Abstract
Analysis of one of the vital functions of retinal pigment epithelial (RPE) cells, the phagocytosis of spent aged distal fragments of photoreceptor outer segments (POS) can be performed in vitro. Photoreceptor outer segments with stacks of membranous discs containing the phototransduction machinery are continuously renewed in the retina. Spent POS are eliminated daily by RPE cells. Rodent, porcine/bovine and human RPE cells recognize POS from various species in a similar manner. To facilitate performing large series of experiments with little variability, a large stock of POS can be isolated from porcine eyes and stored frozen in aliquots. This protocol takes advantage of the characteristic of photopigments that display an orange color when kept in the dark. Under dim red light, retinae are collected in a buffer from opened eyecups cut in halves. The retinal cell suspension is homogenized, filtered and loaded onto a continuous sucrose gradient. After centrifugation, POS are located in a discrete band in the upper part of the gradient that has a characteristic orange color. POS are then collected, spun, resuspended sequentially in wash buffers, counted and aliquoted. POS obtained this way can be used for phagocytosis assays and analysis of protein activation, localization or interaction at various times after POS challenge. Alternatively, POS can be labeled with fluorophores, e.g., FITC, before aliquoting for subsequent fluorescence quantification of POS binding or engulfment. Other possible applications include the use of modified POS or POS challenge combined with stress conditions to study the effect of oxidative stress or aging on RPE cells.
Keywords: Immunology, Issue 94, Retina, photoreceptor, outer segment, sucrose gradient, purification, ultracentrifugation, phagocytosis assay, retinal pigment epithelium
Introduction
In the retina, vision is triggered by isomerization of photosensitive molecules called opsins, before being transformed into a signal that can be transmitted between neurons up to the visual areas in the brain. These molecules are embedded in stacks of membranous disks resembling pancakes that constitute the outer segment portions of photoreceptor cells (PRs). Being subjected to constant exposure to light and therefore considerable levels of oxidative stress, PRs continuously renew their outer segments to limit potential oxidative damage. Photoreceptor outer segments are in close contact with apical microvilli of the neighboring retinal pigment epithelial (RPE) cells. RPE cells constitute the outer part of the blood-retinal barrier and ensure numerous tasks that are crucial for photoreceptors health and function1, such as quenching light rays via melanin pigments, re-isomerization of the photoreactive opsin component retinal, providing nutrients and growth factors, participating in PR metabolite disposal.
In addition, RPE cells eliminate spent POS and recycle their components, a daily occupation that is regulated by the circadian rhythm in mammalian retina2,3. The clearance of shed POS is absolutely necessary for PR survival. When it is completely abrogated, POS debris accumulate and PRs degenerate causing rapid vision loss4,5. If the rhythmic profile is lost and replaced by a constant activity, PR and RPE defects accumulate with age6. Therefore, it is very important to characterize the molecular regulation of RPE phagocytosis in vitro in order to understand phenotypes linked to its dysfunction. Interestingly, the molecular machinery in RPE cells is very similar to the one used by macrophages to clear apoptotic cells and both are dependent on recognition of exposed phosphatidylserine on phagocytic debris7-9. Still, RPE cells and macrophages regulate phagocytosis differently, as macrophages opt for immediate elimination of apoptotic cells at encounter time while RPE cells rhythmically engulf POS only once a day despite their permanent contact with outer segments. This suggests specific regulation mechanisms that are not yet fully understood.
Many of the molecules implicated in the RPE phagocytic machinery have been identified or validated thanks to the use of isolated POS and cell culture phagocytosis assays. The alphavbeta5 integrin receptor located at the RPE apical cell surface, in coordination with its ligand MFG-E8, binds specifically to POS10-12, which are then internalized via the MerTK tyrosine kinase receptor13-15. The CD36 scavenger receptor has been shown to participate in POS intake and influence its speed16,17, and might serve as a sensor of oxidized phospholipids at the POS surface18. Internalization needs the recruitment of F-actin cytoskeleton-associated proteins such as annexin 219, myosin II20 and myosin VIIA21,22. Native or oxidized POS in vitro are also utilized to understand aging phenotypes of RPE cells in vivo linked to accumulation of poorly digested oxidized POS23-28. The generation of RPE cells derived from stem cells has initiated a new application for isolated POS that are used to prove functionality of cells before they are transplanted to animals or patients29,30,27.
First described by Molday and colleagues in 198731, the protocol for isolation of bovine POS combines an ultracentrifugation step of retinal homogenates on continuous sucrose gradients with observation of the characteristic orange appearance of unbleached retinal photopigment (carrying 11-cis retinal). In the past 10 years, due to precautions taken in order to minimize risk of mad cow disease, use of porcine eyes has become increasingly prominent. The protocol described here shows how to obtain large amounts of POS from porcine or bovine eyes that can be aliquoted and stored for extended periods of time. This eliminates the need to prepare POS from rodent eyes, which requires using a large number of animals per POS preparation and assay32,33,21,22. In addition, details about POS labeling before storage using fluorescent molecules are given, to quantify and visualize POS in a simplified and comparable manner for some applications compared to labeling POS after the phagocytosis assay32,10. Therefore, these large stocks allow for reproducibility and ease of use in many different types of experiments.
Protocol
This POS isolation experiment is time consuming and may require up to 12 hr to complete if POS are labeled before storage. The protocol has been adapted from a paper published by R.S. Molday and colleagues in 198731 and modified by S.C. Finnemann and colleagues in 199710.
Animals were handled according to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. Protocols were reviewed and approved by the Charles Darwin Ethics Committee from University Pierre and Marie Curie-Paris 06 and the Fordham University Institutional Animal Care and Use Committee.
1. General Set Up
Obtain 80 fresh pig or cow eyes as fresh as soon after slaughtering as possible and keep them chilled in the dark. Provide instructions to the provider so the company proceeds the same way. This protocol is optimized for 80 eyes. To proceed with more eyes and get bigger stocks (e.g.,, from 160 eyes), double the solution quantities and perform 2 rounds of ultracentrifugation; pool samples later at the collection step (see step 2.2.2).
Set up on the bench the red safelight lamp, absorbent pads, wipes (Materials table). Prepare 15 cm dishes and biohazard bags for waste collection (unused eye parts, vitreous…). Wear tight-fitting labcoat, gloves, sleeve protectors and goggles.
- Precautions:
- Pre-chill all solutions then keep them cold during all steps. Keep eyes and gradients chilled on ice as much as possible.
- Keep eyes and derived tissues in the dark as much as possible until the collection step after the ultracentrifugation to avoid photobleaching of visual pigments and loss of the orange-pink color of the active form of the photopigments.
2. Protocol Actions
- Prepare solutions and gradients:
- Thaw taurine stock completely using a 37 °C water bath.
- Prepare all solutions from stock solutions (Table 1, Stock solutions) and mix thoroughly the sucrose with other ingredients using a magnetic stirrer for each solution.
- Prepare 15 ml of the homogenization solution to a final concentration of 20% sucrose, 20 mM tris acetate pH7.2, 2 mM MgCl2, 10 mM glucose and 5 mM taurine.
- Prepare the 25% sucrose solution to a final concentration of 25% sucrose (using 70% sucrose stock), 20 mM tris acetate pH 7.2, 10 mM glucose and 5 mM taurine.
- Prepare the 60% sucrose solution to a final concentration of 60% sucrose (using sucrose powder), 20 mM tris acetate pH7.2, 10 mM glucose and 5 mM taurine.
- Prepare the Wash 1 solution to a final concentration of 20 mM tris acetate pH 7.2 and 5 mM taurine.
- Prepare the Wash 2 solution to a final concentration of 10% sucrose, 20 mM tris acetate pH 7.2 and 5 mM taurine.
- Prepare the Wash 3 solution to a final concentration of 10% sucrose, 20 mM sodium phosphate pH 7.2 and 5 mM taurine.
- Steady the gradient maker on a magnetic stirrer and insert a stir bar in the chamber closest to the exit where the 60% solution will be inserted. Use a cut-off P200 pipet tip placed at the exit of the tubing to achieve proper casting speed.
- Cast linear gradients delicately by diluting and stirring the 60% sucrose solution with the 25% one using 12 ml from each solution (i.e., total 6 tubes for 80 eyes) in pre-chilled ultracentrifuge tubes. Stir the gradually-diluting 60% sucrose solution well. Steady the speed of gradient flow, pouring the solution smoothly and not too fast. NOTE: In order to get a continuous, linear gradient, keep the pipet tip opening right at the surface of the solution for the entire gradient formation.
- Let the gradients sit on ice for around 1 hr for stabilization. Keep the gradients chilled until loaded. Do not shake the tubes to prevent disruption of the gradient.
- Tissue collection:
- Collect tissues under dim red light. Take an eye into one hand and poke the front with edge of razor blade while holding the eye away (to avoid splashing; Figure 1). Use the razor blade to cut the anterior eyeball into 2 halves (cornea plus at least 5 mm into the sclera). Remove the lens and flip the eyecup inside out so that it can be held over the tip of one finger thus exposing the retina.
- Using the razor blade at an angle, gently scrape the retina, detaching easily and appearing as a pinkish layer, off the tapetum surface and cut at the optic nerve head (Figure 1). Collect all retinas in 2 x 50 ml tubes each containing 15 ml of homogenization solution on ice.
- Retina homogenization:
- Shake the suspension vigorously by hand for 2 min in order to disrupt the different cell layers, break POS off the rest of the PR cell, fragment POS and homogenize the retina suspension.
- Filter 3x through a double-layer of clean gauze to remove large tissue fragments and collect the flow-through in clean 50 ml tubes (Figure 1). The retinal suspension being quite thick, to maximize the yield, gently press on the gauze to release remaining fragmented tissues after each filtration.
- Photoreceptor outer segment fragment (POS) isolation:
- Gently lay the crude retina prep, around 6 - 7 ml per tube, over 6 x 30 ml ultracentrifuge tubes each containing 24 ml of fresh chilled continuous 25 - 60% sucrose gradient (Figure 1). Balance the opposing tubes as appropriate for the rotor to be used. Ultracentrifuge at 106,000 x g for 50 min at 4 °C.
- Remove most of the solution above the orange-pink band in the upper third of gradient by aspiration (Figure 2A). Collect the orange-pink band with a cut-off P1000 tip into a beaker. Discard the rest after neutralizing using bleach (biological waste).
- Dilute with ~4 - 5 volumes of ice-cold Wash 1 solution. Separate into as many 30 ml tubes as needed. Centrifuge at 3,000 x g for 10 min at 4 °C. Carefully discard the supernatant.
- Resuspend pellets in 10 ml Wash 2 solution and spin at 3,000 x g for 10 min as detailed above. Resuspend pellets in 15 ml Wash 3 and spin again at 3,000 x g for 10 min. Combine suspensions from several pellets to reduce the number of tubes needed before centrifugation in Wash 2 and Wash 3 solutions.
- POS aliquoting without labeling:
- Resuspend POS in 10 - 20 ml DMEM. Predilute 10 µl in 490 µl DMEM (1:50) and count elongated as well as whirled POS in a cell counting chamber (Figure 2B). Calculate yield and concentration in POS particles per mL. NOTE: The yield varies, depending mostly on the age and strain of pigs/cows and size of the eyes, e.g., a good preparation ranges from 5 - 8 x 109 POS from 80 pig eyes.
- Decide on the final DMEM volume (typically 10 - 20 ml) and add sucrose to yield a final concentration of 2.5% sucrose.
- Prepare aliquots of the desired size, e.g., 5 x 107 POS per tube for a 2 ml resuspension volume for a 96-well experiment at 40 µl/well. Spin 1 aliquot for 5 min at 2,300 x g at RT and evaluate pellet size. Prepare various aliquot sizes for different application volumes as POS should NOT be frozen and thawed twice.
- Freeze POS aliquots at -80 °C until further use. NOTE: Aliquots are stable for many months. In our hands, unlabeled or labeled POS can be stored for at least one year.
- POS labeling and aliquoting: NOTE: We typically use FITC to label POS for quantification of phagocytosis assays, but other dyes may be used as well depending on needs.
- Resuspend 1 10 mg FITC vial at a 2.5 mg/ml concentration in 0.1 M Na carbonate buffer pH 9.5 by mixing 2.6 ml 0.1 M NaHCO3 pH 8.4 with 1.4 ml 0.1 M Na2CO3 pH 11.5 (Table 2, Stock solutions). Rotate for 1 hr at RT while protecting from light and spin for 5 min at maximum speed to pellet non-resuspended FITC solids.
- Resuspend the POS pellet in 10 ml of Wash 3 solution (or DMEM) and add 2 ml of FITC solution for 80 eyes. Store leftover FITC frozen at -80 °C. Rotate for at least 1.5 hr at RT. Protect from light using aluminum foil.
- Wash labeled POS twice using Wash 3 solution as described in section 2.3.4, then once or twice in DMEM until almost no free FITC is seen in the supernatant fraction. Resuspend POS in DMEM and proceed as for unlabeled POS for counting and aliquoting (section 2.4).
- Using POS in experiments:
- Thaw POS at RT, and keep them in the dark as much as possible if they are fluorescently-labeled. Spin for 5 min at 2,300 x g at RT. Aspirate the supernatant.
- Immediately resuspend in appropriate volume of assay solution as dictated by the experiment. In case of different times of POS challenge, store resuspended POS at RT in the dark between time-points for several hours.
Representative Results
The combination of the linear sucrose gradient and the ultracentrifugation allows the separation of the different components of the retinal suspension by density. Heavy larger retinal debris and RPE cells sink to or near the bottom of the gradient (Figure 2A). Lighter POS and lighter individual cells or cell debris from the retina migrate as separate bands to reach the top half of the gradient by the end of the centrifugation period. By keeping the eyes and the samples in the dark until after the centrifugation step, the band containing POS can be immediately recognized by its bright orange color. The width and intensity of this band depends on gradient quality and tube height (89 mm height is preferred). Typically, when isolated properly POS should appear elongated, straight or bent when observed on a microscope slide (Figure 2B). Depending on the focus plane on the microscope, they can appear either dark or shiny. If not resuspended properly, they will stay in clumps. If they are damaged (see paragraph below), they will appear as much smaller pieces, a little like a ‘dusty’ background.
Problems that can arise are mostly related to three issues. The first possible issue is that the shaking step of the retinal homogenates is not vigorous enough, which leads to POS remaining attached to PRs thus to decreased yield. The second possible issue is linked to poor gradient casting or gradient quality loss to insufficient stabilization time before loading or excessive tube shaking at any step before the collection. In this case, the bands cannot be properly distinguished and POS cannot be collected in the corresponding tube. Finally, the third potential issue arises if the pH of any of the solutions is wrong: POS membranes may disintegrate and only small POS debris appears on the microscope when visualizing them for yield assessment.
Purified POS can be used for a number of different applications related to studying the phagocytic function of RPE cells. RPE cells are usually used as cell lines or in primary culture from animal models 32,6,12,18-21. More recently, RPE cells derived from reprogrammed stem cells like iPSC or ESC have been used29,30,27,34. Isolated POS serve to test directly the phagocytic ability of mutant RPE cells; e.g., POS are readily phagocytosed by primary RPE cells from wild-type mice, whereas cells from beta5 integrin and MFG-E8 knockout mice phagocytose less POS in the same amount of time6,12. Quantification of POS phagocytosis can be done either by counting FITC-labeled POS manually on microscope pictures6 (Figure 3A), using equipment capable of reading fluorescence intensity10,24,15,35 or a flow cytometer after cell trypsinization36,27. More recently, POS intake and degradation has been evaluated on immunoblots probed for opsins37,38,27.
In vivo, the POS uptake process occurs sequentially as recognition/binding precedes synchronized internalization6. The different phases of uptake can also distinguished in vitro by using appropriate experimental procedures39. Pulse-chase experiments can be performed using temperature switch: POS binding occurs at 20 °C while internalization needs a temperature of 37 °C to proceed32,40,7. This distinction also exists in macrophages, which can recognize POS as well and in which the molecular machinery to eliminate apoptotic cells is very similar to the RPE's machinery7. After FITC-POS phagocytosis, binding and internalization can be quantified in separate samples by quenching the FITC-fluorescence of surface-bound POS during pre-incubation with trypan blue before fixing the cells10. In trypan blue-treated cells, only internal POS will be visualized, and bound POS can be quantified by subtracting internal POS from total POS fluorescence counts. When phagocytosis is evaluated on immunoblots obtained using lysates collected after POS challenge, an equivalent treatment with EDTA before lysis will allow the detachment of POS bound at the cell surface for internal versus total POS phagocytosis comparison37,38.
Tremendous advances have been made in the identification of the phagocytic machinery thanks to experiments employing purified POS. Protein recruitment can be assessed by immunofluorescence co-localization assays13-15,19-21,37,41(Figure 3B). Protein recruitment or activation can be validated on immunoblots after immunoprecipitation or by checking for phosphorylation7,10,12-15,17,19,20,27,35,37,41 (Figure 3C). More open-ended studies on overall gene expression changes after different times of POS challenge have also been performed42.
Isolated POS can also be used to study POS elimination by RPE cells. Indeed, during normal aging and/or when POS are not digested properly, POS degradation products accumulate gradually as lipofuscin deposits and can lead to pathologies due to oxidation mechanisms24,27. Hence, understanding the effects of light and related oxidative damage may require POS challenge of RPE cells18,26. Finally, repeated feeding with normal27 or oxidized25,28 POS can be used to induce cumulative effects in RPE cells in culture in order to understand pathological mechanisms that develop in vivo over longer periods of time.
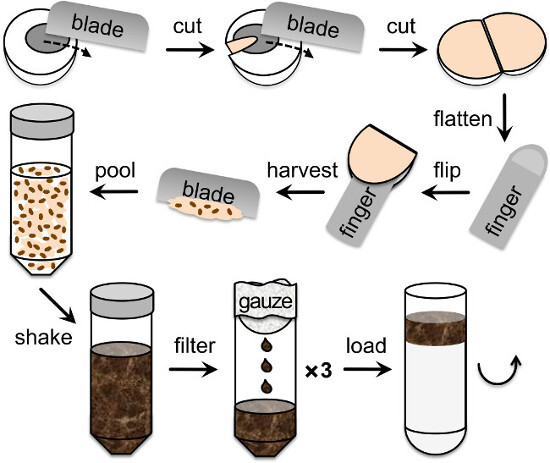 Figure 1. Retina isolation from porcine eyes. Drawing showing the different steps of the eye dissection in order to collect and homogenize the retina before the ultracentrifugation step on continuous sucrose gradients, starting top left. A 60 mm wide blade is used to cut the eye sequentially on one side and then the other in order to be able to stretch it inside-out on a finger tip, thus exposing the retina. Next, the retina is collected by scrapping it from the tapetum with the blade. Pooled retinae are then shaken thoroughly in homogenization buffer, filtered 3 times through double layers of gauze and delicately poured on top of the linear 25 - 60% sucrose gradient.
Figure 1. Retina isolation from porcine eyes. Drawing showing the different steps of the eye dissection in order to collect and homogenize the retina before the ultracentrifugation step on continuous sucrose gradients, starting top left. A 60 mm wide blade is used to cut the eye sequentially on one side and then the other in order to be able to stretch it inside-out on a finger tip, thus exposing the retina. Next, the retina is collected by scrapping it from the tapetum with the blade. Pooled retinae are then shaken thoroughly in homogenization buffer, filtered 3 times through double layers of gauze and delicately poured on top of the linear 25 - 60% sucrose gradient.
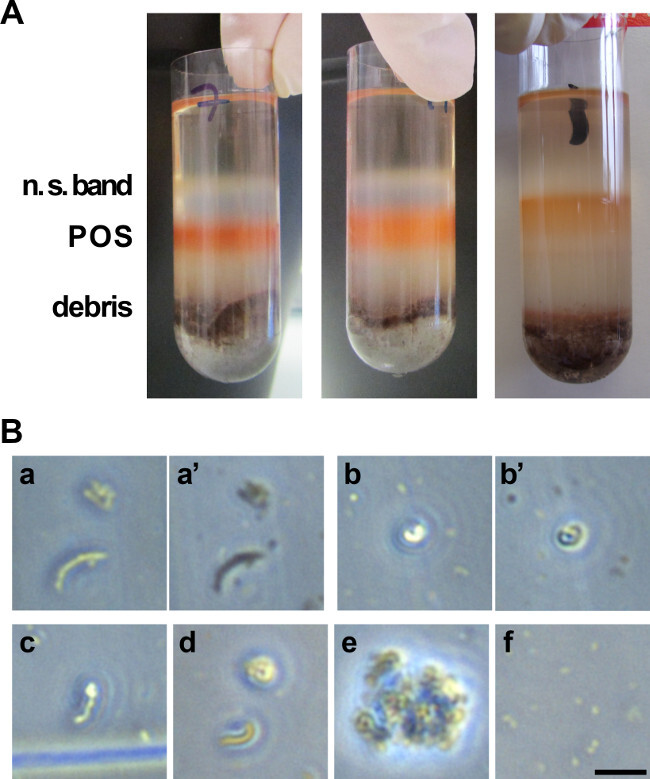 Figure 2. POS purification from porcine eyes. (A) Photograph of the various layers observed on a 25 - 60% sucrose gradient after ultracentrifugation of retina homogenates. The orange band corresponds to POS to be collected. The white upper band corresponds to non-POS related materials, as well as larger debris migrating near or at the bottom of the tube. (B) Picture of isolated POS as observed on a bright field microscope on a counting cell for yield assessment. POS exist in elongated (a, a’, c, d) and whirled (a, a’, b, b’, d) forms. Some elongated POS can display a coiled extremity (c). When changing the plane of focus, POS appearance shifts from shiny to dark (compare a and a’, b and b’). Panel e illustrates POS that have not been resuspended properly and reside in clumps. Panel f shows POS that have been damaged and are not usable, only small pieces are detectable. Scale 10 µm. Please click here to view a larger version of this figure.
Figure 2. POS purification from porcine eyes. (A) Photograph of the various layers observed on a 25 - 60% sucrose gradient after ultracentrifugation of retina homogenates. The orange band corresponds to POS to be collected. The white upper band corresponds to non-POS related materials, as well as larger debris migrating near or at the bottom of the tube. (B) Picture of isolated POS as observed on a bright field microscope on a counting cell for yield assessment. POS exist in elongated (a, a’, c, d) and whirled (a, a’, b, b’, d) forms. Some elongated POS can display a coiled extremity (c). When changing the plane of focus, POS appearance shifts from shiny to dark (compare a and a’, b and b’). Panel e illustrates POS that have not been resuspended properly and reside in clumps. Panel f shows POS that have been damaged and are not usable, only small pieces are detectable. Scale 10 µm. Please click here to view a larger version of this figure.
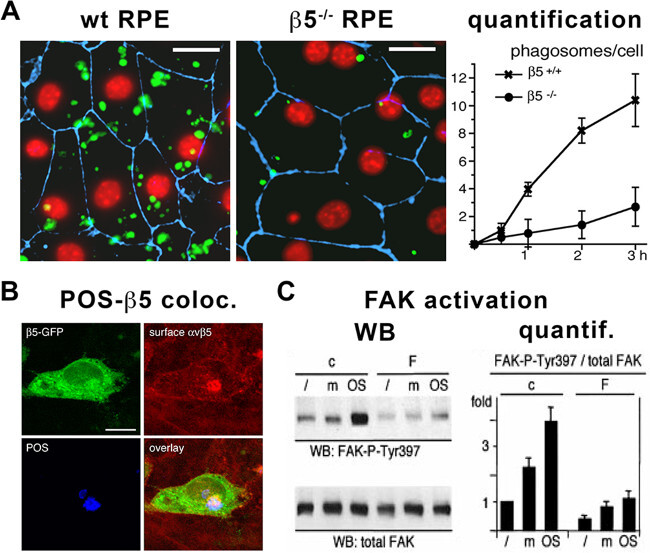 Figure 3. Analysis of RPE phagocytosis using purified POS. (A) Confocal microscope picture and corresponding quantification of the number of phagosomes per cell with time showing that FITC-labeled POS (green) have been less phagocytosed by RPE primary cells isolated from beta5 integrin knockout (β5-/-) compared to wild-type (wt or β5+/+) control mice as indicated. Nuclei (red) were labeled using DAPI and cell junctions (blue) using the tight junction marker ZO-1. Scale bars 10 µm. Reproduced from ©Nandrot et al., 2004, originally published in The Journal of Experimental Medicine 200(12):1539-1545. (B) Confocal microscope pictures showing co-localization (yellow-magenta, bottom right panel) between β5-GFP fusion proteins (green, top left panel), surface αvβ5 integrin receptors (red, top right panel) and POS (blue, bottom left panel). Scale bar 10 µm. Reproduced from Nandrot et al., 2012, originally published in Biology of the Cell 104(6):326-341, ©Portland Press Limited. (C) Immunoblots (WB) and corresponding quantification (quantif.) showing that POS challenge (POS) increases phosphorylation of FAK on the Tyr397 residue when compared without treatment (/) or challenged with medium alone (m) in control cells (c) while cells expressing an inactivated form of FAK (F) do not react, as indicated. Reproduced from Finnemann, 2003, originally published in EMBO Journal 22(16):4143-4154. Please click here to view a larger version of this figure.
Figure 3. Analysis of RPE phagocytosis using purified POS. (A) Confocal microscope picture and corresponding quantification of the number of phagosomes per cell with time showing that FITC-labeled POS (green) have been less phagocytosed by RPE primary cells isolated from beta5 integrin knockout (β5-/-) compared to wild-type (wt or β5+/+) control mice as indicated. Nuclei (red) were labeled using DAPI and cell junctions (blue) using the tight junction marker ZO-1. Scale bars 10 µm. Reproduced from ©Nandrot et al., 2004, originally published in The Journal of Experimental Medicine 200(12):1539-1545. (B) Confocal microscope pictures showing co-localization (yellow-magenta, bottom right panel) between β5-GFP fusion proteins (green, top left panel), surface αvβ5 integrin receptors (red, top right panel) and POS (blue, bottom left panel). Scale bar 10 µm. Reproduced from Nandrot et al., 2012, originally published in Biology of the Cell 104(6):326-341, ©Portland Press Limited. (C) Immunoblots (WB) and corresponding quantification (quantif.) showing that POS challenge (POS) increases phosphorylation of FAK on the Tyr397 residue when compared without treatment (/) or challenged with medium alone (m) in control cells (c) while cells expressing an inactivated form of FAK (F) do not react, as indicated. Reproduced from Finnemann, 2003, originally published in EMBO Journal 22(16):4143-4154. Please click here to view a larger version of this figure.
| Stock solution | Storage temperature |
| 70% sucrose | 4 °C |
| 100 mM glucose | 4 °C |
| 1 M MgCl2 | RT |
| 0.5 M taurine | -20 °C |
| 200 mM tris acetate pH 7.2 | RT |
| 200 mM sodium phosphate pH 7.2 | RT |
| 0.1 M NaHCO3 pH 8.4 | RT |
| 0.1 M Na2CO3 pH 11.5 | RT |
Table 1. Recipes of stock solutions and associated storage temperatures.
Discussion
Three steps or conditions are crucial for an optimized purification: quality of gradient casting and delicate gradient tubes manipulation, keeping tissues chilled and in the dark until the collection step, strength of shaking of retinal homogenates to obtain proper POS isolation from the rest of the PR cell. If some issues arise in seeing the orange band properly, they are most likely due to one of the three reasons above (see also the second paragraph of the Results section). Some modification of certain conditions is possible, like the use of a 27 - 50% sucrose gradient concentration32 or of Optiprep step gradients33,21.
Some caution needs to be taken regarding aliquots for experiments as POS should not be frozen and thawed twice. Therefore, it is useful to prepare various sizes for different application volumes.
POS pre-labeling should be used with caution. Covalent labeling generates very highly fluorescent POS that make co-localization studies difficult. Moreover, pre-labeling followed by freeze-thaw may not maintain POS surface characteristics and alter POS immunogenicity. Hence, for co-localization assays it is advisable to store unlabeled POS and label them after thaw immediately before using them for an experiment10,13-15,32.
Preparing large-scale POS stocks is economical: a one-day preparation yields reliable experimental material for numerous experiments. Moreover, using rodent retinas to prepare POS for the same number of assays would require very large numbers of animals. Like synthetic particles like beads, a frozen stock of POS will provide a highly consistent tool to test directly the phagocytic ability of RPE cells. Unlike synthetic beads targeting other particle ingestion mechanism such as endocytosis not reacted to POS phagocytosis43,44, POS isolated using our protocol are physiologically-relevant phagocytic particles: (1) Isolated POS expose phosphatidylserines, a critical “eat me” signal recognized by the RPE’s phagocytic machinery; (2) POS can be efficiently digested by RPE cells after uptake and they thus allow studying all phases of RPE phagocytosis. A possible drawback to the use of porcine or bovine POS could be any species difference between POS as most cell culture phagocytosis assays do not use porcine RPE cells. For instance, the overall cone to rod ratio varies between species – from approximately 2 - 3% in rodents to 5% in humans and 20% in pigs – and cones are concentrated in the macular region in humans. However, current data on the phagocytic machinery show that POS particles are recognized by RPE cell receptors due to their ability to bind soluble ligands, such as the integrin ligand MFG-E89,12 and MerTK ligands Gas6 and Protein S45. These ligand proteins bind to phosphatidylserines regardless of species7-9,21,22,31-33 but may bridge to RPE receptors in a species-specific manner. Potential pitfalls from using porcine/bovine POS for RPE uptake assays can just be avoided by supplementing soluble ligands during assays that are tailored to the origin of the RPE to be used.
More recently, generation of RPE cells derived from reprogrammed stem cells like ES cells or iPS have instigated a renewed interest for isolated POS27,29,30,34. These RPE cells may be derived from healthy donors or from patients carrying specific mutant genes. They can either be explored functionally to characterize the precise cellular phenotypic effect of the mutations present, or they require to be validated for their phagocytic function before potential transplantation therapies27,30,34. These new approaches open a new demand for POS purified on a large scale, as RPE phagocytosis constitutes one of RPE cell main functions and reproducible tests are recommended for reliable comparison between sets of cells.
Disclosures
The authors declare that they have no competing financial interest.
Acknowledgments
This work was supported by Agence Nationale de la Recherche (Jeunes Chercheuses/Jeunes Chercheurs to EFN), Fondation Voir et Entendre and Fondation Bettencourt Schueller (Young Investigator Grants to EFN), Centre National de la Recherche Scientifique (CNRS, permanent position for EFN), and The National Eye Institute of the National Institutes of Health (R01-EY13295 to SCF). Additionally, the Institut de la Vision is funded by Institut National de la Santé et de la Recherche Médicale, Université Pierre et Marie Curie-Paris 6, Centre National de la Recherche Scientifique and Départment de Paris.
References
- Strauss O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005;85(3):845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J. Cell Biol. 1969;42(2):392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 1976;194(4269):1071–1074. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, LaVail MM. Inherited retinal dystrophy: primary defect in pigment epithelium determined with experimental rat chimeras. Science. 1976;192(4241):799–801. doi: 10.1126/science.1265483. [DOI] [PubMed] [Google Scholar]
- Nandrot E, et al. Homozygous deletion in the coding sequence of the c-mer gene in RCS rats unravels general mechanisms of physiological cell adhesion and apoptosis. Neurobiol. Dis. 2000;7(6 pt B):586–599. doi: 10.1006/nbdi.2000.0328. [DOI] [PubMed] [Google Scholar]
- Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, Finnemann SC, editors. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin. J. Exp. Med. 2004;200(12):1539–1545. doi: 10.1084/jem.20041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC, Rodriguez-Boulan E. Macrophage and retinal pigment epithelium phagocytosis: apoptotic cells and photoreceptors compete for alphavbeta3 and alphavbeta5 integrins, and protein kinase C regulates alphavbeta5 binding and cytoskeletal linkage. J. Exp. Med. 1999;190(6):861–874. doi: 10.1084/jem.190.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343(6254):170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- Ruggiero L, Connor MP, Chen J, Langen R, Diurnal Finnemann SC. localized exposure of phosphatidylserine by rod outer segment tips in wild-type but not Itgb5-/- or Mfge8-/- mouse retina. Proc. Natl. Acad. Sci. U. S. A. 2012;109(21):8145–8148. doi: 10.1073/pnas.1121101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC, Bonilha VL, Marmorstein AD, Rodriguez-Boulan E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires alphavbeta5 integrin for binding but not for internalization. Proc. Natl. Acad. Sci. U.S.A. 1997;94(24):12932–12937. doi: 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Clegg DO. Integrin alphavbeta5 participates in the binding of photoreceptor rod outer segments during phagocytosis by cultured human retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 1998;39(9):1703–1712. [PubMed] [Google Scholar]
- Nandrot EF, Anand M, Almeida D, Atabai K, Sheppard D, Finnemann SC. Essential role for MFG-E8 as ligand for alphavbeta5 integrin in diurnal retinal phagocytosis. Proc. Natl. Acad. Sci. U. S. A. 2007;104(29):12005–12010. doi: 10.1073/pnas.0704756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Yasumura D, Matthes MT, LaVail MM, Vollrath D. Mertk triggers uptake of photoreceptor outer segments during phagocytosis by cultured retinal pigment epithelial cells. J. Biol. Chem. 2002;277(19):17016–17022. doi: 10.1074/jbc.M107876200. [DOI] [PubMed] [Google Scholar]
- Finnemann SC. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J. 2003;22(16):4143–4154. doi: 10.1093/emboj/cdg416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandrot EF, Silva KE, Scelfo C, Finnemann SC. Retinal pigment epithelial cells use a MerTK-dependent mechanism to limit the phagocytic particle binding activity of αvβ5 integrin. Biol. Cell. 2012;104(6):326–341. doi: 10.1111/boc.201100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryeom SW, Sparrow JR, Silverstein RL. CD36 participates in the phagocytosis of rod outer segments by retinal pigment epithelium. J. Cell Sci. 1996;109(2):387–395. doi: 10.1242/jcs.109.2.387. [DOI] [PubMed] [Google Scholar]
- Finnemann SC, Silverstein RL. Differential roles of CD36 and alphavbeta5 integrin in photoreceptor phagocytosis by the retinal pigment epithelium. J. Exp. Med. 2001;194(9):1289–1298. doi: 10.1084/jem.194.9.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, et al. Light-induced oxidation of photoreceptor outer segment phospholipids generates ligands for CD36-mediated phagocytosis by retinal pigment epithelium: a potential mechanism for modulating outer segment phagocytosis under oxidant stress conditions. J. Biol. Chem. 2006;281(7):4222–4230. doi: 10.1074/jbc.M509769200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AL, et al. Annexin A2 regulates phagocytosis of photoreceptor outer segments in the mouse retina. Mol. Biol. Cell. 2009;20(17):3896–3904. doi: 10.1091/mbc.E08-12-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick DJ, Feng W, Vollrath D. Mertk drives myosin II redistribution during retinal pigment epithelial phagocytosis. Invest. Ophthalmol. Vis. Sci. 2009;50(5):2427–2435. doi: 10.1167/iovs.08-3058. [DOI] [PubMed] [Google Scholar]
- Gibbs D, Kitamoto J, Williams DS. Abnormal phagocytosis by retinal pigmented epithelium that lacks myosin VIIa, the Usher syndrome 1B protein. Proc. Natl. Acad. Sci. U. S. A. 2003;100(11):6481–6486. doi: 10.1073/pnas.1130432100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs D, Diemer T, Khanobdee K, Hu J, Bok D, Williams DS. Function of MYO7A in the human RPE and the validity of shaker1 mice as a model for Usher syndrome 1B. Invest. Ophthalmol. Vis. Sci. 2010;51(2):1130–1135. doi: 10.1167/iovs.09-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy CJ, Rakoczy PE, Constable IJ. Lipofuscin of the retinal pigment epithelium: a review. Eye (Lond.) 1995;9(Pt 6):763–771. doi: 10.1038/eye.1995.192. [DOI] [PubMed] [Google Scholar]
- Finnemann SC, Leung LW, Rodriguez-Boulan E. The lipofuscin component A2E selectively inhibits phagolysosomal degradation of photoreceptor phospholipid by the retinal pigment epithelium) Proc. Natl. Acad. Sci. U. S. A. 2002;99(6):3842–3847. doi: 10.1073/pnas.052025899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano E, Tomita H, Ishiguro S, Isago H, Tamai M. Nitric oxide-induced accumulation of lipofuscin-like materials is caused by inhibition of cathepsin. S. Curr. Eye Res. 2006;31(7-8):607–616. doi: 10.1080/02713680600744851. [DOI] [PubMed] [Google Scholar]
- Vives-Bauza C, et al. The age lipid A2E and mitochondrial dysfunction synergistically impair phagocytosis by retinal pigment epithelial cells. J. Biol. Chem. 2008;283(36):24770–24780. doi: 10.1074/jbc.M800706200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, et al. Functional analysis of serially expanded human iPS cell-derived RPE cultures. Invest. Ophthalmol. Vis Sci. 2013;54(10):6767–6778. doi: 10.1167/iovs.13-11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Tzekov R, McDowell JH, Smith WC, Tang S, Kaushal S. Formation of lipofuscin-like material in the RPE Cell by different components of rod outer segments. Exp. Eye Res. 2013;112:57–67. doi: 10.1016/j.exer.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr AJ, et al. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One. 2009;4(12):8152. doi: 10.1371/journal.pone.0008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustremant C, et al. Human induced pluripotent stem cells reveal early developmental molecular correlates with a probable Leber congenital amaurosis type I. Cell. Reprogram. 2013;15(3):233–246. doi: 10.1089/cell.2012.0076. [DOI] [PubMed] [Google Scholar]
- Molday RS, Hicks D, Peripherin Molday L. A rim-specific membrane protein of rod outer segment discs. Invest. Ophthalmol. Vis. Sci. 1987;28(1):50–61. [PubMed] [Google Scholar]
- Chaitin MH, Hall MO. Defective ingestion of rod outer segments by cultured dystrophic rat pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 1983;24(7):812–820. [PubMed] [Google Scholar]
- Tsang SH, et al. Role for the target enzyme in deactivation of photoreceptor G protein in vivo. Science. 1998;282(5386):117–121. doi: 10.1126/science.282.5386.117. [DOI] [PubMed] [Google Scholar]
- Carr AJ, et al. Molecular characterization and functional analysis of phagocytosis by human embryonic stem cell-derived RPE cells using a novel human retinal assay. Mol. Vis. 2009;15:283–295. [PMC free article] [PubMed] [Google Scholar]
- Dun Y, Vargas J, Brot N, Finnemann SC. Independent roles of methionine sulfoxide reductase A in mitochondrial ATP synthesis and as antioxidant in retinal pigment epithelial cells. Free Radic. Biol. Med. 2013;65:1340–1351. doi: 10.1016/j.freeradbiomed.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YT, Wang Y, Chen P, Xu HF. Age-related maculopathy susceptibility 2 participates in the phagocytosis functions of the retinal pigment epithelium. Int. J. Ophthalmol. 2012;5(2):125–132. doi: 10.3980/j.issn.2222-3959.2012.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Finnemann SC. Essential diurnal Rac1 activation during retinal phagocytosis requires αvβ5 integrin but not tyrosine kinases focal adhesion kinase or Mer tyrosine kinase. Mol. Biol. Cell. 2013;23(6):1104–1114. doi: 10.1091/mbc.E11-10-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Finnemann SC. Analysis of photoreceptor outer segment phagocytosis by RPE cells in culture. Methods Mol. Biol. 2013;935:285–295. doi: 10.1007/978-1-62703-080-9_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni F, Safa H, Finnemann SC. Understanding photoreceptor outer segment phagocytosis: Use and utility of RPE cells in culture. Exp Eye Res. 2014;126:51–60. doi: 10.1016/j.exer.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MO, Abrams T. Kinetic studies of rod outer segment binding and ingestion by cultured rat RPE cells. Exp. Eye Res. 1987;45(6):907–922. doi: 10.1016/s0014-4835(87)80105-7. [DOI] [PubMed] [Google Scholar]
- Bulloj A, Duan W, Finnemann SC. PI 3-kinase independent role for AKT in F-actin regulation during outer segment phagocytosis by RPE cells. Exp. Eye Res. 2013;113:9–18. doi: 10.1016/j.exer.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowers I, et al. Changes in retinal pigment epithelial gene expression induced by rod outer segment uptake. Invest. Ophthalmol. Vis. Sci. 2004;45(7):2098–2106. doi: 10.1167/iovs.03-0863. [DOI] [PubMed] [Google Scholar]
- Edwards RB, Szamier RB. Defective phagocytosis of isolated rod outer segments by RCS rat retinal pigment epithelium in culture. Science. 1977;197(4307):1001–1003. doi: 10.1126/science.560718. [DOI] [PubMed] [Google Scholar]
- Philp NJ, Bernstein MH. Phagocytosis by retinal pigment epithelium explants in culture. Exp. Eye Res. 1981;33(1):47–53. doi: 10.1016/s0014-4835(81)80080-2. [DOI] [PubMed] [Google Scholar]
- Burstyn-Cohen T, Lew ED, Través PG, Burrola PG, Hash JC, Lemke G. Genetic dissection of TAM receptor-ligand interaction in retinal pigment epithelial cell phagocytosis. Neuron. 2012;76(6):1123–1132. doi: 10.1016/j.neuron.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


