Abstract
Background
Obesity is associated with advanced cardiovascular disease. However, some studies have reported the “obesity paradox” after percutaneous coronary intervention (PCI). The relationship between body mass index (BMI) and clinical outcomes after PCI has not been thoroughly investigated, especially in Asian populations.
Methods
We studied 10,142 patients who underwent PCI at 15 Japanese hospitals participating in the JCD-KICS registry from September 2008 to April 2013. Patients were divided into four groups according to BMI: underweight, BMI <18.5 (n=462); normal, BMI ≥18.5 and <25.0 (n=5,945); overweight, BMI ≥25.0 and <30.0 (n=3,100); and obese, BMI ≥30.0 (n=635).
Results
Patients with a high BMI were significantly younger (p<0.001) and had a higher incidence of coronary risk factors such as hypertension (p<0.001), hyperlipidemia (p<0.001), diabetes mellitus (p<0.001), and current smoking (p<0.001), than those with a low BMI. Importantly, patients in the underweight group had the worst in-hospital outcomes, including overall complications (underweight, normal, overweight, and obese groups: 20.4%, 11.5%, 8.4%, and 10.2%, p<0.001), in-hospital mortality (5.8%, 2.1%, 1.2%, and 2.7%, p<0.001), cardiogenic shock (3.5%, 2.0%, 1.5%, and 1.6%, p=0.018), bleeding complications (10.0%, 4.5%, 2.6%, and 2.8%, p<0.001), and receiving blood transfusion (7.6%, 2.7%, 1.6%, and 1.7%, p<0.001). BMI was inversely associated with bleeding complications after adjustment by multivariate logistic regression analysis (odds ratio, 0.95; 95% confidence interval, 0.92–0.98; p=0.002). In subgroup multivariate analysis of patients without cardiogenic shock, BMI was inversely associated with overall complications (OR, 0.98; 95% CI, 0.95–0.99; p=0.033) and bleeding complications (OR, 0.95; 95% CI, 0.91–0.98; p=0.006). Furthermore, there was a trend that BMI was moderately associated with in-hospital mortality (OR, 0.94; 95% CI, 0.88–1.01; p=0.091).
Conclusions
Lean patients, rather than obese patients are at greater risk for in-hospital complications during and after PCI, particularly for bleeding complications.
Introduction
Obesity is an independent risk factor of advanced cardiovascular disease and mortality [1–3]. Some previous studies have reported that obesity is associated with adverse cardiovascular events after percutaneous coronary intervention (PCI) [4–6]. However, various studies performed in Western countries have reported that obese patients have better short- and long-term outcomes after PCI than non-obese patients [7–15]. This phenomenon is well known as an “obesity paradox”, not only among patients with coronary artery disease (CAD), but also in those with heart failure [16,17]. However, the precise mechanisms of this phenomenon are still unclear [18–20]. Additionally, there are few data regarding the obesity paradox especially in Asian populations, because few studies have been conducted in Asia.
Patients with CAD in Asian countries have different characteristics compared with those in Western countries (e.g., older age, lower body mass index (BMI), frequently smoke, and have less traditional risk factors, except for diabetes mellitus) [21,22]. In addition, relationships between cardiovascular risk factors and cardiovascular disease may differ in Asian populations and Western populations [23]. In particular, one of the biggest differences between populations is physique. The average BMI in patients with CAD is remarkably lower in Asian countries compared with Western countries [21,22]. Moreover, the impact of BMI on the incidence of cardiovascular disease may differ in Asian populations and Western populations. Lu et al. reported higher hazard ratios per 5 kg/m2 BMI increase for coronary heart disease and stroke in Asian cohorts than in Western cohorts [3]. Previous studies have suggested that lower cut-off points for BMI should be adopted in Asian than in Western countries [23]. Furthermore, Japanese patients with CAD tend to have more bleeding complications during and after PCI compared with Western populations [21,22], and undergo complex procedures because surgical revascularization is less preferred by patients.
Because the risk profiles and procedural preference of Japanese patients with CAD differ from those in Western populations, investigation of the obesity paradox in Japan is important. This study aimed to investigate the impact of BMI on in-hospital complications in patients undergoing PCI in a Japanese multicenter PCI registry.
Material and Methods
Study design
The Japan Cardiovascular Database (JCD) is a large, ongoing, prospective, multicenter cohort study that was designed to record clinical background and outcome data for PCI patients in Japan [24–28]. Data for approximately 200 variables are continuously being collected in this study. Participating hospitals are instructed to record data from consecutive hospital visits for PCI and to register these data into an internet-based database system.
Entered data were checked for completeness and internal consistency. Quality assurance of the data was achieved through automatic system validation and reporting of data completeness, education, and training for dedicated clinical research coordinators specifically trained for the present PCI registry. The senior study coordinator (I.U.) and exclusive on-site auditing by investigators (S.K. and H.M.) ensured proper registration of each patient.
PCI with any commercially available coronary device was included. The decision to perform PCI was made according to the investigators’ clinical assessment of the patients. This study did not mandate specific interventional or surgical techniques, such as vascular access, use of specific stents, or closure devices.
Major teaching hospitals within the metropolitan Tokyo area were selected for this study. Patients were enrolled based on the individual PCI event, and all consecutive PCI procedures during the study period were registered, including cases of failure. Patients aged <18 years were excluded from the study.
The majority of clinical variables in the JCD are defined according to the National Cardiovascular Data Registry. This registry is sponsored by the American College of Cardiology for conducting comparative research to determine factors that can lead to disparities in PCI management. The National Cardiovascular Data Registry is a large PCI registry system with over 1,000,000 entries for ischemic heart disease and over 500,000 entries for PCI that were collected from more than 500 institutions in the United States [29].
Information disclosure
The study protocol was approved by the institutional review board committee at Keio University, School of Medicine in Japan. All of the participants provided written informed consent for the present study. Before the launch of the JCD registry, information on the objectives of the present study, its social significance, and an abstract were provided for clinical trial registration with the University Hospital Medical Information Network. This Network is recognized by the International Committee of Medical Journal Editors as an “acceptable registry,” according to a statement issued in September 2004 (UMIN R000005598).
Study population
We analyzed data from 10,788 consecutive patients who underwent PCI at 15 Japanese hospitals participating in the JCD-KICS registry from September 2008 to April 2013. For the present analysis, 646 patients were excluded because of missing data on basic information, including sex, height, and/or body weight. We divided the remaining 10,142 patients into four groups according to BMI. BMI was defined as weight in kilograms divided by the square of the height in meters. The National Heart, Lung, and Blood Institute and the World Health Organization have introduced a weight classification for BMI. According to this classification, patients with a BMI of 18.5–24.9 kg/m2 are considered normal, those with a BMI of 25–30 kg/m2 are considered overweight, and those with a BMI >30 kg/m2 are considered obese [4]. Patients were divided into four groups according to BMI in the present study: underweight, BMI <18.5; normal, BMI ≥18.5 and <25.0; overweight, BMI ≥25.0 and <30.0; and obese, BMI ≥30.0.
Clinical, angiographic, and procedural complications were prospectively entered into the JCD-KICS registry database. The choice of access site was based on the preference of the interventional cardiologist. Although the sizes of the sheath and guiding catheter were not protocol mandated in this cohort, the commonly used size was 6–8 Fr in transfemoral intervention, and 6 Fr in transradial intervention (TRI). All of the patients underwent periprocedural anticoagulation via heparin based on institutional dosing instructions during PCI. A bolus dose of 5000–10000 IU was usually administered and additional doses were provided based on an activated clotting time of > 300 seconds during PCI. We did not have a mandated protocol for hemostasis after the PCI procedures. Details of post-procedural management were left to the primary operators’ discretion. The recommended antiplatelet therapy was long-term 81 mg aspirin daily and a thienopyridine (75 mg clopidogrel or 200 mg ticlopidine daily). The loading dose of clopidogrel was 300 mg, and dual antiplatelet therapy was continued for at least 12 months after drug-eluting stent implantation, and 1 month after bare-metal stent implantation.
The endpoints were defined as in-hospital mortality and other complications. Complications were defined as all complications as follows: severe coronary artery dissection or coronary perforation; myocardial infarction after PCI; cardiac shock or heart failure; cerebral bleeding or stroke; and bleeding complications. Severe coronary artery dissection was defined as an intimal tear of the coronary artery, leading to impaired blood flow (final thrombolysis in myocardial infarction flow grade <3) on an angiogram. Myocardial infarction was defined as the new occurrence of a biomarker-positive myocardial infarction after PCI. Bleeding complications in this registry were further defined as those requiring blood transfusion, prolonging hospital stay, or causing a decrease in hemoglobin of >3.0 g/dL. Furthermore, bleeding complications were divided into puncture-site bleeding, retroperitoneal bleeding, gastrointestinal bleeding, genitourinary bleeding, or other bleeding. Hematomas >10 cm for femoral access or >2 cm for radial access also qualified as access site bleeding.
Data analysis
Continuous variables are expressed as mean ± standard deviation (SD). Categorical variables are expressed as a percentage. Continuous variables were compared using the Student’s t-test, and the differences between categorical variables were examined using the chi-squared test. Univariate logistic regression analysis was performed to specify the odds ratio (OR) for overall complications, in-hospital mortality, and bleeding complications within 72 hours. Multivariate logistic regression analysis was then performed to investigate independent predictors for overall complications, in-hospital mortality, and bleeding complications. Variables in these models were selected based on univariate p values <0.05 and overall clinical significance. Variables that were entered in these models included age, sex, BMI, hyperlipidemia, diabetes mellitus, current smoking, previous PCI, previous myocardial infarction, previous heart failure, cerebrovascular disease, peripheral artery disease, chronic obstructive pulmonary disease, hemodialysis, ST elevation myocardial infarction (STEMI), non-STEMI, unstable angina, stable angina, cardiogenic shock, use of intra-aortic balloon pumping (IABP), TRI, transfemoral intervention, three-vessel disease, left main trunk lesion, type C lesion, chronic total occlusion, and use of a rotablator. All statistical calculations and analyses were performed using JMP version 10.0 (SAS Institute, Cary, NC, USA). A p value of <0.05 was considered statistically significant.
Results
Baseline clinical characteristics
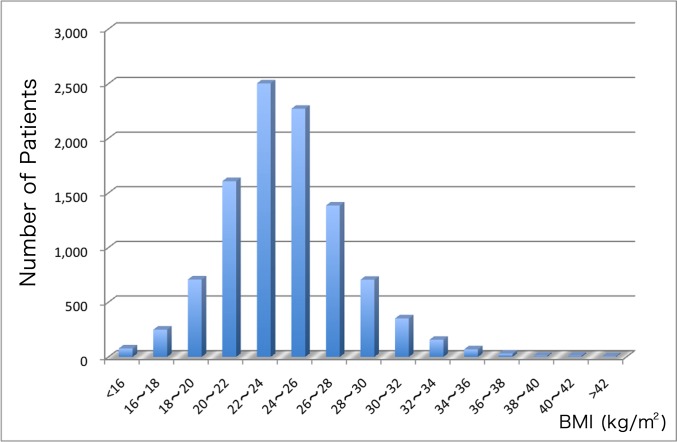
The distribution of BMI for the 10,142 study patients is shown in Fig 1. The average BMI in the total cohort was 24.2. The baseline clinical characteristics of the study patients according to BMI are shown in Table 1. Of 10,142 patients, 4.5% (n = 462) were underweight, 58.6% (n = 5,945) were normal weight, 30.6% (n = 3,100) were overweight, and 6.3% (n = 635) were obese. Patients with a high BMI were significantly younger (p<0.001), had a higher incidence of coronary risk factors such as hypertension (p<0.001), hyperlipidemia (p<0.001), and diabetes mellitus (p<0.001), and had a higher rate of previous PCI (p<0.001) and current smoking habit (p<0.001) than those with a low BMI. On the other hand, patients with a low BMI were older (p<0.001), more often female (p<0.001), and more likely to have previous heart failure (p<0.001), peripheral artery disease (p<0.001), higher baseline serum creatinine level (p<0.001), and end-stage renal disease (p<0.001) than those with a high BMI. Furthermore, patients with a low BMI were more likely to present with acute coronary syndrome, especially with STEMI (p<0.001), unstable angina (p = 0.031) and cardiogenic shock (p<0.001) compared with those with a high BMI. The rate of administration of antiplatelet agents was not significantly different among the BMI groups.
Fig 1. Distribution of BMI values.
The distribution of BMI for 10,142 patients is shown.
Table 1. Baseline Clinical Characteristics.
| Underweight (BMI<18.5, n = 462) | Normal (18.5≦BMI<25.0, n = 5945) | Overweight (25.0≦BMI<30.0, n = 3100) | Obese (30.0≦BMI, n = 635) | P-value | |
|---|---|---|---|---|---|
| Age, years | 74.7±10.0 | 69.4±10.1 | 65.4±10.6 | 59.2±11.8 | <0.001 |
| Female gender | 200(43.3%) | 1233(20.7%) | 512(16.5%) | 135(21.3%) | <0.001 |
| Height (cm) | 157.9±9.8 | 161.7±8.7 | 163.1±8.6 | 164.1±9.5 | <0.001 |
| Weight (kg) | 42.9±6.1 | 59.0±8.0 | 71.7±8.2 | 88.0±13.5 | <0.001 |
| Hypertension | 317(68.6%) | 4213(70.9%) | 2481(80.0%) | 539(84.9%) | <0.001 |
| Hyperlipidemia | 219(47.4%) | 3801(63.9%) | 2261(72.9%) | 498(78.4%) | <0.001 |
| Diabetes mellitus | 156(33.8%) | 2375(40.0%) | 1438(46.4%) | 372(58.6%) | <0.001 |
| Insulin use | 39(8.4%) | 469(7.9%) | 295(9.5%) | 102(16.1%) | <0.001 |
| Current smoking | 121(26.2%) | 1982(33.3%) | 1201(38.7%) | 284(44.7%) | <0.001 |
| Family History | 40(8.7%) | 748(12.6%) | 439(14.2%) | 109(17.2%) | <0.001 |
| Previous PCI | 146(31.6%) | 2316(39.0%) | 1280(41.3%) | 280(44.1%) | <0.001 |
| Previous CABG | 36(7.8%) | 325(5.5%) | 147(4.7%) | 35(5.5%) | 0.048 |
| Previous HF | 72(15.6%) | 552(9.3%) | 212(6.8%) | 58(9.1%) | <0.001 |
| Previous MI | 121(26.2%) | 1610(27.1%) | 866(27.9%) | 184(29.0%) | 0.598 |
| CVD | 61(13.2%) | 559(9.4%) | 250(8.1%) | 45(7.1%) | <0.001 |
| PAD | 77(16.7%) | 518(8.7%) | 193(6.2%) | 33(5.2%) | <0.001 |
| COPD | 25(5.4%) | 204(3.4%) | 72(2.3%) | 6(0.9%) | <0.001 |
| STEMI | 150(32.5%) | 1423(23.9%) | 628(20.3%) | 132(20.8%) | <0.001 |
| non-STEMI | 42(9.1%) | 482(8.1%) | 220(7.1%) | 52(8.2%) | 0.255 |
| Unstable angina | 101(21.9%) | 1098(18.5%) | 551(17.8%) | 96(15.1%) | 0.031 |
| Stable angina | 76(16.5%) | 1573(26.5%) | 938(30.3%) | 189(29.8%) | <0.001 |
| Cardiogenic shock | 39(8.4%) | 239(4.0%) | 83(2.7%) | 26(4.1%) | <0.001 |
| Serum creatinine (mg/dl) | 1.8±2.3 | 1.3±1.9 | 1.2±1.7 | 1.3±1.9 | <0.001 |
| Hemodialysis | 61(13.2%) | 272(4.6%) | 84(2.7%) | 28(4.4%) | <0.001 |
| Aspirin | 442(95.7%) | 5735(96.5%) | 3022(97.5%) | 615(96.9%) | 0.113 |
| Clopidogrel | 325(70.4%) | 4523(76.1%) | 2378(76.7%) | 492(77.5%) | 0.084 |
| Ticlopidine | 15(3.3%) | 183(3.1%) | 110(3.6%) | 29(4.6%) | 0.311 |
Values are presented as n (%) or mean ± SD, as indicated.
BMI = body mass index; PCI = percutaneous coronary intervention; CABG = coronary artery bypass grafting; HF = heart failure; MI = myocardial infarction; CVD = cerebrovascular disease; PAD = peripheral artery disease; COPD = chronic obstructive pulmonary disease; STEMI = ST elevation myocardial infarction.
Angiographic and Procedural Data
Angiographic and procedural data are shown in Table 2. TRI was performed more frequently in patients with a high BMI (p = 0.002). However, transfemoral intervention was performed more frequently in patients with a low BMI (p = 0.005). There was no difference in the frequency of use of a closure device in each group. The rotablator and IABP were used more frequently in patients with a low BMI than in those with a high BMI (p = 0.003).
Table 2. Angiographical and Procedural Data.
| Underweight (BMI<18.5, n = 462) | Normal (18.5≦BMI<25.0, n = 5945) | Overweight (25.0≦BMI<30.0, n = 3100) | Obese (30.0≦BMI, n = 635) | P-value | |
|---|---|---|---|---|---|
| Two-vessel disease | 142(30.7%) | 1933(32.5%) | 1007(32.5%) | 233(36.7%) | 0.135 |
| Three-vessel disease | 124(26.8%) | 1432(24.1%) | 751(24.2%) | 169(26.6%) | 0.315 |
| Bifurcation lesion | 127(27.5%) | 1702(28.6%) | 839(27.1%) | 196(30.9%) | 0.183 |
| LMT lesion | 20(4.3%) | 258(4.3%) | 87(2.8%) | 22(3.5%) | 0.003 |
| CTO lesion | 33(7.1%) | 349(5.9%) | 209(6.7%) | 48(7.6%) | 0.161 |
| Type C lesion | 163(35.3%) | 1761(29.6%) | 918(29.6%) | 219(34.5%) | 0.006 |
| Transradial Intervention | 116(25.1%) | 1862(31.3%) | 1026(33.1%) | 218(34.3%) | 0.002 |
| Transfemoral Intervention | 336(72.7%) | 3939(66.3%) | 2011(64.9%) | 403(63.5%) | 0.005 |
| Drug-eluting stent | 304(65.8%) | 4183(70.4%) | 2237(72.2%) | 436(68.7%) | 0.018 |
| Sirolimus-eluting stent | 22(4.8%) | 349(5.9%) | 173(5.6%) | 31(4.9%) | 0.584 |
| Paclitaxel-eluting stent | 24(5.2%) | 208(3.5%) | 117(3.8%) | 24(3.8%) | 0.303 |
| Zotarolimus-eluting stent | 47(10.2%) | 487(8.2%) | 272(8.8%) | 47(7.4%) | 0.307 |
| Everolimus-eluting stent | 176(38.1%) | 2648(44.5%) | 1393(44.9%) | 275(43.3%) | 0.044 |
| Biolimus-eluting stent | 37(8.0%) | 445(7.5%) | 231(7.4%) | 49(7.7%) | 0.973 |
| Bare-metal stent | 105(22.7%) | 1363(22.9%) | 670(21.6%) | 158(24.9%) | 0.265 |
| Rotablator | 32(6.9%) | 251(4.2%) | 108(3.5%) | 21(3.3%) | 0.003 |
| Thrombus aspiration | 104(22.5) | 1195(20.1) | 600(19.4) | 139(21.9) | 0.260 |
| IVUS use | 367(79.4%) | 4803(80.8%) | 2539(81.9%) | 511(80.5%) | 0.449 |
| IABP use | 46(10.0%) | 465(7.8%) | 191(6.2%) | 40(6.3%) | 0.003 |
| Closure device | 68(14.7%) | 831(14.0%) | 453(14.6%) | 89(14.0%) | 0.851 |
Values are presented as n (%) or mean ± SD, as indicated.
LMT = left main trunk; CTO = chronic total occlusion; IVUS = intravascular ultrasound; IABP = intra-aortic balloon pumping.
Complications
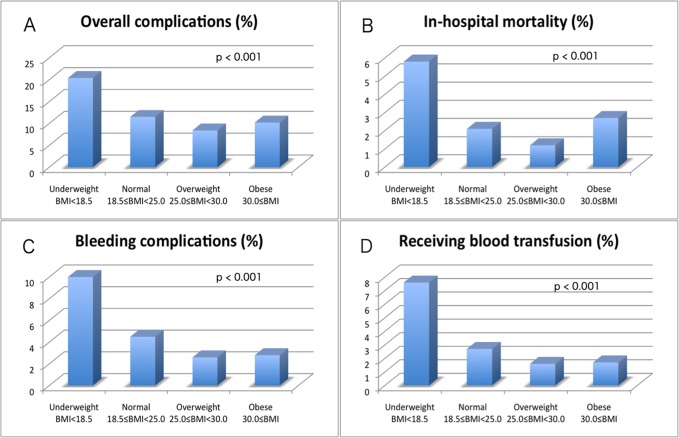
In-hospital complications are shown in Fig 2 and Table 3. Importantly, patients in the underweight group had the worst in-hospital outcomes, including overall complications (p<0.001), in-hospital mortality (p<0.001), heart failure after PCI (p = 0.048), cardiogenic shock (p = 0.018), bleeding complications within 72 hours (p<0.001), and a higher incidence of receiving blood transfusion (p<0.001).
Fig 2. Relationship between BMI and in-hospital complications.
In-hospital complication rates by BMI groups in 10,142 patients are shown. A: Overall complications; B: in-hospital mortality; C: bleeding complications within 72 hours; and D: rates of receiving blood transfusion.
Table 3. Complications.
| Underweight (BMI<18.5, n = 462) | Normal (18.5≦BMI<25.0, n = 5945) | Overweight (25.0≦BMI<30.0, n = 3100) | Obese (30.0≦BMI, n = 635) | P-value | |
|---|---|---|---|---|---|
| Overall complications | 94(20.4%) | 681(11.5%) | 261(8.4%) | 65(10.2%) | <0.001 |
| In-hospital mortality | 27(5.8%) | 127(2.1%) | 38(1.2%) | 17(2.7%) | <0.001 |
| Severe dissection | 10(2.1%) | 66(1.1%) | 26(0.8%) | 15(2.4%) | 0.002 |
| Coronary perforation | 8(1.7%) | 54(0.9%) | 29(0.9%) | 8(1.3%) | 0.309 |
| Myocardial infarction after PCI | 10(2.2%) | 135(2.3%) | 61(2.0%) | 13(2.1%) | 0.819 |
| Heart failure after PCI | 10(2.2%) | 121(2.0%) | 40(1.3%) | 8(1.3%) | 0.048 |
| Cardiogenic shock | 16(3.5%) | 117(2.0%) | 45(1.5%) | 10(1.6%) | 0.018 |
| Cardiac tamponade | 3(0.7%) | 22(0.4%) | 6(0.2%) | 1(0.2%) | 0.244 |
| Cerebral bleeding | 3(0.7%) | 2(0.1%) | 1(0.1%) | 0(0%) | <0.001 |
| Cerebral infarction | 5(1.1%) | 23(0.4%) | 11(0.4%) | 1(0.2%) | 0.086 |
| Bleeding complications(<72h) | 46(10.0%) | 266(4.5%) | 81(2.6%) | 18(2.8%) | <0.001 |
| Puncture site bleeding | 13(2.8%) | 66(1.1%) | 25(0.8%) | 5(0.8%) | 0.001 |
| Puncture site hematoma | 7(1.5%) | 60(1.0%) | 25(0.8%) | 2(0.3%) | 0.154 |
| Retroperitoneal bleeding | 1(0.2%) | 11(0.2%) | 1(0.1%) | 0(0%) | 0.185 |
| Gastrointestinal bleeding | 2(0.4%) | 24(0.4%) | 8(0.3%) | 3(0.5%) | 0.687 |
| Genitourinary bleeding | 2(0.4%) | 8(0.1%) | 3(0.1%) | 2(0.3%) | 0.225 |
| Other bleeding | 12(2.6%) | 64(1.1%) | 20(0.7%) | 4(0.6%) | 0.001 |
| New hemodialysis | 5(1.1%) | 53(0.9%) | 26(0.8%) | 5(0.8%) | 0.951 |
| Transfusion | 35(7.6%) | 163(2.7%) | 49(1.6%) | 11(1.7%) | <0.001 |
Values are presented as n (%).
PCI = percutaneous coronary intervention.
The results of multivariate logistic regression analysis on overall complications, in-hospital mortality, and bleeding complications within 72 hours in the total cohort are shown in Tables 4, 5 and 6. When BMI was entered as a continuous variable, it was not an independent predictor of overall complications (OR, 0.99; 95% CI, 0.97–1.01; p = 0.247) and in-hospital mortality (OR, 0.98; 95% CI, 0.94–1.03; p = 0.411), but was inversely associated with bleeding complications after adjustment for confounding variables (OR, 0.95; 95% CI, 0.92–0.98; p = 0.002). Because cardiogenic shock is known to be such a strong predictor of mortality and complications after PCI, we performed subgroup multivariate analysis in patients without cardiogenic shock (n = 9755, Tables 7, 8 and 9). In subgroup multivariate analysis, BMI was inversely associated with overall complications (OR, 0.98; 95% CI, 0.95–0.99; p = 0.033) and bleeding complications (OR, 0.95; 95% CI, 0.91–0.98; p = 0.006) after adjustment for confounding variables. Furthermore, there was a trend that BMI was moderately associated with in-hospital mortality (OR, 0.94; 95% CI, 0.88–1.01; p = 0.091).
Table 4. Multivariate Logistic Regression Analysis on Overall Complications in the Total Cohort.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age | 1.03 | 1.02–1.03 | <0.001 | 1.02 | 1.01–1.03 | <0.001 |
| Female gender | 1.62 | 1.41–1.87 | <0.001 | 1.45 | 1.23–1.71 | <0.001 |
| BMI | 0.94 | 0.92–0.95 | <0.001 | 0.99 | 0.97–1.01 | 0.247 |
| Previous HF | 1.84 | 1.52–2.21 | <0.001 | 1.52 | 1.22–1.89 | <0.001 |
| PAD | 1.38 | 1.11–1.69 | 0.004 | 1.30 | 1.02–1.64 | 0.036 |
| COPD | 1.67 | 1.21–2.25 | 0.002 | 1.53 | 1.07–2.14 | 0.020 |
| Hemodialysis | 1.74 | 1.34–2.24 | <0.001 | 1.48 | 1.09–1.97 | 0.011 |
| STEMI | 2.64 | 2.32–3.01 | <0.001 | 2.08 | 1.66–2.61 | <0.001 |
| non-STEMI | 1.42 | 1.14–1.74 | 0.002 | 1.45 | 1.09–1.92 | 0.010 |
| Cardiogenic Shock | 9.04 | 7.32–11.17 | <0.001 | 2.39 | 1.84–3.09 | <0.001 |
| IABP use | 9.73 | 8.27–11.45 | <0.001 | 4.90 | 4.02–5.96 | <0.001 |
| Transradial Intervention | 0.39 | 0.33–0.46 | <0.001 | 0.61 | 0.39–0.98 | 0.040 |
| Three-vessel disease | 1.57 | 1.37–1.80 | <0.001 | 1.19 | 1.02–1.39 | 0.024 |
| Type C lesion | 1.74 | 1.53–1.98 | <0.001 | 1.41 | 1.21–1.64 | <0.001 |
| CTO | 1.46 | 1.15–1.82 | 0.002 | 1.42 | 1.08–1.85 | 0.013 |
| Rotablator | 1.95 | 1.50–2.51 | <0.001 | 1.68 | 1.25–2.24 | <0.001 |
OR = odds ratio; CI = confidence interval; BMI = body mass index; HF = heart failure; PAD = peripheral artery disease; COPD = chronic obstructive pulmonary disease; STEMI = ST elevation myocardial infarction; IABP = intra-aortic balloon pumping; CTO = chronic total occlusion.
Table 5. Multivariate Logistic Regression Analysis on In-hospital Mortality in the Total Cohort.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age | 1.07 | 1.05–1.09 | <0.001 | 1.07 | 1.05–1.09 | <0.001 |
| BMI | 0.90 | 0.87–0.94 | <0.001 | 0.98 | 0.94–1.03 | 0.411 |
| Hyperlipidemia | 0.35 | 0.27–0.46 | <0.001 | 0.60 | 0.43–0.83 | 0.002 |
| Previous HF | 3.02 | 2.14–4.19 | <0.001 | 2.42 | 1.53–3.76 | <0.001 |
| Hemodialysis | 4.00 | 2.65–5.85 | <0.001 | 7.31 | 4.14–12.66 | <0.001 |
| STEMI | 5.65 | 4.27–7.53 | <0.001 | 3.90 | 2.16–7.45 | <0.001 |
| non-STEMI | 2.25 | 1.52–3.25 | <0.001 | 2.96 | 1.51–6.01 | 0.002 |
| Cardiogenic Shock | 30.8 | 22.9–41.5 | <0.001 | 5.74 | 3.89–8.50 | <0.001 |
| IABP use | 24.5 | 18.4–32.9 | <0.001 | 6.91 | 4.71–10.15 | <0.001 |
OR = odds ratio; CI = confidence interval; BMI = body mass index; HF = heart failure; STEMI = ST elevation myocardial infarction; IABP = intra-aortic balloon pumping.
Table 6. Multivariate Logistic Regression Analysis on Bleeding Complications within 72 Hours in the Total Cohort.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age | 1.04 | 1.03–1.05 | <0.001 | 1.01 | 1.00–1.03 | 0.011 |
| Female gender | 2.35 | 1.91–2.88 | <0.001 | 2.13 | 1.68–2.69 | <0.001 |
| BMI | 0.89 | 0.87–0.92 | <0.001 | 0.95 | 0.92–0.98 | 0.002 |
| Previous PCI | 0.54 | 0.43–0.68 | <0.001 | 0.70 | 0.52–0.94 | 0.016 |
| Previous HF | 2.14 | 1.62–2.78 | <0.001 | 1.55 | 1.13–2.10 | 0.007 |
| PAD | 1.69 | 1.24–2.26 | 0.001 | 1.45 | 1.03–2.03 | 0.035 |
| COPD | 1.97 | 1.24–2.99 | 0.005 | 1.88 | 1.14–2.97 | 0.015 |
| Hemodialysis | 2.79 | 1.98–3.83 | <0.001 | 2.14 | 1.45–3.11 | <0.001 |
| STEMI | 2.27 | 1.85–2.77 | <0.001 | 1.59 | 1.19–2.14 | 0.002 |
| non-STEMI | 2.09 | 1.56–2.75 | <0.001 | 1.92 | 1.35–2.70 | <0.001 |
| Cardiogenic Shock | 7.99 | 6.10–10.38 | <0.001 | 2.45 | 1.74–3.42 | <0.001 |
| IABP use | 7.64 | 6.11–9.51 | <0.001 | 3.50 | 2.63–4.63 | <0.001 |
| Transradial Intervention | 0.33 | 0.25–0.44 | <0.001 | 0.55 | 0.29–1.12 | 0.551 |
| Closure device | 0.91 | 0.67–1.21 | 0.522 | |||
| CTO | 1.64 | 1.16–2.27 | 0.006 | 1.78 | 1.20–2.61 | 0.005 |
| Rotablator | 2.63 | 1.84–3.67 | <0.001 | 2.25 | 1.50–3.31 | <0.001 |
OR = odds ratio; CI = confidence interval; BMI = body mass index; PCI = percutaneous coronary intervention; HF = heart failure; PAD = peripheral artery disease; COPD = chronic obstructive pulmonary disease; STEMI = ST elevation myocardial infarction; IABP = intra-aortic balloon pumping; CTO = chronic total occlusion.
Table 7. Multivariate Logistic Regression Analysis on Overall Complications in Patients without Cardiogenic Shock.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age | 1.03 | 1.02–1.04 | <0.001 | 1.02 | 1.01–1.03 | <0.001 |
| Female gender | 1.73 | 1.49–2.02 | <0.001 | 1.49 | 1.26–1.76 | <0.001 |
| BMI | 0.93 | 0.91–0.95 | <0.001 | 0.98 | 0.95–0.99 | 0.033 |
| Previous HF | 1.80 | 1.46–2.20 | <0.001 | 1.41 | 1.12–1.75 | 0.003 |
| PAD | 1.41 | 1.12–1.76 | 0.003 | 1.31 | 1.02–1.66 | 0.034 |
| COPD | 1.51 | 1.05–2.10 | 0.026 | 1.36 | 0.92–1.95 | 0.114 |
| Hemodialysis | 1.78 | 1.35–2.33 | <0.001 | 1.43 | 1.05–1.92 | 0.025 |
| STEMI | 2.07 | 1.79–2.40 | <0.001 | 2.02 | 1.69–2.40 | <0.001 |
| non-STEMI | 1.32 | 1.03–1.65 | 0.025 | 1.38 | 1.06–1.77 | 0.017 |
| IABP use | 7.87 | 6.47–9.55 | <0.001 | 5.57 | 4.52–6.86 | <0.001 |
| Transradial Intervention | 0.43 | 0.36–0.51 | <0.001 | 0.61 | 0.50–0.73 | <0.001 |
| Three-vessel disease | 1.53 | 1.32–1.78 | <0.001 | 1.15 | 0.98–1.35 | 0.087 |
| Type C lesion | 1.74 | 1.51–2.00 | <0.001 | 1.44 | 1.22–1.69 | <0.001 |
| CTO | 1.51 | 1.18–1.92 | 0.001 | 1.40 | 1.05–1.84 | 0.020 |
| Rotablator | 2.25 | 1.73–2.92 | <0.001 | 1.66 | 1.23–2.21 | 0.001 |
OR = odds ratio; CI = confidence interval; BMI = body mass index; HF = heart failure; PAD = peripheral artery disease; COPD = chronic obstructive pulmonary disease; STEMI = ST elevation myocardial infarction; IABP = intra-aortic balloon pumping; CTO = chronic total occlusion.
Table 8. Multivariate Logistic Regression Analysis on In-hospital Mortality in Patients without Cardiogenic Shock.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age | 1.10 | 1.08–1.13 | <0.001 | 1.09 | 1.07–1.12 | <0.001 |
| BMI | 0.82 | 0.77–0.87 | <0.001 | 0.94 | 0.88–1.01 | 0.091 |
| Hyperlipidemia | 0.40 | 0.27–0.58 | <0.001 | 0.70 | 0.46–1.05 | 0.087 |
| Previous HF | 3.69 | 2.35–5.63 | <0.001 | 2.47 | 1.46–4.06 | 0.001 |
| Hemodialysis | 5.40 | 3.24–8.61 | <0.001 | 7.82 | 4.25–13.96 | <0.001 |
| STEMI | 4.74 | 3.24–6.96 | <0.001 | 6.12 | 3.78–10.11 | <0.001 |
| non-STEMI | 1.93 | 1.07–3.25 | 0.030 | 2.74 | 1.38–5.21 | 0.005 |
| IABP use | 15.35 | 10.34–22.64 | <0.001 | 9.01 | 5.81–13.91 | <0.001 |
OR = odds ratio; CI = confidence interval; BMI = body mass index; HF = heart failure; STEMI = ST elevation myocardial infarction; IABP = intra-aortic balloon pumping.
Table 9. Multivariate Logistic Regression Analysis on Bleeding Complications within 72 Hours in Patients without Cardiogenic Shock.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age | 1.04 | 1.03–1.05 | <0.001 | 1.02 | 1.01–1.04 | <0.001 |
| Female gender | 2.29 | 1.77–2.96 | <0.001 | 1.82 | 1.38–2.38 | <0.001 |
| BMI | 0.89 | 0.86–0.93 | <0.001 | 0.95 | 0.91–0.98 | 0.006 |
| Previous PCI | 0.60 | 0.45–0.78 | <0.001 | 0.69 | 0.51–0.92 | 0.012 |
| Previous HF | 2.12 | 1.50–2.93 | <0.001 | 1.55 | 1.07–2.20 | 0.021 |
| PAD | 1.27 | 0.82–1.88 | 0.273 | |||
| COPD | 1.59 | 0.83–2.74 | 0.151 | |||
| Hemodialysis | 2.97 | 1.97–4.34 | <0.001 | 2.20 | 1.40–3.34 | <0.001 |
| STEMI | 1.61 | 1.22–2.11 | <0.001 | 1.65 | 1.19–2.29 | 0.003 |
| non-STEMI | 1.98 | 1.36–2.80 | <0.001 | 1.97 | 1.31–2.89 | 0.001 |
| IABP use | 4.52 | 3.22–6.21 | <0.001 | 3.07 | 2.15–4.32 | <0.001 |
| Transradial Intervention | 0.37 | 0.26–0.51 | <0.001 | 0.55 | 0.38–0.78 | <0.001 |
| Closure device | 1.01 | 0.70–1.42 | 0.944 | |||
| CTO | 2.06 | 1.38–2.99 | <0.001 | 2.39 | 1.57–3.54 | <0.001 |
| Rotablator | 3.42 | 2.29–4.95 | <0.001 | 2.67 | 1.73–4.01 | <0.001 |
OR = odds ratio; CI = confidence interval; BMI = body mass index; PCI = percutaneous coronary intervention; HF = heart failure; PAD = peripheral artery disease; COPD = chronic obstructive pulmonary disease; STEMI = ST elevation myocardial infarction; IABP = intra-aortic balloon pumping; CTO = chronic total occlusion.
Notably, variables that were independent predictors for overall complications, in-hospital mortality, and bleeding complications in the total cohort included age, previous heart failure, hemodialysis, STEMI, non-STEMI, cardiogenic shock, and use of IABP. TRI was an independent predictor of preventing overall complications (OR, 0.61; 95% CI, 0.39–0.98; p = 0.040). Use of a closure device was not a predictor of reducing bleeding complications by univariate analysis (OR, 0.91; 95% CI, 0.67–1.21; p = 0.522).
Discussion
The major findings of this study were that lean patients, rather than obese patients, were at greater risk for in-hospital complications during and after PCI in one of the largest, contemporary, multicenter registries in Japan. Our dataset included more than 10,000 patients. This allowed us to analyze the various in-hospital outcomes in each BMI group.
One of the biggest differences between Japanese patients with CAD and those in Western countries is physique. The average BMI is remarkably lower in Japanese CAD patients compared with those in Western countries. Wang et al. reported a comparative study of Asian versus non-Asian White Americans with non-STEMI. In their study, BMI was significantly lower in Asian patients than in non-Asian White Americans (24.9 vs 27.8 kg/m2, p<0.001) [21]. Consistent with their study, the average BMI was 24.2 in our cohort. In addition, previous studies have reported that more than 70% of patients were overweight or obese in Western PCI registries [4,8,12]. However, only 36.8% of the patients (3,735/10,142) were overweight or obese in our study. Our data regarding BMI in Japanese CAD patients are consistent with previous studies in Japan [30,31]. Some previous studies have reported that patients with a BMI >40 kg/m2 are considered extremely obese [32–34]. However, because the present Japanese study group included only 18 patients (0.2%) with a BMI >40 kg/m2, they were included in the highest BMI subgroup [10]. Previous studies in Western countries have reported that lean patients and extremely obese patients are at greater risk for adverse outcomes after PCI [7,10,14,20,35,36]. In our study, complication rates showed a reverse J-shape relation with a peak in risk in the lowest BMI group (Fig 2), but not a bi-modal (U-shaped) relation with a peak in risk in the lowest and highest BMI groups, as observed in Western registries [20,35,36]. The small number of extremely obese patients in Japan is one of the major reasons for the unique obesity paradox that is observed in this country.
The precise mechanism of the obesity paradox remains unclear. However, there are some possible explanations for this phenomenon. Previous studies have shown that obese patients tend to have more aggressive and invasive therapy for CAD at a younger age [10,20,32,37]. Our data are consistent with those previous studies, and patients with a high BMI were younger than those with a low BMI in our study. Younger age at the time of PCI in patients with a high BMI may be one of the reasons for the obesity paradox [5,31]. However, Gruberg et al. reported that the relationship between BMI and mortality rate after PCI, and analysis by age groups showed that 1-year mortality rate was higher in patients with a normal BMI for all age groups, except for those younger than 50 years old [12]. Furthermore, obese patients tend to have a high rate of guideline-based optimal medical therapy, including statins, angiotensin-converting enzyme inhibitors, and beta-blockers [7,8,32]. Cessation of smoking, cardiac rehabilitation, and dietary counseling are more frequently enforced in overweight and obese patients than in lean patients [8,38]. Furthermore, patients with a low BMI tend to have more disease-induced cachexia induced by carcinoma, smoking, chronic obstructive pulmonary disease, chronic heart failure, and insulin-dependent diabetes mellitus. These comorbidities have been suggested as a possible explanation for the obesity paradox [10,12,16,17].
Potential overdosing of antiplatelets or anticoagulants, and differences in platelet biology have been reported as reasons for the high risk of bleeding with a low BMI [9,39]. Notably, in our study, almost all of the Japanese patients underwent PCI with a unified regimen of aspirin and clopidogrel during and after the procedure. This was because other agents, such as prasugrel and ticagrelor were not available at the time of this study in Japan. A unified regimen of antiplatelets for all CAD patients with various BMIs may affect the obesity paradox in bleeding complications after PCI [13]. Wang et al. reported that Asian patients with non-STEMI had a significantly higher risk of bleeding compared with non-Asian white patients [21]. They also reported that Asian patients were more likely to receive excess doses of antithrombotic agents compared with non-Asians. Appropriate doses of antiplatelets or anticoagulants may reduce bleeding complications. Furthermore, patients with a low BMI were older (p<0.001), more likely to have end-stage renal disease (p<0.001) and systemic atherosclerotic disease including cerebrovascular disease (p<0.001) and peripheral artery disease (p<0.001), than those with a high BMI in our cohort. These results are consistent with previous studies [9,13,14,40], and indicate that patients with a low BMI in our present study tended to have progressive atherosclerosis in the arterial system of the whole body. Arterial stiffness due to progressive atherosclerosis might be associated with a higher risk of bleeding in patients with a low BMI [9,14], although we performed multivariate analysis to adjust for possible confounding variables.
Because bleeding complications are associated with short- and long-term adverse outcomes after PCI [39,41], efforts for reducing bleeding complications are important. Bivalirudin, TRI, and use of a closure device are considered as bleeding avoidance strategies [42]. Bivalirudin is not available in Japan. Therefore, appropriate use of a closure device and TRI may be useful for reducing bleeding complications after PCI. In our study, there was no difference in the frequency of using a closure device in each BMI group, and use of a closure device was not a predictor of reducing bleeding complications by univariate analysis. TRI has been reported as a useful method for reducing bleeding complications compared with conventional transfemoral intervention [24,43]. In our study, TRI was an independent predictor of preventing overall complications in the total cohort. TRI was also associated with a small risk of bleeding in a subgroup analysis of patients without cardiogenic shock in multivariate logistic regression analysis. Approximately one-third of all of the PCIs in our dataset were performed with the transradial approach. Furthermore, TRI is performed more frequently in patients with a high BMI than in those with a low BMI [36]. Although TRI is more commonly performed in Japan than in Western countries [24], more frequent use of radial access in patients with a low BMI for reducing bleeding complications should be considered. Because patients with a low BMI have small vessels compared with patients with a high BMI, an unfavorable arterial sheath size has been reported as a possible explanation for increased access site bleeding complications in those with a low BMI [7,40,44]. Kang et al. reported that a high BMI was associated with a large diameter of the coronary arteries, and was associated with a large stent area after intravascular ultrasound-guided stent implantation [45]. They concluded that a high BMI is not associated with worse outcomes after drug-eluting stent implantation, despite more comorbidities, greater plaque burden, and more plaque rupture. A large vessel diameter in patients with a high BMI is one of the potential causes of the obesity paradox after PCI. Endovascular techniques and devices have evolved over the years, and smaller sheaths, guiding catheters, stents, and balloons have become available in recent years. However, physicians should be aware that lean patients are at greater risk for complications during and after PCI.
Obesity is an independent risk factor of advanced cardiovascular disease and mortality [1–3]. Although the obesity paradox may be a real phenomenon, physicians should be aware that patients with an increased body mass remain at high risk for development of CAD and poor outcomes over the long term [2,3,32]. Current guidelines recommend weight reduction to a BMI <25 as a second prevention for patients with CAD [46,47]. However, there is no clear evidence that a reduction in weight improves the prognosis of patients after PCI. Further long-term studies are needed in the future regarding this important issue.
Study limitations
The first limitation of our study is that it was an observational clinical trial. The study population was heterogenous, including patients with different severities of coronary artery disease, ranging from acute coronary syndrome with cardiogenic shock to stable angina. Although we performed multivariate logistic regression analysis to adjust for possible confounding variables, some selection bias might not have been completely adjusted for in our statistical model, and the heterogeneity of the patients may have affected the incidence of complications in each of the BMI groups. Furthermore, we excluded 646 patients with missing data of basic information, including sex, height and/or body weight, which might have affected selection bias. Another limitation is that the duration of antiplatelet therapy and the size of the sheaths and guiding catheters were not recorded, and patients with cancer or other serious comorbidities were not excluded in our registry. These factors might have been associated with the rate of bleeding complications. Finally, the impact of BMI and in-hospital bleeding complications on long-term clinical outcomes in patients who undergo PCI should be investigated in our registry in the future.
Conclusions
Lean patients, rather than obese patients are at greater risk for in-hospital complications during and after PCI, particularly for bleeding complications.
Acknowledgments
We thank all of the investigators, clinical coordinators and institutions involved in the JCD-KICS study.
Investigators: Toshiki Kuno, Hiroyuki Motoda, Masaki Kodaira (Ashikaga Red Cross Hospital), Yutaka Okada (Eiju General Hospital), Soushin Inoue, Iwao Nakamura (Hino Municipal Hospital), Shunsuke Takagi, Takashi Matsubara (Hiratsuka City Hospital), Masashi Takahashi, Keishu Li, Koichiro Sueyoshi (Kawasaki City Municipal Hospital), Taku Inohara, Fumiaki Yashima, Atsushi Anzai, Ryota Tabei, Kentaro Hayashida, Takashi Kawakami, Hideaki Kanazawa, Shunsuke Yuasa, Yuichiro Maekawa, Akio Kawamura (Keio University School of Medicine), Masahiro Suzuki, Keisuke Matsumura (National Hospital Organization Saitama National Hospital) Yukinori Ikegami, Jun Fuse, Munehisa Sakamoto, Yukihiko Momiyama (National Hospital Organization Tokyo Medical Center), Ayaka Endo, Tasuku Hasegawa, Toshiyuki Takahashi, Susumu Nakagawa (Saiseikai Central Hospital), Takashi Yagi, Kenichiro Shimoji, Shigetaka Noma (Saiseikai Utsunomiya Hospital), Masahito Munakata, Shiro Ishikawa, Takashi Koyama (Saitama City Hospital), Atsushi Mizuno, Yutaro Nishi (St Luke’s International Hospital Heart Center), Daisuke Shinmura, Kazunori Moritani, Masaru Shibata (Tachikawa Kyosai Hospital), Kimi Koide, Yoshinori Mano, Takahiro Oki (Tokyo Dental College Ichikawa General Hospital), Koji Negishi, Yusuke Jo, and Takahiro Koura (Yokohama Municipal Hospital)
Clinical Coordinators: Junko Susa, Ayano Ishikawa, Hiroaki Nagayama, Miho Umemura, Itsuka Saito, Saori Sugiyama, and Ikuko Ueda
Data Availability
All relevant data are within the paper.
Funding Statement
This research was supported by a grant from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (KAKENHI No. 21790751). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. [DOI] [PubMed] [Google Scholar]
- 2. Chen Y, Copeland WK, Vedanthan R, Grant E, Lee JE, Gu D, et al. Association between body mass index and cardiovascular disease mortality in east Asians and south Asians: pooled analysis of prospective data from the Asia Cohort Consortium. BMJ. 2013;347:f5446 10.1136/bmj.f5446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383:970–983. 10.1016/S0140-6736(13)61836-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sarno G, Garg S, Onuma Y, Buszman P, Linke A, Ischinger T, et al. The impact of body mass index on the one year outcomes of patients treated by percutaneous coronary intervention with Biolimus- and Sirolimus-eluting stents (from the LEADERS Trial). Am J Cardiol. 2010;105:475–479. 10.1016/j.amjcard.2009.09.055 [DOI] [PubMed] [Google Scholar]
- 5. Sarno G, Raber L, Onuma Y, Garg S, Brugaletta S, van Domburg RT, et al. Impact of body mass index on the five-year outcome of patients having percutaneous coronary interventions with drug-eluting stents. Am J Cardiol. 2011;108:195–201. 10.1016/j.amjcard.2011.03.023 [DOI] [PubMed] [Google Scholar]
- 6. Wang ZJ, Zhou YJ, Liu YY, Yu M, Shi DM, Zhao YX, et al. Obesity and cardiovascular thrombotic events in patients undergoing percutaneous coronary intervention with drug-eluting stents. Heart. 2009;95:1587–1592. 10.1136/hrt.2009.172395 [DOI] [PubMed] [Google Scholar]
- 7. Angeras O, Albertsson P, Karason K, Ramunddal T, Matejka G, James S, et al. Evidence for obesity paradox in patients with acute coronary syndromes: a report from the Swedish Coronary Angiography and Angioplasty Registry. Eur Heart J. 2013;34:345–353. 10.1093/eurheartj/ehs217 [DOI] [PubMed] [Google Scholar]
- 8. Lancefield T, Clark DJ, Andrianopoulos N, Brennan AL, Reid CM, Johns J, et al. Is there an obesity paradox after percutaneous coronary intervention in the contemporary era? An analysis from a multicenter Australian registry. JACC Cardiovasc Interv. 2010;3:660–668. 10.1016/j.jcin.2010.03.018 [DOI] [PubMed] [Google Scholar]
- 9. Delhaye C, Wakabayashi K, Maluenda G, Belle L, Ben-Dor I, Gonzalez MA, et al. Body mass index and bleeding complications after percutaneous coronary intervention: does bivalirudin make a difference? Am Heart J. 2010;159:1139–1146. 10.1016/j.ahj.2010.03.011 [DOI] [PubMed] [Google Scholar]
- 10. Benderly M, Boyko V, Goldbourt U. Relation of body mass index to mortality among men with coronary heart disease. Am J Cardiol. 2010;106:297–304. 10.1016/j.amjcard.2010.03.078 [DOI] [PubMed] [Google Scholar]
- 11. Oreopoulos A, Padwal R, Norris CM, Mullen JC, Pretorius V, Kalantar-Zadeh K. Effect of obesity on short- and long-term mortality postcoronary revascularization: a meta-analysis. Obesity (Silver Spring). 2008;16:442–450. 10.1038/oby.2007.36 [DOI] [PubMed] [Google Scholar]
- 12. Gruberg L, Weissman NJ, Waksman R, Fuchs S, Deible R, Pinnow EE, et al. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol. 2002;39:578–584. [DOI] [PubMed] [Google Scholar]
- 13. Mak KH, Bhatt DL, Shao M, Haffner SM, Hamm CW, Hankey GJ, et al. The influence of body mass index on mortality and bleeding among patients with or at high-risk of atherothrombotic disease. Eur Heart J. 2009;30:857–865. 10.1093/eurheartj/ehp037 [DOI] [PubMed] [Google Scholar]
- 14. Byrne J, Spence MS, Fretz E, Mildenberger R, Chase A, Berry B, et al. Body mass index, periprocedural bleeding, and outcome following percutaneous coronary intervention (from the British Columbia Cardiac Registry). Am J Cardiol. 2009;103:507–511. 10.1016/j.amjcard.2008.10.027 [DOI] [PubMed] [Google Scholar]
- 15. Ndrepepa G, Fusaro M, Cassese S, Guerra E, Schunkert H, Kastrati A. Relation of Body Mass Index to Bleeding During Percutaneous Coronary Interventions. Am J Cardiol. 2014;115:434–440. 10.1016/j.amjcard.2014.11.022 [DOI] [PubMed] [Google Scholar]
- 16. Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. 2008;156:13–22. 10.1016/j.ahj.2008.02.014 [DOI] [PubMed] [Google Scholar]
- 17. Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M. An obesity paradox in acute heart failure: analysis of body mass index and inhospital mortality for 108,927 patients in the Acute Decompensated Heart Failure National Registry. Am Heart J. 2007;153:74–81. [DOI] [PubMed] [Google Scholar]
- 18. Mobeirek AF, Al-Habib K, Al-Faleh H, Hersi A, Kashour T, Ullah A, et al. Absence of obesity paradox in Saudi patients admitted with acute coronary syndromes: insights from SPACE registry. Annals of Saudi medicine. 2014;34:38–45. 10.5144/0256-4947.2014.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herrmann J, Gersh BJ, Goldfinger JZ, Witzenbichler B, Guagliumi G, Dudek D, et al. Body mass index and acute and long-term outcomes after acute myocardial infarction (from the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction Trial). Am J Cardiol. 2014;114:9–16. 10.1016/j.amjcard.2014.03.057 [DOI] [PubMed] [Google Scholar]
- 20. Witassek F, Schwenkglenks M, Erne P, Radovanovic D. Impact of Body Mass Index on mortality in Swiss hospital patients with ST-elevation myocardial infarction: does an obesity paradox exist? Swiss medical weekly. 2014;144:w13986 10.4414/smw.2014.13986 [DOI] [PubMed] [Google Scholar]
- 21. Wang TY, Chen AY, Roe MT, Alexander KP, Newby LK, Smith SC Jr, et al. Comparison of baseline characteristics, treatment patterns, and in-hospital outcomes of Asian versus non-Asian white Americans with non-ST-segment elevation acute coronary syndromes from the CRUSADE quality improvement initiative. Am J Cardiol. 2007;100:391–396. [DOI] [PubMed] [Google Scholar]
- 22. Kohsaka S, Kimura T, Goto M, Lee VV, Elayda M, Furukawa Y, et al. Difference in patient profiles and outcomes in Japanese versus American patients undergoing coronary revascularization (collaborative study by CREDO-Kyoto and the Texas Heart Institute Research Database). Am J Cardiol. 2010;105:1698–1704. 10.1016/j.amjcard.2010.01.349 [DOI] [PubMed] [Google Scholar]
- 23. Sasayama S. Heart disease in Asia. Circulation. 2008;118(25):2669–2671. 10.1161/CIRCULATIONAHA.108.837054 [DOI] [PubMed] [Google Scholar]
- 24. Numasawa Y, Kohsaka S, Miyata H, Kawamura A, Noma S, Suzuki M, et al. Safety of transradial approach for percutaneous coronary intervention in relation to body mass index: a report from a Japanese multicenter registry. Cardiovasc Interv Ther. 2013;28:148–156. 10.1007/s12928-012-0138-8 [DOI] [PubMed] [Google Scholar]
- 25. Numasawa Y, Kohsaka S, Miyata H, Kawamura A, Noma S, Suzuki M, et al. Use of Thrombolysis in Myocardial Infarction Risk Score to predict bleeding complications in patients with unstable angina and non-ST elevation myocardial infarction undergoing percutaneous coronary intervention. Cardiovasc Interv Ther. 2013;28:242–249. 10.1007/s12928-013-0162-3 [DOI] [PubMed] [Google Scholar]
- 26. Ohno Y, Maekawa Y, Miyata H, Inoue S, Ishikawa S, Sueyoshi K, et al. Impact of periprocedural bleeding on incidence of contrast-induced acute kidney injury in patients treated with percutaneous coronary intervention. J Am Coll Cardiol. 2013;62:1260–1266. 10.1016/j.jacc.2013.03.086 [DOI] [PubMed] [Google Scholar]
- 27. Kuno T, Numasawa Y, Miyata H, Takahashi T, Sueyoshi K, Ohki T, et al. Impact of coronary dominance on in-hospital outcomes after percutaneous coronary intervention in patients with acute coronary syndrome. PLoS One. 2013;8:e72672 10.1371/journal.pone.0072672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Numasawa Y, Kohsaka S, Miyata H, Noma S, Suzuki M, Ishikawa S, et al. Gender differences in in-hospital clinical outcomes after percutaneous coronary interventions: an insight from a Japanese multicenter registry. PLoS One. 2015;10:e0116496 10.1371/journal.pone.0116496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mehta SK, Frutkin AD, Lindsey JB, House JA, Spertus JA, Rao SV, et al. Bleeding in patients undergoing percutaneous coronary intervention: the development of a clinical risk algorithm from the National Cardiovascular Data Registry. Circ Cardiovasc Interv. 2009;2:222–229. 10.1161/CIRCINTERVENTIONS.108.846741 [DOI] [PubMed] [Google Scholar]
- 30. Kaneko H, Yajima J, Oikawa Y, Tanaka S, Fukamachi D, Suzuki S, et al. Obesity paradox in Japanese patients after percutaneous coronary intervention: An observation cohort study. J Cardiol. 2013;62:18–24. 10.1016/j.jjcc.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 31. Kosuge M, Kimura K, Kojima S, Sakamoto T, Ishihara M, Asada Y, et al. Impact of body mass index on in-hospital outcomes after percutaneous coronary intervention for ST segment elevation acute myocardial infarction. Circ J. 2008;72:521–525. [DOI] [PubMed] [Google Scholar]
- 32. Steinberg BA, Cannon CP, Hernandez AF, Pan W, Peterson ED, Fonarow GC. Medical therapies and invasive treatments for coronary artery disease by body mass: the "obesity paradox" in the Get With The Guidelines database. Am J Cardiol. 2007;100:1331–1335. [DOI] [PubMed] [Google Scholar]
- 33. Payvar S, Kim S, Rao SV, Krone R, Neely M, Paladugu N, et al. In-Hospital Outcomes of Percutaneous Coronary Interventions in Extremely Obese and Normal-Weight Patients: Findings From the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol. 2013;62:692–696. 10.1016/j.jacc.2013.05.058 [DOI] [PubMed] [Google Scholar]
- 34. Das SR, Alexander KP, Chen AY, Powell-Wiley TM, Diercks DB, Peterson ED, et al. Impact of body weight and extreme obesity on the presentation, treatment, and in-hospital outcomes of 50,149 patients with ST-Segment elevation myocardial infarction results from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol. 2011;58:2642–2650. 10.1016/j.jacc.2011.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–678. [DOI] [PubMed] [Google Scholar]
- 36. Cox N, Resnic FS, Popma JJ, Simon DI, Eisenhauer AC, Rogers C. Comparison of the risk of vascular complications associated with femoral and radial access coronary catheterization procedures in obese versus nonobese patients. Am J Cardiol. 2004;94:1174–1177. [DOI] [PubMed] [Google Scholar]
- 37. Niraj A, Pradhan J, Fakhry H, Veeranna V, Afonso L. Severity of coronary artery disease in obese patients undergoing coronary angiography: "obesity paradox" revisited. Clin Cardiol. 2007;30:391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Diercks DB, Roe MT, Mulgund J, Pollack CV Jr, Kirk JD, Gibler WB, et al. The obesity paradox in non-ST-segment elevation acute coronary syndromes: results from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the American College of Cardiology/American Heart Association Guidelines Quality Improvement Initiative. Am Heart J. 2006;152:140–148. [DOI] [PubMed] [Google Scholar]
- 39. Ahmed B, Piper WD, Malenka D, VerLee P, Robb J, Ryan T, et al. Significantly improved vascular complications among women undergoing percutaneous coronary intervention: a report from the Northern New England Percutaneous Coronary Intervention Registry. Circ Cardiovasc Interv. 2009;2:423–429. 10.1161/CIRCINTERVENTIONS.109.860494 [DOI] [PubMed] [Google Scholar]
- 40. Gurm HS, Brennan DM, Booth J, Tcheng JE, Lincoff AM, Topol EJ. Impact of body mass index on outcome after percutaneous coronary intervention (the obesity paradox). Am J Cardiol. 2002;90:42–45. [DOI] [PubMed] [Google Scholar]
- 41. Ndrepepa G, Berger PB, Mehilli J, Seyfarth M, Neumann FJ, Schomig A, et al. Periprocedural bleeding and 1-year outcome after percutaneous coronary interventions: appropriateness of including bleeding as a component of a quadruple end point. J Am Coll Cardiol. 2008;51:690–697. 10.1016/j.jacc.2007.10.040 [DOI] [PubMed] [Google Scholar]
- 42. Daugherty SL, Thompson LE, Kim S, Rao SV, Subherwal S, Tsai TT, et al. Patterns of use and comparative effectiveness of bleeding avoidance strategies in men and women following percutaneous coronary interventions: an observational study from the national cardiovascular data registry. J Am Coll Cardiol. 2013;61:2070–2078. 10.1016/j.jacc.2013.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brueck M, Bandorski D, Kramer W, Wieczorek M, Holtgen R, Tillmanns H. A randomized comparison of transradial versus transfemoral approach for coronary angiography and angioplasty. JACC Cardiovasc Interv. 2009;2:1047–1054. 10.1016/j.jcin.2009.07.016 [DOI] [PubMed] [Google Scholar]
- 44. Gurm HS, Whitlow PL, Kip KE. The impact of body mass index on short- and long-term outcomes inpatients undergoing coronary revascularization. Insights from the bypass angioplasty revascularization investigation (BARI). J Am Coll Cardiol. 2002;39:834–840. [DOI] [PubMed] [Google Scholar]
- 45. Kang SJ, Mintz GS, Witzenbichler B, Metzger DC, Rinaldi MJ, Duffy PL, et al. Effect of obesity on coronary atherosclerosis and outcomes of percutaneous coronary intervention: grayscale and virtual histology intravascular ultrasound substudy of assessment of dual antiplatelet therapy with drug-eluting stents. Circ Cardiovasc Interv. 2015;8(1). 10.1161/CIRCINTERVENTIONS.114.002079 [DOI] [PubMed] [Google Scholar]
- 46. Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE Jr, Ettinger SM, et al. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American Academy of Family Physicians, Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;57:e215–367. 10.1016/j.jacc.2011.02.011 [DOI] [PubMed] [Google Scholar]
- 47. Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernandez-Aviles F, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007;28:1598–1660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.