Abstract
The basic design used in our human fear-conditioning studies on disrupting reconsolidation includes testing over different phases across three consecutive days. On day 1 - the fear acquisition phase, healthy participants are exposed to a series of picture presentations. One picture stimulus (CS1+) is repeatedly paired with an aversive electric stimulus (US), resulting in the acquisition of a fear association, whereas another picture stimulus (CS2-) is never followed by an US. On day 2 - the memory reactivation phase, the participants are re-exposed to the conditioned stimulus without the US (CS1-), which typically triggers a conditioned fear response. After the memory reactivation we administer an oral dose of 40 mg of propranolol HCl, a β-adrenergic receptor antagonist that indirectly targets the protein synthesis required for reconsolidation by inhibiting the noradrenaline-stimulated CREB phosphorylation. On day 3 - the test phase, the participants are again exposed to the unreinforced conditioned stimuli (CS1- and CS2-) in order to measure the fear-reducing effect of the manipulation. This retention test is followed by an extinction procedure and the presentation of situational triggers to test for the return of fear. Potentiation of the eye blink startle reflex is measured as an index for conditioned fear responding. Declarative knowledge of the fear association is measured through online US expectancy ratings during each CS presentation. In contrast to extinction learning, disrupting reconsolidation targets the original fear memory thereby preventing the return of fear. Although the clinical applications are still in their infancy, disrupting reconsolidation of fear memory seems to be a promising new technique with the prospect to persistently dampen the expression of fear memory in patients suffering from anxiety disorders and other psychiatric disorders.
Keywords: Behavior, Issue 94, Fear memory, reconsolidation, noradrenergic β-blocker, human fear conditioning, startle potentiation, translational research.
Introduction
Our brains are programmed to learn. Humans are well equipped with the ability to learn the potential dangers in life and perhaps even more important to learn the predictors of danger. Pavlovian aversive conditioning is an excellent tool to study associative fear learning not only in humans but also across a wide range of organisms1,2. This procedure involves presenting an innocuous biologically neutral conditioned stimulus (CS, e.g., a tone or picture) with a noxious or harmful unconditioned stimulus (US), typically a mild electrical shock. If the CS becomes a reliable predictor of the US, the CS will elicit species-typical conditioned behavioral responses (e.g., freezing in rats and potentiated startle reflex in humans), which are conceptualized as expressions of fear (see 3 for critical comments on this terminology). Not only does aversive conditioning research add to a better understanding of the molecular and cellular processes of associative fear learning and memory4 but it also provides a foundation for understanding the etiology and course of anxiety disorders5. Though it bears mentioning that anxiety disorders do not necessarily result from direct conditioning experiences such as traumatic events. They may also result from indirect or vicarious fear learning experiences6. But irrespective of the learning history, associative fear memory lies at the core of anxiety disorders. The Pavlovian conditioning paradigm has not only proven its utility in understanding the origin of anxiety disorders, it is also an excellent translational model to develop and advance treatment for anxiety disorders5.
In the laboratory the two most extensively studied procedures to reduce learned fear are (1) extinction and (2) disruption of reconsolidation. Even though extinction training — i.e., the repeated unreinforced re-exposure to the CS7,8 — is an effective anxiolytic strategy, animal and human fear conditioning studies reliably show that the conditioned fear response can easily return by re-exposure to unsignaled USs (i.e., reinstatement), a context change (i.e., renewal), or the passage of time (i.e., spontaneous recovery)7,9,10. A consensus has been building that extinction learning does not erase the original fear memory but instead reflects the formation of a new inhibitory memory. As a consequence, the fear memory may resurface resulting in a return of fear even after originally successful fear extinction. The various sources of relapse are still a major challenge for clinical practice.
An alternative approach to diminish conditioned fear responding is the more recently (re)discovered procedure of disrupting memory reconsolidation by pharmacological agents. This procedure is very promising as it not only diminishes conditioned fear responding but it even seems to erase associative fear memory, which might eventually solve the problem of relapse.
Memory reconsolidation refers to a two-phase process by which previously consolidated memories transfer to a transient destabilized state at retrieval and require a time-dependent restabilization to persist14-18. Gene transcription, protein and RNA synthesis are necessary for this restabilization and offer a window of opportunity for amnestic agents to affect the fear memory. Amnesia for learned fear has been demonstrated in animals by drugs (e.g., anisomycin) targeting the required protein synthesis14,19,20 or the release of neurotransmitters21,22. Protein synthesis may also be disturbed by the noradrenergic beta-blocker propranolol, which is supposed to inhibit the noradrenaline-stimulated CREB phosphorylation23-25. The fear reducing effects of propranolol have been demonstrated in animals and humans26-32. In a series of discriminative fear-conditioning studies, we consistently demonstrated that propranolol (40 mg) administered prior to or after memory reactivation effectively reduced the conditioned startle fear response and prevented the return of fear in healthy participants.
Proof of Principle
The basic design used in our human fear-conditioning studies on disrupting reconsolidation includes testing over different phases across three consecutive days separated by at least 24 hr in order to (1) support (re)consolidation of the memories and (2) allow the drug to wash out before testing. On day 1 - the fear acquisition phase, healthy participants are exposed to a series of picture presentations. One picture stimulus (CS1+) is repeatedly paired with an aversive electric stimulus (US), resulting in the acquisition of a fear association, whereas another picture stimulus (CS2-) is never followed by an US. On day 2 - the memory reactivation phase, re-exposure to the unreinforced conditioned stimulus (CS1-) typically triggers a conditioned fear response. In this phase, we systemically administer 40 mg propranolol HCl, a β-adrenergic receptor antagonist that indirectly targets the protein synthesis required for reconsolidation25. In view of the peak plasma concentrations of propranolol33, we administered an oral dose of propranolol 90 min prior to the reactivation of the fear memory in our first experiments. However, for a more optimal test of reconsolidation, the amnestic agent should be administered after the memory reactivation. Therefore, in our latter experiments we always administered propranolol after memory reactivation with very similar results. On day 3 - the test phase, the retention of the fear memory is tested 24 hr after the intervention (i.e., first test trial day 3), followed by an extinction procedure and situational triggers to test for the return of fear (i.e., reinstatement, renewal, spontaneous recovery, rapid reacquisition). For determining whether the effect of propranolol requires active retrieval of the fear memory, the drug should be administered to another fear-conditioned group without reactivation of the memory (i.e., propranolol no-reactivation condition)27,28. We used the potentiation of the startle reflex as a measure for conditioned fear responding and online US expectancy ratings as an index for contingency learning. The potentiation of the startle reflex is conceived as a specific and reliable index of fear34, subserved by the amygdala35. The most commonly used startle-eliciting stimulus is the “startle probe”, a loud noise that is presented through headphones during a stimulus or in the interval between two stimulus presentations (i.e., intertrial intervals)36. Stronger startle responses to the loud noise during the fear conditioned stimulus (CS1+) as compared to the control stimulus (CS2-) reflects the fearful state of the participant elicited by the feared stimulus (CS1+).
In a series of 10 consecutive experiments in independent samples 27-32,37 we consistently replicated our original finding where disrupting reconsolidation of fear memory by a beta-adrenergic blocker propranolol HCl effectively reduced the conditioned startle reflex and prevented the return of fear27. The observations that exposure to primary reinforcers (i.e., reinstatement), a change in context (i.e., renewal) or simple the passage of time (i.e., spontaneous recovery) did not lead to the re-emergence of conditioned fear responding as is generally observed after extinction training, support the superiority of disrupting reconsolidation over extinction learning. In addition to these retrieval techniques, reacquisition learning did not reveal any savings of the previously learned fear response. In sum, these findings suggest that disrupting reconsolidation of associative fear memory by propranolol effectively reduced the emotional expression of fear memory.
Furthermore, we tested several boundary and necessary conditions for disrupting reconsolidation: (1) The memory reactivation session seems procedurally similar to extinction training (i.e., unreinforced exposure), but it should involve less unreinforced trials than extinction training because noradrenergic beta-blockers (i.e., propranolol) may also interfere with the formation of extinction memory instead of targeting the original fear memory38,39; (2) Memory retrieval is not sufficient for memory reconsolidation18,31,40,41. Propranolol only reduced the conditioned fear responding when there was something to be learned during the retrieval session31,32. A discrepancy between what has already been learned and what can be learned on a given retrieval session (i.e., prediction error) seems to be necessary for inducing reconsolidation of associative fear memory32. The results indicate that the occurrence of a prediction error is a necessary condition for reconsolidation and provide a useful instrument for developing and optimizing reconsolidation-based treatments for patients suffering from anxiety disorders.
Protocol
Ethics statement: Our physician-approved protocol meets the requirements of the Ethical Committee of the Department of Psychology at the University of Amsterdam for testing human participants.
1. Attachment of the Fear Potentiated Startle and Shock Electrodes
Ensure the experimental setting consists out of a sound-attenuated participants’ room and a separated experimenter room.
Use two computers with four screens for recording and monitoring physiological responses as well as for presenting the experimental script to the participants.
Attach the double-sided adhesive collars to the EMG electrodes.
Clean the participant’s skin below the right or left eye and on the forehead using an alcohol swab. Instruct the participants to keep their eyes closed to avoid potential eye irritation.
Fill the center of the EMG electrodes with highly conductive electrolyte gel using a syringe. Do not try to fill the electrodes by applying small amounts of gel successively as this increases the risk of air bubbles within the gel, which may affect the recordings negatively.
Attach one of the EMG electrodes below the lower eyelid of the right or left eye in line with the pupil in forward gaze. Attach the second one approximately 1 - 2 cm lateral to the first following the curvature of the lower eyelid. Attach a ground reference on the forehead approximately 1 cm below the hairline. Optimal placement of the electrodes relative to the muscle may require individual adjustments.
Apply a drop of the conductive gel on the shock electrodes and attach them on the left upper wrist using nylon Velcro strap. Make sure the strap is not on too tight.
Adjust the intensity of the 2-msec electric shock for each participant individually. Inform the participants that the shock should be “uncomfortable but not painful”.
Manually trigger the shocks. Start at an intensity of 1 mA and continue triggering single shocks — gradually increasing the intensity in 2 or 3 mA increments — until the participant indicates that the shock is “uncomfortable but not painful”.
Write down the final shock level and maintain this level throughout the remainder of the experiment.
2. Fear Acquisition - Day 1
Duration about 45 min.
Instruct the participants about the delivery of shocks and the possible administration of propranolol HCl. Further inform them that propranolol HCl is used to treat hypertension. Obtain written informed consent.
Screen the participants regarding their health and any conditions that would contra-indicate taking a single dose of 40 mg of propranolol HCl: pregnancy, seizure disorder, respiratory disorder, cardiovascular diseases, cardiovascular diseases in first-line relatives, diabetes, liver or kidney disorder, current use of an anti-depressant drug, or psychosis.
Obtain blood pressures and heart rate measurements using an electronic sphygmomanometer. Ask the participants to remove their arm from the sleeves and put the inflatable cuff securely on the upper arm. Instruct the participants to relax and sit with their arm slightly bent on the same level as their heart and resting comfortably on a table or other flat surface. BP should be above 90/60 mmHg and heart rate above 60 BPM for inclusion in the experiment.
Administer the State-Trait Anxiety Inventory42 and the Anxiety Sensitivity Index43 when a participant is medically cleared. Also administer the Spider Phobic Questionnaire44 when using pictures of spiders as the conditioned stimuli.
Position the participants in front of a computer screen and attach the EMG and shock electrodes (see section 1 "Attachment of the Fear Potentiated Startle and Shock Electrodes").
- Use two different learning procedures — see steps 2.6.1 and 2.6.2. Inform the participants about the two different pictures they will see on the computer screen. Instruct them to monitor the relationship between the pictures they are seeing and when a shock is received.
- Non-Asymptotic Learning: Inform the participants that one of the pictures will be followed by the electric shock in most cases whereas the other will never be followed by the shock.
- Asymptotic Learning: Inform the participants which of the two pictures will always be followed by the electric shock and which picture will never be followed by the shock (i.e., instructed fear learning).
Inform the participants about the loud noises they will hear through the headphones. Order them to pay no attention to the noises.
Instruct the participants to report the expectancy of an electric shock during the presentation of all pictures by shifting a cursor on continuous rating scales by using a mouse and to push the left mouse button within 5 sec following stimulus onset in order to confirm their expectancy. Ensure scales consist of 11-points labeled from “certainly no electric shock” through “uncertain” to “certainly an electric shock”. Present the scales continuously at the bottom of the computer screen in order to support the participants to focus their attention on the pictures. When the pictures disappear the cursor should automatically return to the “uncertain” position.
Direct the participants to keep their eyes on the screen and sit as still as possible with both arms placed in a relaxed position with wrists up on the desk in front of them. Put on the headphones. Dim the lights and shut the door. Start the experiment from the other room.
During fear acquisition one of the two pictures co-terminates with the shock: CS1, whereas the other one is never followed by the shock: CS2. Make use of fear relevant stimuli in order to strengthen the fear association during acquisition. For instance pictures of spiders: IAPS numbers 1200 - 1201.
Present both the CS1 and CS2 for 8 sec. Loud noises or startle probes — 40 msec and 104 dB — are presented 7 sec after CS onset; and for the CS1, these are followed by the 2-msec electric shock 500 msec later.
- Start the actual fear learning by presenting ten baseline startle probes to diminish initial startle reactivity:
- Non-Asymptotic Learning: Present a total of 8 CS1 trials on a 75% reinforcement scheme: 6 CS1 trials are reinforced by a shock — the first and the fifth trial are unreinforced. Further present 8 CS2 trials as well as 8 NA trials during the inter-trial-intervals.
- Asymptotic Learning: Present a total of 3 CS1 trials on a 100% reinforcement scheme: all CS1 trials are reinforced by a shock. Further present 3 CS2 trials as well as 3 noise alone startle probes (NA) during the inter-trial-intervals.
Counterbalance the assignment of the pictures as CS1 and CS2 across participants. Randomize the order of trial type within blocks of CS1 - CS2 - NA such that no trial-type repeats more than two times. Vary the inter-trial-intervals — during which participants view a black screen — between 15 sec 20 sec and 25 sec with a mean of 20 sec.
After completion of the experiment detach the electrodes and let the participants clean off the gel.
Instruct the participants to judge the pleasantness of the shock on 11-point rating scales ranging from minus 5 (i.e., unpleasant) to 5 (i.e., pleasant).
Explicitly instruct the participants to remember what they had learned during fear acquisition in order to augment retention of the CS-US contingency the following days as well as to prevent them from erroneously expecting a different contingency scheme during subsequent testing. Furthermore, to facilitate the salivary samples the next day, instruct the participants to refrain from exercise caffeine and alcohol during the 12 hr and to avoid food and drinks other than water as well as smoking and tooth brushing during the 2 hr prior to the next session.
Clean the electrodes thoroughly with water after usage: get rid of all the gel without scratching any portion of the AgCl layer. After cleaning do not wipe off the water but use a different set of electrodes on the next participant and allow the other set to dry over time.
3. Memory Reactivation - Day 2
Duration about 105 min.
Randomly assign the participants to either the propranolol HCl or placebo pill condition with the restriction that conditions are matched on STAI scores as close as possible given that trait anxiety determines the fear-reducing effects of disrupting reconsolidation45. Match the participants on SPQ scores as well when using pictures of spiders as the conditioned stimuli.
Obtain blood pressures and heart rate measurements as well as salivary samples: instruct the participants just to place the swab in their mouths for about 3 min. Keep the Salivettes at minus 25°C after removal.
For the propranolol no-reactivation condition go to step 4.8.1.
Position the participants in front of the computer screen and attach the EMG and shock electrodes (see section 1 "Attachment of the Fear Potentiated Startle and Shock Electrodes"). Report to the participants that the intensity of shock will remain the same.
Inform the participants that the same two pictures will be presented on the computer screen and ask them to remember what they had learned the previous day — one of the pictures will again be followed by the electric shock in either most (i.e., non-asymptotic learning) or all (i.e., asymptotic learning) cases whereas the other will never be followed by the shock. Instruct the participants to report the expectancy of an electric shock during the presentation of all pictures. Put on the headphones and dim the lights. Shut the door and start the experiment from the other room.
Begin the memory reactivation by presenting 10 baseline startle probes to diminish initial startle reactivity. Next present a single unreinforced CS1 trial followed by a single NA trial.
Detach the participants from the experimental set-up and let them clean off the gel. Seat them behind the other table.
- Administer “double-blind” an oral dose of placebo pill or 40 mg of propranolol HCl. Pills should be blinded by a pharmacy or colleague independent of the study. Further a physician on call with knowledge of the protocol is considered necessary after drug administration.
- Administer “single-blind” an oral dose of 40 mg of propranolol HCl for the propranolol no-reactivation condition.
Insert a resting period of about 90 min. Offer the participants magazines to read. Clean the electrodes in the meantime.
Again obtain BP and heart rate measurements as well as salivary samples.
4. Test - Day 3
Duration about 45 min.
Obtain BP and heart rate measurements.
Position the participants in front of the computer screen and attach the EMG and shock electrodes. Again report to the participants that the intensity of shock will remain the same.
Instruct the participants that the same two pictures will be presented on the computer screen. Again instruct the participants to report the expectancy of an electric shock during the presentation of all pictures. Put on the headphones and dim the lights. Shut the door and start the experiment from the other room.
Start the experiment by presenting 10 baseline startle probes. During extinction present about 12 unreinforced CS1 and CS2 trials as well as 12 NA trials. After extinction participants get three unsignaled USs: reinstatement. Again present the participants with about 4 unreinforced CS1 and CS2 trials as well as 4 NA trials following the unsignaled USs: reinstatement testing.
Detach the electrodes and let participants clean off the gel. Pay the participants a small amount for their participation in the experiment. Clean the electrodes thoroughly with water.
Representative Results
Manipulation check propranolol: Contrary to pill placebo, both the systolic and diastolic BP as well as the salivary alpha amylase should decrease 90 min after the propranolol intake during memory reactivation, which indicates that the drug manipulation exerted its intended physiological effect. BP and salivary alpha amylase should again return to baseline levels at the test on day 3.
US expectancy ratings: With the current protocol you may not expect any effects of the propranolol treatment on the US expectancy ratings. Irrespective of the pharmacological treatment, the differential US expectancy ratings should increase during fear acquisition and decrease during extinction learning 48 hr later. Moreover the presentation of the unsignaled USs is expected to result in a return of the differential expectancy ratings (Figure 1A-C).
Prerequisite for reconsolidation: In order to trigger the process of reconsolidation, the memory trace should firstly be destabilized as a necessary condition for the restabilization phase. For fear memory destabilization, an expectation of threat at the moment of reactivation (i.e., intact CS1-US expectancy) is necessary31 though not sufficient. Pursuing on the idea that the function of reconsolidation is to update the memory trace to an ever-changing environment, the memory reactivation should also involve a prediction error (PE: a mismatch between what is expected and what actually happens). Given that the US expectancy ratings remain unaffected by the propranolol manipulation, changes in threat expectations from acquisition to test (PE) may serve as an independent indicator of memory destabilization (see 32 for more details). But only when the memory reactivation follows asymptotic learning, these changes in threat expectation may be observed. In case of partially reinforced non-asymptotic learning, a shift in threat expectancies is not necessary for memory destabilization. Hence a retrieval trial may also trigger reconsolidation without a change in expectancies after non-asymptotic learning.
Startle fear responding: Successful fear acquisition on day 1 is demonstrated by an increase in differential startle fear responding (i.e., CS1 vs. CS2) from the first to the last trial of acquisition (Figure 1D-F). Higher startle fear responding to the stimulus that was previously coupled with the shock (CS1) compared to noise alone during memory reactivation on day 2 further indicates that the fear memory is well consolidated.
During the test phase on day 3 you may expect intact startle fear responding in the placebo group: startle responding to the CS1 should be higher compared to the CS2 at the beginning of test. Extinction training subsequently reduces the differential startle responding (i.e., CS1 vs. CS2). Moreover the presentation of the unsignaled USs should result in a return of fear responding to the CS1 compared to the CS2 (Figure 1D).
Contrary to pill placebo, the administration of propranolol HCl is expected to result in an elimination of the differential startle fear responding (i.e., CS1 vs. CS2) at the beginning of the test phase on day 3. Furthermore you may expect that the unsignaled USs will not recover the startle fear responding (Figure 1E).
Note that propranolol should not have any fear-reducing effects when the pill is administered without memory reactivation on day 2 (Figure 1F).
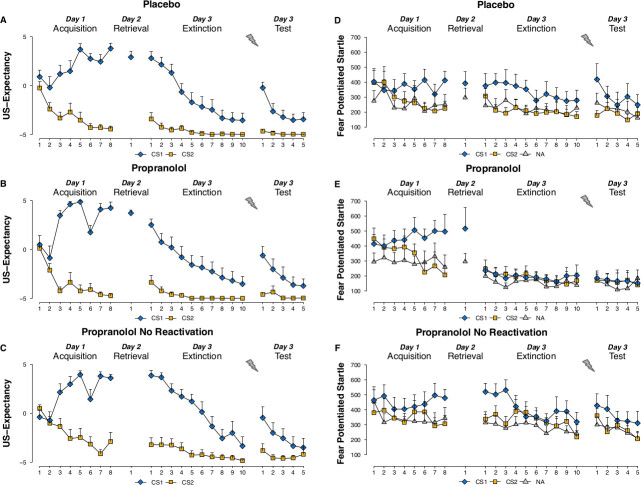 Figure 1.US expectancy ratings to the CS1 and CS2 trials and startle fear responses to the CS1, CS2 and noise alone (NA) trials during acquisition, memory reactivation, extinction and test for the placebo (A,D), propranolol (B,E), and propranolol no- reactivation (C,F) groups. R-CS1- refers to an unreinforced reactivation trial. Please click here to view a larger version of this figure.
Figure 1.US expectancy ratings to the CS1 and CS2 trials and startle fear responses to the CS1, CS2 and noise alone (NA) trials during acquisition, memory reactivation, extinction and test for the placebo (A,D), propranolol (B,E), and propranolol no- reactivation (C,F) groups. R-CS1- refers to an unreinforced reactivation trial. Please click here to view a larger version of this figure.
Discussion
In a series of studies we consistently demonstrate that, irrespective of gender, 40 mg of the noradrenergic beta-blocker propranolol either administered prior to or after memory reactivation effectively neutralized the conditioned fear responding (i.e., defensive startle response). None of the four mechanisms of relapse — reinstatement, renewal, spontaneous recovery and rapid reacquisition — were observed after disrupting reconsolidation with propranolol27-32,37. It is noteworthy that the fear-neutralizing effects were only observed for the defensive startle response, but neither for the threat expectancy rating nor for the skin conductance. The data for the electrodermal conditioning are not reported here as the general patterns for the SCR did not significantly deviate from the expectancy ratings. In human fear conditioning research, multiple indices of conditioned responding (e.g., US-expectancy, SCR, startle response, pupil dilation, neural activity) are usually obtained for reasons of cross-validation46. However, there is now convincing evidence that these different response levels do not necessarily act in concert and may even dissociate from each other3,27-32,34,37,47. Note that the startle response is an automatic defensive reflex, which is potentiated in response to a CS that is associated with a US of negative valence and can generally not be observed with USs of neutral or positive valence47,48 (e.g., vibrotactile stimulation or reaction time task). Hence, potentiation of the defensive startle reflex is a reliable and specific index of aversive conditioning34. In contrast, SCR conditioning can occur irrespective of the valence of the US47,48. Given that affective valence of the US does not modify SCR, electrodermal conditioning is a non-specific measure of anticipatory arousal. We therefore believe that the SCR is less suited as behavioral measure in human aversive conditioning research.
If we speculate on translating these findings into clinical practice, several issues and potential limitations are to be considered. First, it may be questioned whether a pharmacological manipulation by the noradrenergic beta-blocker is really necessary to disrupt the process of reconsolidation or whether a behavioral procedure aimed to interfere with reconsolidation would yield a similar neutralizing effect. Even though a one-session treatment of a low dose of propranolol is clearly nontoxic, an entirely behavioral procedure is always preferable over a pharmacological intervention. There is indeed an alternative method where extinction training is presented within the window of reconsolidation49. Several studies failed however to replicate these original findings by Schiller et al.29,50-52 but see 53,54. In addition to these conflicting results, another potential limitation of the extinction within reactivation procedure would be that in clinical practice the fear response is generally not extinguished in a one-session exposure treatment. For instance, imaginary exposure for patients with PTSD traditionally takes ten sessions before the fear subsides55. Thus, even acknowledging the obvious disadvantages of a simple pharmacological treatment in comparison to an entirely behavioral intervention, we believe that the noradrenergic manipulation of memory reconsolidation seems to be more feasible than extinction within the reconsolidation window.
A second issue concerns the optimal conditions to trigger memory reconsolidation. There is growing evidence that the mechanisms mediating the behavioral expression of fear are clearly dissociated from the mechanisms mediating the process of reconsolidation31,32,56-60. For instance, recent animal studies uncovered differential and dissociable receptors in the basolateral amygdala mediating the expression, destabilization and restabilization of previously conditioned fear memories61,62. A behavioral expression of fear memory is not only dissociated from processes mediating memory reactivation (i.e., access to a memory trace), it seems also not being indispensable for reconsolidation to occur61. As such, fear expression during memory reactivation is not informative on whether the memory trace enters a labile phase. Given that memory destabilization is a prerequisite for the noradrenergic beta-blocker to interfere with the restabilization process, an important question is how we can infer successful memory destabilization in clinical practice. A host of findings indicate that a crucial factor in inducing reconsolidation and in demarcating reconsolidation from either memory retrieval or the consolidation of a novel memory38,63 is the degree of prediction error induced during memory retrieval31,32. But given that no objective criterion is available to determine the optimal degree of prediction error in clinical practice, the current laboratory findings cannot easily be translated to treatment protocols.
Another challenge for translating the neuroscience literature into clinical practice concerns the ecological validity of the paradigm. The evidence for disrupting reconsolidation has mainly been shown in animals and humans for relatively new (one day old) and simple fear memories (i.e., tone shock; picture shock). It is not self-evident that disrupting reconsolidation of older, stronger and broader memory networks such as in patients with PTSD is as effective as it has been shown in the laboratory for cued fear conditioning. Also with respect to the dependent variable it is still unclear whether the observations from the animal and human laboratory studies generalize to patients with anxiety disorders. The fear reducing effects are thus far mainly demonstrated for the behavioral expression of aversive conditioning (i.e., freezing behavior in rodents or defensive startle reflex in humans), with only one exception where we demonstrated that also the subjective feelings of distress were significantly neutralized by noradrenergic blockade of memory reconsolidation37. It may be questioned whether these fear-reducing effects in the laboratory are indicative of the typical experiences of fear and avoidance behavior characteristic of patients with anxiety disorders. Future research should investigate whether the current findings indeed generalize to avoidance behaviors, one of the central symptoms of anxiety disorders.
In sum, although the Pavlovian aversive conditioning procedure is an excellent tool to study the basic mechanisms of fear learning and memory, we cannot easily translate the laboratory findings into clinical practice. The insights that we have been acquiring on the optimal, boundary and necessary conditions for memory reconsolidation should only be considered as a starting point for the development of reconsolidation-based treatments. On the other hand, the extensive research on extinction training has resulted in extinction-based exposure interventions, which still belong to the most effective treatments for anxiety disorders and other related disorders. Given that the noradrenergic blockade of memory reconsolidation overshadows the anxiolytic effect of extinction learning, disrupting reconsolidation points to a promising new intervention to effectively reduce excessive and irrational fears.
Disclosures
Authors have nothing to disclose.
Acknowledgments
This work is supported by a VICI grant (Merel Kindt) from the Netherlands Organization for Scientific Research.
References
- LeDoux JE. The Emotional Brain. New York: Simon & Schuster; 1996. [Google Scholar]
- Rescorla RA, Holland PC. Behavioral studies of associative learning in animals. Ann. Rev. Psychol. 1982;33:265–308. [Google Scholar]
- LeDoux JE. Coming to terms with fear. PNAS. 2014;111(8):2871–2878. doi: 10.1073/pnas.1400335111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. The biology of memory: a forty-year perspective. J. Neurosci. 2009;29(41):12748–12756. doi: 10.1523/JNEUROSCI.3958-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorders: It’s not what you thought it was. Am. Psychol. 2006;6(1):10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- Rachman S. The conditioning theory of fear acquisition: a critical examination. Beh. Res. Ther. 1977;15(5):375–387. doi: 10.1016/0005-7967(77)90041-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles CR. Contextual control of the extinction of conditioned fear. Learn. Mot. 1979;10(4):455–466. [Google Scholar]
- Rescorla RA. Experimental Extinction. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 2001. [Google Scholar]
- Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned context. J. Exp. Psychol. 1975;1(1):88–98. [PubMed] [Google Scholar]
- Baum M. Spontaneous recovery from the effects of flooding in animals. Beh. Res. Ther. 1988;26(2):185–186. doi: 10.1016/0005-7967(88)90118-0. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigm of Pavlovian learning. Psychol. Bull. 1993;114(1):80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion: Clues from the brain. Ann. Rev. Psychol. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol. Psych. 2002;52(10):976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406(6797):722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn. Mem. 2000;7(2):73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidation, Or, How stable is the engram? Ann. Rev. Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat. Rev. 2007;8(4):262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Lee JLC. Reconsolidation: maintaining memory relevance. Trends. Neurosci. 2009;32(8):413–420. doi: 10.1016/j.tins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, Doyere V, Nader K, LeDoux JE. Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. PNAS. 2006;130(9):3428–3433. doi: 10.1073/pnas.0507168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyere V, Debiec J, Monfils M-H, Schafe GE, LeDoux JE. Synapse-specific reconsolidation of distinct fear memories in the lateral amygdala. Nat. Neurosci. 2007;10(4):414–416. doi: 10.1038/nn1871. [DOI] [PubMed] [Google Scholar]
- Canal CE, Chang Q, Gold PE. Amnesia produced by altered release of neurotransmitters after intra-amygdala injections of a protein synthesis inhibitor. PNAS. 2007;104(30):12500–12505. doi: 10.1073/pnas.0705195104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Gold PE. Intrahippocampal infusions of anisomycin produce amnesia: Contribution of increased release of norepinephrine, dopamine. 2009;16(5):308–314. doi: 10.1101/lm.1333409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockers R, et al. Desensitization of the beta-adrenergic response in human brown adipocytes. Endocrinology. 1998;139(6):2676–2684. doi: 10.1210/endo.139.6.6050. [DOI] [PubMed] [Google Scholar]
- Chaundhry A, Granneman JG. Differential regulation of function responses by beta-adrenergic receptor subtypes in brown adipocytes. Am. J. Physiol. 1999;227(1 Pt 2):137–153. doi: 10.1152/ajpregu.1999.277.1.R147. [DOI] [PubMed] [Google Scholar]
- Thonberg H, Frederiksson JM, Nedergaard J, Cannon B. A novel pathway for adrenergic stimulation of camp-response-element-binding protein (CREB) phosphorylation: mediation via alpha-adrenoceptors and protein kinase C activation. Biochem. J. 2002;264(1):73–79. doi: 10.1042/bj3640073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala) Neuroscience. 2004;129(2):267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responding and preventing the return of fear. Nat. Neurosci. 2009;12(3):256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Dissociating response systems: erasing fear from memory. Neurobiol. Learn. Mem. 2010;94(1):30–41. doi: 10.1016/j.nlm.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Disrupting reconsolidation: pharmacological and behavioral manipulations. Learn. Mem. 2011;18(6):357–366. doi: 10.1101/lm.2148511. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Stimulation of the noradrenergic system during memory formation impairs extinction learning but not the disruption of reconsolidation. Neuropsychopharmacology. 2012;37(5):1204–1215. doi: 10.1038/npp.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M. Retrieval per se is not sufficient to trigger reconsolidation of human fear memory. Neurobiol. Learn. Mem. 2012;97(3):338–345. doi: 10.1016/j.nlm.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M. Prediction error governs pharmacologically induced amnesia for learned fear. Science. 2013;339(6121):830–833. doi: 10.1126/science.1231357. [DOI] [PubMed] [Google Scholar]
- Gilman AG, Goodman LS. Goodman and Gilman’s the pharmacological basis of therapeutics. New York: McGraw-Hill; 1996. [Google Scholar]
- Hamm AO, Weike AI. The neuropsychology of fear learning and fear regulation. Int. J. Psychophysiol. 2005;57(1):5–14. doi: 10.1016/j.ijpsycho.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear potentiated startle. Am. Psychol. 2006;61(8):741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Landis C, Hunt WA. The startle pattern. New York: Ferrar; 1939. [Google Scholar]
- Soeter M, Kindt M. Erasing fear for an imagined threat event. Psychoneuroendocrinology. 2012;37(11):1769–1779. doi: 10.1016/j.psyneuen.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Reconsolidation of associative fear memory is not restricted to exact replica of the original learning experience. In Press doi: 10.3389/fnbeh.2015.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M. Prediction error uncovers the transition from retrieval to reconsolidation, to new learning. In Press doi: 10.1101/lm.035493.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos MGN, Beckers T, Kindt M. The effects of noradrenergic blockade on extinction in humans. Biol. Psychol. 2012;89(3):598–605. doi: 10.1016/j.biopsycho.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Eisenberg M, Kobilo T, Berman DE, Dudai Y. Stability of retrieved memory: inverse correlation with trace dominance. Science. 2003;301(5636):1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- Pedreira ME, Perez-Cuesta KM, Maldonado H. Mismatch between what is expected and what actually occurs triggers memory reconsolidation or extinction. Learn. Mem. 2004;11(5):579–585. doi: 10.1101/lm.76904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcato C, Argibay PF, Pedreira ME, Maldonado H. Human reconsolidation does not always occur when a memory is retrieved: the relevance of the reminder structure. Neurobiol. Learn. Mem. 2009;91(1):50–57. doi: 10.1016/j.nlm.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Luthene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press; 1970. [Google Scholar]
- Peterson RA, Reiss S. Anxiety Sensitivity Index Manual. International Diagnostic System. Worthington. 1992.
- Klorman R, Weerts TC, Hastings JE, Melamed GBG, Lang PJ. Psychometric description of some specific fear quistionnaires. Behav. Ther. 1974;5(3):401–409. [Google Scholar]
- Soeter M, Kindt M. High trait anxiety: a challenge for disrupting fear memory reconsolidation. PloSONE. 2013;8(11):e75239. doi: 10.1371/journal.pone.0075239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Well SM, Visser RM, Scholte HS, Kindt M. Neural substrates of individual differences in human fear learning: evidence from concurrent fMRI, fear-potentiated startle and US-expectancy data. Cog. Affec. Beh. Neurosci. 2012;12(3):499–512. doi: 10.3758/s13415-012-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp OV, Sheridan J, Siddle DAT. Human blink startle during aversive and nonaversive Pavlovian conditioning. J. Exp. Psychol. 1994;20(4):380–389. [PubMed] [Google Scholar]
- Hamm AO, Vaitl D. Affective learning: awareness and aversion. Psychophysiology. 1996;33(6):698–710. doi: 10.1111/j.1469-8986.1996.tb02366.x. [DOI] [PubMed] [Google Scholar]
- Schiller D, et al. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2012;463(7277):49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M, Soeter M. Reconsolidation in a human fear conditioning study: a test of extinction as updating mechanism. Biol. Psychol. 2013;92(1):43–50. doi: 10.1016/j.biopsycho.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Golkar A, et al. Are fear memories erasable? - Reconsolidation of learned fear with fear-relevant and fear-irrelevant stimuli. Front. Behav. Neurosci. 2012;6:1–11. doi: 10.3389/fnbeh.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WYM, Leung HT, Westbrook F, McNally GP. Effects of recent exposure to a conditionied stimulus on extinction of Pavlovian fear conditioning. Learn. Mem. 2010;17(10):512–521. doi: 10.1101/lm.1912510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agren T, Furmakr T, Eriksson E, Redrikson M. Human fear reconsolidation and ellelic differences in serotonergic and dopaminergic genes. Trans. Psych. 2012;2:e76. doi: 10.1038/tp.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyarzun JP, et al. Updating Fearful Memories with Extinction Training during Reconsolidation: A Human Study using Auditory Aversive Stimuli. PloSONE. 2012;7(6):e38849. doi: 10.1371/journal.pone.0038849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnen A, Hagenaars M. Fear activation and habituation patterns as early process predictors of response to prolonged exposure treatment in PTSD. J. Traum. Stress. 2002;15(5):359–367. doi: 10.1023/A:1020177023209. [DOI] [PubMed] [Google Scholar]
- Balderas I, Rodriguez-Ortiz CJ, Bermudez-Rattoni F. Retrieval and reconsolidation of object recognition memory are independent processes in the periphinal cortex. Neurosci. 2013;253:398–405. doi: 10.1016/j.neuroscience.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Ben Mamou C, Gamache K, Nader K. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat. Neurosci. 2006;9(10):1237–1239. doi: 10.1038/nn1778. [DOI] [PubMed] [Google Scholar]
- Caffaro PA, Suarez LD, Blake MG, Delorenzi A. Dissociation between memory reactivation and its behavioral expression: scopolamine interferes with memory expression without disrupting long-term storage. Neurobiol. Learn. Mem. 2012;98(3):235–245. doi: 10.1016/j.nlm.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Coccoz V, Maldonado H, Delorenzi A. The enhancement of reconsolidation with a naturalistic mild stressor improves the expression of declarative memory in humans. Neurosci. 2011;185:61–72. doi: 10.1016/j.neuroscience.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Ortiz CJ, Balderas I, Garcia-DeLaTorre P, Bermudez-Rattoni F. Taste aversion memory reconsolidation is independent of its retrieval. Neurobiol. Learn. Mem. 2012;98(3):215–219. doi: 10.1016/j.nlm.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Barreiro KA, Suarez LD, Lynch VM, Molina VA, Delorenzi A. Memory expression is independent of memory labilization-reconsolidation. Neurobiol. Learn. Mem. 2013;106:283–291. doi: 10.1016/j.nlm.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Milton AL, Merlo E, Ratano P, Gregory BL, Dumbreck JK, Everitt BJ. Double dissociation of the requirement for GluN2B and GluN2A-containing NMDA receptors in the destabilization and restabilization of a reconsolidation memory. J. Neurosci. 2013;33(3):1109–1115. doi: 10.1523/JNEUROSCI.3273-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo E, Milton AL, Goozee ZY, Theobald DE, Everitt BJ. Reconsolidation and extinction are dissociable and mutually exclusive processes: behavioral and molecular evidence. J. Neurosci. 2014;34(7):2422–2431. doi: 10.1523/JNEUROSCI.4001-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


