Abstract
Free radical scavenging activity (FRSA), total phenolic content (TPC), and total flavonoid content (TFC) of in vitro cultured and field grown Withania somnifera (Ashwagandha) roots were investigated. Withanolides analysis and comprehensive metabolic profiling between 100% methanol extracts of in vitro and field grown root tissues was performed using high performance thin layer chromatography (HPTLC) and gas chromatography-mass spectrometry (GC-MS), respectively. Significantly higher levels of FRSA, TPC, and TFC were observed in in-vitro cultured roots compared with field grown samples. In addition, 30 day-cultured in vitro root samples (1MIR) exhibited a significantly higher FRSA (IC50 81.01 μg/mL), TPC (118.91 mg GAE/g), and TFC (32.68 mg CE/g) compared with those in 45 day-cultured samples (1.5MIR). Total of 29 metabolites were identified in in vitro cultured and field grown roots by GC-MS analysis. The metabolites included alcohols, organic acids, purine, pyrimidine, sugars, and putrescine. Vanillic acid was only observed in the in vitro cultured root samples, and higher level of the vanillic acid was observed in 1MIR when compared to 1.5MIR. Therefore, it is suggested that 1MIR might serve as an alternative to field grown roots for the development of medicinal and functional food products.
Introduction
Withania somnifera, commonly known as Ashwagandha, is a traditional medicinal herb that has been used since ancient times. Various forms of the herb including decoctions, infusions, ointments, powders, and syrups are used as medicine all over the world for patients of all age groups without any side effects, even during pregnancy [1–5]. Various bioactive constituents of this plant have been reported to possess adaptogenic, anticancer, anticonvulsant, immunomodulatory, antioxidative, and neurological effects [6].
The major biochemical constituents of this plant include steroidal alkaloids and steroidal lactones, which constitute a class of compounds known as withanolides, naturally occurring C28- steroidal lactones built on an intact or rearranged ergostane framework, in which C-22 and C-26 are appropriately oxidized to form a six-membered lactone ring [7,8]. Among withanolides, withanolide A and withaferin A were reported to be dominant metabolites, distributed among numerous plant tissues at varying concentrations, and were shown to possess many therapeutic properties [9–11]. Despite the therapeutic advantages that continuously attract the attention of pharmacologists, the annual production of this plant is not sufficient to meet the global demand [12]. Therefore, an in vitro culture system could serve as an alternative to field grown plants for the production of medically valuable compounds. In vitro cultures have a tendency to produce secondary metabolites faster than field-grown plants, owing to their active growth [13].
In the last decade the metabolomic analysis of medicinal plants has been well established [14–16]. Metabolomics in this field has been used for quality control [17–19], identification of compounds [20,21], and correlation of a plant’s metabolome with biological activity [22,23]. The improvement in the analytical performance greatly facilitates the development of plant metabolomics. Metabolomics platforms including nuclear magnetic resonance (NMR) spectrometry, liquid chromatography mass spectrometry (LC-MS), and gas chromatography mass spectrometry (GC-MS) detect a wide range of metabolites resulting in comprehensive data sets. Among these techniques, GC-MS facilitates the identification and robust quantification of various metabolites from plant extract. Furthermore, GC-MS has long been used for metabolomics approach and thus the protocols and the libraries were well established [24]. Although LC-MS has a relatively broad coverage of metabolites compared with GC-MS, it has a distinct drawbacks, such as lower reproducibility of retention times and more susceptible to ion suppression effects, which hamper quantification [15].
There have been no reports regarding the comprehensive metabolic profiling and free radical scavenging activity as well as total phenolics contents (TPC) and total flavonoids content (TFC) of field-grown and in vitro cultured W. somnifera roots. Thus, the purpose of this study was to investigate the metabolic profiles and free radical scavenging activities of field and in vitro cultured W. somnifera roots.
Materials and Methods
Solvents and Chemicals
HPLC-grade methanol, water, Murashige and Skoog (MS) powder, pyridine, 1,1-diphenyl-2-picrylhydrazyl (DPPH), ascorbic acid, dimethyl sulfoxide (DMSO), Folin-Ciocalteu’s reagent, sodium carbonate, gallic acid, sodium nitrite, aluminum chloride, Catechin, and methoxyamine were purchased from Sigma (St. Louis, MO, USA). BSTFA [N,O-bis(trimethylsilyl)trifluoroacetamide containing 1% trimethyl chlorosilane (TMCS)] was obtained from Alfa Aesar (Ward Hill, MA, USA), and 2-Chloronaphtahalene (internal standard for GC-MS) was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan).
Plant Materials
Seeds of W. somnifera (L.) Dunal “Jawahar” were obtained from the Central Institute of Medicinal and Aromatic Plants (Lucknow). They were surface sterilized as per the procedure described by Murthy et al.[25] and were inoculated in Murashige and Skoog (MS) solid basal medium supplemented with 2% sucrose for germination, and were subsequently incubated in the dark at 25°C. Shoots from in vitro germinated seedlings were cultured in basal MS media and were maintained at 25°C for a 16 h photoperiod. These plants were maintained in MS basal medium for at least four generations.
For root induction, fully grown leaf explants grown in vitro on MS medium were excised and trimmed into pieces of about 1 cm2 and were inoculated on a MS solid medium (0.8% agar) supplemented with 3% sucrose and 2 mg L-1 IBA+0.5 mg L-1 IAA [26]. The pH of the medium was adjusted to 5.6 ± 0.2 prior to sterilization. The inoculated explants were incubated at 25°C under a 16 h photoperiod for root induction.
For mass production, 500 mg of the root tips and branches from in vitro-induced adventitious roots were inoculated into one liter of liquid MS media containing 3% sucrose, without any plant hormone in a 2 L bubble column bioreactor (Biopia, Republic of Korea), which aseptically aerated with 0.1 vvm aeration rate using a mini aerator (Biopia, Republic of Korea) through 0.20 μm hydrophobic PTFE (Sartorius stedim) membrane filter. The temperature was maintained at 25°C and the media was changed once every 15 days. Three bioreactors were used to obtain triplicate biological replications. After 30 days (1MIR: 1 month in vitro root, in vitro cultured root for 30 days) and 45 days (1.5MIR: 1.5 month in vitro root, in vitro cultured root for 45 days) of inoculation, in vitro root tissues were harvested for further analysis.
To obtain field grown root samples, the same seeds were sown in the field following standard cultivation practices. The seeds were sown in the month of August, which has been known as ideal plantation time, in the field of Avinashilingam Institute, Coimbatore, Tamilnadu. The field lies between 11°1’ North latitude and 76°57’ East longitude. The city receives an annual rainfall of around 700 mm from September to October with the north east and south west monsoons. The seeds are sown about 2 cm deep into the soil at a distance of about 50 cm. The method of sowing was preferred as it promotes the development of a health root system compared to being sown using the broad casting method. The temperature range was 28–30°C. There was no application of fungicides or organic fertilizers. The field was irrigated twice in a week. The root samples grown in the field were harvested after 60 days (2MFR: 2 month field-grown root, field grown root for 60 days) and 150 days (5MFR: 5 month field-grown root, field grown root for 150 days) for further analysis.
Withanolide A and Withaferin A Analysis
One gram of freeze-dried and powdered root material was extracted with 50 ml of 100% methanol. The extraction was carried out four times. Each time the extract was sonicated for 20 min, then kept in shaker for 2 hr at 100 rpm and filtered using Whatmann No. 1 filter paper. All extracts were then pooled, filtered and evaporated to dryness using a rotary vacuum evaporator (Roteva, Mumbai) in a water bath at 40°C. The residue was dissolved in 10 ml of HPLC grade methanol and stored at -20°C until further analysis. Withanolide A and withaferin A standards were obtained from Chromodex (city, USA). Standard stock solutions of withanolide A and withaferin A (1.0 mg/ml) were prepared using HPLC grade methanol and stored in a refrigerator at 4°C. From the stock solutions, working solutions (0.1 mg/ml) were prepared by dilution with HPLC grade methanol. High performance thin layer chromatography (HPTLC) analysis was carried out using toluene:ethyl acetate:formic acid in the ratio 5:5:1 (v/v/v). Chromatography was performed at 25±2°C on precoated aluminium plates (20x10 cm/10x10cm and 0.2 mm thickness). The withanolide-A and withaferin-A standards at concentration of 0.1 mg/ml were applied with the concentration range from 200 to 1000 ng per band for quantification. A volume of 20 μl of samples dissolved in HPLC grade methanol along with the standards were applied to the plates as 6/8 mm bands, 8 mm from the bottom, 15 mm from the side, under a stream of nitrogen, by means of a CAMAG Linomat V semiautomatic sample applicator (Camag Chemie, Muttenz, Switzerland) fixed with a 100 μl HPTLC syringe (Hamilton Co., NV, USA). The spraying rate was 150 nLs-1. Linear ascending development to a distance of 80 mm was carried out on 10x10 cm/20x20 cm twin trough chamber saturated with the mobile phase, pre-saturated with the solvent for 30 min. After running, the plates were removed from the chamber, air dried and visualized at 254 and 366 nm. Densitometric scanning was performed with Camag TLC scanner III (Camag Chemie, Muttenz, Switzerland) controlled by CAMAG CATS 4 integration software at 235 nm for withanolide A and withaferin A. The slit dimensions were 4x0.3/6x0.3 mm and the scanning speed was 20 mm s-1. The plates were derivatized in anisaldehyde-sulphuric acid reagent (conc. sulfuric acid: methanol: glacial acetic acid: anisaldehyde in the ratio of 5:85:10:0.5) for 2 seconds and kept in hot-air oven for 10 min at 110°C for detection of spots. The Rf values of the resolved spots were noted. The amount of withaferin A and withanolide A was computed from peak areas in all samples.
Free Radical Scavenging Activity
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity was determined using a previously reported method with minor modifications [27]. The samples extracted with 100% methanol and ascorbic acid (positive control) were dissolved in dimethyl sulfoxide (DMSO). An aliquot (20 μL) of each of the extracted samples was introduced into a 96 well plate, and 180 μL of 100 mM DPPH solution was added. After standing for 30 min at room temperature, the absorbance was measured at 517 nm using microplate spectrophotometer (xMark, Biorad, Berkeley, CA). DMSO was utilized as the blank. The free radical scavenging activity was expressed as IC50 (the concentration of the Withania somnifera roots sample (μg/mL) required to scavenge 50% of DPPH), and calculated from the calibration curve prepared by free radical scavenging activity percentage of the various concentrations of the extracts (10–500 μg/mL). The IC50 of the reference antioxidant ascorbic acid (1–20 μg/mL) was calculated by the same method as described above.
Determination of Total Phenolic Content
The total phenolic content (TPC) was estimated by the modified Folin-Ciocalteu method [28]. All extracted samples were dissolved in DMSO. A 0.5 mL aliquot of each sample (1 mg/mL concentration) was transferred into a test tube, and 4.5 mL of distilled water and 0.5 mL of Folin-Ciocalteu’s reagent were added. After standing for 2 min at room temperature, 1.5 mL of 20% (v/w) sodium carbonate solution was added. The mixture was allowed to react in the dark for 1.5 h, and the absorbance at 765 nm was subsequently measured using microplate spectrophotometer (xMark, Biorad, Berkeley, CA). A calibration curve for gallic acid was prepared in the concentration range of 100–500 μg/mL. The results were expressed as milligrams of gallic acid equivalent per gram of the dried extract.
Determination of Total Flavonoid Content
The total flavonoid content (TFC) was determined as per the method described previously [29]. All extracted samples were dissolved in DMSO. Briefly, 0.5 mL of each sample (1 mg/mL concentration) was mixed with 0.3 mL of 5% sodium nitrite. After standing for 5 min at room temperature, 0.3 mL of 10% aluminum chloride was added, and the sample was incubated for 6 min. Finally, 2 mL of 1M sodium hydroxide was added and the total volume of the mixture was adjusted to 5 mL by adding 1.9 mL of deionized water. Catechin was used as the standard for the calibration curve (10–100 μg/mL). The results were expressed as milligrams of catechin equivalent per gram of the dried extract.
GC-MS Analysis
Dried samples (20 mg) of young and mature roots of in vitro cultured and field grown W. somnifera were extracted with 1 mL of 100% methanol prior to metabolite analysis by GC-MS. The extracts were sonicated for 30 min, followed by centrifugation at 2,000 rpm for 5 min. The supernatant was filtered through a 0.45 μm filter (PTFE, Sartorius Stedim Biotech, Göttingen, Germany). To perform derivatization of the extracted sample, 100 μL of each sample was transferred into a GC vial and dried with a flow of nitrogen gas. 30 μL of methoxylamine hydrochloride (200 μg/mL) in pyridine, 50 μL of BSTFA (N,O-bis(trimethylsilyl)trifluoroacetamide; Alfa Aesar, Ward Hill, MA, USA) containing 1% TMCS (trimethyl chlorosilane), and 10 μL of 2-chloronaphthalene (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan; 200 μg/mL in pyridine as an internal standard) were added to dried vials. After derivatization, the samples were incubated for 60 min at 60°C, and were subsequently subjected to GC-MS analysis.
GC-MS analysis was performed using a 7890A Agilent GC system (Agilent Technologies, CA, USA) equipped with a 5975C mass selective detector (Agilent Technologies) and automatic sampler (7683 B series, Agilent Technologies). Electron impact ionization mode with an ionization energy of 70 eV was used for GC-MS detection. Analytes were separated on a fused silica capillary column with 5% phenyl methylpolysiloxane (DB-5, Agilent Technologies; dimensions were 30 m × 0.25 mm i.d. × 0.25 μm film thickness). Helium was used as the carrier gas at a constant flow rate of 1.0 mL/min. The inlet temperature was set to 250°C, with an injection volume of 1.0 μL and split ratio of 1:10. The mass range was 50–700 Da and data were obtained in mass selective detector with the full scan mode. The oven temperature was set to 70°C and was programmed to increase to 150°C (at 5°C/min) then 250°C (at 3°C/min; hold 2 min), and finally 320°C (at 10°C/min; hold 3 min).
Data Processing
Pearson correlation test was conducted using IBM SPSS Statistics 19 software (IBM, Somers, NY). To quantitatively compare the global metabolic profile of all samples, raw datasets from GC-MS analysis were processed as described previously [30]. Mass spectra were deconvoluted using the AMDIS (Automated Mass Spectral Deconvolution and Identification System, http://chemdata.nist.gov/mass-spc/amdis/) and ELU files were created and analyzed with an online peak-filtering algorithm (SpectConnect, http://spectconnect.mit.edu). The metabolites were identified by comparing the mass spectra with those of the NIST-Wiley Mass Spectra Library. Then the normalization was performed by dividing the peak intensity of each compound by that of the internal standard for relative quantification of the metabolites in each sample. Significant differences in metabolite levels were detected by one-way analysis of variance (ANOVA) using Statistical Package for the Social Sciences (SPSS) software (version 19, IBM, Somers, NY) followed by Tukey’s significant-difference test. The level of statistical significance was set at p<0.05. Principal component analysis (PCA) was performed using SIMCA-P+ software (version 13.0, Umetrics, Umeå, Sweden). All data were mean-centered, with scaling to unit variance as a preprocessing method.
Results and Discussion
1. Withaferin and Withanolide Content
In this study, two major withanolide contents in in vitro cultured and field grown roots of W. somnifera were analyzed using high performance thin layer chromatography. The accumulation pattern of withaferin A and withanolide A varied according to the type of tissue and the culture period. Significantly lower levels of withaferin A was observed in in vitro cultured roots than field grown roots (Table 1). Only leaf tissues preferentially accumulate withaferin A whereas in vitro adventitious root tissues accumulate prodigious quantities of withanolide A [31]. These results were coincide with the previous report that withaferin A accumulation was tightly linked only to leaf tissue rather root [32]. However, withanolide A contents in in vitro cultured roots were comparable to those of field grown roots. Significantly higher content of withanolide A (380.0±0.4 μg/g DW) was observed in 1.5MIR (in vitro roots cultured for 45 days) than 1MIR (in vitro roots cultured for 30 days). Among the field grown roots, higher content of withanolide A of 380.0±0.9 μg/g was obtained in 2MFR than 5MFR. Especially, withanolide A contents of 2MFR and 1.5MIR samples were similar to each other (380 μg /mL). Considering the cultivation time, in vitro culture showed a better productivity than field grown samples. Hence, in vitro raised root cultures could serve as an alternative to field grown root for the production of withanolide A.
Table 1. Pattern of withanolide accumulation in in vitro and field grown tissues quantified using HPTLC.
| Sample a | Withanolide A (μg/g dried weight) | Withaferin A (μg/g dried weight) |
|---|---|---|
| 2MFR | 380.0±0.9 a | 180.0±1.2 a |
| 5MFR | 287.0±0.9b | 175.6±0.6b |
| 1MIR | 101.0±0.4c | 2.4±0.8c |
| 1.5MIR | 380.0±0.4 a | 5.36±1.0d |
The data presented in the figure are the mean ± standard deviation of three replicates obtained from three independent experiments. Different letters in the same column indicate a significant difference (p<0.05). Data represents Mean ± Standard deviation of three replicates obtained from three independent experiments.
a2MFR, 2 months field-grown root; 5MFR, 5 months field-grown root; 1MIR, 1MIR, 1 month in vitro root; 1.5 months in vitro root.
2. Free Radical Scavenging Activity, Total Phenolic and Flavonoid Content
It was previously reported that the methanol extracts of W. somnifera roots exhibited higher antioxidant activities compared to extracts from chloroform, acetone, and ethyl acetate [33]. Therefore, the free radical scavenging activity (FRSA) was investigated using methanol extracts of W. somnifera root samples. As listed in Table 2, the FRSA of the root samples revealed that 1MIR exhibited the highest activity with an IC50 value of 81.01 μg/mL, followed by 1.5MIR (IC50 101.60 μg/mL), 5MFR (IC50 188.40 μg/mL), and 2MFR (IC50 117.27 μg/mL). Overall, the root samples cultivated in vitro showed significantly higher antioxidant potentials than the field samples. When we compare the root samples of 1MIR and 2MFR, it was revealed that 1MIR cultivated for a shorter period than 2MFR had a 1.3 times higher FRSA than that of 2MFR. Interestingly, the FRSA of in vitro cultured root samples decreased with longer cultivation periods (1MIR > 1.5MIR), while that of the field samples increased with longer cultivation periods (5MFR > 2MFR). The differences in FRSA according to cultivation period might be attributed to metabolic changes of active compounds in the samples. It has been reported that the FRSA of plant materials is strongly correlated with the amount of phenolics and flavonoids [34]. Therefore, the amount of phenolics and flavonoids in the samples was investigated.
Table 2. Free radical scavenging activity (FRSA), total phenolic contents (TPC) and total flavonoid content (TFC) of W. somnifera roots grown in vitro and in the field.
| Sample a | FRSA IC 50 (μg/mL) | TPC (mg GAE/g) | TFC (mg CE/g) |
|---|---|---|---|
| 2MFR | 188.40 ± 3.83 a | 79.25 ± 0.84 a | 14.78 ± 0.33 a |
| 5MFR | 117.27 ± 1.31b | 95.45 ± 2.20b | 19.84 ± 0.67b |
| 1MIR | 81.01 ± 1.60c | 118.91 ± 2.85c | 32.68 ± 0.46c |
| 1.5MIR | 101.60 ± 2.23d | 104.40 ± 5.40d | 22.03 ± 0.19d |
| Ascorbic acid | 7.39 ± 0.08e | - | - |
Each value is shown as the mean ± SD (N = 7). Different letters in the same column indicate a significant difference (p<0.05). GAE: gallic acid equivalent, CE: catechin equivalent.
a2MFR, 2 months field-grown root; 5MFR, 5 months field-grown root; 1MIR, 1MIR, 1 month in vitro root; 1.5 months in vitro root.
The total phenolic content (TPC) of the root samples was determined according to the Folin-Ciocalteu method and was expressed as gallic acid equivalents (GAE) per gram of extracted sample. The total flavonoid content (TFC) of the root sample was evaluated using a calibration curve with a catechin standard and was expressed as catechin equivalents (CE) per gram of extracted sample. The TPC and TFC of the root samples are summarized in Table 2. The TPC ranged from 79.3 to 118.9 GAE mg/g, and 1MIR (118.9 GAE mg/g) yielded the highest TPC followed by 1.5MIR (104.4 GAE mg/g), 5MFR (95.5 GAE mg/g), and 2MFR (79.3 GAE mg/g). Additionally, the TFC ranged from 14.8 to 32.7 CE mg/g, and 1MIR (32.7 CE mg/g) showed the highest TPC followed by 1.5MIR (22.0 CE mg/g), 5MFR (19.9 CE mg/g), and 2MFR (14.8 CE mg/g). Significantly higher TPC and TFC were observed in in vitro cultured roots, indicating that they could be used as an alternative to field root samples.
To elucidate the relationship between antioxidant activity, TPC, and TFC, Pearson’s correlation test was performed using the data set listed in S1 Table and the results are presented in Table 3. For correlation analysis, the IC50 values of FRSA were transformed into their reciprocal values (1/IC50). The FRSA of root samples were positively correlated with TPC (r = 0.974), and TFC (r = 0.953) with statistical significance of p<0.01 (Table 3). The significant correlations could be due to the use of methanol, which was reported to be an efficient solvent to extract phenolics and flavonoids from W. somnifera [35]. In addition, a significant correlation (r = 0.946, p<0.01) was found between TPC and TFC, indicating that flavonoids were the major components of phenolics in the root samples. It was reported that the gene cluster for phenylpropanoid biosynthesis accounted for the largest group in biosynthesis of secondary metabolites in W. somnifera roots [36]. It seems that the phenylpropanoid pathway might be more activated in in-vitro cultured W. somnifera roots. It was reported that 8-week-old callus induced from W. somnifera leaves dramatically accumulated proteins, soluble sugars, and lipids, but had less TPC than the leaves and roots of field plants [37]. In addition, it was reported that roots of W. somnifera cultured in solid medium showed higher levels of TPC and TFC than those acclimatized and grown in a greenhouse [38]. However, there had been no reports regarding investigation of FRSA and comparative metabolic profiling between in vitro cultured and field grown roots of W. somnifera. In this study, in vitro cultured roots showed a higher FRSA, TPC, and TFC than field grown roots.
Table 3. Pearson’s correlation coefficients (p<0.01) between antioxidant activity (FRSA), total phenolic content (TPC), and total flavonoid content (TFC) of the extracts from roots of W. somnifera.
| FRSA | TPC | TFC | |
|---|---|---|---|
| FRSA | 1 | 0.974 | 0.953 |
| TPC | 1 | 0.946 | |
| TFC | 1 |
3. Identification of Metabolites and Principal Component Analysis (PCA)
As shown in Table 4, the following 29 metabolites were identified in in vitro cultured and field grown roots of W. somnifera: alcohols (glycerol, mannitol, myo-inositol, and xylitol), amino acids (β-alanine, asparagine, aspartic acid, glutamic acid, glutamine, glycine, lysine, phenylalanine, proline, serine, and threonine), organic acids (aconitic acid, citric acid, fumaric acid, glucaric acid, gluconic acid, glucuronic acid, glyceric acid, malic acid, succinic acid, and xylonic acid), phenolic acid (vanillic acid), sugars (fructose, and glucose), and putrescine.
Table 4. Metabolites identified in methanol extracts of W. somnifera roots by GC-MS.
| Class | Metabolites | RT(min) | Fragmentation ion (m/z) | TMS | KEGG ID |
|---|---|---|---|---|---|
| Alcohols | Glycerol | 10.23 | 73, 117, 147, 205, 308(M+) | 3 TMS | C00116 |
| Mannitol | 28.54 | 73, 147, 205, 319, 614(M+) | 6 TMS | C00392 | |
| Myo-inositol | 33.36 | 73, 147, 217, 305, 612(M+) | 6 TMS | C00137 | |
| Xylitol | 22.20 | 73, 147, 217, 307, 512(M+) | 5 TMS | C00379 | |
| Amino acids | β-Alanine | 14.16 | 73, 174, 248, 290, 305(M+) | 3TMS | C00099 |
| Asparagine | 20.66 | 73, 116, 231, 333, 348(M+) | 3 TMS | C00152 | |
| Aspartic acid | 16.59 | 73, 100, 147, 218, 349(M+) | 3 TMS | C00049 | |
| Glutamic acid | 19.30 | 73, 147, 246, 348, 363(M+) | 3 TMS | C00025 | |
| Glutamine | 23.78 | 73, 156, 245, 347, 362(M+) | 3 TMS | C00064 | |
| Glycine | 10.96 | 73, 147, 174, 276, 291(M+) | 3 TMS | C00037 | |
| Lysine | 28.35 | 73, 174, 317, 419, 434(M+) | 4 TMS | C00047 | |
| Phenylalanine | 37.99 | 73, 192, 218, 294, 309(M+) | 2 TMS | C00079 | |
| Proline | 10.74 | 73, 142, 216, 244, 259(M+) | 2 TMS | C00148 | |
| Serine | 12.47 | 73, 147, 204, 218, 321(M+) | 3 TMS | C00065 | |
| Threonine | 13.12 | 73, 117, 218, 291, 335(M+) | 3 TMS | C00188 | |
| Organic acids | Aconitic acid | 23.17 | 73, 147, 229, 375, 390(M+) | 3 TMS | C00417 |
| Citric acid | 25.24 | 73, 147, 273, 465, 480(M+) | 4 TMS | C00158 | |
| Fumaric acid | 12.25 | 73, 147, 217, 245, 260(M+) | 2 TMS | C00122 | |
| Glucaric acid | 32.00 | 73, 147, 333, 627, 642(M+) | 6 TMS | C00818 | |
| Gluconic acid | 30.67 | 73, 147, 205, 333, 628(M+) | 6 TMS | C00257 | |
| Glucuronic acid | 42.08 | 73, 147, 204, 292, 554(M+) | 5 TMS | C00191 | |
| Glyceric acid | 11.67 | 73, 147, 189, 292, 322(M+) | 3 TMS | C00258 | |
| Malic acid | 15.81 | 73, 147, 233, 335, 350(M+) | 3 TMS | C00149 | |
| Succinic acid | 11.27 | 73, 129, 147, 247, 262(M+) | 2 TMS | C00042 | |
| Xylonic acid | 19.57 | 73, 147, 217, 349, 364(M+) | 3 TMS | C05411 | |
| Phenolic acid | Vanillic acid | 23.43 | 73, 223, 267, 297, 312(M+) | 2 TMS | C06672 |
| Sugars | Fructose | 25.11 | 73, 103, 217, 307, 569(M+) | 5 TMS | C00095 |
| Glucose | 27.45, 28.87 | 73, 147, 204, 525, 540(M+) | 6 TMS | C00031 | |
| Other | Putrescine | 22.47 | 73, 174, 214, 361, 376(M+) | 4 TMS | C00134 |
Each base peak in the mass spectra is identified by bold type. KEGG ID means that the metabolite could be identified in the KEGG pathway. M+, molecular ion peak; TMS, trimethylsilylation; p-value < 0.05.
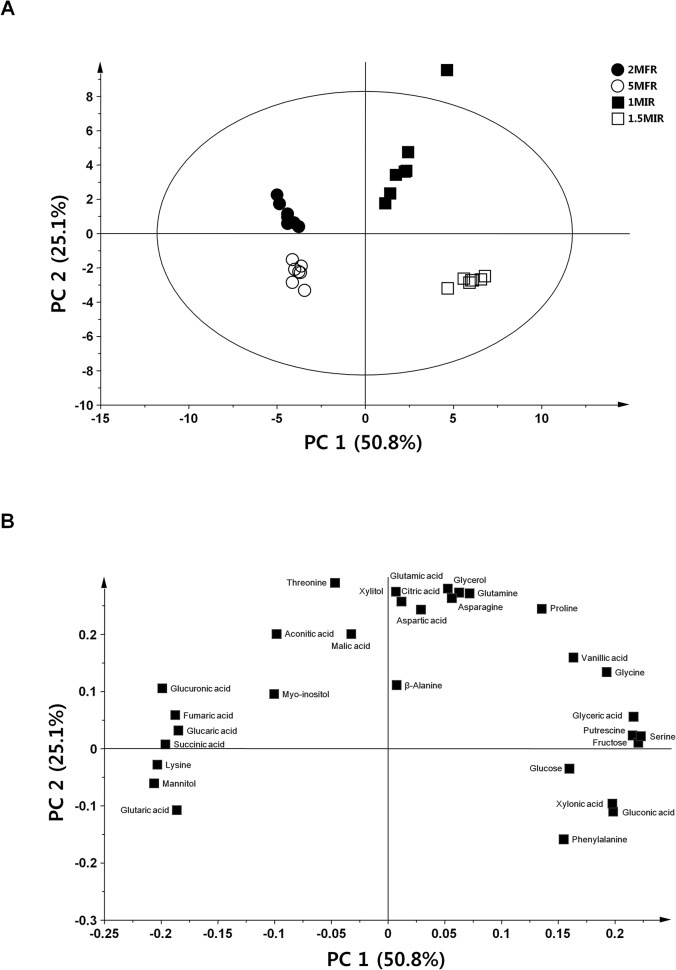
The PCA derived score plot derived from the analysis of W. somnifera roots is shown in Fig 1A. Each root sample was clearly separated by PC 1, accounting for 50.8% of the total variation, and by PC 2, accounting for 25.1% of the variation. Loading plots corresponding to the score plots was shown in Fig 1B. Most of amino acids were present at higher levels in the field root samples (2MFR and 5MFR) than in the in vitro root samples (1MIR and 1.5MIR). Among them, a phenylalanine as a precursor of polyphenol was contained much in in vitro root sample than in field root sample. Furthermore, it was revealed that significantly higher levels of vanillic acid, glucose, and fructose were existed in 1MIR and 1.5MIR root samples than in the field root samples.
Fig 1. Principal component analysis (PCA) of the metabolomes derived from in vitro cultured and field grown roots of W. somnifera.
(A) PCA-derived score plots. (B) PCA-derived loading plots. PC: principal component.
4. Comparison of Metabolic Profiles of in vitro Cultured and Field Grown W. somnifera Roots
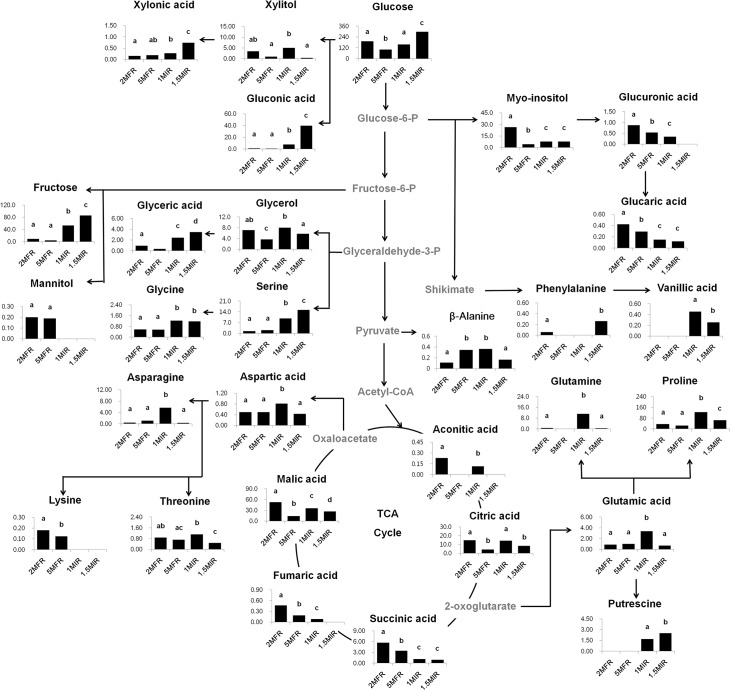
The relative levels of various metabolites were investigated, and biochemical changes throughout the metabolic pathway were shown in Fig 2 and S2 Table. ANOVA was performed to assess the statistical significance on relative level of each metabolite among different samples (p<0.05). Myo-inositol levels of 2MFR were significantly higher than those in in vitro grown root samples (1MIR and 1.5MIR). Accumulation of myo-inositol in the cytosol was observed during salt stress. Field-grown plants are adversely affected by salinity, a major environmental stress factor that limits agricultural production [39]. The increased level of myo-inositol in field grown 2MFR samples as compared to that of in vitro cultured samples might be attributed to a tolerance to decreased water levels or stress induced by high salinity levels. It has been also reported that myo-inositol could serve as a substrate for the production of d-ononitol and d-pinitol, which mediate tolerance to drought or high salt conditions [40]. Decreased myo-inositol levels of the root samples with longer cultivation periods could be due to the production of D-ononitol and D-pinitol from myo-inositol to adapt osmotic stress.
Fig 2. Schematic diagram of the metabolic pathway and relative levels of major compounds detected in W. somnifera root extracts.
This was modified from the pathways presented in the KEGG database (http://www.genome.jp/kegg/). ANOVA was performed to assess the statistical significance of differences between samples (p-value < 0.05). Data are presented as mean values and error bars represent the standard deviation. Different letters represent the statistical significance of metabolite levels.
Proline was the most abundant amino acids in root samples. It has been reported that the accumulation of proline ameliorate osmotic stress [41]. In addition, proline has been known to protect membranes from damage induced by reactive oxygen species and has been associated with the prevention of protein denaturation, and the preservation of the structure and activity of essential enzymes [42–44]. Moreover, the functional significance of proline accumulation has been known to provide sufficient energy to sustain rapid cell growth [43]. It was found that amino acids can induce rhizogenesis. It was reported that proline especially increased the rooting percentage and number of roots per rooted explants of sweet cherry (Prunus avium L.) [45]. The significantly higher level of proline in 1MIR sample could be related to initial root growth of in vitro cultured W. somnifera root.
Vanillic acid was only detected in in vitro cultured roots (1MIR and 1.5MIR) and the levels of vanillic acid were higher in 1MIR than 1.5MIR. The biosynthesis of phenolic metabolites such as vanillic acid has been known to be stimulated by activation of phenylpropanoid biosynthetic pathway through proline linked pentose phosphate pathway. It has been proposed that the proline biosynthesis was closely associated with the pentose phosphate pathway [44]. The accumulation of vanillic acid in in vitro cultured root samples might be stimulated by the pentose phosphate pathway linked to increased proline levels. In addition, it is well known that vanillic acid has a significant antioxidative activity from in vitro experiments [46,47]. The highest level of vanillic acid in in vitro sample 1MIR than other samples could contribute to the most active antioxidant activity of 1MIR sample.
The levels of soluble sugars, including fructose and glucose, were significantly different between the field grown and in vitro cultured samples. The sugar levels of in vitro cultured root samples were much higher than those of field grown root samples. Li et al (2013) [48] observed similar result of high sugar levels released by peanut root cultures and concluded that accumulation of sugar molecules could provide abundant nutrition for growth and development.
Conclusion
Withanolides, signature metabolite of W. somnifera appear to be present in varying concentration at different period of growth. Further, enhancement of withanolide accumulation can be done upon exposure of in vitro root cultures to elicitors and abiotic stress conditions, since, field grown materials are highly affected by genotype and adverse environmental conditions. The present study compared the free radical scavenging activity (FRSA), total phenolic (TPC), total flavonoid contents (TFC), and metabolic profiles of field-grown and in vitro cultured root samples of W. somnifera. In vitro cultured roots showed a higher FRSA, TPC, and TFC than field grown roots. Specifically, roots cultivated for 30 days (1MIR) showed higher FRSA than those cultivated for 45 days (1.5MIR). As the correlations between the FRSA, TPC, TFC were found to be statistically significant (p<0.01), phenolic compounds including flavonoids appear to be responsible for the highest antioxidant activity of 1MIR. The results of this study highlighted the potential utilization of in vitro-cultured W. somnifera roots as alternative resources to field-grown roots, as they exhibited relatively higher antioxidant activities and shorter cultivation periods. Moreover, in vitro-cultured W. somnifera roots had advantages for the production of useful metabolites such as withanolide A and vanillic acid. This study implies that in vitro cultured roots of W. somnifera can be used for the development of biopharmaceuticals or functional foods.
Supporting Information
Seven independent sample extracts of W. somnifera roots cultivated under different environment were prepared and determined.
(DOCX)
The relative levels of each metabolite were obtained by dividing the percentage area corresponding to each metabolite by the percentage area of the internal standard. Different letters in the same row indicate a significant difference. Mean ± SD values (n = 7) are shown. “ND” means “not detected.”
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Mid-career Researcher Program (NRF-2012R1A2A2A02011748) through NRF grant funded by the MSIP of Republic of Korea and Major Research Project by University Grants Commission Government of India (F.No.34-278/2008 (SR)).
References
- 1. Archana R, Namasivayam A. Antistressor effect of Withania somnifera . J Ethnopharmacol. 1998;64: 91–93. [DOI] [PubMed] [Google Scholar]
- 2. Davis L, Kuttan G. Effect of Withania somnifera on DMBA induced carcinogenesis. J Ethnopharmacol. 2001;75: 165–168. [DOI] [PubMed] [Google Scholar]
- 3. Kumar A, Kaul M, Bhan M, Khanna PK, Suri K. Morphological and chemical variation in 25 collections of the Indian medicinal plant, Withania somnifera (L.) Dunal (Solanaceae). Genet Resour Crop Evol. 2007;54: 655–660. [Google Scholar]
- 4. Gupta GL, Rana A. Withania somnifera (Ashwagandha): a review. Pharmacogn Rev. 2007;1: 129–136. [Google Scholar]
- 5. Sharma S, Dahanukar S, Karandikar S. Effects of long term administration of the roots of ashwagandha and shatavari in rats. Indian Drugs. 1985;29: 133–139. [Google Scholar]
- 6. Chatterjee S, Srivastava S, Khalid A, Singh N, Sangwan RS, Sidhu OP, et al. Comprehensive metabolic fingerprinting of Withania somnifera leaf and root extracts. Phytochemistry. 2010;71: 1085–1094. 10.1016/j.phytochem.2010.04.001 [DOI] [PubMed] [Google Scholar]
- 7. Ray AB, Gupta M. Withasteroids, a growing group of naturally occurring steroidal lactones In: Cardenas J, Esquivel B, Gupta M, Rray AB, Rodriquez-Hahn L, editors. Progress in the chemistry of organic natural products. Wien: Springer; 1994. pp. 1–106. [DOI] [PubMed] [Google Scholar]
- 8. Mirjalili MH, Moyano E, Bonfill M, Cusido RM, Palazón J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules. 2009;14: 2373–2393. 10.3390/molecules14072373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jayaprakasam B, Nair MG. Cyclooxygenase-2 enzyme inhibitory withanolides from Withania somnifera leaves. Tetrahedron. 2003;59: 841–849. [Google Scholar]
- 10. Ichikawa H, Takada Y, Shishodia S, Jayaprakasam B, Nair MG, Aggarwal BB. Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-kappaB NF-kB. activation and NF-kB-regulated gene expression. Mol Cancer Ther. 2006;5: 1434–1445. [DOI] [PubMed] [Google Scholar]
- 11. Praveen N, Naik P, Manohar S, Murthy H. Distribution of withanolide A content in various organs of Withania somnifera (L.) Dunal. Int J Pharma Bio Sci. 2010;1: 1–5. [Google Scholar]
- 12. Sharada M, Ahuja A, Suri K, Vij S, Khajuria R, Verma V, et al. Withanolide production by in vitro cultures of Withania somnifera and its association with differentiation. Biol Plant. 2007;51: 161–164. [Google Scholar]
- 13. Sivanandhan G, Arun M, Mayavan S, Rajesh M, Mariashibu T, Manickavasagama M, et al. Chitosan enhances withanolides production in adventitious root cultures of Withania somnifera (L.) Dunal. Ind Crops Prod. 2012;37: 124–129. [Google Scholar]
- 14. Kim HK, Choi YH, Verpoorte R. NMR-based metabolomic analysis of plants. Nat Protoc. 2010;5: 536–549. 10.1038/nprot.2009.237 [DOI] [PubMed] [Google Scholar]
- 15. Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat Protoc. 2006;1: 387–396. [DOI] [PubMed] [Google Scholar]
- 16. Sumnet LW, Lei ZL, Nikolau BJ, Saito K. Modern plant metabolomics: advanced natural product gene discoveries, improved technologies, and future prospects. Nat Prod Rep. 2015;32: 212–229. 10.1039/c4np00072b [DOI] [PubMed] [Google Scholar]
- 17. Frédérich M, Wauters JN, Tits M, Jason C, de Tullio P, Van der Heyden Y, et al. Quality assessment of Polygonum cuspidatum and Polygonum multiflorum by 1H NMR metabolite fingerprinting and profiling analysis. Planta medica. 2011;77: 81–86. 10.1055/s-0030-1250132 [DOI] [PubMed] [Google Scholar]
- 18. Tarachiwin L, Katoh A, Ute K, Fukusaki E. Quality evaluation of Angelica acutiloba Kitagawa roots by 1H NMR-based metabolic fingerprinting. J Pharmaceut Biomed. 2008;48: 42–48. [DOI] [PubMed] [Google Scholar]
- 19. van der Kooy F, Maltese F, Choi YH, Kim HK, Verpoorte R. Quality control of herbal material and phytopharmaceuticals with MS and NMR based metabolic fingerprinting. Planta medica. 2009;75: 763–775. 10.1055/s-0029-1185450 [DOI] [PubMed] [Google Scholar]
- 20. Murch SJ, Rupasinghe HV, Goodenowe D, Saxena PK. A metabolomic analysis of medicinal diversity in Huang-qin (Scutellaria baicalensis Georgi) genotypes: discovery of novel compounds. Plant Cell Rep. 2004;23: 419–425. [DOI] [PubMed] [Google Scholar]
- 21. Wolfender JL, Glauser G, Boccard J, Rudaz S. MS-based plant metabolomic approaches for biomarker discovery. Nat Prod Commun. 2009;4: 1417–1430. [PubMed] [Google Scholar]
- 22. Cho SK, Yang SO, Kim SH, Kim H, Ko JS, Riu KZ, et al. Classification and prediction of free-radical scavenging activities of dangyuja (Citrus grandis Osbeck) fruit extracts using 1H NMR spectroscopy and multivariate statistical analysis. J Pharmaceut Biomed. 2009;49: 567–571. [DOI] [PubMed] [Google Scholar]
- 23. Kim Y, Hyun SH, Park HE, Choi HK. Metabolic profiling, free-radical scavenging and tyrosinase inhibitory activities of Lemna minor whole plants cultivated in various concentrations of proline and sucrose. Process Biochem. 2012;47: 62–68. [Google Scholar]
- 24. Cox DG, Oh J, Keasling A, Colson KL, Hamann MT. The utility of metabolomics in natural product and biomarker characterization. Biochim Biophys Acta Gen Subj. 2014;1840: 3460–3474. 10.1016/j.bbagen.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murthy HN, Dijkstra C, Anthony P, White DA, Davey MR, Power JB, et al. Establishment of Withania somnifera hairy root cultures for the production of withanolide A. J Integr Plant Biol. 2008;50: 975–981. 10.1111/j.1744-7909.2008.00680.x [DOI] [PubMed] [Google Scholar]
- 26. Wasnik NG, Muthusamy M, Chellappan S, Vaidhyanathan V, Pulla R, Purushotham PM, et al. Establishment of in vitro toot cultures and analysis of secondary metabolites in Indian ginseng-Withania somnifera . Korean J Plant Resour. 2009;22: 584–591. [Google Scholar]
- 27. Vaz JA, Barros L, Martins A, Santos-Buelga C, Vasconcelos MH, Ferreira ICFR. Chemical composition of wild edible mushrooms and antioxidant properties of their water soluble polysaccharidic and ethanolic fractions. Food Chem. 2011;126: 610–616. [Google Scholar]
- 28. Hithamani G, Srinivasan K. Effect of domestic processing on the polyphenol content and bioaccessibility in finger millet (Eleusine coracana) and pearl millet (Pennisetum glaucum). Food Chem. 2014;164: 55–62. 10.1016/j.foodchem.2014.04.107 [DOI] [PubMed] [Google Scholar]
- 29. Lahouar L, Arem AE, Ghrairi F, Chahdoura H, Ben SH, Felah ME, et al. Phytochemical content and antioxidant properties of diverse varieties of whole barley (Hordeum vulgare L.) grown in Tunisia. Food Chem. 2014;145: 578–583. 10.1016/j.foodchem.2013.08.102 [DOI] [PubMed] [Google Scholar]
- 30. Styczynski MP, Moxley JF, Tong LV, Walther JL, Jensen KL, Stephanopoulos GN. Systematic identification of conserved metabolites in GC/MS data for metabolomics and biomarker discovery. Anal Chem. 2007;79: 966–973. [DOI] [PubMed] [Google Scholar]
- 31. Sabir F, Sangwan NS, Chaurasiya ND, Misra LN, Sangwan RS. In vitro withanolide production by Withania somnifera L. cultures. Z Naturforsch C. 2008;63: 409–412. [DOI] [PubMed] [Google Scholar]
- 32. Praveen N, Murthy HN. Production of withanolide-A from adventitious root cultures of Withania somnifera . Acta Physiol Plant. 2010;32: 1017–1022. [Google Scholar]
- 33. Yadava SA, Hakkim L, Sathishkumar F, Sathishkumar R. Antioxidant activity of Withania somnifera (L.) Dunal by different solvent extraction methods. J Pharm Res. 2011;4: 1428–1430. [Google Scholar]
- 34. Demiray S, Pintado M, Castro P. Evaluation of phenolic profiles and antioxidant activities of Turkish medicinal plants: Tilia argentea, Crataegi folium leaves and Polygonum bistorta roots. World Acad Sci Eng Technol. 2009;54: 312–317. [Google Scholar]
- 35. Visweswari G, Christopher R, Rajendra W. Phytochemical screening of active secondary metabolites present in Withania somnifera root: role in traditional medicine. Int J Pharm Sci Res. 2013;4: 2770–2776. [Google Scholar]
- 36. Gupta P, Goel R, Pathak S, Srivastava A, Singh SP, Sangwan RS, et al. De novo assembly, functional annotation and comparative analysis of Withania somnifera leaf and root transcriptomes to identify putative genes involved in the withanolides biosynthesis. PLoS One. 2013;8: e62714 10.1371/journal.pone.0062714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Singh S, Tanwer BS, Khan M. Callus induction and in vivo and in vitro comparative study of primary metabolites of Withania Somnifera . Adv App Sci Res. 2011;2: 47–52. [Google Scholar]
- 38. Dewir Y, Chakrabarty D, Lee S, Hahn E, Paek KY. Indirect regeneration of Withania somnifera and comparative analysis of withanolides in in vitro and greenhouse grown plants. Biol Plant. 2010;54: 357–360. [Google Scholar]
- 39. Läuchli A, Grattan S. Plant growth and development under salinity stress In: Jenks MA, Hasegawa PM, Jain SM., editors. Advances in molecular breeding toward drought and salt tolerant crops. Dordrecht: Springer, 2007. pp. 1–32. [Google Scholar]
- 40. Vernon DM, Bohnert HJ. A novel methyl transferase induced by osmotic stress in the facultative halophyte Mesembryanthemum crystallinum . EMBO J. 1992;11: 2077–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Delauney AJ, Verma DPS. Proline biosynthesis and osmoregulation in plants. Plant J. 1993;4: 215–223. [Google Scholar]
- 42. Rajendrakumar CS, Reddy BV, Reddy AR. Proline-protein interactions: protection of structural and functional integrity of M 4 lactate dehydrogenase. Biochem Biophys Res Commun. 1994;201: 957–963. [DOI] [PubMed] [Google Scholar]
- 43. Saradhi PP, AliaArora S, Prasad K. Proline accumulates in plants exposed to UV radiation and protects them against UV-induced peroxidation. Biochem Biophys Res Commun. 1995;209: 1–5. [DOI] [PubMed] [Google Scholar]
- 44. Shetty K. Biotechnology to harness the benefits of dietary phenolics, focus on Lamiaceae . Asia Pac J Clin Nutr. 1997;6: 162–171. [PubMed] [Google Scholar]
- 45. Sapropoulou V, Dimassi-Theriou K, Therios I. In vitro rooting and biochemical parameters in the cherry rootstocks CAB-6 and Gisela 6 using L-methionine. Turk J Agric For. 2013;37: 688–698. [Google Scholar]
- 46. Sang S, Lapsley K, Jeong WS, Lachance PA, Ho CT, Rosen RT. Antioxidative phenolic compounds isolated from almond skins (Prunus amygdalus Batsch). J Agr Food Chem. 2002;50: 2459–2463. [DOI] [PubMed] [Google Scholar]
- 47. Tai A, Sawano T, Ito H. Antioxidative properties of vanillic acid esters in multiple antioxidant assays. Biosci Biotechnol Biochem. 2012;76: 314–318. [DOI] [PubMed] [Google Scholar]
- 48. Li XG, Zhang TL, Wang XX, Hua K, Zhao L, Han ZM. The composition of root exudates from two different resistant peanut cultivars and their effects on the growth of soil-borne pathogen. Int J Biol Sci. 2013;9: 164–173. 10.7150/ijbs.5579 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Seven independent sample extracts of W. somnifera roots cultivated under different environment were prepared and determined.
(DOCX)
The relative levels of each metabolite were obtained by dividing the percentage area corresponding to each metabolite by the percentage area of the internal standard. Different letters in the same row indicate a significant difference. Mean ± SD values (n = 7) are shown. “ND” means “not detected.”
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.