Abstract
Background
The possibility that hyperglycemia accounts for the 2–3 fold higher risk of ischemic heart disease (IHD) in type 2 diabetes was explored by assessing the effect of intensive glucose lowering on IHD in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial.
Methods
10,251 people (mean age = 62) with type 2 diabetes (mean duration = 10 years, mean A1c = 8.3%) were allocated to intensive or standard glycemic control targeting an A1c <6% or 7–7.9% respectively. This intervention’s effect on IHD (fatal or non-fatal myocardial infarction, coronary revascularization, unstable angina, and new angina) was assessed during a mean active treatment period of 3.7 years followed by an additional 1.2 years.
Results
Fewer participants allocated to the intensive versus standard care group experienced a myocardial infarction during both active treatment (HR = 0.80; 95% CI 0.67 – 0.96; P = 0.015) and full (active and additional) follow-up (HR = 0.84; 95% CI 0.72 – 0.97; P = 0.02). Similar findings were observed for a composite IHD outcome of myocardial infarction, coronary revascularization or unstable angina (HR = 0.89; 95% CI 0.79–0.99 and HR = 0.87; 95% CI 0.79 – 0.96 during active treatment and full follow-up respectively)and for coronary revascularization (HR = 0.84; 95% CI 0.75–0.94), and unstable angina (HR = 0.81; 95% CI 0.67–0.97) during full follow-up. Adding A1C levels achieved during active treatment attenuated the significant hazard ratios to neutrality.
Conclusions
Glucose elevation is a modifiable risk factor for IHD in middle aged people with type 2 diabetes and other IHD risk factors.
Introduction
People with type 2 diabetes have a 2–3 fold higher incidence of ischemic heart disease (IHD) than people without diabetes, even after accounting for other IHD risk factors1, 2. Reasons for this relationship remain unclear. However as diabetes is defined on the basis of an elevated glucose level3, and as progressively higher A1c levels are related to progressively higher incidence of IHD4, an elevated glucose level may be an important contributing factor. This possibility is supported by the observation that 10 years of more versus less intensive glucose lowering reduced the 20 year risk of myocardial infarction (MI) by 15% in people with newly diagnosed type 2 diabetes5. It is also supported by a meta-analysis of data from the 4 large outcomes trials of more versus less intense glucose lowering (conducted by the investigators of these trials) which reported a 15% lower incidence of total MIs (95% CI 6–24) during a mean follow-up period of 4.4 years6.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial was a large North American trial of more versus less intense glucose lowering that was conducted in people with established type 2 diabetes and additional risk factors for cardiovascular disease. As previously reported the intervention had a nonsignificant effect on the primary composite cardiovascular outcome. However it also modestly reduced the incidence of nonfatal myocardial infarctions and increased the risk of death and particularly death from cardiovascular causes. The increased cardiovascular mortality remains unexplained, and exploratory analyses to date have not implicated severe hypoglycemia7, the degree or speed of glucose lowering8 or other potential causes9–11. Conversely the reduced rate of ischemic heart disease in ACCORD remains unexplored. The effect of the ACCORD glycemia intervention on indices of IHD including both fatal and nonfatal MI, angina and new onset angina, and the degree to which any of the above effects may be accounted for by the effect of the glycemia intervention on the A1c level during the active treatment period is therefore reported in this paper.
Methods
Design
The design and results of the ACCORD trial have been previously published12, 13. Briefly, 10,251 men and women aged 40–79 years with type 2 diabetes of mean duration 10 years, a mean glycated hemoglobin (A1c) level of 8.3%, and either previous cardiovascular events or risk factors for cardiovascular disease were recruited from 77 clinical centers in the United States and Canada. Participants were randomly allocated to either intensive glucose-lowering targeting an A1c level <6% or to standard glucose lowering targeting an A1c level of 7–7.9%; the same medications were available to achieve these targets in both groups. These participants were concomitantly enrolled in either a blood pressure trial (in which participants were randomly allocated to either more versus less intense blood pressure lowering)14 or a lipid trial (in which participants all had statin therapy optimized and were then allocated to the addition of either fenofibrate versus placebo)15 using a double factorial design. Follow-up occurred at least every 4 months to facilitate attainment and maintenance of the therapeutic goals and to ascertain the occurrence of outcomes and adverse effects. The ACCORD trial was sponsored by the National Heart, Lung and Blood Institute (NHLBI) and all participants provided written informed consent.
Outcomes and Course of the Trial
The prespecified primary outcome of the ACCORD trial was the first occurrence of nonfatal myocardial infarction, nonfatal stroke or cardiovascular death. Cardiovascular death was defined (in order of observed incidence during follow-up) as: death that was unexpected or presumed to be due to cardiovascular disease; fatal congestive heart failure; fatal myocardial infarction; fatal stroke: “other” cardiovascular disease; fatal procedure; or a fatal arrhythmia12, 13. Secondary outcomes included each component of the primary outcome; death from any cause; an expanded composite comprising the primary outcome or revascularization or hospitalization for heart failure; a composite of cardiovascular death, or nonfatal myocardial infarction or unstable angina; fatal or nonfatal stroke; and fatal or non-fatal congestive heart failure. The ACCORD definitions of the foregoing outcomes are available in a supplementary appendix to a prior publication15. Coronary revascularization was defined as a percutaneous coronary intervention (with or without a stent) or coronary artery bypass surgery. Unstable angina was defined as self-reported new, accelerated or rest angina, plus ischemia on the electrocardiogram and/or evidence of stenosis on angiography, and new onset angina was defined as the first time an event was recorded on the case report form as “new onset exertional angina”. The primary outcome and its’ components were adjudicated centrally by adjudicators unaware of treatment group.
Accruing data were carefully scrutinized by the Data Safety and Monitoring Board which recommended that the intervention be discontinued after they detected an increased all-cause and cardiovascular mortality in the intensive glycemia group. This recommendation was accepted, and participants who had been allocated to the intensive glycemia regimen were switched to the standard regimen on February 5, 2008 after a mean follow-up period of 3.7 years. These participants continued to be followed for a mean of 1.2 years within the factorialized blood pressure or lipid ACCORD trials during which outcomes continued to be ascertained.
Statistical Analysis
All statistical analyses were done at the ACCORD Coordinating Center using SAS 9.3. Continuous data were summarized as means and standard deviations, medians and interquartile ranges, or counts and percentages. Event rates were estimated as the number of events divided by the total person years of follow-up and expressed as N per 100 person years. Analyses were conducted using an intent-to-treat approach based on all outcomes accrued from randomization until the date of transition (February 5, 2008), and from randomization until the end of follow-up. The mean or median A1c, blood pressure, LDL and serum creatinine levels, and the use of cardioprotective drugs at these 2 time-points were counted based on the last data that were available prior to these time-points. Cumulative incidence plots accounting for death as a competing risk16 were used to estimate the cumulative proportion of participants who had each event type during these 2 follow-up periods.
Analyses for this report of the effect of the glycemia intervention on IHD focused on fatal or nonfatal myocardial infarction; coronary revascularization; unstable angina; new onset angina; and 2 composite outcomes: a) the first occurrence of a myocardial infarction, coronary revascularization or unstable angina; and b) the first occurrence of a myocardial infarction or unstable angina. Fine and Gray’s approach17 for fitting proportional subdistribution hazard models to survival data with competing risks was used to estimate the hazard ratio and 95% confidence intervals during these 2 follow-up periods. These models included a term representing glycemia group assignment plus terms accounting for participation in the blood pressure or lipid trial, assignment to the intensive blood pressure intervention in the blood pressure trial, assignment to fenofibrate in the lipid trial, and the presence or absence of prior cardiovascular disease. To determine whether any effect of the intervention on analyzed outcomes could be attributable to the A1c difference achieved during the active (pre-transition) treatment period, an exploratory analysis was conducted in which the A1c levels achieved during this period were included as time-dependent covariates (i.e. the covariate changed when new results were obtained every 4 months) in the proportional subdistribution hazard models and the hazard ratios were re-estimated. P-values of < 0.05 were considered to be significant.
The funder (National Heart Lung and Blood Institute) participated in the design of the ACCORD study. All of the trial data were collected, stored and analyzed by the trial coordinating center at Wake Forest University on behalf of the investigators. All authors vouch for the accuracy and completeness of the reported data and are responsible for the decision to submit the manuscript.
Results
Participants were followed for a mean of 3.7 years until the date of transition and mean of 4.8 years until the end of follow-up. As noted in Table 1, the mean age at randomization was 62 years, 39% were women, 18% reported a prior myocardial infarction, 11% reported prior angina, and 22% reported a prior revascularization. At the end of both the active glycemia treatment period (mean of 3.7 years) and the full follow-up period (mean of 4.8 years), participants allocated to the intensive or standard glycemia intervention had similar mean blood pressures, LDL cholesterol levels and serum creatinine levels, and similar proportions of participants in each group were using statins, renin-angiotensin system drugs (i.e. ACE inhibitors or angiotensin receptor blockers) and aspirin (Table 1). As previously published12 a greater number of every class of glucose lowering drug (and combinations of drugs) was used in the intensive versus standard group at the end of the active treatment period.
Table 1.
Ischemic Heart Disease Risk Factors and Event Rates During ACCORD
| Intensive (N=5128) |
Standard (N=5123) |
|
|---|---|---|
| Baseline Age (yrs) | 62 (7) | 62 (7) |
| Females | 1983 (38.7%) | 1969 (38.4%) |
| Prior Myocardial Infarction at Baseline | 909 (17.7%) | 925 (18.1%) |
| Prior Angina at Baseline | 608 (11.9%) | 560 (10.9%) |
| Prior Revascularization at Baseline | 1185 (23.1%) | 1112 (21.7%) |
| Until Transition | ||
| Last A1c, Mean (SD) | 6.6 (1.0) | 7.7 (1.1) |
| Last A1c, Median (IQR) | 6.4 (6.0 – 7.0) | 7.5 (7.0 – 8.2) |
| Median Yrs Follow-up (IQR)* | 3.7 (2.8 – 4.5) | 3.7 (2.9 – 4.5) |
| Mean Yrs Follow-up (SD)* | 3.7 (1.4) | 3.7 (1.4) |
| Total Person Years of Follow-up | 19,497 | 19,535 |
| Last ACE-I or ARB use | 1830 (37.0) | 1874 (37.6%) |
| Last Statin use | 3829 (74.7) | 3833 (74.9) |
| Last ASA use | 3020 (58.9) | 3002 (58.6) |
| Last SBP (mm) | 128 (17) | 129 (17) |
| Last DBP (mm) | 67 (11) | 68 (11) |
| Last LDL (mg/dl) | 91 (34) | 91 (35) |
| Last Serum Creatinine (mg/dl) | 1.1 (0.4) | 1.1 (0.4) |
| Until Study End | ||
| LastA1c, Mean (SD) | 7.3 (1.2) | 7.8 (1.2) |
| Last A1c, Median (IQR) | 7.1 (6.5 – 7.9) | 7.6 (7.0 – 8.4) |
| Median Yrs Follow-up (IQR)** | 4.8 (4.0 – 5.7) | 4.8 (4.0 – 5.7) |
| Mean Yrs Follow-up (SD)** | 4.8 (1.6) | 4.7 (1.6) |
| Total Person Years of Follow-up | 25,048 | 25,162 |
| Last ACE-I or ARB use | 1855 (37.2) | 1915 (38.3%) |
| Last Statin use | 3819 (74.5) | 3843 (75.0) |
| Last ASA use | 3133 (61.1) | 3156 (61.6) |
| Last SBP (mm) | 129 (18) | 129 (17) |
| Last DBP (mm) | 68 (11) | 68 (10) |
| Last LDL (mg/dl) | 89 (34) | 89 (34) |
| Last Serum Creatinine (mg/dl) | 1.1 (0.4) | 1.1 (0.5) |
defined as time from randomization until initial occurrence of primary outcome, censoring date, or last day of pre-transition period (February 4, 2008);
defined as time from randomization until initial occurrence of primary outcome, censoring date, or exit visit.
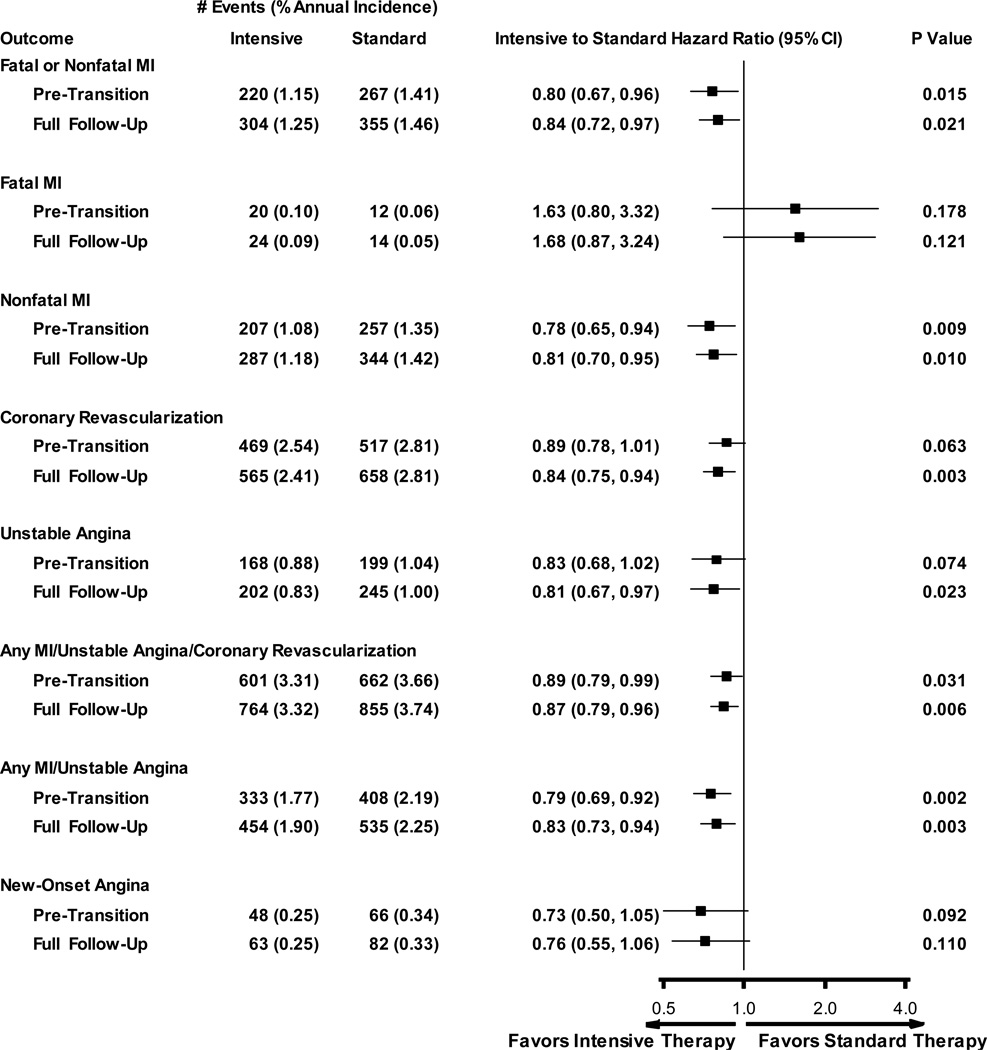
The entire follow-up period included up to 26% more IHD events than the active period alone. As illustrated in Figure 1, fewer participants allocated to the intensive group versus the standard care group experienced a myocardial infarction (i.e. fatal or nonfatal) both during the active treatment period (1.15/100 person-years versus 1.41 per 100 person-years) and during the entire follow-up period (1.25/100 person-years versus 1.46 per 100 person-years). Compared to the standard group, participants in the intensive group were 20% less likely to have a myocardial infarction during the active treatment period (HR = 0.80; 95% CI 0.67 – 0.96; P = 0.015) and 16% less likely to have one during the entire follow-up period (HR = 0.84; 95% CI 0.72 – 0.97; P = 0.02). As noted in Figure 1 similar findings were observed for the composite IHD outcome of the first occurrence of a myocardial infarction, coronary revascularization or unstable angina (HR = 0.89; 95% CI 0.79–0.99 and HR = 0.87; 95% CI 0.79 –0.96 for the 2 treatment periods respectively) and for coronary revascularization (HR 0.84; 95% CI 0.75–0.94) and unstable angina (HR 0.81; 95% CI 0.67–0.97) for just the entire treatment period. Similar findings were also noted for the first occurrence of a nonfatal myocardial infarction, and for the composite of any myocardial infarction or unstable angina. No significant effect of the intervention was noted with respect to a fatal myocardial infarction or new angina. Cumulative incidence curves for myocardial infarction, coronary revascularization, unstable angina and the composite of these 3 IHD outcomes are shown in Figure 2 (Supplemental Figures 1–8 in the online appendix display the numbers at risk for each plot).
Figure 1. Ischemic Heart Disease Incidence and Hazard Ratios.
Forest Plot of Incidence and Hazard Ratios. The number of events, annual incidence (%/year), hazard ratios with 95% confidence intervals and P values are show for each treatment group from baseline until the end of the intervention trial (i.e. pre-transition), and from baseline until the end of the ACCORD trial (i.e. active plus additional follow-up). All analyses use the intent-to-treat approach, include the initial occurrence of each listed event, and account for competing risks due to deaths. Unstable angina includes new-onset angina, accelerated angina or rest angina. The numbers of the composite outcome of any MI or unstable angina shown in the figure differ from those in a prior report12 due to a typographic error; the numbers in the prior report referred to non-stroke CV death, or nonfatal MI or unstable angina.
Figure 2.
Event Curves. The incidence of events in the intensive and standard glycemia groups from the time of randomization until the transition date (accounting for competing risk due to death) and until the end of the ACCORD trial is shown.
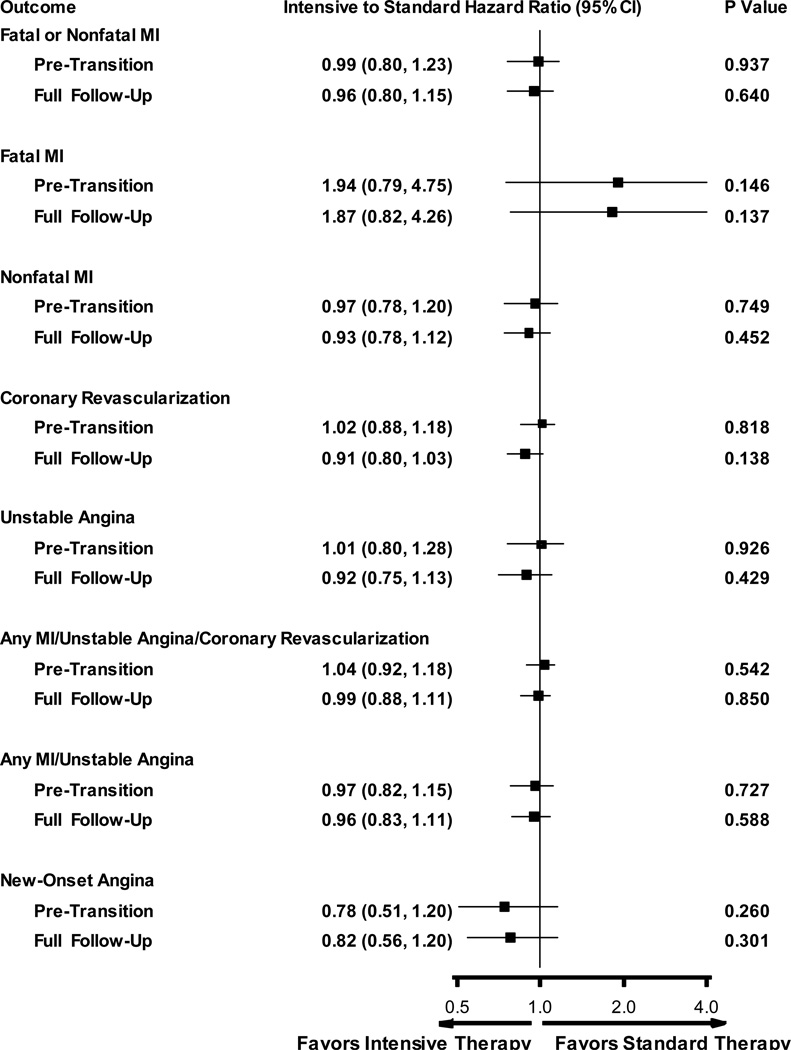
Adding the A1c level measured during the active treatment period as a time-dependent covariate attenuated all of the significant hazard ratios to neutrality and did not change the significance of the non significant hazard ratios (Figure 3).
Figure 3. Ischemic Heart Disease Incidence and Hazard Ratios After Adjustment for Pre-transition A1c Levels.
Forest Plot and Hazard Ratios Adjusted for Pre-transition A1c Levels. Hazard ratios adjusted for the pre-transition A1c levels as a time-varying covariate and account for competing risk due to death.
Discussion
These analyses summarize the effect of approximately 3.7 years of targeting an A1c <6% versus 7 – 7.9% on IHD using life style approaches plus drugs that were available until 2008. They show that randomization to intensive glucose lowering significantly reduced the 5 year incidence of a composite IHD outcome by 13%, and specifically reduced the incidence of any myocardial infarction (i.e. fatal or non-fatal) by 16%, nonfatal myocardial infarction by 19%, coronary revascularization by 16%, and unstable angina by 19%.
These analyses were not prespecified in the ACCORD protocol. Nevertheless, the suggestion that glucose lowering using a variety of approaches reduces incident IHD is consistent with findings from other large outcomes trials that glucose lowering reduces the incidence of fatal and non-fatal myocardial infarction in type 2 diabetes6. The fact that this effect was observed at the end of the active treatment period and was even stronger after a further 1.2 years of follow-up is consistent with at least one other outcomes trial conducted in people with type 2 diabetes5. Moreover, the observation that adjusting for the A1c levels collected during the pre-transition period renders all of these effects statistically insignificant is consistent with the hypothesis that the degree of glucose lowering or some closely related factor may account for the effect of the intervention on IHD. Finally the effect was consistent across different measures of IHD. When viewed in light of a recent study showing that individuals with genetic markers of hyperglycemia had higher glucose levels and were also more likely to develop cardiovascular disease18 than those without these makers, these findings suggest that glucose elevation is a modifiable risk factor for IHD.
The beneficial effect of the intensive glycemia intervention on IHD is at odds with the ACCORD finding of increased death from cardiovascular causes with the intensive versus standard intervention. It is also at odds with the observation in the current analysis that the intensive intervention reduced all of the indices of IHD except for fatal MI. One possible explanation for the discrepancy is suggested by the previously published epidemiologic analysis of ACCORD which reported that much of the mortality in the intensive group occurred in those people whose A1c levels did not decrease from baseline, despite allocation to the intensive glycemic intervention7. When viewed in light of the current analysis showing that glucose lowering accounts for reduced IHD, those individuals whose glucose levels did not fall in response to the intervention may have been harmed by persistent, futile attempts to lower glucose levels due to unknown behavioural or biologic factors. Alternatively, as suggested in a recent editorial, the mortality signal in ACCORD may have been a chance finding11 – a possibility supported by the inability to identify a specific explanation to date7, 8, 10. It is also possible that some of the fatal MIs were misclassified as cardiovascular deaths not due to an MI. Regardless of the explanation, the fact more than 80% of the cardiovascular deaths were adjudicated as not being attributable to a myocardial infarction (i.e. most of them were either unexpected deaths or due to presumed cardiovascular disease)12, 13, and the reduced risk of IHD events with the intensive glycemia intervention demonstrated by these analyses suggest that IHD may not be related to the mortality signal observed in the ACCORD intensive group.
It is important to note that these analyses were not preplanned and as such the current findings may have occurred by chance and hence may not be reproducible in other studies. Indeed, the large number of statistical tests conducted, and the overlap between the various indices of IHD mean that the reported findings may well have occurred by chance alone. Moreover the relatively few fatal myocardial infarctions did not provide enough power to clearly estimate the effect of the intervention on fatal IHD or to rule out the possibility of divergent effects on fatal versus nonfatal IHD. Strengths of these analyses include the randomized design, the large number of nonfatal events, and the high ascertainment of outcomes.
Whereas these findings suggest that glucose lowering interventions can reduce IHD, they do not nullify the prior observation that any overall cardiovascular benefits of 3.7 years of intensive glycemic control are outweighed by fatal harms. Nevertheless, they are consistent with the hypothesis that dysglycemia is causally related to IHD and strongly indicate the need for further elucidation of this relationship.
Supplementary Material
Research in Context Panel.
Systematic Review
The effect of intensive glucose lowering on cardiovascular outcomes in people with type 2 diabetes remains unclear. To date, only 4 large outcomes trials were identified that allocated people with type 2 diabetes to either more intensive versus less intensive glucose lowering and assessed the effect of the intervention on a variety of cardiovascular outcomes. A meta-analysis of data from these 4 trials reported that myocardial infarctions (i.e. fatal or nonfatal) were reduced by 15% (95% CI 6–24) during a mean follow-up period of 4.4 years6 whereas the meta-analyzed effect on strokes (fatal or nonfatal) did not differ from neutrality.
Interpretation
The effect of intensive glycemic control on all of the indices of ischemic heart disease that were measured in the ACCORD trial is analyzed in this report. Intensive glycemic control reduced the risk of myocardial infarctions, coronary revascularization, unstable angina, and a composite of myocardial infarction, coronary revascularization or unstable angina during a mean active treatment period of 3.7 years and a total follow-up period of 4.9 years. Further elucidation of this relationship may identify individuals in whom the benefit of such an approach would clearly outweigh any harms.
Acknowledgments
ACCORD was sponsored by the National Heart, Lung, and Blood Institute with support from other NIH institutes, and the protocol was reviewed, approved in advance, and monitored by an Independent Data, Safety and Management Board and by ethics committees at each center.
HCG reports grants from NHLBI, Sanofi, and Lilly and personal fees from Sanofi, Hoffman LaRoche, Bayer, Novo Nordisk, GSK, Astra Zeneca, Bristol Myers Squibb, and Boehringer Ingelheim. FI-B reports grants from NIH and Novo Nordisk. JL reports grants from NIH, Andromeda, Boehringer Ingelheim, GI Dynamics, Halozyme, , Hoffman LaRoche, Immune Tolerance Network, Jaeb Center for Health Research, Johnson & Johnson, Lexicon, Lilly, Merck, Novo Nordisk, Orexigen, Phase Bio, Sanofi, Tolerx, and personal fees from Dexcon, Sanofi, Takeda, Valeritas, and Vivus. CM reports grants from Novo Nordisk, Merck Frosst, GlaxoSmith Kline, Sanofi, Bristol Myers Squibb, Hoffman LaRoche and Eli Lilly and personal fees and non-financial support from Novo Nordisk. HAL reports grants from Sanofi, Lilly, Boehringer Ingelheim and Amylin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of Contributions
All authors participated in acquiring and interpreting the data. HCG and MEM wrote the first draft of the manuscript, MEM completed the statistical analyses, and F I-B, JL, CM, HAL, and GLB revised the manuscript and approved the final draft.
Declaration of Interests
MEM and GLB report no other disclosures.
Reference List
- 1.Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di AE, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seshasai SR, Kaptoge S, Thompson A, Di AE, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njolstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–S74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year Follow-up of Intensive Glucose Control in Type 2 Diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 6.Turnbull FM, Abraira C, Anderson RJ, Byington RP, Chalmers JP, Duckworth WC, Evans GW, Gerstein HC, Holman RR, Moritz TE, Neal BC, Ninomiya T, Patel AA, Paul SK, Travert F, Woodward M. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52(11):2288–2298. doi: 10.1007/s00125-009-1470-0. [DOI] [PubMed] [Google Scholar]
- 7.Riddle MC, Ambrosius WT, Brillon DJ, Buse JB, Byington RP, Cohen RM, Goff DC, Jr, Malozowski S, Margolis KL, Probstfield JL, Schnall A, Seaquist ER. Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care. 2010;33(5):983–990. doi: 10.2337/dc09-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonds DE, Miller ME, Bergenstal RM, Buse JB, Byington RP, Cutler JA, Dudl RJ, Ismail-Beigi F, Kimel AR, Hoogwerf B, Horowitz KR, Savage PJ, Seaquist ER, Simmons DL, Sivitz WI, Speril-Hillen JM, Sweeney ME. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909. doi: 10.1136/bmj.b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riddle MC. Counterpoint: Intensive glucose control and mortality in ACCORD--still looking for clues. Diabetes Care. 2010;33(12):2722–2724. doi: 10.2337/dc10-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pop-Busui R, Evans GW, Gerstein HC, Fonseca V, Fleg JL, Hoogwerf BJ, Genuth S, Grimm RH, Corson MA, Prineas R. Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33(7):1578–1584. doi: 10.2337/dc10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lachin JM. Point: Intensive glycemic control and mortality in ACCORD--a chance finding? Diabetes Care. 2010;33(12):2719–2721. doi: 10.2337/dc10-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC, Jr, Probstfield JL, Cushman WC, Ginsberg HN, Bigger JT, Grimm RH, Jr, Byington RP, Rosenberg YD, Friedewald WT. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818–828. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginsberg HN, Elam MB, Lovato LC, Crouse JR, III, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC, Jr, Cushman WC, Simons-Morton DG, Byington RP. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 18.Benn M, Tybjaerg-Hansen A, McCarthy MI, Jensen GB, Grande P, Nordestgaard BG. Nonfasting glucose, ischemic heart disease, and myocardial infarction: a Mendelian randomization study. J Am Coll Cardiol. 2012;59(25):2356–2365. doi: 10.1016/j.jacc.2012.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.