the host lung 7 days after implantation. Scale bars 1 mm.
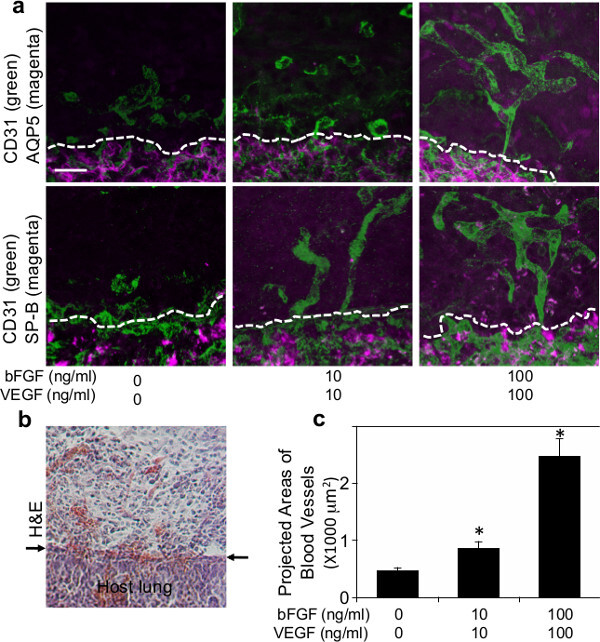 Figure 2: (a) Fluorescence micrographs showing formation of vascular networks (CD31 positive; green) and recruited type I (AQP5-positive; magenta) or type II (SP-B positive; magenta) lung epithelial cells inside the fibrin gel supplemented with various concentrations of VEGF and bFGF (0, 10 and 100 ng/ml each) 7 days after implantation. Dashed lines indicate the interface between implanted fibrin gel and host lung. Scale bar: 20 µm. (b) Light micrograph of H&E staining showing infiltration of host cells into the fibrin gel 7 days after implantation. Arrows indicate the interface between the gel and host lung. Scale bar: 20 µm. (c) Graph showing projected areas of newly formed blood vessels in the fibrin gels that are supplemented with various concentrations of VEGF and bFGF (0, 10 and 100 ng/ml each) 7 days after implantation.
Figure 2: (a) Fluorescence micrographs showing formation of vascular networks (CD31 positive; green) and recruited type I (AQP5-positive; magenta) or type II (SP-B positive; magenta) lung epithelial cells inside the fibrin gel supplemented with various concentrations of VEGF and bFGF (0, 10 and 100 ng/ml each) 7 days after implantation. Dashed lines indicate the interface between implanted fibrin gel and host lung. Scale bar: 20 µm. (b) Light micrograph of H&E staining showing infiltration of host cells into the fibrin gel 7 days after implantation. Arrows indicate the interface between the gel and host lung. Scale bar: 20 µm. (c) Graph showing projected areas of newly formed blood vessels in the fibrin gels that are supplemented with various concentrations of VEGF and bFGF (0, 10 and 100 ng/ml each) 7 days after implantation.
Discussion
This article introduces a new method to implant biomaterials on the lung surface of living adult mouse. With this system, host lung-derived angiogenesis is successfully recapitulated inside the material. This system allows researchers to explore crosstalk between endothelial cells, other cells (e.g., epithelial cells, mesenchymal cells, immune cells) and various ECM components that are required for local angiogenesis 50-53 and alveolar regeneration 24,54. Although conventional in vivo subcutaneous hydrogel implantation has been used extensively for angiogenesis research 37-39, those methods do not recapitulate organ-specific angiogenesis. This system, in which hydrogel is implanted directly on the lung surface, will enable researchers to explore the roles of the lung-specific microenvironment in angiogenesis and alveolar regeneration in adult mouse lung. These gels can be fabricated from various ECM-rich biomaterials (e.g., collagens, fibrins) that can be supplemented with various chemical factors (e.g., angiogenic factors, growth factors) 55,56, progenitor cells and/or iPS cells. In addition to chemical factors, mechanical forces also control angiogenesis 23,37. The stiffness of fibrin gel changes in a fibrinogen concentration-dependent manner 57 and manipulating the fibrinogen concentration may affect angiogenesis not only through chemical signals but also through physical cues 58,59. Therefore, physicochemical properties of the fibrin gels may need to be optimized carefully to recapitulate physiological organ-specific angiogenesis in the future. Wound healing after scraping the visceral pleura also produces an endogenous fibrin clot, which includes various types of host cells and promotes the healing process and tissue regeneration. This natural clot may interact with the exogenously implanted fibrin gel, and hence control angiogenesis in the implanted gel. Fluorescently labeled fibrinogen may enable researchers to distinguish between natural fibrin clot and implanted fibrin gel and explore these mechanisms. Although this is a powerful method to characterize angiogenesis in adult mouse lungs, application to the study of lung development and diseases in neonatal mice would likely present technical challenges.
The ultimate goal of this study is to recruit functional blood vessels into fibrin gels implanted on diseased lungs and to use the matrix as a medical device to restore functional lung structures. Possible communications between host cells and the vascular and alveolar structures inside the gels as well as the functionality of these structures should be explored in future experiments. Since VEGF levels in the lungs are decreased in patients with BPD 60 and emphysema 61, adding VEGF to the matrix may improve recruitment of blood vessels into the matrix implanted on these diseased lungs. Mechanical properties also differ between healthy and diseased lungs 23,62. For example, expression of matrix metalloproteinases and lysyl oxidase, which control degradation and crosslinking of collagens, respectively, are altered in various lung diseases including COPD and pulmonary fibrosis 63-67. In diseased lungs, certain lineages of progenitors for lung endothelial and epithelial cells are depleted 68. Thus, manipulating these factors (angiogenic factors, ECMs, ECM stiffness) or implanting fibrin gels supplemented with progenitor cells 69 will likely lead to the formation of functional blood vessels inside the matrix and recovery of lung function in various pathological conditions. Since chemical factors can be supplemented inside the fibrin gels to modulate local angiogenesis, this system can also be utilized explore specific environmental cues that may normalize diseased lungs in chronic lung diseases.
In summary, this article introduces a method to implant fibrin hydrogel on the lung surface of living mouse, which enables researchers to characterize lung-specific angiogenesis in vivo. Modification of various factors (e.g., time course, concentrations and combinations of angiogenic factors, various kinds of hydrogels, physicochemical properties of hydrogels) in this system, will unveil the mechanisms of angiogenesis and regeneration in the lung. Thus, this system will significantly advance scientific knowledge of basic vascular biology, tissue engineering, as well as pulmonary medicine.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
This work was supported by funds from American Heart Association (A.M.), U.S. Department of Defense (BC074986), and Boston Children’s Hospital Faculty Career Development Fellowship (T.M., A.M.). The authors thank Amanda Jiang and Elisabeth Jiang for technical assistance.
References
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Campos JL, Calero C, Quintana-Gallego E. Symptom variability in COPD: a narrative review. Int J Chron Obstruct Pulmon Dis. 2013;8:231–238. doi: 10.2147/COPD.S42866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley B, Collard HR, King TE. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- Ferrer M, et al. Chronic obstructive pulmonary disease stage and health-related quality of life. The Quality of Life of Chronic Obstructive Pulmonary Disease Study Group. Ann Intern Med. 1997;127:1072–1079. doi: 10.7326/0003-4819-127-12-199712150-00003. [DOI] [PubMed] [Google Scholar]
- Reardon JZ, Lareau SC, ZuWallack R. Functional status and quality of life in chronic obstructive pulmonary disease. Am J Med. 2006;119:32–37. doi: 10.1016/j.amjmed.2006.08.005. [DOI] [PubMed] [Google Scholar]
- De Vries J, Kessels BL, Drent M. Quality of life of idiopathic pulmonary fibrosis patients. Eur Respir J. 2001;17:954–961. doi: 10.1183/09031936.01.17509540. [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest. 2000;117:5S–9S. doi: 10.1378/chest.117.2_suppl.5s. [DOI] [PubMed] [Google Scholar]
- Orens JB, Garrity ER. General overview of lung transplantation and review of organ allocation. Proc Am Thorac Soc. 2009;6:13–19. doi: 10.1513/pats.200807-072GO. [DOI] [PubMed] [Google Scholar]
- Benden C. Specific aspects of children and adolescents undergoing lung transplantation. Curr Opin Organ Transplant. 2012;17:509–514. doi: 10.1097/MOT.0b013e3283564fba. [DOI] [PubMed] [Google Scholar]
- Lyu DM, Zamora MR. Medical complications of lung transplantation. Proc Am Thorac Soc. 2009;6:101–107. doi: 10.1513/pats.200808-077GO. [DOI] [PubMed] [Google Scholar]
- Trulock EP, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult lung and heart-lung transplantation report-2007. J Heart Lung Transplant. 2007;26:782–795. doi: 10.1016/j.healun.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Weiss DJ. Current status of stem cells and regenerative medicine in lung biology and diseases. Stem Cells. 2013;32:16–25. doi: 10.1002/stem.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaedi M, et al. Human iPS cell-derived alveolar epithelium repopulates lung extracellular matrix. J Clin Invest. 2013;123:4950–4962. doi: 10.1172/JCI68793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott HC, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- Petersen TH, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuyl M, et al. Angiogenic factors stimulate tubular branching morphogenesis of sonic hedgehog-deficient lungs. Dev Biol. 2007;303:514–526. doi: 10.1016/j.ydbio.2006.11.029. [DOI] [PubMed] [Google Scholar]
- Galambos C, deMello DE. Molecular mechanisms of pulmonary vascular development. Pediatr Dev Pathol. 2007;10:1–17. doi: 10.2350/06-06-0122.1. [DOI] [PubMed] [Google Scholar]
- McGrath-Morrow SA, et al. Vascular endothelial growth factor receptor 2 blockade disrupts postnatal lung development. Am J Respir Cell Mol Biol. 2005;32:420–427. doi: 10.1165/rcmb.2004-0287OC. [DOI] [PubMed] [Google Scholar]
- White AC, Lavine KJ, Ornitz DM. FGF9 and SHH regulate mesenchymal Vegfa expression and development of the pulmonary capillary network. Development. 2007;134:3743–3752. doi: 10.1242/dev.004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Wang K, Ferrara N, Vu TH. Vascular endothelial growth factor co-ordinates proper development of lung epithelium and vasculature. Mech Dev. 2005;122:877–886. doi: 10.1016/j.mod.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol. 2005;67:623–661. doi: 10.1146/annurev.physiol.67.040403.102229. [DOI] [PubMed] [Google Scholar]
- Mammoto T, et al. LRP5 Regulates Development of Lung Microvessels and Alveoli through the Angiopoietin-Tie2 Pathway. PLoS ONE. 2012;7:e41596. doi: 10.1371/journal.pone.0041596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto T, Jiang E, Jiang A, Mammoto A. ECM structure and tissue stiffness control postnatal lung development through the LRP5-Tie2 signaling system. American Journal of Respiratory Cell and Molecular Biology. 2013;49:1009–1018. doi: 10.1165/rcmb.2013-0147OC. [DOI] [PubMed] [Google Scholar]
- Ding BS, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crivellato E. The role of angiogenic growth factors in organogenesis. Int J Dev Biol. 2011;55:365–375. doi: 10.1387/ijdb.103214ec. [DOI] [PubMed] [Google Scholar]
- Sakurai MK, et al. Vascular endothelial growth factor accelerates compensatory lung growth after unilateral pneumonectomy. Am J Physiol Lung Cell Mol Physiol. 2007;292:742–747. doi: 10.1152/ajplung.00064.2006. [DOI] [PubMed] [Google Scholar]
- Panigrahy D, et al. Epoxyeicosanoids promote organ and tissue regeneration. Proc Natl Acad Sci U S A. 2013;110:13528–13533. doi: 10.1073/pnas.1311565110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkel NF, Douglas IS, Nicolls M. Angiogenesis in chronic lung disease. Chest. 2007;131:874–879. doi: 10.1378/chest.06-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanumegowda C, Farkas L, Kolb M. Angiogenesis in pulmonary fibrosis: too much or not enough. Chest. 2012;142:200–207. doi: 10.1378/chest.11-1962. [DOI] [PubMed] [Google Scholar]
- Levental KR, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto A, Mammoto T, Ingber DE. Mechanosensitive mechanisms in transcriptional regulation. J Cell Sci. 2012;125:3061–3073. doi: 10.1242/jcs.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann D, et al. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008;117:2051–2060. doi: 10.1161/CIRCULATIONAHA.107.716886. [DOI] [PubMed] [Google Scholar]
- Merchante N, et al. Liver stiffness predicts clinical outcome in human immunodeficiency virus/hepatitis C virus-coinfected patients with compensated liver cirrhosis. Hepatology. 2012;56:228–238. doi: 10.1002/hep.25616. [DOI] [PubMed] [Google Scholar]
- Ding BS, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler IJ. Angiogenic heterogeneity: regulation of neoplastic angiogenesis by the organ microenvironment. J Natl Cancer Inst. 2001;93:1040–1041. doi: 10.1093/jnci/93.14.1040. [DOI] [PubMed] [Google Scholar]
- Folkman J. How is blood vessel growth regulated in normal and neoplastic tissue? G.H.A. Clowes memorial Award lecture. Cancer Res. 1986;46:467–473. [PubMed] [Google Scholar]
- Mammoto A, et al. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457:1103–1108. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinda KM. In vivo matrigel migration and angiogenesis assay. Methods Mol Biol. 2009;467:287–294. doi: 10.1007/978-1-59745-241-0_17. [DOI] [PubMed] [Google Scholar]
- Norrby K. In vivo models of angiogenesis. J Cell Mol Med. 2006;10:588–612. doi: 10.1111/j.1582-4934.2006.tb00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto T, Jiang A, Jiang E, Mammoto A. Platelet rich plasma extract promotes angiogenesis through the angiopoietin1-Tie2 pathway. Microvasc Res. 2013;89:15–24. doi: 10.1016/j.mvr.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- Bensaid W, et al. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials. 2003;24:2497–2502. doi: 10.1016/s0142-9612(02)00618-x. [DOI] [PubMed] [Google Scholar]
- Teichert-Kuliszewska K, et al. Biological action of angiopoietin-2 in a fibrin matrix model of angiogenesis is associated with activation of Tie2. Cardiovasc Res. 2001;49:659–670. doi: 10.1016/s0008-6363(00)00231-5. [DOI] [PubMed] [Google Scholar]
- Lafleur MA, Handsley MM, Knauper V, Murphy G, Edwards DR. Endothelial tubulogenesis within fibrin gels specifically requires the activity of membrane-type-matrix metalloproteinases (MT-MMPs) J Cell Sci. 2002;115:3427–3438. doi: 10.1242/jcs.115.17.3427. [DOI] [PubMed] [Google Scholar]
- Collen A, et al. Aberrant fibrin formation and cross-linking of fibrinogen Nieuwegein, a variant with a shortened Aalpha-chain, alters endothelial capillary tube formation. Blood. 2001;97:973–980. doi: 10.1182/blood.v97.4.973. [DOI] [PubMed] [Google Scholar]
- Mammoto T, et al. Mechanochemical Control of Mesenchymal Condensation and Embryonic Tooth Organ Formation. Dev Cell. 2011;21:758–769. doi: 10.1016/j.devcel.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KC, Leach JK. A reproducible, high throughput method for fabricating fibrin gels. BMC Res Notes. 2012;5:423. doi: 10.1186/1756-0500-5-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matar AF, Hill JG, Duncan W, Orfanakis N, Law I. Use of biological glue to control pulmonary air leaks. Thorax. 1990;45:670–674. doi: 10.1136/thx.45.9.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thetter O. Fibrin adhesive and its application in thoracic surgery. Thorac Cardiovasc Surg. 1981;29:290–292. doi: 10.1055/s-2007-1023498. [DOI] [PubMed] [Google Scholar]
- Rahbarghazi R, et al. Juxtacrine and paracrine interactions of rat marrow-derived mesenchymal stem cells, muscle-derived satellite cells, and neonatal cardiomyocytes with endothelial cells in angiogenesis dynamics. Stem Cells Dev. 2013;22:855–865. doi: 10.1089/scd.2012.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucera S, Biziato D, De Palma M. The interplay between macrophages and angiogenesis in development, tissue injury and regeneration. Int J Dev Biol. 2011;55:495–503. doi: 10.1387/ijdb.103227sn. [DOI] [PubMed] [Google Scholar]
- Joensuu K, et al. Interaction between marrow-derived human mesenchymal stem cells and peripheral blood mononuclear cells in endothelial cell differentiation. Scand J Surg. 2011;100:216–222. doi: 10.1177/145749691110000314. [DOI] [PubMed] [Google Scholar]
- Takakura N. Role of intimate interactions between endothelial cells and the surrounding accessory cells in the maturation of blood vessels. J Thromb Haemost. 2011;9 Suppl 1:144–150. doi: 10.1111/j.1538-7836.2011.04275.x. [DOI] [PubMed] [Google Scholar]
- Plantier L, Boczkowski J, Crestani B. Defect of alveolar regeneration in pulmonary emphysema: role of lung fibroblasts. Int J Chron Obstruct Pulmon Dis. 2007;2:463–469. [PMC free article] [PubMed] [Google Scholar]
- Belair DG, Murphy WL. Specific VEGF sequestering to biomaterials: influence of serum stability. Acta Biomater. 2013;9:8823–8831. doi: 10.1016/j.actbio.2013.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C, Inman E, Spaethe R, Helgerson S. Fibrin-based biomaterials to deliver human growth factors. Thromb Haemost. 2003;89:573–582. [PubMed] [Google Scholar]
- Stolzing A, Colley H, Scutt A. Effect of age and diabetes on the response of mesenchymal progenitor cells to fibrin matrices. Int J Biomater. 2011;2011:378034. doi: 10.1155/2011/378034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vailhe B, Ronot X, Tracqui P, Usson Y, Tranqui L. In vitro angiogenesis is modulated by the mechanical properties of fibrin gels and is related to alpha(v)beta3 integrin localization. In Vitro Cell Dev Biol Anim. 1997;33:763–773. doi: 10.1007/s11626-997-0155-6. [DOI] [PubMed] [Google Scholar]
- Kniazeva E, Kachgal S, Putnam AJ. Effects of extracellular matrix density and mesenchymal stem cells on neovascularization in vivo. Tissue Eng Part A. 2011;17:905–914. doi: 10.1089/ten.tea.2010.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angio CT, Maniscalco WM. The role of vascular growth factors in hyperoxia-induced injury to the developing lung. Front Biosci. 2002;7:1609–1623. doi: 10.2741/A865. [DOI] [PubMed] [Google Scholar]
- Kasahara Y, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311–1319. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen CA. Roles for proteinases in the pathogenesis of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3:253–268. doi: 10.2147/copd.s2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demedts IK, et al. Elevated MMP-12 protein levels in induced sputum from patients with COPD. Thorax. 2006;61:196–201. doi: 10.1136/thx.2005.042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq I, et al. Association of MMP-2 polymorphisms with severe and very severe COPD: a case control study of MMPs-1, 9 and 12 in a European population. BMC Med Genet. 2010;11:7. doi: 10.1186/1471-2350-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer PF, et al. MMP-9, TIMP-1 and inflammatory cells in sputum from COPD patients during exacerbation. Respir Res. 2005;6:151. doi: 10.1186/1465-9921-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute-Bello G, et al. Essential role of MMP-12 in Fas-induced lung fibrosis. Am J Respir Cell Mol Biol. 2007;37:210–221. doi: 10.1165/rcmb.2006-0471OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumar P, Gupta S, Sarkar S, Sen S. Upregulation of lysyl oxidase and MMPs during cardiac remodeling in human dilated cardiomyopathy. Mol Cell Biochem. 2008;307:159–167. doi: 10.1007/s11010-007-9595-2. [DOI] [PubMed] [Google Scholar]
- Gomperts BN, Strieter RM. Stem cells and chronic lung disease. Annu Rev Med. 2007;58:285–298. doi: 10.1146/annurev.med.58.081905.134954. [DOI] [PubMed] [Google Scholar]
- Lau AN, Goodwin M, Kim CF, Weiss DJ. Stem cells and regenerative medicine in lung biology and diseases. Mol Ther. 2012;20:1116–1130. doi: 10.1038/mt.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]


