Abstract
Objective. Since oligodendrocyte progenitor cells (OPCs) are the target cells of neonatal hypoxic-ischemic encephalopathy (HIE), the present study was aimed at investigating the protective effects of N-acetyl-l-cysteine (NAC), a well-known antioxidant and precursor of glutathione, in OPCs as well as in neonatal rats. Methods. In in vitro study, protective effects of NAC on KCN cytotoxicity in F3.Olig2 OPCs were investigated via MTT assay and apoptotic signal analysis. In in vivo study, NAC was administered to rats with HIE induced by hypoxia-ischemia surgery at postnatal day 7, and their motor functions and white matter demyelination were analyzed. Results. NAC decreased KCN cytotoxicity in F3.Olig2 cells and especially suppressed apoptosis by regulating Bcl2 and p-ERK. Administration of NAC recovered motor functions such as the using ratio of forelimb contralateral to the injured brain, locomotor activity, and rotarod performance of neonatal HIE animals. It was also confirmed that NAC attenuated demyelination in the corpus callosum, a white matter region vulnerable to HIE. Conclusion. The results indicate that NAC exerts neuroprotective effects in vitro and in vivo by preserving OPCs, via regulation of antiapoptotic signaling, and that F3.Olig2 human OPCs could be a good tool for screening of candidates for demyelinating diseases.
1. Introduction
Cerebral palsy (CP) resulting from hypoxic-ischemic encephalopathy (HIE) is one of the most devastating neurological diseases in children exhibiting diverse neurobehavioral symptoms including motor, perceptual, visual, behavioral, and cognitive disorders [1, 2]. HIE during delivery and/or intrauterine infection has been reported to be an important feature in many cases with white matter injury (WMI) showing periventricular leukomalacia (PVL) [1, 3]. Oligodendrocyte progenitor cells (OPCs) and immature oligodendrocytes were found to be particularly susceptible to free radicals and inflammatory cytokines induced by hypoxic-ischemic insults [1, 2, 4]. Therefore, loss of OPCs and immature oligodendrocytes during their maturation process of the central nervous system (CNS) is a key feature of HIE [1, 4].
Earlier, we established a human OPC line (HB1.F3.Olig2) by transducing HB1.F3 human neural stem cell (NSC) line with a retroviral vector encoding Olig2, an essential regulator of oligodendrocyte development, and confirmed the characteristics for the oligodendrocyte lineage cells [5, 6]. Since OPCs are the target cells of HIE [1, 2, 4], we hypothesized that compounds displaying protective activity against hypoxic cytotoxicity of OPCs would be more effective in HIE animal models than those screened from neuronal culture.
In the treatment of HIE, current therapeutic strategies are very limited and restricted to supportive intensive care such as clinical hypothermia [7–9]. Recently, experimental administration of antioxidative, anti-inflammatory, and neuroprotective compounds such as vitamin C (ascorbic acid), N-acetyl-l-cysteine (NAC), minocycline, and erythropoietin was found to attenuate WMI and delay the progress of physical dysfunction [10]. However, there are controversial results on the effects of vitamin C, minocycline, and erythropoietin in animal models or in humans [11–13].
Vitamin C and NAC are well-known broad spectrum antioxidants used as drugs and functional heath foods. NAC, an antidote of acetaminophen overdose, especially has been proposed as a candidate for treatment of vascular and nonvascular neurological disorders [14]. Indeed, it was reported that NAC prevented the decrease in myelin-related gene expression of primary oligodendrocytes exposed to cytokines [15], which was focused on multiple sclerosis (MS), another adult demyelinating disease, using mature oligodendrocytes. Paintlia et al. [16] demonstrated that NAC treatment attenuated lipopolysaccharide- (LPS-) induced degeneration of OPCs in developing rat brain. Although there are no clear differences in the pathogenic mechanisms and factors between CPs caused by hypoxia during delivery and intrauterine infection, their study is mainly describing anti-inflammatory activity of NAC. In the present study, we assessed the protective effects of NAC and vitamin C against the cytotoxicity of potassium cyanide (KCN), a well-known hypoxia-inducing neurotoxin [17] as a model of hypoxic OPCs damage.
As animal models for HIE, ischemia (carotid artery ligation) followed by hypoxia (8% O2 for 2 h) caused demyelination and neurobehavioral abnormalities in rats [18–20]. In order to confirm our hypothesis, we assessed in vitro cytoprotective effects of NAC in F3.Olig2 human OPCs as well as the neuroprotective activities of NAC in HI animals.
2. Materials and Methods
2.1. F3.Olig2 Human OPCs
An immortalized NSC line (F3) was established from primary cultures of a 15-week gestational human fetal brain by infecting with a retroviral vector encoding v-myc oncogene [21]. The Clinical Research Screening Committee involving Human Subjects of the University of British Columbia (Ethics Committee) approved the use of the fetal tissue, and the fetal tissues were obtained from the Anatomical Pathology Department of Vancouver General Hospital. Subsequently, F3.Olig2 cells were obtained by transducing F3 NSCs with a retroviral vector encoding Olig2. The characteristics of F3.Olig2 cells as oligodendrocyte lineage cells were confirmed with immunoreactivity to O4 and CNPase, specific markers for OPCs [5, 6].
2.2. MTT Assay for KCN Cytotoxicity
The protective effects of NAC and vitamin C against KCN cytotoxicity were determined via 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. Briefly, F3.Olig2 cells (1 × 106 cells/mL) were seeded in each well containing 100 μL of Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 1% d-glutamine, 100 μg/mL gentamicin, and 2.5 U/mL amphotericin B in a 96-well plate. After 2 h incubation, the cells were treated with various concentrations (0.25–7.5 mM) of NAC or vitamin C, followed immediately by KCN (5 mM). After 18 h incubation and washing twice with fresh medium, 10 μL of MTT (5 mg/mL DMEM, filtered) was added and incubated for additional 2 h. The medium was discarded, and the formazan blue formed in the living cells was dissolved with 50 μL of dimethyl sulfoxide. The optical density was measured at 570 nm in 30 min using an ELISA reader (Molecular Devices, Sunnyvale, CA, USA). The experiments were performed 5 times, and mean values were presented.
2.3. Flow Cytometric Analysis of Annexin V
In order to investigate antiapoptotic effects, F3.Olig2 cells (2 × 106) were treated with various concentrations of NAC (0.5–5 mM) or vitamin C (0.15 mM), followed by KCN (5 mM) for 18 h. Induction of apoptosis was measured by flow cytometry using an Annexin V-FITC/PI double staining kit according to the manufacturer's indications (Enzo Life Sciences, Lörrach, Germany). In brief, cells (1 × 106 cells/mL) were suspended in 1x binding buffer, and Annexin V (1 μL) and propidium iodide (PI, 2.5 μL) were added to 96 μL cell suspension. The mixture was kept on ice for 10 min in the dark and analyzed by FACScan (Becton Dickinson, Mountain View, CA, USA). The experiments were performed 5 times, and representative analytical data and mean values were presented.
2.4. Western Blot Analysis of Apoptosis-Related Molecules
F3.Olig2 cells were lysed in 1% RIPA buffer containing protease and phosphatase inhibitors (Roche, Mannheim, Germany) and whole cell lysates were separated by 10% SDS-PAGE. After electrophoresis, proteins were transferred onto polyvinylidene fluoride membranes and the membranes were blocked with 5% skim milk in Tris-buffered saline solution containing 0.1% Tween-20. The membrane was then immunoblotted with primary antibodies such as anti-Bcl2, anti-Bad, anti-extracellular signal-regulated protein kinase (ERK), anti-phosphor-ERK (p-ERK), and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), which was followed by incubation with horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibodies (Cell Signaling Technology, Beverly, MA, USA). Blots were developed using an ECL solution and exposed to X-ray film (Amersham Bioscience, Piscataway, NJ, USA). The experiments were performed 5 times, and representative bands and mean densities normalized to GAPDH were presented.
2.5. Hypoxic-Ischemic Encephalopathy Animal Model and Treatment
Pregnant Sprague-Dawley rats were purchased from Daehan-Biolink (Eumseong, Chungbuk, Korea). The animals (n = 7/group) were maintained at a constant temperature (23 ± 2°C), relative humidity of 55 ± 10%, and 12 h light/dark cycle and fed with standard rodent chow and purified water ad libitum. Neonates were obtained from natural delivery, and male pups of postnatal day (PND) 7 underwent HI; that is, their left common carotid artery was occluded and placed in an 8% oxygen/92% nitrogen incubator (36°C) for 2 h [19, 20]. The rats were intraperitoneally administered with NAC (100 mg/kg) 30 min prior to the carotid artery occlusion surgery at PND 7 and once a day to PND 44. Control animals only underwent the Sham operation and vehicle treatment. All experimental procedures were approved and carried out in accordance with the Institutional Animal Care and Use Committee of Laboratory Animal Research Center at Chungbuk National University, Cheongju, Chungbuk, Korea.
2.6. Measurement of Neurobehavioral Functions
2.6.1. Cylinder Test
For the evaluation of forelimb use asymmetry by brain damage, the ratio of right (impaired) forelimb (contralateral to left carotid artery occlusion) use was analyzed at PND 20, 30, and 40 [20]. Each animal was individually placed in a glass cylinder (21 cm in diameter, 34 cm in height) for 3 min. The weight-bearing forepaw to contact the cylinder wall during a full rear was recorded as left (normal), right (impaired), or both. Paw preference was calculated as ((normal forepaw − impaired forepaw)/(normal forepaw + impaired forepaw + both) × 100%).
2.6.2. Locomotor Activity
Spontaneous activities and exploratory behaviors were evaluated using a video tracking system (Smart v2.5; Panlab Technology, Barcelona, Spain), connected to a CCTV (Samsung, Changwon, Gyeongnam, Korea) at PND 20, 30, and 40 [20, 22]. Rats were placed in a quiet chamber with a dim light. The types of movement, that is, resting, slow-moving, and fast-moving times, were recorded for 5 min, and the ratio was analyzed.
2.6.3. Rotarod Performance
Motor balance and coordination were evaluated using a rotarod test system (Panlab Technology) at PND 20, 30, and 40 [20]. Rats were placed on a rotating rod at a constant speed of 12 rpm, and the time it took for the rats to fall off the rod was recorded. The average latency was calculated from 3 consecutive measurements.
2.7. Immunohistochemistry in Brain Sections
In order to confirm the integrity of host myelin at PND 45, the rat brain was perfusion-fixed with 10% paraformaldehyde solution and postfixed in the same solution for 48 h, followed by cryoprotection in 30% sucrose for 72 h. Paraffin-embedded sections were stained with Luxol fast blue (LFB) for examination of myelins. Coronal cryosections in 30 μm thickness were prepared and processed for immunostaining for myelin basic protein (MBP). Brain sections were incubated with primary antibody specific for MBP (1 : 200; rabbit polyclonal, Chemicon, Temecula, CA, USA) overnight at 4°C, followed by Alexa Fluor 594-conjugated anti-rabbit IgG (1 : 1,000; Molecular Probes, Eugene, OR, USA) for 2 h at room temperature [20]. All samples were evaluated immediately after staining and photographed with a laser-scanning confocal microscope (LSM710; Zeiss, New York, NY, USA). In order to quantify the immunoreactivity of host MBP, the photographs were analyzed with a Digital Image Analyzer (Image Inside; Focus, Seoul, Korea) for the red intensity, and expressed as a % of the control (normal) group.
2.8. Statistical Analysis
Data are presented as mean ± standard error. The statistical significance was determined by one-way analysis of variance, followed by post hoc Tukey's multiple-comparison test. P values < 0.05 were considered to be statistically significant.
3. Results
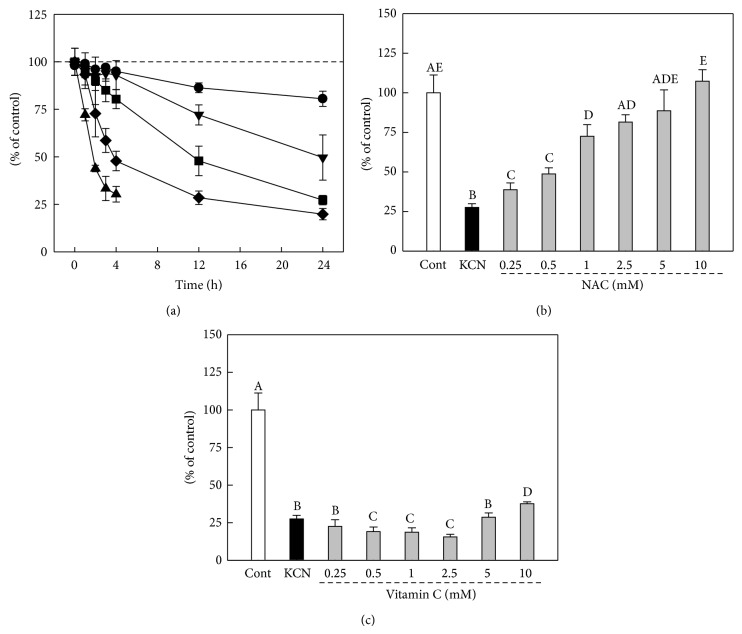
KCN induced cell death of F3.Olig2 OPCs in a concentration- and time-dependent manner as determined by in vitro MTT assay. KCN induced mortalities of 52.2, 71.5, and 80.2% at 4, 12, and 24 h with 5 mM KCN, respectively (Figure 1(a)). We selected 18 h exposure to 5 mM KCN to assess the protective effects of NAC over KCN cytotoxicity. NAC (0.25–10 mM) significantly reversed the cytotoxicity of KCN in a concentration-dependent manner, leading to 72.5% survivability of the cells at 1 mM, compared to 27.6% in vehicle-treated cells (Figure 1(b)). In contrast, vitamin C did not show a protective activity on the KCN cytotoxicity, further decreasing the cell survivability at 0.5–2.5 mM (Figure 1(c)).
Figure 1.
Effects of NAC and vitamin C on KCN cytotoxicity in F3.Olig2 human oligodendrocyte progenitor cells. (a) Cytotoxicity of KCN in MTT assay. F3.olig2 cells were with various concentrations (●: 0.5 mM, ▼: 1 mM, ■: 2.5 mM, ◆: 5 mM, and ▲: 7.5 mM) of KCN for 18 h. (b, c) F3.Olig2 cells were treated with KCN (5 mM) alone or in combination with NAC (b) or vitamin C (c) and assayed after 18 h incubation. Values with different superscript letters represent a significant difference, P < 0.05.
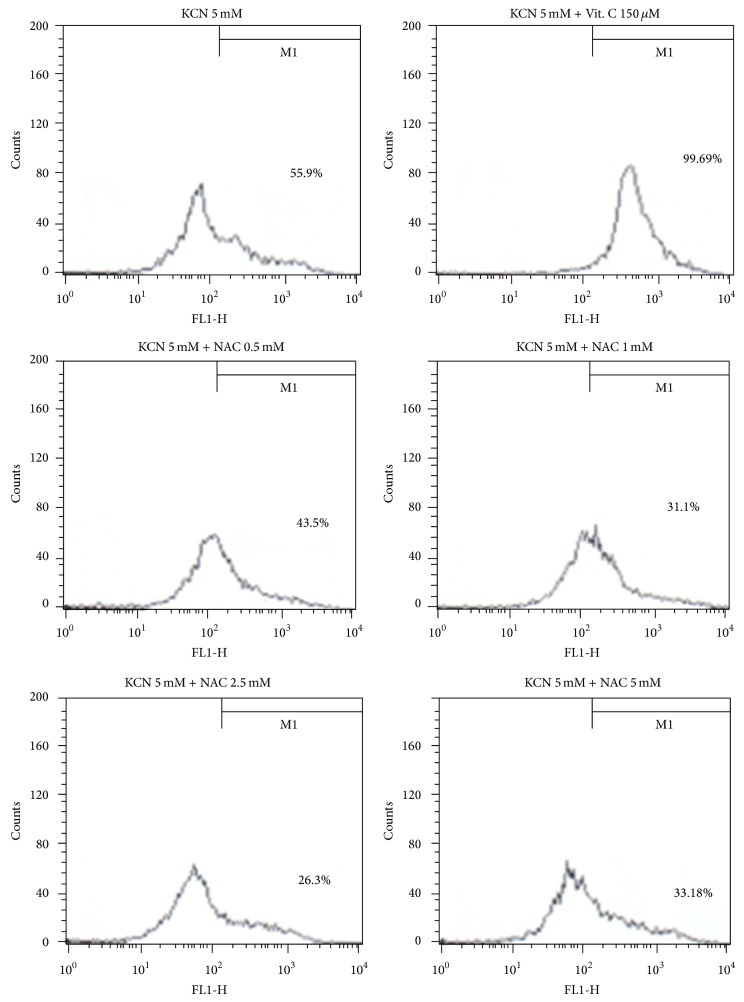
Exposure to KCN (5 mM) caused apoptotic death of F3.Olig2 OPCs, leading to 56.8% Annexin V/PI double positivity (Figure 2, Table 1). Notably, NAC at 0.5–2.5 mM decreased the ratio of apoptotic cells in a concentration-dependent manner, although a higher concentration (5 mM) did not further decrease the ratio. In contrast, vitamin C even a low concentration (0.15 mM) significantly elevated cell death, indicative of an enhanced apoptosis similarly in MTT assay.
Figure 2.
Representative flow cytometric analysis of Annexin V/PI in F3.Olig2 human oligodendrocyte progenitor cells. F3.Olig2 cells were treated with KCN (5 mM) alone or in combination with vitamin C or NAC for 18 h.
Table 1.
| Treatment (mM) | Annexin V positivity (%) | Bcl2 (relative to GAPDH) | Bad (relative to GAPDH) | p-ERK (relative to GAPDH) |
|---|---|---|---|---|
| Vehicle | 3.6 ± 2.7a | 0.28 ± 0.13a | 0.001 ± 0.001a | 0.097 ± 0.015a |
| KCN (5.0) | 56.8 ± 4.8b | 0.11 ± 0.06b | 0.312 ± 0.124b | 0.065 ± 0.011b |
| +vitamin C (0.15) | 98.6 ± 7.4c | 0.21 ± 0.11ab | 0.282 ± 0.152b | 0.068 ± 0.082ab |
| +NAC (0.5) | 42.7 ± 3.4d | 0.37 ± 0.14ac | 0.521 ± 0.206b | 0.124 ± 0.113a |
| +NAC (1.0) | 32.3 ± 5.1df | 0.68 ± 0.20d | 0.341 ± 0.093b | 0.156 ± 0.108c |
| +NAC (2.5) | 26.1 ± 2.8e | 0.91 ± 0.26d | 0.238 ± 0.062b | 0.202 ± 0.077c |
| +NAC (5.0) | 31.3 ± 4.2ef | 0.82 ± 0.22d | 0.110 ± 0.046c | 0.235 ± 0.086c |
Values (n = 5) in the same column with different superscript letters represent a significant difference, P < 0.05.
In order to elucidate the change in apoptosis-related signals, we analyzed the expression of Bcl2, Bad, ERK, and p-ERK in F3.Olig2 cells. KCN markedly increased the expression of Bad, a proapoptotic molecule, while it decreased Bcl2 and p-ERK, the antiapoptotic proteins (Figure 3). NAC (0.5–5 mM) upregulated the expression of Bcl2 and p-ERK in a concentration-dependent manner, to levels higher than in normal cells at 1–5 mM, while vitamin C (0.15 mM) did not affect the expression of signaling molecules in F3.Olig2 OPCs following KCN exposure (Table 1).
Figure 3.
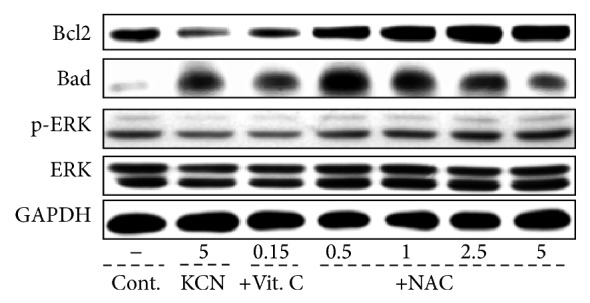
Representative western blot analysis of apoptosis-related proteins in F3.Olig2 human oligodendrocyte progenitor cells. F3.Olig2 cells were treated with KCN (5 mM) alone or in combination with vitamin C or NAC for 18 h.
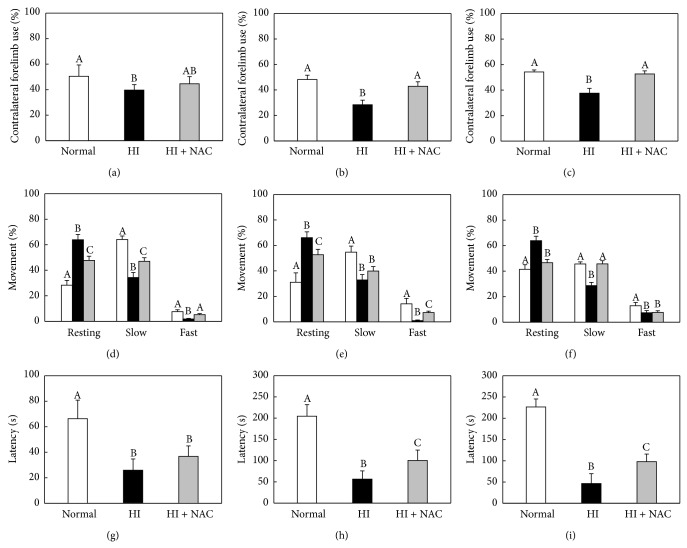
Based on the different effects of NAC and vitamin C on the KCN cytotoxicity in F3.Olig2 cells, we selected NAC as a candidate compound for the treatment of HI model animals. In the cylinder test, normal animals used their left and right forelimbs in similar ratios (50 : 50%) at PND 20, 30, and 40 (Figures 4(a)–4(c)). However, rats subjected to HI at PND 7 showed significantly decreased (<40%) use of the contralateral forelimb at PND 20, 30, and 40. Such a reduced use of the contralateral forelimb was near fully recovered at PND 30 and 40 by daily intraperitoneal administration of NAC (100 mg/kg) from PND 7.
Figure 4.
Recovery of motor functions of HI rats by NAC. Rats were subjected to hypoxic-ischemic (HI) surgery at postnatal day (PND) 7, and behavioral functions in cylinder test (a–c), locomotor activity (d–f), and rotarod performance (g–i) were assessed at PND 20 (a, d, and g), 30 (b, e, and h), and 40 (c, f, and i). White: normal animals, black: HI alone, and gray: HI + NAC. Values with different superscript letters represent a significant difference, P < 0.05.
Normal animals exhibited active movement in locomotor activity analysis, in which the sum (60–70%) of slow-moving and fast-moving times was longer than resting time (30–40%) at all PND 20–40 (Figures 4(d)–4(f)). However, the resting time greatly increased in HI rats (>65%), leading to significant decreases in moving times from PND 20 to PND 40. By comparison, treatment with NAC markedly recovered the HI-induced decrease in global activity.
HI caused impairment of motor coordination in rotarod performance, leading to a marked reduction in the latency time by 60–80% at PND 20–40 (Figures 4(g)–4(i)). Interestingly, however, such decreased rotarod performances were significantly improved at PND 30 and PND 40 by NAC treatment.
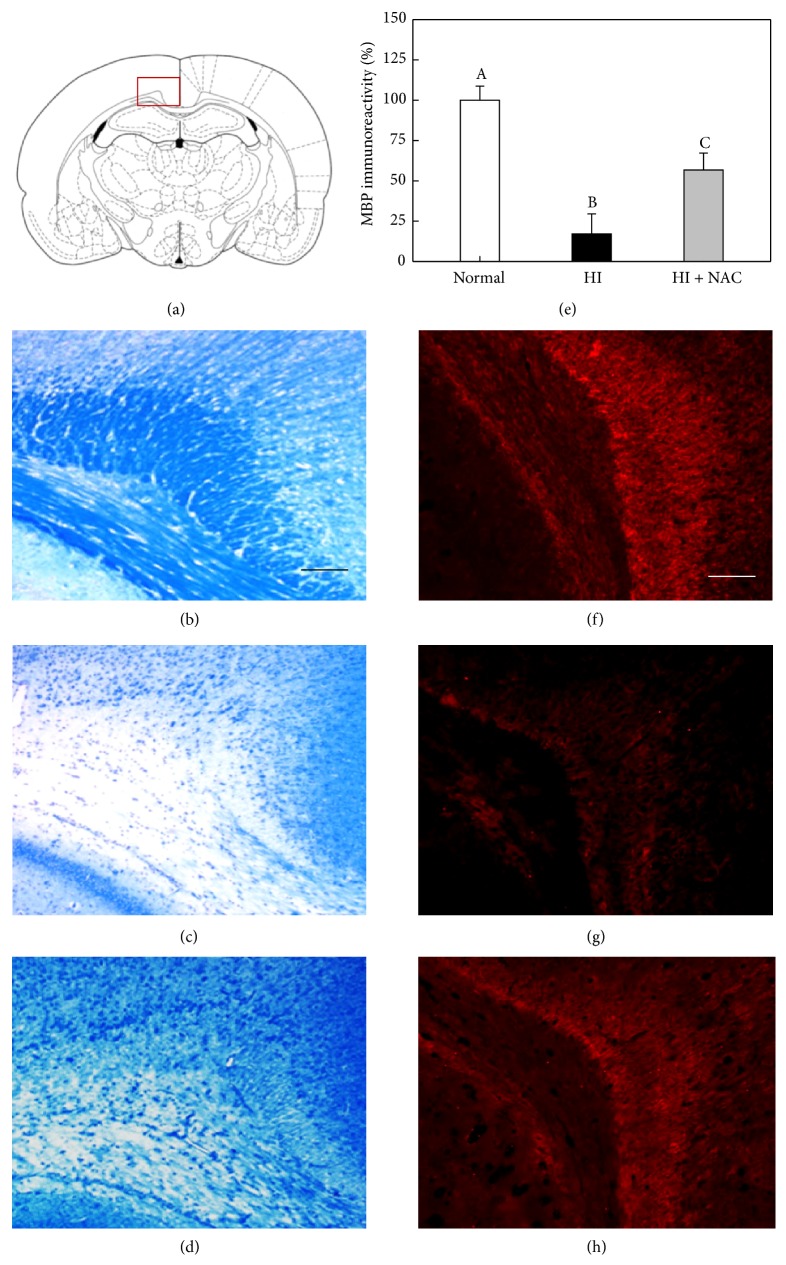
Normal animals exhibited a good integrity of myelins in the corpus callosum, a white matter region vulnerable to HI injury, revealing strong LFB-staining intensity as well as MBP immunoreaction (Figures 5(b) and 5(f)) [20]. By comparison, the LFB-staining intensity and MBP immunoreactivity were markedly reduced in HI animals as observed at PND 45, indicative of a severe demyelination and loss of host oligodendrocytes (Figures 5(c), 5(g), and 5(e)). Notably, however, the HI-induced loss of myelins and MBP was significantly attenuated by NAC treatment (Figures 5(d), 5(h), and 5(e)).
Figure 5.
Recovery of LFB-stained myelins and MBP immunoreactivity in rat corpus callosum by NAC. Integrity of myelins was analyzed with LFB staining and MBP immunoreactivity at postnatal day 45. The square box in (a) represents an examined area of corpus callosum. The brain tissues were stained with LFB (b–d) or MBP antibody (f–h). (b, f) Normal animals, (c, g) HI alone, and (d, h) HI + NAC. Scale bars = 500 μm. Values with different superscript letters in (e) represent a significant difference in MBP immunoreactivity from (f–h), P < 0.05.
4. Discussion
It is well known that damage of OPCs and preoligodendrocytes during the maturation process of the CNS is a key factor for HIE, and the loss of OPCs and hypomyelination of the CNS due to perinatal HI or intrauterine infection are major features of PVL [1, 2, 4]. Although there have been studies on drug screening in vitro using primary oligodendrocytes [15] or neuronal cell lines such as PC12 cells [17], here we suggest human F3.Olig2 OPCs as an appropriate cell system to screen new drug candidates for treatment of PVL.
Among diverse candidate compounds [10], vitamin C has been suggested to be neuroprotective in a neonatal HI model [23]. However, vitamin C is known to act as a prooxidant in the presence of transition metal ions and was shown to cause neurotoxicity in vitro in rodent cortical neurons [24]. In addition to the cytotoxicity in cortical neurons, aggravating effects of vitamin C on the cytotoxicity and apoptosis induced by KCN were observed in F3.Olig2 OPCs. In fact, there are controversial results on the effects of vitamin C in human CP patients [11].
In previous studies, NAC exerted anti-inflammatory and antioxidative potentials in the diverse CNS diseases [25–30]. In cases of multiple sclerosis and traumatic brain injury, NAC suppressed TNF-α-mediated via downregulating the activation of nuclear factor-κB (NF-κB) [25, 26]. Also, in the neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS), Parkinson disease (PD), Huntington disease (HD), and Alzheimer disease (AD), NAC reduced tissue injury and endothelial cell apoptosis by enhancing antioxidant enzymes and/or inhibiting lipid peroxidation [25, 27–29]. NAC also improved microcirculation and tissue oxygenation that may facilitate tissue regeneration in the degenerative diseases [30]. Accordingly, NAC has attracted investigators' attention for its efficacy in diverse vascular and nonvascular neurological disorders [14] including both intrauterine infection and HI models of PVL [9, 10]. In fact, NAC suppressed inflammatory cytokines in amniotic fluid and placenta in LPS-treated rats [31] and displayed neuroprotective activity in LPS-sensitized rat pups exposed to HI [32]. In addition, NAC improved platelet aggregation and hemodynamics in the brain of piglets with hypoxia-reperfusion (IR) injury [33, 34]. Notably, NAC enhanced the efficacy of hypothermia in reducing infarct volume after focal HI brain injury in neonatal rats [35].
Interestingly, NAC prevented endotoxin-induced degeneration of OPCs and hypomyelination in developing rat brain in an intrauterine infection model of PVL [16]. In the present study, we demonstrated neuroprotective effects of NAC both in vitro and in vivo; that is, NAC prevented KCN-induced cytotoxicity of F3.Olig2 human OPCs and hypomyelination in developing rat brain in an HI model of PVL. Although more detailed studies on the mechanism(s) of NAC for the preservation of myelins in vivo remained to be clarified, it is proposed that NAC may protect host OPCs against hypoxic damage as confirmed in in vitro study. In contrast to the aggravating effects of vitamin C, NAC decreased KCN cytotoxicity; that is, NAC preserved F3.Olig2 cell viability impaired by KCN as measured by MTT assay. It is believed that such a protective effect of NAC might be in part due to the regulatory activity related to survival including Bcl2 and p-ERK. NAC upregulated Bcl2/p-ERK prosurvival proteins in a concentration-dependent manner and downregulated Bad proapoptotic protein at a high concentration (5 mM), which may led to a higher survivability of KCN-exposed F3.Olig2 human OPCs. However, is it suggested that NAC preserves mitochondrial function and blocks apoptotic signaling at different concentration ranges, as seen in MTT and Annexin V analyses, respectively.
In spite of extensive studies on the candidate therapeutics for PVL, there are very limited clinical studies on the efficacy of chemical drugs in preterm or perinatal PVL patients. In a randomized clinical trial of preterm newborns, continuous intravenous infusion of NAC (16–32 mg/kg) for 6 days after birth reduced the incidence of PVL, although this was a very preliminary study [36]. It is worthy to note that hypothermia plus NAC combination therapy improves infarct volume, myelin expression, and functional outcomes after focal HI injury in neonatal rats [35].
It should be emphasized that timely delivery of neuroprotective treatment is the most important in neonates suffering from hypoxic injury or intrauterine infection [9]. In a piglet IR model, intravenous injection of NAC (150 mg/kg bolus and 20 or 100 mg/kg/h for 24 h) improved hemodynamics and neural oxidative stress, in addition to platelet aggregation [33, 34]. In the present study, daily intraperitoneal injection of NAC (100 mg/kg) recovered neurobehavioral dysfunction in neonatal HI rats, along with the neuroprotective effect preserving a part of MBP reactivity in the corpus callosum. For an enhanced efficacy of NAC, a dose-range-finding study with diverse administration routes including continuous intravenous infusion is required. Moreover, it is expected that the optimized treatment of NAC in combination with hypothermia or other neuroprotectants could be a promising strategy for the effective intensive care of PVL patients.
To date, there are no effective regimens for the prevention of HIE in CP patients. In the present study, we used F3.Olig2 human OPCs for the first time to screen candidate compounds for PVL. We showed protective effects of NAC, but aggravating potential of vitamin C on KCN cytotoxicity in the F3.Olig2 cells. Furthermore, NAC recovered motor function of HI rats by attenuating the loss of myelins and MBP in the corpus callosum. Therefore, it is suggested that F3.Olig2 human OPCs could serve as a good cell system for the screening of demyelinating diseases and that NAC could be a good candidate for the treatment for HI brain injury.
Acknowledgments
This research was supported by High Value-Added Food Technology Development Program, Ministry of Agriculture, Food and Rural Affairs (MAFRA; Grant no. 113034-3). The authors also thank Eun-Jung Kim, Christine Sungmin Kim, Alice Chang, Solar Sora Kim, and Diana Kim (Dr. Kim Laboratory, UBC Hospital) for their continuous supports during in vitro experiments.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Dongsun Park and Kyungha Shin equally contributed to this work.
References
- 1.Deng W., Pleasure J., Pleasure D. Progress in periventricular leukomalacia. Archives of Neurology. 2008;65(10):1291–1295. doi: 10.1001/archneur.65.10.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi E. K., Park D., Kim T. K., et al. Animal models of periventricular leukomalacia. Laboratory Animal Research. 2011;27(2):77–84. doi: 10.5625/lar.2011.27.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volpe J. J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. The Lancet Neurology. 2009;8(1):110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Back S. A., Han B. H., Luo N. L., et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. The Journal of Neuroscience. 2002;22(2):455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn S.-M., Byun K., Kim D., et al. Olig2-induced neural stem cell differentiation involves downregulation of Wnt signaling and induction of Dickkopf-1 expression. PLoS ONE. 2008;3(12) doi: 10.1371/journal.pone.0003917.e3917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang D. H., Kim B. G., Kim E. J., et al. Transplantation of human neural stem cells transduced with Olig2 transcription factor improves locomotor recovery and enhances myelination in the white matter of rat spinal cord following contusive injury. BMC Neuroscience. 2009;10, article 117:p. 117. doi: 10.1186/1471-2202-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azzopardi D. V., Strohm B., Edwards A. D., et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. The New England Journal of Medicine. 2009;361(14):1349–1358. doi: 10.1056/nejmoa0900854. [DOI] [PubMed] [Google Scholar]
- 8.Edwards A. D., Brocklehurst P., Gunn A. J., et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. The British Medical Journal. 2010;340(7743, article c363) doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cilio M. R., Ferriero D. M. Synergistic neuroprotective therapies with hypothermia. Seminars in Fetal & Neonatal Medicine. 2010;15(5):293–298. doi: 10.1016/j.siny.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rees S., Harding R., Walker D. The biological basis of injury and neuroprotection in the fetal and neonatal brain. International Journal of Developmental Neuroscience. 2011;29(6):551–563. doi: 10.1016/j.ijdevneu.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aly H., Abd-Rabboh L., El-Dib M., et al. Ascorbic acid combined with ibuprofen in hypoxic ischemic encephalopathy: a randomized controlled trial. Journal of Perinatology. 2009;29(6):438–443. doi: 10.1038/jp.2009.1. [DOI] [PubMed] [Google Scholar]
- 12.Tsuji M., Wilson M. A., Lange M. S., Johnston M. V. Minocycline worsens hypoxic-ischemic brain injury in a neonatal mouse model. Experimental Neurology. 2004;189(1):58–65. doi: 10.1016/j.expneurol.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 13.van der Kooij M. A., Groenendaal F., Kavelaars A., Heijnen C. J., van Bel F. Combination of deferoxamine and erythropoietin: therapy for hypoxia-ischemia-induced brain injury in the neonatal rat? Neuroscience Letters. 2009;451(2):109–113. doi: 10.1016/j.neulet.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Bavarsad Shahripour R., Harrigan M. R., Alexandrov A. V. N-acetylcysteine (NAC) in neurological disorders: mechanisms of action and therapeutic opportunities. Brain and Behavior. 2014;4(2):108–122. doi: 10.1002/brb3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jana M., Pahan K. Redox regulation of cytokine-mediated inhibition of myelin gene expression in human primary oligodendrocytes. Free Radical Biology & Medicine. 2005;39(6):823–831. doi: 10.1016/j.freeradbiomed.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paintlia M. K., Paintlia A. S., Barbosa E., Singh I., Singh A. K. N-acetylcysteine prevents endotoxin-induced degeneration of oligodendrocyte progenitors and hypomyelination in developing rat brain. Journal of Neuroscience Research. 2004;78(3):347–361. doi: 10.1002/jnr.20261. [DOI] [PubMed] [Google Scholar]
- 17.Satpute R. M., Hariharakrishnan J., Bhattacharya R. Alpha-ketoglutarate and N-acetyl cysteine protect PC12 cells from cyanide-induced cytotoxicity and altered energy metabolism. NeuroToxicology. 2008;29(1):170–178. doi: 10.1016/j.neuro.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Girard S., Kadhim H., Beaudet N., Sarret P., Sébire G. Developmental motor deficits induced by combined fetal exposure to lipopolysaccharide and early neonatal hypoxia/ischemia: a novel animal model for cerebral palsy in very premature infants. Neuroscience. 2009;158(2):673–682. doi: 10.1016/j.neuroscience.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 19.Park D., Kim T. K., Choi Y. J., et al. Experimental models of cerebral palsy in infant rats. Laboratory Animal Research. 2010;26(4):345–351. doi: 10.5625/lar.2010.26.4.345. [DOI] [Google Scholar]
- 20.Park D., Lee S. H., Bae D. K., et al. Transplantation of human adipose tissue-derived mesenchymal stem cells restores the neurobehavioral disorders of rats with neonatal hypoxic-ischemic encephalopathy. Cell Medicine. 2013;5(1):17–28. doi: 10.3727/215517913x658936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee H. J., Kim K. S., Kim E. J., et al. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells. 2007;25(5):1204–1212. doi: 10.1634/stemcells.2006-0409. [DOI] [PubMed] [Google Scholar]
- 22.Park D., Yang Y.-H., Bae D. K., et al. Improvement of cognitive function and physical activity of aging mice by human neural stem cells over-expressing choline acetyltransferase. Neurobiology of Aging. 2013;34(11):2639–2646. doi: 10.1016/j.neurobiolaging.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 23.Miura S., Ishida-Nakajima W., Ishida A., et al. Ascorbic acid protects the newborn rat brain from hypoxic-ischemia. Brain and Development. 2009;31(4):307–317. doi: 10.1016/j.braindev.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Hisanaga K., Sagar S. M., Sharp F. R. Ascorbate neurotoxicity in cortical cell culture. Annals of Neurology. 1992;31(5):562–565. doi: 10.1002/ana.410310516. [DOI] [PubMed] [Google Scholar]
- 25.Stanislaus R., Gilg A. G., Singh A. K., Singh I. N-acetyl-L-cysteine ameliorates the inflammatory disease process in experimental autoimmune encephalomyelitis in Lewis rats. Journal of Autoimmune Diseases. 2005;2(1, article 4) doi: 10.1186/1740-2557-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen G., Shi J., Hu Z., Hang C. Inhibitory effect on cerebral inflammatory response following traumatic brain injury in rats: a potential neuroprotective mechanism of N-Acetylcysteine. Mediators of Inflammation. 2008;2008:8. doi: 10.1155/2008/716458.716458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louwerse E. S., Weverling G. J., Bossuyt P. M. M., Meyjes F. E. P., de Jong J. M. B. V. Randomized, double-blind, controlled trial of acetylcysteine in amyotrophic lateral sclerosis. Archives of Neurology. 1995;52(6):559–564. doi: 10.1001/archneur.1995.00540300031009. [DOI] [PubMed] [Google Scholar]
- 28.Martínez M., Martínez N., Hernández A. I., Ferrándiz M. L. Hypothesis: can N-acetylcysteine be beneficial in Parkinson's disease? Life Sciences. 1999;64(15):1253–1257. doi: 10.1016/s0024-3205(98)00472-x. [DOI] [PubMed] [Google Scholar]
- 29.Sen O., Caner H., Aydin M. V., et al. The effect of mexiletine on the level of lipid peroxidation and apoptosis of endothelium following experimental subarachnoid hemorrhage. Neurological Research. 2006;28(8):859–863. doi: 10.1179/016164106X115099. [DOI] [PubMed] [Google Scholar]
- 30.Cuzzocrea S., Mazzon E., Costantino G., et al. Beneficial effects of n-acetylcysteine on ischaemic brain injury. British Journal of Pharmacology. 2000;130(6):1219–1226. doi: 10.1038/sj.bjp.0703421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beloosesky R., Gayle D. A., Amidi F., et al. N-acetyl-cysteine suppresses amniotic fluid and placenta inflammatory cytokine responses to lipopolysaccharide in rats. The American Journal of Obstetrics and Gynecology. 2006;194(1):268–273. doi: 10.1016/j.ajog.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 32.Wang Q., Tang X. N., Yenari M. A. The inflammatory response in stroke. Journal of Neuroimmunology. 2007;184(1-2):53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan E., Obaid L., Johnson S. T., Bigam D. L., Cheung P.-Y. N-acetylcysteine administration improves platelet aggregation in hypoxia-reoxygenation injury. Proceedings of the Western Pharmacology Society. 2007;50:53–57. [PubMed] [Google Scholar]
- 34.Liu J.-Q., Lee T.-F., Chen C., Bagim D. L., Cheung P.-Y. N-acetylcysteine improves hemodynamics and reduces oxidative stress in the brains of newborn piglets with hypoxia-reoxygenation injury. Journal of Neurotrauma. 2010;27(10):1865–1873. doi: 10.1089/neu.2010.1325. [DOI] [PubMed] [Google Scholar]
- 35.Jatana M., Singh I., Singh A. K., Jenkins D. Combination of systemic hypothermia and N-acetylcysteine attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatric Research. 2006;59(5):684–689. doi: 10.1203/01.pdr.0000215045.91122.44. [DOI] [PubMed] [Google Scholar]
- 36.Ahola T., Lapatto R., Raivio K. O., et al. N-acetylcysteine does not prevent bronchopulmonary dysplasia in immature infants: a randomized controlled trial. The Journal of Pediatrics. 2003;143(6):713–719. doi: 10.1067/s0022-3476(03)00419-0. [DOI] [PubMed] [Google Scholar]