Abstract
Obstruction of the kidney may affect native or transplanted kidneys and results in kidney injury and scarring. Presented here is a model of obstructive nephropathy induced by unilateral ureteric obstruction (UUO), which can either be irreversible (UUO) or reversible (R-UUO). In the irreversible UUO model, the ureter may be obstructed for variable periods of time in order to induce increasingly severe renal inflammation and interstitial fibrotic scarring. In the reversible R-UUO model the ureter is obstructed to induce hydronephrosis, tubular dilation and inflammation. After a suitable period of time the ureteric obstruction is then surgically reversed by anastomosis of the severed previously obstructed ureter to the bladder in order to allow complete decompression of the kidney and restoration of urinary flow to the bladder. The irreversible UUO model has been used to investigate various aspects of renal inflammation and scarring including the pathogenesis of disease and the testing of potential anti-inflammatory or anti-fibrotic therapies. The more challenging model of R-UUO has been used by some investigators and does offer significant research potential as it allows the study of inflammatory and immune processes and tissue remodeling in an injured and scarred kidney following the removal of the injurious stimulus. As a result, the R-UUO model offers investigators the opportunity to explore the resolution of kidney inflammation together with key aspects of tissue repair. These experimental models are of relevance to human disease as patients often present with obstruction of the renal tract that requires decompression and are commonly left with significant residual kidney impairment that has no current treatment options and may lead to eventual end stage kidney failure.
Keywords: Medicine, Issue 94, Mouse, Unilateral Ureteric Obstruction, Irreversible, Reversible, Kidney, Hydronephrosis, Inflammation, Fibrosis
Introduction
The overall aim of the experimental model described here is to induce obstructive nephropathy by unilateral ureteric obstruction (UUO), which can either be irreversible (UUO) or reversible (R-UUO). A simple irreversible model of UUO is presented in which the left ureter is permanently obstructed by ligation with a suture or by the application of a ligating clip. This results in marked dilatation of the ureter together with reduced renal blood flow and glomerular filtration. Renal histology demonstrates tubular dilatation and increasingly severe interstitial renal inflammation and fibrosis. Irreversible UUO is a useful model and has been adopted by many researchers in the study of both renal inflammation and fibrosis.1-4 Although the irreversible model of UUO requires some surgical expertise it is relatively straightforward and is often used to seek insights into the pathogenesis of interstitial renal injury and the ensuing fibrosis. Also presented is a less frequently used R-UUO model using a modification of the method originally described by Tapmeier et al.5 The R-UUO model has much future potential for the study of inflammatory and immune processes, cellular and tissue regeneration as well as the subsequent tissue remodeling following the removal of an injurious stimulus.
The more challenging R-UUO model has been used by a limited number of investigators with some groups employing a significantly different surgical technique to that described here6,7 though with interesting results. In the R-UUO model presented, the ureter is ligated to induce complete ureteric obstruction for a period of time sufficient to induce the level of injury and fibrosis desired: 7 days of UUO was chosen in the method described here. The ureteric obstruction is reversed and the kidney is allowed to decompress for a period of time determined by the investigator before the mice are culled and the kidney removed for analysis: 7 days of decompression was chosen in the method described here though a longer period would be chosen if the resolution of inflammation and fibrosis was being studied. Although the method described here requires significant surgical expertise, it offers several advantages over other R-UUO models. The application of soft walled plastic tubing to the obstructed ureter prevents excessive ureteric dilation and this facilitates the subsequent manipulation and anastomosis of the ureter. Furthermore, in the described R-UUO model the ureter is divided thereby allowing the removal of any residual urinary sediment and debris. This confirms that the remaining ureter lumen is de-obstructed and patent prior to anastomosis to the bladder.
Experiments incorporating both the irreversible and reversible UUO models can provide researchers with a powerful insight into the molecular and cellular mechanisms of both injury and subsequent resolution and regeneration. Thus, the model of R-UUO described here would be highly relevant to researchers interested in post-inflammatory tissue remodeling and how this can be modulated.
Protocol
General Guidance: Animal experiments were performed in accordance with the guidelines and regulations imposed by the Animals (Scientific Procedures) Act 1986. This protocol and the accompanying video protocol are for both a standard UUO and a R-UUO, which can be performed on many mouse strains. In the accompanying video, both procedures are performed on male C57BL/6 mice aged 8 weeks. The data presented in the representative results section were obtained from male FVB/n mice.
NOTE: This protocol and accompanying video details how to perform a standard UUO and R-UUO utilizing the left ureter, however the same techniques can easily be applied to the right ureter.
1. Animal Preparation and Laparotomy
Perform all procedures with sterile (autoclaved) instruments and consumables.
Inject ketamine hydrochloride (70mg/kg) and medetomidine hydrochloride (1mg/kg) intraperitoneally to anesthetize the mouse. Note: The duration of the resulting anesthetic plane is 4 hr and no supplemental anesthesia is required.
Confirm the depth of anesthesia by loss of reflexes e.g., toe pinch.
Remove all hair surrounding the incision area and prepare the abdominal skin for surgery via application of a dilute chlorhexidine solution.
Place the mouse on a heated surgical pad in a supine position and fix the limbs to the pad using low-tack adhesive tape.
During the procedure, monitor the mouse for signs of thermal burns as a result of the heated surgical pad. If possible use a non-electric heat source.
Administer analgesic by a subcutaneous injection of buprenorphine hydrochloride (0.06mg/kg) and apply eye lubricant to prevent corneal drying.
Make a midline laparotomy and an incision of the avascular linea alba using tissue separating scissors to gain access to the peritoneal cavity.
Drape the mouse and insert a colibri retractor into the incision.
2. Unilateral Ureteric Obstruction
Using sterilized cotton buds expose the left ureter by displacing the intestines towards the right side of the abdominal cavity and cover them with moistened drapes.
Using angled forceps isolate and lift the left ureter.
To create a ureteric obstruction, ligate the left ureter twice with 6/O black braided silk suture anywhere between the bladder and renal pelvis. For long term experiments, use absorbable suture for all abdominal surgeries. Alternatively, a ligating clip can be applied to the ureter.
To isolate the bladder from the ureter, divide the ureter between the two sutures.
Carefully replace the intestines into the peritoneal cavity.
Follow the steps listed in Section 4 – Post-Operative Recovery and Care to close the incision and reverse the anesthesia.
3. Preparation for Reversible Unilateral Ureteric Obstruction
Prepare a mouse for surgery and isolate the left ureter as detailed in Steps 1.1 to 2.2 above.
To create a ureteric obstruction that can be reversed, ligate the left ureter twice with 6/O black braided silk suture close to the bladder. Leave one end of the upper suture long as this will be used to anchor the soft walled plastic tubing in place around the ureter.
Make a longitudinal slit in a 5mm length of soft walled plastic tubing such that it can be splayed open to allow it to be applied to the ureter. Any soft walled silicone plastic tubing, with an internal diameter of 1mm and external diameter of 2mm, can be used.
Place the soft walled plastic tubing gently around the ureter. Ensure that the long suture emerges from the center of the slit in the tubing once it is closed around the ureter.
Place a length of 6/O black braided silk suture around the soft walled tubing enclosing the ureter and tie once. Now place the long end of the suture, emerging from the center of the slit in the tubing, longitudinally across the tubing such that it lies on top of the previously tied suture.
Tie the suture located around the tubing twice to anchor the tubing and the long suture end in place around the ureter. Note: To prevent adhesion formation on the ureter and the soft walled plastic tubing, an adhesion reduction solution can be applied to the area around the tubing.
Carefully replace the intestines into the peritoneal cavity.
4. Post-Operative Recovery and Care
Close the peritoneum with blanket stitch using 5/O black braided silk suture and approximate the skin using metallic skin clips.
To minimize the risk of post-operative infection, apply an antiseptic such as iodine/alcohol solution to the abdominal skin.
Partially reverse anesthesia with atipamezole hydrochloride (2mg/kg) subcutaneously.
Administer fluids by a subcutaneous injection of 1 ml warmed saline.
Monitor the mouse until it has recovered consciousness.
Allow the mouse to recover in a heated box kept at 29 °C for 24 hr. Moistened food can also be provided to encourage fluid and nutrition intake.
Leave the mouse to recover to induce a desired level of obstruction, typically 7 days.
For long-term recovery experiments, provide ongoing analgesics. If the mouse is to be recovered for longer than 7 days, remove the skin clips 7 days following surgery.
Once the desired level of obstruction has been induced, either reverse the UUO, as described below, or euthanize the mouse by cervical dislocation and collect the kidneys for histopathology analysis.
5. Reversible Unilateral Ureteric Obstruction
To perform a R-UUO prepare a mouse, which has undergone preparation for R-UUO, for surgery as described in steps 1.1 to 1.7.
If present, remove the skin clips and divide or remove the sutures in the peritoneum to gain access to the abdominal cavity. Note: If the ureter has been obstructed for a long-term experiment skin clips should have been removed 7 days following application.
Prepare the mouse and isolate the left ureter as described in steps 1.8 to 2.2.
Using angled forceps, free the soft walled plastic tubing from any granulomatous tissue which may have formed.
Cut the suture holding the plastic tubing around the obstructed ureter with a scalpel and remove the tubing.
Confirm successful UUO by assessing for the presence of hydronephrosis in the left kidney, the kidney should also appear pale.
Divide the ureter between the sutures.
Place the remaining length of ureter, attached to the kidney, on a small piece of sterile gauze. This will be used to collect the urinary sediment and dead cells that will drain from the ureter and renal pelvis once the suture is removed.
Divide the ureter above, but near, the suture and allow the kidney to drain onto the gauze. Leave the lower suture, closest to the bladder, intact. This is to ensure that no urine will leak from the bladder and into the peritoneal cavity.
Once the ureter and renal pelvis have been drained, apply a long 6/O black braided silk suture to the end of the remaining length of ureter. This will be used to aid the ureter to bladder anastomosis performed later.
- Follow the steps below to anastomose the remaining length of ureter into the bladder:
- Turn the ureter such that it lies anterior to its original position and lies over the kidney.
- Place a single 9/O polyamide monofilament tacking suture 2 mm from the end of the ureter such that it emerges in the direction of the bladder. Take care to ensure that the suture remains in the muscular coat and does not enter the ureter lumen. The tacking suture will be used to anchor the ureter in the bladder.
- To create a channel through the bladder, pass a 21G needle diagonally through the bladder such that it exits towards the frontal (ventral) wall of the bladder.
- Rest an eyed needle in the bevel of the 21G needle. Use the 21G needle to guide the eyed needle through the bladder. The eyed needle will be used to take the ureter through the bladder.
- Pass the 9/O tacking suture, applied to the ureter in step 5.11.2, through the first incision in the bladder and out through the bladder wall adjacent to the entry point. Once the ureter is passed through the bladder this will be tied to anchor the ureter to the bladder.
- Place the long 6/O black braided silk suture applied to the end of the ureter in step 5.10 through the eyed needle.
- Carefully withdraw the eyed needle out of the bladder whilst ensuring that the ureter is also pulled through the bladder.
- Once the ureter emerges from the bladder remove the eyed needle and apply a clamp to the long suture at the end of the ureter to prevent retraction back into the bladder.
- To anchor the ureter within place in the bladder, tie the 9/O polyamide monofilament tack suture, applied in step 5.11.5.
- Apply additional single 9/O polyamide monofilament tie sutures as described in steps 5.11.2 and 5.11.5 at two locations around the entry point to firmly anchor the ureter within the bladder.
- Using the back of opened scissors, push the bladder wall back slightly to expose more of the emergent ureter.
- Divide the ureter above the long suture located at the end of the ureter. The ureter should retract into the body of the bladder. It is usual to observe urine flowing out of the opening in the bladder, confirming the open lumen of the ureter.
- Close the exit wound in the bladder with a 9/O polyamide monofilament suture.
Carefully replace the intestines into the peritoneal cavity.
Provide post-operative care as detailed in steps 4.1 to 4.8.
Allow the mouse to recover until the kidney is decompressed, typically 7 days.
At the experimental end point, euthanize the mouse by cervical dislocation and collect the kidneys for histopathological analysis.
Representative Results
The appearance of the kidney changes markedly following ureteric obstruction and it becomes paler and tense to palpation with time (Figure 1). There is increasing dilatation of the ureter proximal to the obstruction and the renal pelvis. The kidney becomes increasingly atrophic as the duration of obstructive nephropathy increases with prolonged obstruction resulting in a marked thinning of the renal cortex and medulla. Following the reversal of the ureteric obstruction, the colour of the kidney becomes darker as blood flow increases and tissue oedema resolves. The dilatation of the ureter and renal pelvis of the kidney subsides although they may remain slightly ‘baggy’ as a consequence of the period of prolonged distension (Figure 1). The de-obstructed kidney is typically smaller than the contralateral kidney as an element of tissue atrophy is inevitable although the degree of tissue loss will depend upon the duration of obstruction.
Histology of the normal non-manipulated native kidney reveals glomeruli surrounded by the compact tubules of the tubulointerstitium with many tubules having a discernible lumen evident (Figure 2). Following ureteric obstruction the distension and dilatation of the ureter and renal pelvis (Figure 1) is accompanied by increasing dilatation of the renal tubules that is easily evident on histology of tissue sections (dilated tubules arrowed in Figure 2). The glomeruli exhibit a normal appearance in this model with injury and scarring confined to the tubulointerstitium. Gradual and significant resolution of the tubular distension and dilatation follows the relief of ureteric obstruction though some tubules may remain dilated (Figure 2).
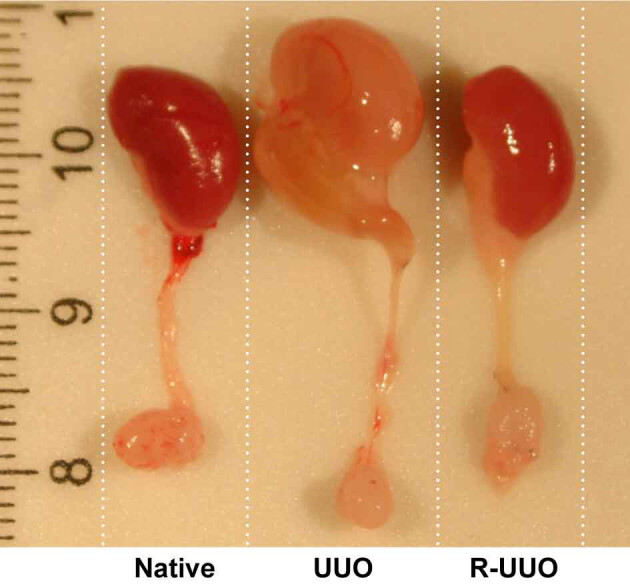 Figure 1. Appearance of native, UUO and R-UUO kidneys, renal pelvis and ureter. Male FVB mice underwent unilateral ureteric obstruction (UUO) for 7 days. Mice were either sacrificed or the UUO was then reversed (R-UUO) and the kidneys decompressed for 7 days before sacrifice. Images illustrate the appearance of the kidney, renal pelvis, ureter and bladder of a native, UUO and R-UUO kidney harvested at the time of sacrifice. In comparison to the native kidney the UUO kidney appears hydronephrotic and pale in colour. Following de-obstruction the R-UUO kidney has decompressed though the renal pelvis and ureter remain slightly ‘baggy’. Notice that the R-UUO ureter is shorter than the UUO and native kidney due to removal of the obstructed portion prior to re-anastomosis into the bladder. Please click here to view a larger version of this figure.
Figure 1. Appearance of native, UUO and R-UUO kidneys, renal pelvis and ureter. Male FVB mice underwent unilateral ureteric obstruction (UUO) for 7 days. Mice were either sacrificed or the UUO was then reversed (R-UUO) and the kidneys decompressed for 7 days before sacrifice. Images illustrate the appearance of the kidney, renal pelvis, ureter and bladder of a native, UUO and R-UUO kidney harvested at the time of sacrifice. In comparison to the native kidney the UUO kidney appears hydronephrotic and pale in colour. Following de-obstruction the R-UUO kidney has decompressed though the renal pelvis and ureter remain slightly ‘baggy’. Notice that the R-UUO ureter is shorter than the UUO and native kidney due to removal of the obstructed portion prior to re-anastomosis into the bladder. Please click here to view a larger version of this figure.
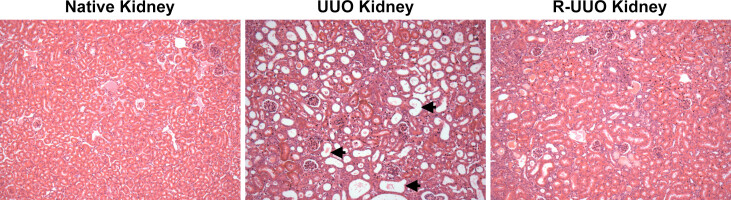 Figure 2. Representative histology of native, UUO and R-UUO kidneys. Male FVB mice underwent unilateral ureteric obstruction (UUO) for 7 days. Mice were either sacrificed or the UUO was then reversed (R-UUO) and the kidneys allowed to decompress for 7 days before sacrifice. Representative photomicrographs (Magnification: x200) of H&E stained kidney sections from control non-manipulated native kidney, UUO kidney and R-UUO kidney. The healthy interstitium of the normal kidney is comprised of normal compact tubules. A number of tubules indicated by the arrows exhibit significant dilatation at day 7 following UUO. In contrast, tubular dilatation is markedly reduced at 7 days following reversal of UUO. Please click here to view a larger version of this figure.
Figure 2. Representative histology of native, UUO and R-UUO kidneys. Male FVB mice underwent unilateral ureteric obstruction (UUO) for 7 days. Mice were either sacrificed or the UUO was then reversed (R-UUO) and the kidneys allowed to decompress for 7 days before sacrifice. Representative photomicrographs (Magnification: x200) of H&E stained kidney sections from control non-manipulated native kidney, UUO kidney and R-UUO kidney. The healthy interstitium of the normal kidney is comprised of normal compact tubules. A number of tubules indicated by the arrows exhibit significant dilatation at day 7 following UUO. In contrast, tubular dilatation is markedly reduced at 7 days following reversal of UUO. Please click here to view a larger version of this figure.
Discussion
Obstruction of the kidney may affect native or transplanted kidneys and results in kidney injury and scarring. The R-UUO model is of relevance to human disease as patients often present with obstruction of the renal tract secondary to prostatic hypertrophy, posterior urethral valves etc. that requires decompression with patients commonly left with significant residual kidney impairment that has no current treatment options and may eventually lead to end stage kidney disease.8,9 The models detailed here enable the study of both irreversible UUO and R-UUO. Irreversible UUO allows the study of inflammation and tissue injury including the role of various leukocytes and mediators1-3,10 whilst study of the R-UUO model enables researchers to explore the resolution of renal injury and associated tissue remodeling.6,7,11-15
Other investigators have used different methods to induce and reverse ureteric obstruction. In the first description of the R-UUO model, the left ureter of male C57BL/6 mice was obstructed with an atraumatic microvascular clamp via a flank incision.6 The clamp was removed after 10 days and the decompressed kidney studied at various time points up to 6 weeks later. In this study, functional physiological studies of the reversed and control contralateral kidney were undertaken to assess the renal blood flow and glomerular filtration rate of each kidney. The use of an atraumatic microvascular clamp to obstruct the ureter with subsequent removal of the clamp has been used by other investigators on 6-8 week old C57BL/6 mice,12,13 10-12 week old male FVB/N mice11 and 5 week old Balb/c mice.16 These studies did not indicate the success rate of the reversal procedure or whether there was any associated mortality. A different technique to induce ureteric obstruction was used by Chaabane et al.17 In this study, the investigators looped a 4-0 wire around the ureter of adult female C57BL/6 mice via a midline laparotomy and then drew both the wire and ureter into a section of silastic catheter in order to fold the ureter. The folded loop of ureter was then held in place within the silastic catheter tubing by a surgical suture. Reversal of obstruction after 7 days was performed by removal of the surgical knots, silastic tubing and wire and the decompressed kidney was removed for analysis 30 days later. Puri et al.,7 found that the ureter does not tolerate a period of prolonged clamping as the subsequent ureteric injury and scarring reduces the incidence of a successful reversal procedure. These investigators therefore adopted a strategy of limiting the period of clamping the ureter in one particular place to a maximum of 2 days.7,14 This entailed a surgical procedure every 2 days to place a new clamp on the right ureter distal to the previous clamp that was then removed. This ensured that the obstruction was maintained and that the ureter was protected from a period of prolonged clamping. Experiments were performed in both 6-8 week old C57BL/6 mice and Balb/c mice though the gender is unclear. In order to assess the function of the de-obstructed right kidney, this group performed either a left nephrectomy or a left ureteric obstruction: this was undertaken at least a week after the reversal procedure in order to minimize mortality. This experimental approach was adopted by another research group who explored the use of MRI imaging to assess renal oxygenation.15 It should also be noted that complete, reversible as well as variable ureteric obstruction has also been described in neonatal rats and mice18,19 as a model of congenital ureteropelvic junction obstruction: an important congenital abnormality that leads to renal atrophy and loss of function.
We used the method of Cochrane et al.,6 in our early experiments but also noted the frequent development of a fibrotic ureteric stricture at the site of the clamp after 7 days of UUO. We therefore explored the method used by Tapmeier et al.5 Tapmeier et al., adopted a strategy of directly anastomosing the severed end of the obstructed right ureter to the bladder in 8 week old female C57BL/6 mice.5 The right ureter was ligated as close as possible to the bladder with reversal of the obstruction being achieved by a direct surgical anastomosis of the severed ureter to the bladder. This approach resulted in >96% success in achieving reversal of obstruction. A left nephrectomy was performed 3 weeks after right ureteric re-implantation in order to make it a functional model and this procedure was well tolerated.
When performing the R-UUO model there are two aspects that we believe are important for successful kidney decompression following the reversal of UUO. It is important to ensure that the ureter is fully de-obstructed before the anastomosis to the bladder is undertaken. Urinary sediment and debris will inevitably collect above the obstruction and this needs to be drained until the production of clean urine is observed. Failure to do so may result in the ureter not being fully patent which may impact upon the success of kidney decompression. Also, the application of soft walled plastic tubing to the obstructed ureter prevents excessive ureteric dilation and this facilitates the subsequent manipulation and anastomosis of the remaining length of de-obstructed ureter into the bladder. It is important that the de-obstructed ureter must be firmly anchored into the bladder in order to prevent any leakage of urine into the peritoneum. Inadequate anchorage or attachment of the ureter may lead to anastomotic failure or breakdown with the subsequent development of ascites or peritonitis if urine infection is also present.
Our laboratory studies are focused upon the intrinsic cell biology of the kidney following reversal of UUO and we have thus not undertaken a right nephrectomy that is required to generate a model that provides information on the function of the de-obstructed kidney. Removal of the contralateral kidney has been undertaken by other investigators in order to generate a functional model5,7,14 and this could be undertaken by a laparotomy or flank approach. An advantage of the R-UUO model is that it may be used in various strains of mice. Indeed it has been used to highlight key differences between the reparative responses of C57BL/6 mice compared to Balb/c mice.7
The UUO and R-UUO model described here offers investigators the opportunity to explore the induction and resolution of kidney inflammation together with key aspects of tissue repair. By following the methodology presented here to induce UUO followed by reversal of ureteric obstruction to allow kidney decompression, researchers will be able to study the various cellular and molecular processes involved in tissue remodeling post injury.
Disclosures
The authors have no competing or conflicting interests to disclose.
Acknowledgments
The present study was supported by grants from Kidney Research UK (ST4/2011), the Cunningham Trust (CT11/14), the Mrs AE Hogg Charitable Trust for Kidney Research and the Renal Endowment Fund of the Royal Infirmary of Edinburgh.
References
- Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009;75:1145–1152. doi: 10.1038/ki.2009.86. [DOI] [PubMed] [Google Scholar]
- Kipari T, et al. Nitric oxide is an important mediator of renal tubular epithelial cell death in vitro and in murine experimental hydronephrosis. Am J Pathol. 2006;169:388–399. doi: 10.2353/ajpath.2006.050964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson NC, et al. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172:288–298. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto K, et al. Effects of liposome clodronate on renal leukocyte populations and renal fibrosis in murine obstructive nephropathy. J Pharmacol Sci. 2009;111:285–292. doi: 10.1254/jphs.09227fp. [DOI] [PubMed] [Google Scholar]
- Tapmeier TT, et al. Reimplantation of the ureter after unilateral ureteral obstruction provides a model that allows functional evaluation. Kidney Int. 2008;73:885–889. doi: 10.1038/sj.ki.5002797. [DOI] [PubMed] [Google Scholar]
- Cochrane AL, et al. Renal structural and functional repair in a mouse model of reversal of ureteral obstruction. J Am Soc Nephrol. 2005;16:3623–3630. doi: 10.1681/ASN.2004090771. [DOI] [PubMed] [Google Scholar]
- Puri TS, et al. Chronic kidney disease induced in mice by reversible unilateral ureteral obstruction is dependent on genetic background. Am J Physiol Renal Physiol. 2010;298:F1024–F1032. doi: 10.1152/ajprenal.00384.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila J, Holmberg C, Kyllonen L, Rintala R, Taskinen S. Long-term risk of end stage renal disease in patients with posterior urethral valves. J Urol. 2011;186:2392–2396. doi: 10.1016/j.juro.2011.07.109. [DOI] [PubMed] [Google Scholar]
- Ravanan R, Tomson CR. Natural history of postobstructive nephropathy: a single-center retrospective study. Nephron Clin Pract. 2007;105:c165–c170. doi: 10.1159/000099007. [DOI] [PubMed] [Google Scholar]
- Bascands JL, Schanstra JP. Obstructive nephropathy: insights from genetically engineered animals. Kidney Int. 2005;68:925–937. doi: 10.1111/j.1523-1755.2005.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HC, et al. Postobstructive regeneration of kidney is derailed when surge in renal stem cells during course of unilateral ureteral obstruction is halted. Am J Physiol Renal Physiol. 2010;298:F357–F364. doi: 10.1152/ajprenal.00542.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson SR, Niederhoff RA, Hruska KA, Austin PF. Endogenous BMP-7 is a critical molecular determinant of the reversibility of obstruction-induced renal injuries. Am J Physiol Renal Physiol. 2011;301:F1293–F1302. doi: 10.1152/ajprenal.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson SR, Niederhoff RA, Hruska KA, Austin PF. The BMP-7-Smad1/5/8 pathway promotes kidney repair after obstruction induced renal injury. J Urol. 2011;185:2523–2530. doi: 10.1016/j.juro.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves LD, et al. Contrasting effects of systemic monocyte/macrophage and CD4+ T cell depletion in a reversible ureteral obstruction mouse model of chronic kidney disease. Clin Dev Immunol. 2013;2013:836–989. doi: 10.1155/2013/836989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque ME, et al. Longitudinal changes in MRI markers in a reversible unilateral ureteral obstruction mouse model: preliminary experience. J Magn Reson Imaging. 2014;39:835–841. doi: 10.1002/jmri.24235. [DOI] [PubMed] [Google Scholar]
- Bai ZM, et al. Arterially transplanted mesenchymal stem cells in a mouse reversible unilateral ureteral obstruction model: in vivo bioluminescence imaging and effects on renal fibrosis. Chinese Med J. 2013;126:1890–1894. [PubMed] [Google Scholar]
- Chaabane W, et al. Renal functional decline and glomerulotubular injury are arrested but not restored by release of unilateral ureteral obstruction (UUO) Am J Physiol Renal Physiol. 2013;304:F432–F439. doi: 10.1152/ajprenal.00425.2012. [DOI] [PubMed] [Google Scholar]
- Thornhill BA, Burt LE, Chen C, Forbes MS, Chevalier RL. Variable chronic partial ureteral obstruction in the neonatal rat: a new model of ureteropelvic junction obstruction. Kidney Int. 2005;67:42–52. doi: 10.1111/j.1523-1755.2005.00052.x. [DOI] [PubMed] [Google Scholar]
- Thornhill BA, Chevalier RL. Variable partial unilateral ureteral obstruction and its release in the neonatal and adult mouse. Methods Mol Biol. 2012;886:381–392. doi: 10.1007/978-1-61779-851-1_33. [DOI] [PubMed] [Google Scholar]


