Abstract
Cryptococcosis is a life-threatening infection caused by pathogenic fungi of the genus Cryptococcus. Infection occurs upon inhalation of spores, which are able to replicate in the deep lung. Phagocytosis of Cryptococcus by macrophages is one of the ways that the disease is able to spread into the central nervous system to cause lethal meningoencephalitis. Therefore, study of the association between Cryptococcus and macrophages is important to understanding the progression of the infection. The present study describes a step-by-step protocol to study macrophage infectivity by C. neoformansin vitro. Using this protocol, the role of host sterols on host-pathogen interactions is studied. Different concentrations of methyl--cyclodextrin (MCD) were used to deplete cholesterol from murine reticulum sarcoma macrophage-like cell line J774A.1. Cholesterol depletion was confirmed and quantified using both a commercially available cholesterol quantification kit and thin layer chromatography. Cholesterol depleted cells were activated using Lipopolysacharide (LPS) and Interferon gamma (IFNγ) and infected with antibody-opsonized Cryptococcus neoformans wild-type H99 cells at an effector-to-target ratio of 1:1. Infected cells were monitored after 2 hr of incubation with C. neoformans and their phagocytic index was calculated. Cholesterol depletion resulted in a significant reduction in the phagocytic index. The presented protocols offer a convenient method to mimic the initiation of the infection process in a laboratory environment and study the role of host lipid composition on infectivity.
Keywords: Immunology, Issue 94, Infection, phagocytosis, Cryptococcus, cholesterol, cyclodextrin, macrophages
Introduction
Phagocytosis is a process by which extracellular entities are internalized by host cells. It is a key weapon in the immune system’s arsenal to defend against pathogens, but the process may often be subverted by pathogens to allow for internalization and spreading throughout the body1. Phagocytosis is mediated by several signaling events that result in attachment and engulfment via rearrangements of the host cell’s cytoskeleton. ‘Professional’ phagocytes are able to recognize and bind to opsonins on the surface of the invading pathogen to signal for attachment and the formation of lamellipodia, which engulf the pathogen and form a phagosome2. Among the so-called ‘professional’ phagocytes are macrophages. Macrophages are highly specialized cells that carry out protective functions that include seeking out and eliminating disease causing agents, repairing damaged tissues, and mediating inflammation, most of these through the process of phagocytosis1,2.
Cryptococcus neoformans is a species of pathogenic yeast that causes a serious disease known as Cryptococcosis. Cryptococcus spores are inhaled by the host and result in a pulmonary infection that is usually asymptomatic. It is thought that exposure is extremely prevalent; a sample of 61 children from the Pediatric Infectious Diseases Clinic at the Bronx-Lebanon Hospital Center found that all those surveyed had antibodies to the cryptococcal polysaccharide glucuronoxylomannan and other studies have shown prevalence in both human immunodeficiency virus (HIV) uninfected and infected adults3,4. Alveolar macrophages are the first line of response to the pulmonary infection and in most cases successfully clear the pathogen. However, in immunocompromised individuals (e.g., HIV and AIDS patients) the yeast is able to survive within the macrophages. In these cases, the macrophages can serve as a niche for the replication of the pathogen and may facilitate its dissemination to the central nervous system (CNS) where the disease becomes fatal5–8. It is thought that macrophages may even deliver the yeast directly into the meninges, helping the yeast to cross the blood brain barrier via the “Trojan horse” model3,9–11. Thus, it is important to understand the process of phagocytosis and the factors that affect it, especially in cryptococcal infections.
Previous work in other pathogen systems point to cholesterol and lipid rafts formed by cholesterol as having an important role to play in phagocytosis12–15. Cholesterol is the most abundant lipid species in mammalian cells and comprises 25 - 50% of the mammalian cell membrane16. It has been found to play a role in modulating the biophysical properties of membranes by changing their rigidity17. Cholesterol and sphingolipids together form lipid microdomains within the membrane known as lipid rafts. Lipid rafts have been found to be involved in the formation of caveolae, as well as providing an isolated domain for certain types of signaling16–18. Due to their small size, it is difficult to study lipid rafts in vivo. One useful way to study the role of lipid rafts is to alter their constituents. Methyl-β-cyclodextrin (MβCD) is a compound that has been found to deplete cholesterol from mammalian membranes and is commonly used to study the role of lipid rafts18.
In this protocol, we present a method to deplete cholesterol from host cell membranes and quantify the effect of the depletion on the ability of the host cells to phagocytose C. neoformans in vitro. This procedure makes use of cell culture techniques on an immortalized macrophage like cell line (J774A.1) as a model for infection. Cholesterol depletion was accomplished by exposure to MβCD, which has a hydrophobic core specific to the size of sterols and is able to act as a sink for cholesterol to draw it out of the membrane19. Cholesterol depletion was measured quantitatively using a commercially available kit and qualitatively using a modified Bligh-Dyer lipid extraction followed by thin layer chromatography (TLC)20. Phagocytosis was measured by infecting the cell line with a culture of opsonized yeast mixed with a cocktail of interferon-γ and lipopolysaccharide for activating the macrophages. Cryptococcus was opsonized using a glucuronoxylomannan (GXM) antibody21–23. Staining and microscopy experiments allowed for visualization of the cells and calculation of the phagocytic index to assess the degree of phagocytosis. Taken together, this protocol describes a basic method that integrates the alteration of lipid composition with a physiological process.
Protocol
1. Cholesterol Depletion of J774A.1 Cells with MβCD
In a sterile biosafety cabinet, seed 105 J774A.1 macrophage-like cells per well on a 96-well cell culture plate in 200 μl of Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S). Incubate at 37 °C and 5% CO2 O/N.
Remove media from the cell monolayer and wash the cells twice with 1x phosphate buffered saline (PBS) that has been filtered or autoclaved.
Add 200 μl of MβCD solution at the desired concentration (10 mM or 30 mM in PBS) or 1x PBS as a control and incubate for 30 min at 37 °C with shaking. Remove supernatant and reserve at RT for quantitative analysis with commercially available kit immediately following the procedure.
Wash the cells two to three times with 1x PBS or serum free DMEM and continue with infection or lyse cells by pipetting two to three times with deionized H2O for analysis with thin layer chromatography or with a kit. NOTE: Following cholesterol depletion a commercially available cholesterol quantification kit can be used. See materials section for details. Follow manufacturer’s instructions as written.
2. Observation of Cholesterol Content by Thin Layer Chromatography (TLC)
Wash a TLC tank twice with acetone and once with a solution of petroleum ether:diethyl ether:acetic acid (65:30:1 by volume). Saturate the tank with the petroleum ether:diethyl ether:acetic acid (65:30:1 by volume) solution and leave O/N. NOTE: Organic solvents should always be used under a fume hood to prevent inhalation of vapor. Gloves and lab coat should be worn at all times. Acetic acid is a strong acid and should be used with caution.
In a sterile biosafety cabinet, seed 106 J774A.1 macrophage-like cells per well on a 6-well cell culture plate in a volume of 5 ml of warm DMEM supplemented with 10% FBS and 1% P/S. Incubate at 37 °C, 5% CO2 O/N.
Deplete macrophages of cholesterol by following steps 1.2 - 1.4, substituting 1 ml for 200 μl where applicable to account for the larger well size.
Add 500 μl of Trypsin-EDTA to each well, incubate for 3 min at 37 °C, and gently scrape cells with a cell scraper.
Transfer into a microfuge tube and add an additional 500 μl of warm DMEM supplemented with 10% FBS and 1% P/S.
Spin the cells for 5 min at 300 x g and remove the supernatant.
Add an additional 500 μl of warm DMEM supplemented with 10% FBS and 1% P/S to the cell pellet and resuspend carefully by pipetting up and down.
Remove 10 μl of cells and count cells on a hemocytometer. Normalize the cell concentrations from each sample and add an equal number of cells to glass tubes. NOTE: At this step, one may choose to combine cells from the same treatment groups to obtain a more concentrated final lipid extract.
Centrifuge cells at 300 x g for 5 min at RT and remove media.
Add 2 ml of methanol and vortex. Then, add 1 ml of chloroform and vortex. Check the phase status to make sure the solution in monophasic. NOTE: Tubes can be stored at 4 °C O/N.
Centrifuge at 1,700 x g for 10 min at RT and transfer the supernatant to a new tube
Add an additional 1 ml of chloroform followed by 1 ml of dH2O. Vortex twice for 30 sec. Centrifuge at 1,700 x g for 5 min at RT.
Weigh a glass tube on a sensitive balance and use a glass Pasteur pipette to transfer the lower phase into the glass tube of known weight.
Dry down lipids in a centrifugal evaporator until dry (approximately 2 hr). Weigh tube with dried lipids and calculate the dry lipid weight. NOTE: Dry lipids can be stored in -20 °C until ready to perform TLC.
Dilute dried lipids in enough chloroform to normalize the concentration of lipid (usually 20 - 50 µl) and load 20 µl of the diluted lipid on a silica TLC plate. Load 20 μg of cholesterol diluted in 20 μl of chloroform as a standard.
Add dried TLC plate to the saturated TLC tank and allow solvent to migrate up to 1 cm before plate edge. Remove TLC plate from tank and allow it to dry about 5 min.
Visualize lipids by placing in an iodine vapor tank to check migration. Remove and allow spots to fade for about 10 - 15 min under the hood. NOTE: Iodine is an inhalation hazard. Always use under a fume hood.
Prepare a solution for neutral lipid staining by combining 60 ml of methanol with 60 ml of deionized H2O, 4 ml of sulfuric acid, and 630 mg of manganese chloride.
Carefully and slowly dip the TLC plate into the neutral lipid staining solution in a tray and remove without sloughing off the silica layer. NOTE: The neutral lipid staining solution can be reused several times and so it can be retrieved from the tray and placed back in a bottle for later use.
Allow plate to dry under the hood at RT until all bubbles has disappeared. Heat the TLC plate on a heat block set to 160 °C and char to the desired color. NOTE: A densitometry program such as Vision Works LS can be used to quantify the charred bands to compare lipid samples.
3. Infection of Macrophages with C. neoformans (H99)
In a sterile biosafety cabinet, seed 105 macrophage-like cells per well on a 96-well cell culture plate in 200 μl of DMEM supplemented with 10% FBS and 1% P/S. Incubate at 37 °C, 5% CO2, 5% CO2 O/N. NOTE: Infection can also be done in glass bottomed confocal dishes for easier imaging; all amounts remain the same.
Grow a culture of C. neoformans (H99) by inoculating 10 ml of YNB with one colony obtained from a struck plate and incubating it O/N at 30 °C with shaking.
- Wash and count C. neoformans (H99) cells.
- Centrifuge C. neoformans O/N culture at 1,700 x g for 10 min at 4 °C.
- Remove media and discard. Wash cells with 5 ml of 1x PBS. Centrifuge at 1,700 x g for 10 min at 4 °C.
- Remove PBS and wash with 5 ml of filtered 1x PBS. Centrifuge at 1,700 x g for 10 min at 4 °C. Repeat this step 2 more times.
- Remove PBS and resuspend in 5 ml of 1x PBS.
- Make a serial dilution in PBS to obtain a 1:500 dilution of the washed culture.
- Add 100 μl of the original sample to 900 μl of 1x PBS to obtain a 1:10 dilution.
- Add 100 μl of 1:10 diluted sample to 900 μl of 1x PBS to obtain a 1:100 dilution.
- Add 200 μl of the 1:100 diluted sample to 800 μl of 1x PBS to obtain a 1:500 dilution
- Take 10 μl of 1:500 dilution and count on hemocytometer to calculate the number of cells.
- Prepare working solution for activating macrophages and opsonizing C. neoformans.
- Dilute LPS and IFNγ 100x from stock solutions by adding 10 μl to 990 μl. NOTE: LPS and IFNγ are used to enhance phagocytic uptake but are not required for phagocytosis. If there is an interest in fungicidal activity of macrophages, perform activation O/N at 37 °C with shaking prior to infection.
- Per sample combine 7.5 μl of diluted LPS, 1.25 μl of diluted IFNγ, 1.25 μl of GXM antibody and the volume of the C. neoformans culture that gives 1.25 x 105 cells. Bring volume up to 250 μl multiplied by number of samples with DMEM supplemented with 10% FBS and 1% P/S.
- Vortex and incubate solution for 20 min at 37 °C with shaking. NOTE: Cholesterol depletion (steps 1.2 - 1.4) can be done concurrently with the opsonization step. Be sure to treat macrophages prior to combining the working solution, as the opsonized cells should optimally be used no longer than 20 min following the step 3.4.3 incubation.
- Infect Macrophages
- Wash macrophages twice with serum free DMEM and add 200 μl of opsonized C. neoformans working solution to each well.
- Incubate for 2 hr at 37 °C.
- Fix and Stain Cells
- Remove media and wash cells 2 times with DMEM.
- Air dry the cell monolayer for 10 min and add 200 μl of ice cold methanol to fix the cells.
- Incubate for 15 min at RT and remove any remaining methanol.
- Add 200 μl of 10x Giemsa and incubate for 5 min at RT.
- Wash 2 - 3 times with deionized water and dry O/N with cap off. NOTE: Imaging can be done the next day or up to a week following staining.
- Visualize and Count
- Using a microscope, count 300 cells per data point (if there are 2 of the same treatment count 150 per well) and note the number of infected macrophages and number of engulfed Cryptococcus cells. NOTE: To ensure even sampling per data point use 2 plates per condition, choose 3 non-overlapping areas per plate and count 50 cells per area.
- Calculate phagocytic index by multiplying the percentage of infected macrophages by the mean number of C. neoformans per macrophage. Normalize phagocytic index by expressing values as a percentage of the 1x PBS treated control. After several trials calculate the mean value and the standard deviation of the mean to determine trends in phagocytic index. Use student t-test to determine significance.
- Take micrographs of cells at 1,000X or 400X magnification.
4. Trypan Blue Assay
In a sterile biosafety cabinet, seed 106 macrophage-like cells per well on a 6-well cell culture plate in a volume of 5 ml of Dulbecco’s minimal essential medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Incubate at 37 °C, 5% CO2 O/N.
Deplete macrophages of cholesterol by following steps 1.2 - 1.4 substituting 2 ml for 200 μl where applicable to account for the larger well size. NOTE: Be sure to include a control treated with 1x PBS as well as a control that is scraped prior to any treatment.
Add 500 μl of 1x PBS to each well and gently scrape cells with a cell scraper. Transfer into a microfuge tube and suspend the cells by gently pipetting up and down.
Remove 10 μl of cells and stain with 1 μl of 4% Trypan blue.
Count cells on a hemocytometer and calculate viability using the following equation: % viability = [1 - (Blue cells / total cells)] x 100. Normalize values to the control that was untreated. After several trials calculate the mean value and the standard deviation to determine trends in viability. Use student t-test to determine significance.
Representative Results
Cholesterol Depletion
Analysis of the supernatant reserved in step 1.3 of the protocol by following the manufacturer’s instructions in the Amplex Red Cholesterol Assay kit yields an elevated concentration of cholesterol in MβCD treated sample as compared to the 1x PBS control. Depending on cell type and MβCD concentration used cholesterol depletion may vary. For J774 treated with 10 mM MβCD, a depletion of approximately 50% was observed. Depletion can be calculated using values obtained from the supernatant and cell lysate collected in step 1.4 (Figure 1).
Cell lysate analyzed using TLC shows a marked decrease in staining of cholesterol in cells treated with increasing concentration of MβCD (Figure 2A). Densitometry analysis of the TLC shows a similar trend to the quantitative assay (Figure 2B). The Bligh-Dyer method gives a crude extract of total lipids and it is essential to allow for adequate separation of lipids in order to identify the correct band utilizing the cholesterol standard.
Infection
After following the infection procedure, cells remain adhered and intact. Cell morphology remains unchanged between treatment groups. A control group that has not been exposed to C. neoformans serves as a checkpoint (Figure 3). It is possible to obtain suboptimal results and may manifest as lysis of cells and other abnormal morphologies. The most likely cause is contamination of the cell line or reagents used in the procedure. Micrographs of optimally infected cells clearly show C. neoformans engulfed within the mammalian cells. Differences in number of phagocytized yeast may be noted by observation between treatment groups (Figure 4). After calculating phagocytic index from 300 macrophage cells per treatment group a reduction in phagocytic index is found in cholesterol depleted cells (Figure 5). The reduction in the phagocytic index does not appear to be dependent on potential differences in macrophage activation, although they may occur. Performing the infection in the absence of macrophage activators, but after treatment with MCD results in a similar reduction of phagocytic index (data not shown).
Trypan Blue
Trypan Blue staining is used to assess the viability of cells after cholesterol depletion. No change in viability is observed between PBS treated and 10mM MCD treated cells. Viability appears to drop off slightly after treatment with 30 mM MCD, which may be expected due to the approximately 75% depletion in cholesterol (an essential lipid) observed in the densitometry analysis (Figure 6 and Figure 2B).
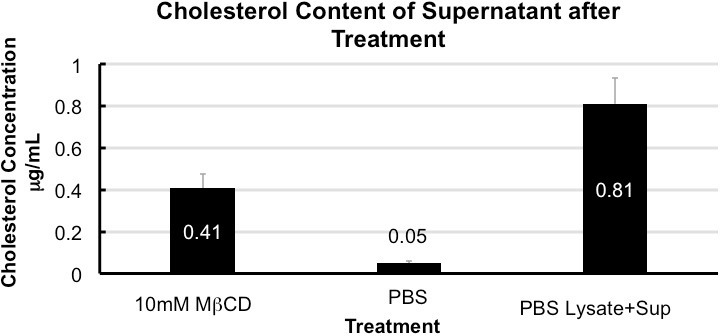 Figure 1. Cholesterol content of supernatant after treatment. Quantification of cholesterol in the supernatant collected from treated cells shows enrichment in MCD when compared to 1x PBS. Cholesterol depletion is 50 ± 5% calculated from total cholesterol in 1x PBS (supernatant + cell lysate). Error bars show standard deviation (n = 5).
Figure 1. Cholesterol content of supernatant after treatment. Quantification of cholesterol in the supernatant collected from treated cells shows enrichment in MCD when compared to 1x PBS. Cholesterol depletion is 50 ± 5% calculated from total cholesterol in 1x PBS (supernatant + cell lysate). Error bars show standard deviation (n = 5).
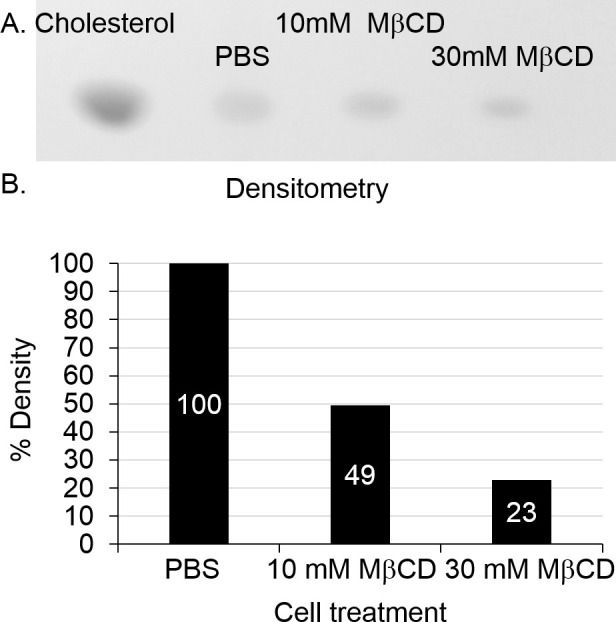 Figure 2. TLC of cholesterol in cell lysate and densitometry. Image of developed TLC plate visualized with MnCl2 charring. A marked decrease in cholesterol is seen after MβCD treatment (A). Densitometry analysis of bands as compared to the PBS treated control (shown as 100%) confirms trend found in Cholesterol quantification assay (B). Please click here to view a larger version of this figure.
Figure 2. TLC of cholesterol in cell lysate and densitometry. Image of developed TLC plate visualized with MnCl2 charring. A marked decrease in cholesterol is seen after MβCD treatment (A). Densitometry analysis of bands as compared to the PBS treated control (shown as 100%) confirms trend found in Cholesterol quantification assay (B). Please click here to view a larger version of this figure.
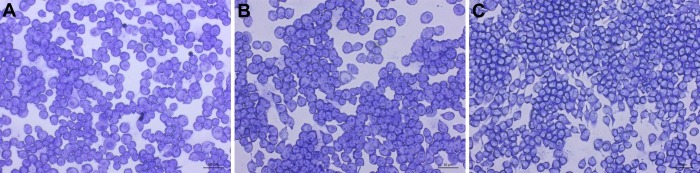 Figure 3. Uninfected control micrographs of treated J774 macrophages. Images of uninfected J774 cells taken at 200X magnification. Scale bar is 50 μm. 1x PBS (A), 10 mM MβCD (B), and 30 mM MβCD (C) treated cells show no change. Please click here to view a larger version of this figure.
Figure 3. Uninfected control micrographs of treated J774 macrophages. Images of uninfected J774 cells taken at 200X magnification. Scale bar is 50 μm. 1x PBS (A), 10 mM MβCD (B), and 30 mM MβCD (C) treated cells show no change. Please click here to view a larger version of this figure.
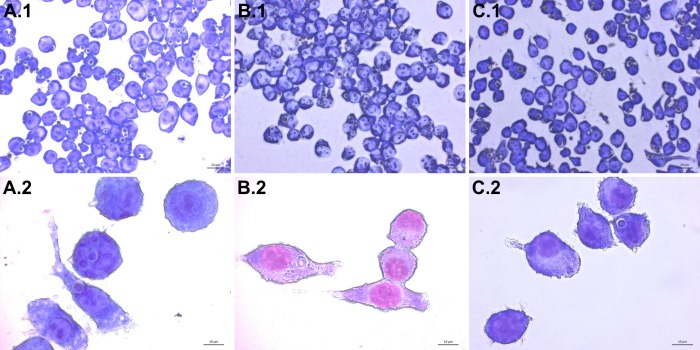 Figure 4. Infection of J774 macrophages with C. neoformans. Images of infected J774 cells taken at 400X (top row A.1 - C.1) and 1,000X (bottom row A.2 - C.2) magnification are shown. Internalized C. neoformans cells appear as blue-violet spheres with a lighter ring surrounding them. Cells treated with 1x PBS (A), 10 mM MβCD (B), and 30 mM MβCD (C) show differences in C. neoformans uptake. Please click here to view a larger version of this figure.
Figure 4. Infection of J774 macrophages with C. neoformans. Images of infected J774 cells taken at 400X (top row A.1 - C.1) and 1,000X (bottom row A.2 - C.2) magnification are shown. Internalized C. neoformans cells appear as blue-violet spheres with a lighter ring surrounding them. Cells treated with 1x PBS (A), 10 mM MβCD (B), and 30 mM MβCD (C) show differences in C. neoformans uptake. Please click here to view a larger version of this figure.
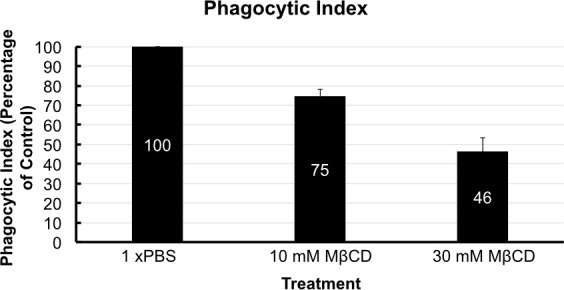 Figure 5. Phagocytic index. Phagocytic index is shown with respect to the control group that was treated with PBS (Marked at 100 for comparison). Phagocytic index was reduced by 25% by 10 mM MβCD treatment and by almost 55% by 30 mM treatment. Error bars show standard deviation of the mean (n = 4).
Figure 5. Phagocytic index. Phagocytic index is shown with respect to the control group that was treated with PBS (Marked at 100 for comparison). Phagocytic index was reduced by 25% by 10 mM MβCD treatment and by almost 55% by 30 mM treatment. Error bars show standard deviation of the mean (n = 4).
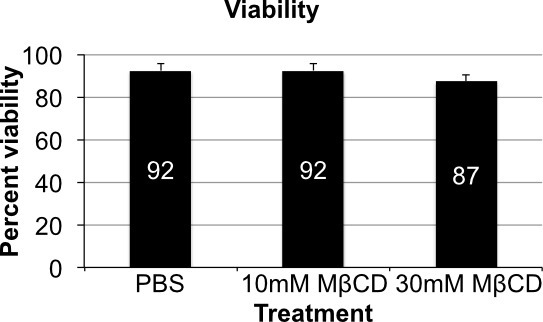 Figure 6. Cell viability. Variations in cell viability by trypan blue assay show little variation when comparing all three treatment groups. There is a slight drop off in viability in the 30 mM MβCD treatment group, which can be expected from depletion of such a major component of the membrane. Error bars show standard deviation (n = 4).
Figure 6. Cell viability. Variations in cell viability by trypan blue assay show little variation when comparing all three treatment groups. There is a slight drop off in viability in the 30 mM MβCD treatment group, which can be expected from depletion of such a major component of the membrane. Error bars show standard deviation (n = 4).
Discussion
In working with this protocol it is important to obtain accurate cell counts when plating mammalian cells and opsonizing C. neoformans cells. This minimizes variation between trials and ensures an accurate 1:1 target to effector ratio throughout the study. It is also critical to coordinate the timing of the cholesterol depletion and infection to prevent the opsonized yeast cells or treated macrophage cells from resting at RT in between the procedures. Long waiting periods could lead to loss of antibody opsonization or the replenishing of depleted cholesterol before infection can begin. If experiments are done with precision the data analysis allows for conclusions to be discerned about the role of cholesterol in phagocytosis.
The limitations of the technique prevent any conclusions as to the specific mechanism by which cholesterol depletion lowers the phagocytic index of macrophage like cells, and it is unclear whether the effect is directly due to cholesterol or due to a secondary mechanism. Further work along this vein investigates other constituents of lipid rafts such as sphingolipids or proteins known to function in phagocytosis such as the Fcγ receptor and the complement receptor 3 2. Modifying this technique to use either antibody opsonization or complement alone could help distinguish a role for cholesterol in one or both of these known pathways. It is also important to remember that MβCD extracts cholesterol based on its hydrophobicity and size, thus sterols of a similar size may also be depleted and will migrate at a similar rate as cholesterol on a TLC. It is also important to note that cholesterol depletion can partially affect macrophage activation, this is unlikely to be responsible for the difference observed in uptake as performing the infection in the absence of IFN- and LPS shows the same reduction in the uptake of C. neoformans (data not shown), but it is of interest when modifying this technique to study the anti-fungal activities of macrophages and the role of activation. This method also does not allow us to discern whether cholesterol depletion has any therapeutic implications in fungal infection. Further work in vivo with cholesterol-lowering drugs and epidemiological studies of patients using cholesterol-lowering drugs could further elucidate a role for cholesterol depletion in the treatment of the disease and may offer a more selective way to inhibit cholesterol accumulation.
This procedure could easily be used to study uptake of other pathogens or solid particles (i.e., glass beads) being phagocytized and allow for the study of basic biology of phagocytosis. Modifications could allow for the study of other aspects of phagocytosis by treating the macrophage cells with enzymes to selectively degrade other membrane components or various drugs and inhibitors which may be of interest. It should also be noted that flow cytometry may present a more accurate and quantitative way to characterize phagocytosis and could be used to replace the direct microscopic count24. Altogether, this is a fairly simple technique that can be used as a starting point for more in depth studies that answer questions about how lipids may play an important part in infection and immune response.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by NIH grants AI56168, AI71142, AI87541 and AI100631 to MDP. Maurizio Del Poeta is Burroughs Wellcome Investigator in Infectious Diseases.
References
- Sarantis H, Grinstein S. Subversion of phagocytosis for pathogen survival. Cell Host & Microbe. 2012;12(4):419–431. doi: 10.1016/j.chom.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Rougerie P, Miskolci V, Cox D. Generation Of Membrane Structures During Phagocytosis And Chemotaxis Of Macrophages: Role And Regulation Of The Actin Cytoskeleton. Immunological Reviews. 1111;256(1):222–239. doi: 10.1111/imr.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Perlin DS, Xue C. Molecular Mechanisms Of Cryptococcal Meningitis. Virulence. 2012;3:173, 181. doi: 10.4161/viru.18685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abadi J, Pirofski L. A Antibodies Reactive With The Cryptococcal Capsular Polysaccharide Glucuronoxylomannan Are Present In Sera From Children With And Without Human Immunodeficiency Virus Infection. The Journal Of Infectious Diseases. 1999;180(3):915–919. doi: 10.1086/314953. [DOI] [PubMed] [Google Scholar]
- Kechichian TB, Shea J, Del Poeta M. Depletion Of Alveolar Macrophages Decreases The Dissemination Of A Glucosylceramide-Deficient Mutant Of Cryptococcus Neoformans In Immunodeficient Mice. Infection And Immunity. 2007;75(10):4792–4798. doi: 10.1128/IAI.00587-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A. Cryptococci At The Brain Gate: Break And Enter Or Use A Trojan Horse. The Journal Of Clinical Investigation. 1172;120(5):1389–1692. doi: 10.1172/JCI42949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrétien F, et al. Pathogenesis Of Cerebral Cryptococcus Neoformans Infection After Fungemia. The Journal Of Infectious Diseases. 2002;186(4):522–530. doi: 10.1086/341564. [DOI] [PubMed] [Google Scholar]
- Luberto C, Martinez-Mariño B, et al. Identification Of App1 As A Regulator Of Phagocytosis And Virulence Of Cryptococcus Neoformans. The Journal Of Clinical Investigation. 1172;112(7):1080–1094. doi: 10.1172/JCI18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcquiston TJ, Williamson PR. Paradoxical Roles Of Alveolar Macrophages In The Host Response To Cryptococcus Neoformans. Journal Of Infection And Chemotherapy: Official Journal Of The Japan Society Of Chemotherapy. 2012;18(1):1–9. doi: 10.1007/s10156-011-0306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Rodas R, Zaragoza O. Catch Me If You Can: Phagocytosis And Killing Avoidance By Cryptococcus Neoformans. FEMS Immunology And Medical Microbiology. 2012;64(2):147–161. doi: 10.1111/j.1574-695X.2011.00871.x. [DOI] [PubMed] [Google Scholar]
- Coelho C, Bocca AL, Casadevall A. The Intracellular Life Of Cryptococcus Neoformans. Annual Review Of Pathology. 9. 2014;219:10–1146. doi: 10.1146/annurev-pathol-012513-104653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M, Peachman KK, Alving CR, Rothwell SW. Immunology And Cell Biology. Vol. 81. Doi: 2003. Depletion Of Cellular Cholesterol Interferes With Intracellular Trafficking Of Liposome-Encapsulated Ovalbumin; pp. 415–423. [DOI] [PubMed] [Google Scholar]
- Sein KK, Aikawa M. The Prime Role Of Plasma Membrane Cholesterol. In The Pathogenesis Of Immune Evasion And Clinical Manifestations Of Falciparum Malaria. Medical Hypotheses. 1998;51(2):10–1016. doi: 10.1016/s0306-9877(98)90102-5. [DOI] [PubMed] [Google Scholar]
- Pucadyil TJ, Tewary P, Madhubala R, Chattopadhyay A. Cholesterol Is Required For Leishmania Donovani Infection: Implications In Leishmaniasis. Molecular And Biochemical Parasitology. 2004;133:145–152. doi: 10.1016/j.molbiopara.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Tagliari L, et al. Membrane Microdomain Components Of Histoplasma Capsulatum Yeast Forms, And Their Role In. Alveolar Macrophage Infectivity. Biochimica Et Biophysica Acta. 2012;1818(3):458–466. doi: 10.1016/j.bbamem.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Crane JM, Tamm LK. Role Of Cholesterol In The Formation And Nature Of Lipid Rafts In Planar And Spherical Model Membranes. Biophysical Journal. 2004;86(5):2965–2979. doi: 10.1016/S0006-3495(04)74347-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, London E. Functions Of Lipid Rafts In Biological Membranes. Annual Review Of Cell And Developmental Biology. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid Rafts And Signal Transduction Nature Reviews. Molecular Cell Biology. 2000;1(1):31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Ilangumaran S, Hoessli DC. Effects Of Cholesterol Depletion By Cyclodextrin On The Sphingolipid Microdomains Of The Plasma Membrane. The Biochemical Journal. 1998;335(Pt 2):433–440. doi: 10.1042/bj3350433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A Rapid Method Of Total Lipid Extraction And Purification) Canadian Journal Of Biochemistry And Physiology. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Lee S, Casadevall A. Antibodies To Cryptococcus Neoformans Glucuronoxylomannan Enhance Antifungal Activity Of Murine Macrophages. Infect. Immun. 1995;63(2):573–579. doi: 10.1128/iai.63.2.573-579.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaw M, Pirofski LA. Antibodies To The Cryptococcus Neoformans Capsular Glucuronoxylomannan Are Ubiquitous. In Serum From HIV+ And HIV- Individuals. Clinical And Experimental Immunology. 1995;99(3):425–432. doi: 10.1111/j.1365-2249.1995.tb05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi K, Mor V, Bairwa NK, Del Poeta M, Mohanty BK. Hydroxyurea Treatment Inhibits Proliferation Of Cryptococcus Neoformans In Mice. Frontiers In Microbiology. 2012;3:187. doi: 10.3389/fmicb.2012.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaka W, et al. Quantitative Analysis Of Phagocytosis And Killing Of Cryptococcus Neoformans By Human Peripheral Blood Mononuclear Cells By Flow Cytometry. Clin. Diagn. Lab. Immunol. 1995;2(6):753–759. doi: 10.1128/cdli.2.6.753-759.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]


