Abstract
Patients with sickle cell disease (SCD) can have recurrent episodes of vaso-occlusive crises, which are associated with severe pain. While opioids are the mainstay of analgesic therapy, in some patients, increasing opioid use results in continued and increasing pain. Many believe that this phenomenon results from opioid-induced tolerance or hyperalgesia or that SCD pain involves non-opioid-responsive mechanisms. Dexmedetomidine, a specific α2-adrenoreceptor agonist, which has sedative and analgesic properties, reduces opioid requirements, and can facilitate opioid withdrawal in clinical settings. We hypothesized that dexmedetomidine would ameliorate the nociception phenotype of SCD mice. Townes and BERK SCD mice, strains known to have altered nociception phenotypes, were used in a crossover preclinical trial that measured nocifensive behavior before and after treatment with dexmedetomidine or vehicle. In a linear dose-effect relationship, over 60-min, dexmedetomidine, compared with vehicle, significantly increased hot plate latency in Townes and BERK mice (P≤0.006). In sickling, but not control mice, dexmedetomidine improved grip force, an indicator of muscle pain (P=0.002). As expected, dexmedetomidine had a sedative effect in sickling and control mice as it decreased wakefulness scores compared with vehicle (all P<0.001). Interestingly, the effects of dexmedetomidine on hot plate latency and wakefulness scores were different in sickling and control mice, i.e., dexmedetomidine-related increases in hotplate latency and decreases in wakefulness scores were significantly smaller in Townes sickling compared to control mice. In conclusion, these findings of beneficial effects of dexmedetomidine on the nociception phenotype in SCD mice might support the conduct of studies of dexmedetomidine in SCD patients.
Keywords: sickle cell, pain, opioid, α2-agonist, hyperalgesia, muscle pain, dexmedetomidine
1. Introduction
Pain is the most common reason why sickle cell disease (SCD) patients seek medical attention and accounts for over 180,000 emergency room visits, 75,000 hospitalizations, and nearly one billion dollars in health care costs yearly(Ballas et al., 2012; Platt et al., 1991; Smith et al., 2008; Steiner and Miller, 2006; Yusuf et al., 2010). When patients are admitted for vaso-occlusive crises, even after several days of hospitalization some report little change in pain severity and after discharge, pain-related re-hospitalization rates remain high(Ballas and Lusardi, 2005; Brousseau et al., 2010). Opioids, the mainstay of SCD-pain therapy, are somewhat effective in alleviating symptoms during acute pain crises, but are often ineffective in treating chronic and neuropathic pain, which are also seen in SCD patients(Brandow et al., 2014; McNicol et al., 2013; Wilkie et al., 2010; Xu et al., 2014). In some patients, escalating doses of opioids can be associated with continued and increasing pain, which many believe results from tolerance, opioid-induced hyperalgesia, or reflects pain due to mechanisms unresponsive to opioid(Ballas et al., 2012; Brush, 2012). Most approaches to treat SCD-pain are based on expert opinion and observational studies rather than clinical trials(Field et al., 2009; Niscola et al., 2009) and often address symptoms rather than SCD-pain mechanisms(Field et al., 2009; Niscola et al., 2009). Therefore, new therapies are needed to improve the treatment of SCD pain.
Humanized SCD mice allow for the conduct of preclinical studies of therapies that might have a role in SCD pain. These animals display thermal, mechanical, and muscle hyperalgesia and sensitization of somatosensory fibers(Cain et al., 2012; Garrison et al., 2012; Hillery et al., 2011; Kenyon et al., 2015; Kohli et al., 2010; Vincent et al., 2013). Interestingly, this mechanical and thermal hyperalgesia is accentuated by hypoxia and reoxygenation, which suggest that the altered nocifensive phenotype in SCD mice could partially result from recurrent ischemia/reperfusion injury associated with vaso-occlusion(Cain et al., 2012; Hebbel, 2014). Therefore, SCD mice are valuable for the study of SCD-pain mechanisms and for the evaluation of novel approaches that might ameliorate ischemia/reperfusion injury and treat SCD-pain.
Animal studies support the investigation of dexmedetomidine, a specific α2-adrenoreceptor agonist (Bol et al., 1999; Kamibayashi and Maze, 2000) in SCD. For example, in visceral pain models, the antinociceptive effects of dexmedetomidine are opioid-receptor-independent and are associated with increased nitric oxide availability(Rangel et al., 2014). In neuropathic pain, the antinociceptive effect of dexmedetomidine results from supraspinal facilitation of inhibitory postsynaptic currents and inhibition of sensory neurons(Funai et al., 2014). Lastly, in several models of organ ischemia/reperfusion injury, dexmedetomidine has been shown to have protective effects(Bell et al., 2014; Bell et al., 2012; Dong et al., 2014; Sahin et al., 2013; Yoshitomi et al., 2012). Therefore, given that ischemia/reperfusion injury underlies SCD complications and that dexmedetomidine has beneficial effects in those settings, one could argue that studies of dexmedetomidine in SCD are warranted. Here we examined the effect of dexmedetomidine in SCD and hypothesized that this α2-adrenoreceptor agonist would ameliorate the nocifensive phenotype in SCD mice.
2. Materials and Methods
The investigational protocol was approved by the Children’s National Health System Institutional Animal Care and Use Committee and all experiments were conducted in compliance with the Guide for the Care and Use of Laboratory Animals, National Institutes of Health.
2.1. Animals
We examined the Townes [B6;129-Hbatm1(HBA)Tow Hbbtm2(HBG1,HBB*)Tow/Hbbtm3(HBG1,HBB)Tow /J (Jackson Laboratory,stock number 013071] (Hanna et al., 2007; Kenyon et al., 2015; Wu et al., 2006) and the BERK strains of humanized SCD mice [Hbatm1Paz Hbbtm1Tow Tg(HBA-HBBs)41Paz/J, stock number 003342] (Paszty et al., 1997). Townes sickling mice do not express mouse hemoglobin and carry mutations that incorporate human hemoglobin. One mutation is designed with the human hemoglobin α-gene (Hbatm1(HBA)Tow, hα) and the second with a 9.7-kb DNA fragment that contains human Aγ-globin gene and sickle hemoglobin (Hbbtm2(HBG1,HBB*)Tow, βS). These animals (hα/hα::βS/βS), here referred to as Townes sickling mice, recapitulate several hematologic phenotypes of human SCD (anemia, reticulocytosis, leukocytosis, sickling) and have liver as well as kidney pathology(Hanna et al., 2007; Wu et al., 2006). Similarly, Townes control mice do not express mouse hemoglobin and carry mutations containing the human α-globin gene and fragments of the human hemoglobin gamma (Aγ) and human wild-type beta globin (Hbbtm3(HBG1,HBB)Tow, βA) genes (hα/hα::βA/βA)(Hanna et al., 2007; Wu et al., 2006)
In some experiments, we also examined another strain of SCD, the BERK sickling mice (Jackson Laboratory, stock number 003342) (Paszty et al., 1997). These animals do not express any mouse hemoglobin and carry copies of a transgene [Tg(HBA-HBBs)41Paz] containing human HBA1 (hemoglobin, alpha 1), HBG2 (hemoglobin, gamma G, fetal component), HBG1 (hemoglobin, gamma A, fetal component), HBD (hemoglobin, delta) and HBBS (hemoglobin, beta, sickle allele) genes(Paszty et al., 1997). We used C57BL/6J as the control strain for BERK sickling mice due to the lack of availability of BERK control mice expressing normal human hemoglobin. Further, because of significant limitations in number of available BERK mice, only females were included in only some experiments in this study. Mice were housed in a temperature-controlled facility (21°C) with a standard 12-h light-dark schedule. Mice from all genotypes were housed together in an attempt to control for estrous cycles. During any given experiment of nocifensive behaviors, a group of SCD and respective control mice were examined.
2.2. Study Design and Experimental Protocol
The experimental design adhered to the suggested framework aimed at increasing the predictive value of preclinical trials (Landis et al., 2012). We conducted a randomized controlled crossover trial where all mice received a single subcutaneous injection of either vehicle (phosphate buffered saline) or various doses of dexmedetomidine (10, 25, 50, or 100 µg/kg) during each experiment. Measurements were obtained at baseline (24 h before) and at 30 and 60 min after drug administration. Between experiments, a minimum of 72-h drug-washout period was observed. In SCD mice, we evaluated nocifensive behavior (current vocalization threshold, hot plate latency, and grip force) and measured the wakefulness score both before and after injections. Experiments were performed between 9:00AM and 02:00 PM in a quiet room with one animal present during interventions. One investigator administered all study drugs and another, who was unaware of the animals genotype or treatment received, obtained the outcome measurements. In order to avoid operator variability, the same investigator obtained given nocifensive behavior measurement for the entirety of the experiments.
2.3. Nocifensive Behavior Studies
Three cohorts of mice were used in this study. One cohort underwent hot plate latency followed by current threshold measurements, another underwent grip force evaluation, and the third was used for wakefulness scores.
2.3.1 Hot plate latency
In order to evaluate the effect of dexmedetomidine on response to noxious thermal stimuli, mice were placed on a hot plate (Harvard Apparatus, Holliston, MA) set at 55°C and latency time for the display of pain-avoiding behaviors (jumping, stomping or repeated lifting or licking of hind or front paws) was measured. Once these behaviors were observed, mice were removed from the hot plate (Le Bars et al., 2001). In order to avoid injuries, animals were allowed to remain on the hot plate for a maximum of 30 seconds. In this crossover design, all animals received one injection of either dexmedetomidine (50 or 100 µg/kg) or vehicle in each of the four experiments.
2.3.2. Sensory nerve fiber evaluation - current vocalization threshold
In the same cohort of animals that underwent the hot plate test, we evaluated somatosensory fiber function using sine-wave electrical stimuli at different frequencies: 2000, 250, and 5 Hz that preferentially stimulate Aβ, Aδ, and C fibers respectively (Finkel et al., 2012; Finkel et al., 2006; Kenyon et al., 2015; Koga et al., 2005). Briefly, sine-wave electrical stimuli generated by a neurostimulator (Neurotron, Inc, Baltimore, MD) and controlled by custom software were delivered to the mouse tail as previously described (Finkel et al., 2012; Finkel et al., 2006; Spornick et al., 2011). Electrical stimuli (2000, 250, and 5Hz) were delivered at increasing intensities for one second (50% duty cycle) and followed by a one-second stimulus-free interval. Between stimulation with different frequencies, there was a one-min rest period. The electrical stimulus amperage that elicited audible vocalization (nocifensive behavior endpoint) or the maximum amperage delivered at each frequency was recorded as the respective current threshold(Finkel et al., 2012; Finkel et al., 2006; Spornick et al., 2011). Current thresholds for each frequency were determined by averaging five consecutive measurements and were obtained in response to 2000, 250, and 5 Hz sequentially. For ease of presentation, the unit of measurement of current threshold is “Unit” (U), which corresponds to 100 times the amperage that elicited audible vocalization. Specifically, 1 current threshold unit equates to 0.01 mAmperes.
2.3.3. Grip Force
In order to determine grip force, a surrogate measure of deep tissue hyperalgesia in SCD mice (Kohli et al., 2010), we used the Grip Strength Meter (GSM, San Diego, Inc., San Diego, CA) as previously described (Kehl et al., 2000). In a crossover design, animals received one subcutaneous injection of vehicle and dexmedetomidine at doses of 25 and 50µg/kg. Prior to the dexmedetomidine trial, animals were trained in the grip force measurement procedures over the course of five sessions. For each measurement, animals were held by the base of the tail and allowed to grasp a steel grip gauge with their forepaws. Then, the mice were gently pulled away from the grip gauges in a steady fashion until the grip was released. The force exerted at the gauge at the time of grip release is determined by the mouse itself and is recorded as the grip force. A similar procedure was used to measure overall grip force except that all four limbs were allowed to grasp two steel grip gauges at the same time. At each time point, forelimb and overall grip forces were the average of five measurements. These measurements were then controlled for mouse body weight.
2.4. Sedation studies
In a separate cohort of animals, sedation studies were conducted using a modification of a previously described technique(Chuck et al., 2006). In a crossover fashion, animals received vehicle and dexmedetomidine at doses of 10, 25, and 50 µg/kg subcutaneously in a volume of 10ml/kg. At each time point before (baseline) and after (30 and 60 min) drug administration, animals were videotaped for 5 min. Another investigator, unaware of treatment allocations or animal genotype, watched the videos and assig ned a wakefulness score as follows: 5- awake: active, engaged in locomotion, rearing (raise itself upright on its hinds legs) or head movements or grooming; 4- awake: inactive, eyes fully open, head up, little to no locomotion, rearing or grooming, normal posture; 3- mild sedation: eyes partly closed, head somewhat down, impaired locomotion including abnormal posture, use of only some limbs, dragging and stumbling; 2-moderate sedation: head mostly or completely down, eyes partially closed, flattened posture, no spontaneous movement; 1- Heavy sedation: eyes mostly closed, loss of righting reflex.
2.5. Statistical analysis
We assessed the effect of period and sequence of crossover intervention on change of study outcomes from baseline. We applied a mixed effect model to assess the interaction between time and treatment (dexmedetomidine by different dosage vs. PBS) at each post-treatment time-point (0.5 and 1 h). At each time point, a significant p value for interaction indicates the significant difference between effect of dexmedetomidine and PBS on change of outcome from baseline. We used mixed models to present the adjusted mean (±S.E.M.) of each outcome during the course of the experiment. We choose the mixed-effect model approach over repeated measures ANOVA because mixed-effect models are more credible for analysis of data with nonlinear trends and non-normally distributed outcomes. In addition, the mixed-effect model provides a robust and powerful strategy for the analysis of unbalanced data and can handle different data patterns (e.g. random coefficient) which are often seen in nociception studies (Liu et al., 2010; Wainwright et al., 2007). We repeated this analysis for different genotypes and then in a combined model we assessed the difference of treatment effect in two genotypes by using an interaction between time*treatment*genotype. All models were checked for outliers. P values <0.05 were reported as significant and all analyses were performed in STATA 13.0 (StataCorp., College Station, TX).
3. Results
All mice enrolled in this study completed the experiments and tolerated dexmedetomidine well. After subcutaneous injection of dexmedetomidine, mice appeared sedated for over one h. There was no complications associated with dexmedetomidine injections at any dose. Table 1 lists number and baseline characteristics of animals enrolled in the study. In concert with prior reports (Kenyon et al., 2015), sickle cell mice both from the Townes and BERK strains have thermal hyperalgesia as indicated by significant decreases in hot plate latency (Table 2). In addition, as we have previously described (Kenyon et al., 2015), Townes mice have increased sensitization of sensory fibers as indicated by decreases in current vocalization thresholds at baseline (Table 2).
Table 1.
Demographics and experimental groups of animals undergoing quantitative sensory testing and grip force test.a
| Variables | Townes Sickle | Townes Controls | BERKs | C57BL/6J |
|---|---|---|---|---|
| Quantitative Sensory Testing (current threshold, hot plate) | ||||
| Number | 20 | 20 | 13 | 10 |
| Age (weeks) | 15 (13–19) | 18 (16–19) | 17 (15–18) | 15 (14–17) |
| Weight (g) | 22.5 (20.0–25.0) | 21.0 (20.5–22.5) | 19.0 (18.0–20.0) | 19.0 (18.0–19.0) |
| Grip Force Test | ||||
| Number | 10 | 10 | 0 | 0 |
| Age (weeks) | 14 (13–18) | 14 (13–18) | ||
| Weight (g) | 19.0 (19.0–20.0) | 19.0 (19.0–20.0) | ||
| Wakefulness Score | ||||
| Number | 9 | 12 | 0 | 0 |
| Age (weeks) | 20 (16–20) | 19 (13–17) | ||
| Weight (g) | 24 (20–25) | 21(19–25) | ||
Results are shown as median (interquartile range) unless otherwise indicated. Age and weight are shown as that at time of baseline measurements
Table 2.
Baseline hot plate latency and current thresholds in sickle cell mice and respective control strains a
| Variables | Townes Sickle |
Townes Controls |
P value | BERKs | C57BL/6J | P value |
|---|---|---|---|---|---|---|
| Hot plate latency (s) | 5.92±0.15 | 7.25±0.31 | 0.0002 | 5.46±0.35 | 8.60±0.22 | <0.001 |
| 2000 Hz (U) | 74±2 | 81±2 | 0.06 | 74±3 | 70±3 | 0.3 |
| 250 Hz (U) | 31±1 | 34±1 | 0.042 | 30±1 | 27±1 | 0.09 |
| 5 Hz (U) | 17±1 | 20±1 | 0.016 | 14±2 | 14±1 | 0.8 |
| Overall Grip Force (g force/b.w.) |
7.22±0.19 | 8.78±0.16 | <0.001 | |||
| Forelimb Grip Force (g force/b.w.) |
3.22±0.19 | 4.48±0.14 | <0.001 |
Results are shown as mean±S.E.M. Hot plate latency measures thermal sensitivity and current thresholds the current that elicits nocifensive behavior. P values represent comparisons between sickle cell mouse and respective control strain, s indicates seconds, U indicates unit [one current threshold unit is equal to 0.01 mAmperes (mA)] and b.w. indicates body weight.
3.1. Effect of dexmedetomidine on quantitative sensory testing in Townes mice
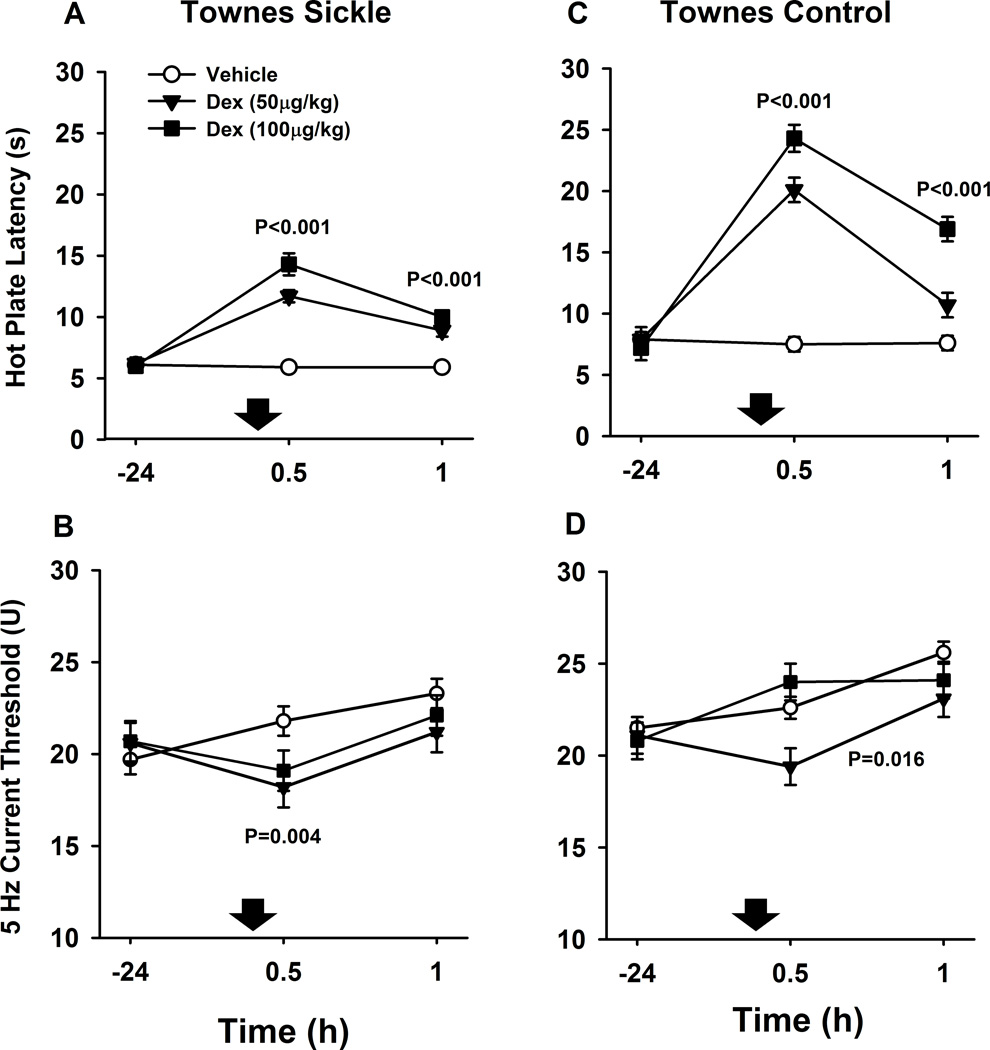
After dexmedetomidine (50 and 100 µg/kg), Townes sickling mice had significantly higher hot plate latencies both at 30 (+8.0±0.6 seconds, mean difference ± S.E.M., P<0.001) and 60 min (+3.9±0.5 s, P<0.001) compared to vehicle injections (Fig. 1A). In addition, after dexmedetomidine (50 and 100 µg/kg), Townes sickling also had significantly lower 5 Hz current thresholds at 30 (−3.3±1.1 U, P=0.004), but not at 60 min (P=0.1) compared to vehicle injections (Fig. 1B).
Fig. 1.
Effect of dexmedetomidine (Dex) on quantitative sensory testing in Townes mice. Data are shown as means±S.E.M. and down arrows indicate administration of dexmedetomidine or vehicle. The unit of measurement of current threshold is “Unit” (U), which corresponds to 100 times the amperage that elicited vocalization (one current threshold unit equates to 0.01 mAmperes). A. Townes sickle mice had significantly higher hot plate latencies at 30 and 60 min after dexmedetomidine (50 and 100 µg/kg combined) compared to vehicle injections (both P<0.001). B. Townes sickle mice had significantly lower 5 Hz current thresholds at 30 (P=0.004), but not at 60 min (P=0.1), after dexmedetomidine (50 and 100 µg/kg combined) compared to vehicle injections. C. Townes control mice also had significantly higher hot plate latencies at 30 and 60 min after dexmedetomidine (50 and 100 µg/kg combined) compared to vehicle injections (both P<0.001). D. Overall, Townes control mice had lower 5 Hz current thresholds over 30 and 60 min after dexmedetomidine (50 and 100 µg/kg combined) compared with vehicle P=0.016, an effect predominantly seen at the 50 µg/kg dose. P values indicate the effect of dexmedetomidine compared with vehicle at indicated time points. Dexmedetomidine injections, compared to vehicle (down arrows), were associated with smaller increases on hot plate latency in Townes sickle than in Townes control mice, P=0.003 for dose*time*genotype interaction (A and B)
After dexmedetomidine (50 and 100 µg/kg combined), Townes control mice also had significantly higher hot plate latencies at 30 (+15.8±0.9 s, mean difference±S.E.M., P<0.001) and 60 min (+5.6±0.8 s, P<0.001) compared to vehicle injections (Fig. 1C). In addition, after dexmedetomidine (50 and 100 µg/kg), Townes control mice had overall lower 5 Hz current thresholds over 30 and 60 min compared with vehicle injections [−1.3 (−2.6, −0.1) overall effect of dexmedetomidine (95% confidence interval), P=0.016, Fig. 1D].
Among Townes sickling and control mice, there were treatment*time*genotype interactions for the effect of dexmedetomidine on hot plate latency, but not on current thresholds. That is, the effect of dexmedetomidine (compared to vehicle) was different in Townes sickling compared to Townes controls. Specifically in Townes sickling, dexmedetomidine was associated with less increases on hot plate latency than in Townes control mice, P=0.003 for treatment*time*genotype interaction (Fig. 1A and 1B). Further, in both Townes sickling and control mice, over the dexmedetomidine dose-range studied (0, 50, and 100 µg/kg), there was a significant linear dose-effect relationship for hot plate latency both at 30 (P<0.001) and 60 min (P<0.001) after injections (Fig. 1A and 1C). Lastly, in both Townes sickling and control mice, dexmedetomidine had no significant effect on 2000 or 250 Hz (Aβ and Aδ myelinated fibers) current thresholds compared with vehicle, all p>0.07 (data not shown).
We also examined the effect of a lower dose of dexmedetomidine on hot plate latency in a different cohort of mice using a longitudinal (not crossover) design. After the injection of dexmedetomidine (10 µg/kg), compared to baseline, there were significant increases in hot plate latency at 30 (P=0.001) and 60 min (P=0.021) in Townes controls and at 30 (P=0.044), but not 60 min (P=0.9), in Townes sickling mice (data not shown).
3.2. Effect of dexmedetomidine on quantitative sensory testing in BERK mice
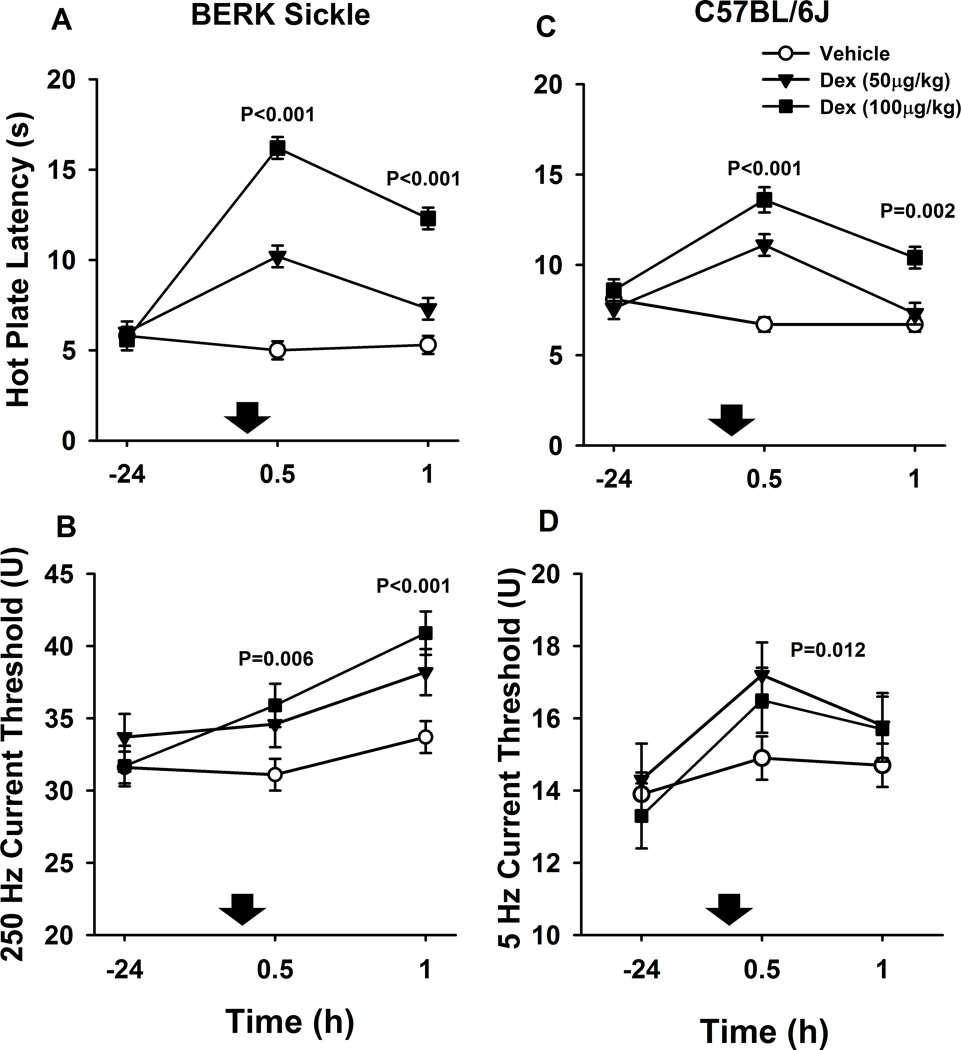
We then examined the effects of dexmedetomidine on another strain of SCD mice. After dexmedetomidine (50 and 100 µg/kg combined), BERK sickling mice had significantly higher hot plate latencies at 30 (+8.3±0.8 seconds, mean difference± S.E.M., P<0.001) and 60 min (+4.7±0.7 s, P<0.001) than after vehicle injections (Fig. 2A). In addition, after dexmedetomidine (50 and 100 µg/kg), BERK sickling mice had significantly higher 250 Hz current thresholds at 30 (+4.2±1.5 U, mean difference± S.E.M., P=0.006) and 60 min (6.0±1.5 U, P<0.001) than after vehicle injections (Fig. 2B). C57BL/6J, used as controls for BERK sickling mice, also had significantly higher hot plate latencies at 30 (+5.5±0.7 s, P<0.001) and 60 min (+2.0±0.6, P=0.002) after dexmedetomidine (50 and 100 µg/kg) than after vehicle injections (Fig. 2C). In addition, C57BL/6J mice had significantly higher 5 Hz current thresholds over 30 and 60 min after dexmedetomidine compared to after vehicle injections [+1.5 (0.3, 2.6) effect of dexmedetomidine (95% confidence interval), P=0.012, Fig. 2D].
Fig. 2.
Effect of dexmedetomidine (Dex) on quantitative sensory testing in BERK mice. Data are shown as means±S.E.M. and down arrows indicate administration of dexmedetomidine or vehicle. The unit of measurement of current threshold is “Unit” (U), which corresponds to 100 times the amperage that elicited vocalization (one current threshold unit equates to 0.01 mAmperes). A. BERK sickle mice had significantly higher hot plate latencies at 30 and 60 min after dexmedetomidine (50 and 100 µg/kg combined) compared to vehicle injections (both, P<0.001). B. BERK sickle mice also had significantly higher 250 Hz current thresholds at 30 (P=0.006) and 60 min (P<0.001) after dexmedetomidine (50 and 100 µg/kg combined) compared to vehicle injections. C. C57BL/6J, here used as controls for BERK sickle mice, also had significantly higher hot plate latencies at 30 (P<0.001) and 60 min (P=0.002) after dexmedetomidine (50 and 100 µg/kg combined) compared to vehicle injections. D. Overall, C57BL/6J mice had significantly higher 5 Hz current thresholds over 30 and 60 min after dexmedetomidine compared to vehicle injections (P=0.012).
In both BERK sickling and C57BL/6J mice, there was a significant linear dose-effect relationship for hot plate latency both at 30 (P<0.001) and 60 min (P<0.001) over the dexmedetomidine dose-range studied (0, 50, and 100 µg/kg), (Fig. 2A and 2C). Similarly, in BERK sickling mice, the effect of dexmedetomidine (0, 50, and 100 µg/kg) on 250 Hz current threshold followed a significant linear dose-effect relationship both at 30 (P=0.008) and 60 min (P<0.001) after subcutaneous injections (Fig. 2B). Lastly, in BERK mice, dexmedetomidine had no significant effect on 2000 and 5 Hz current threshold compared with vehicle, all p≥0.2 (data not shown) and in C57BL/6J, it had no effect on 2000 and 250 Hz, compared with vehicle, all p≥0.1 (data not shown).
3.3. Effect of dexmedetomidine on grip force
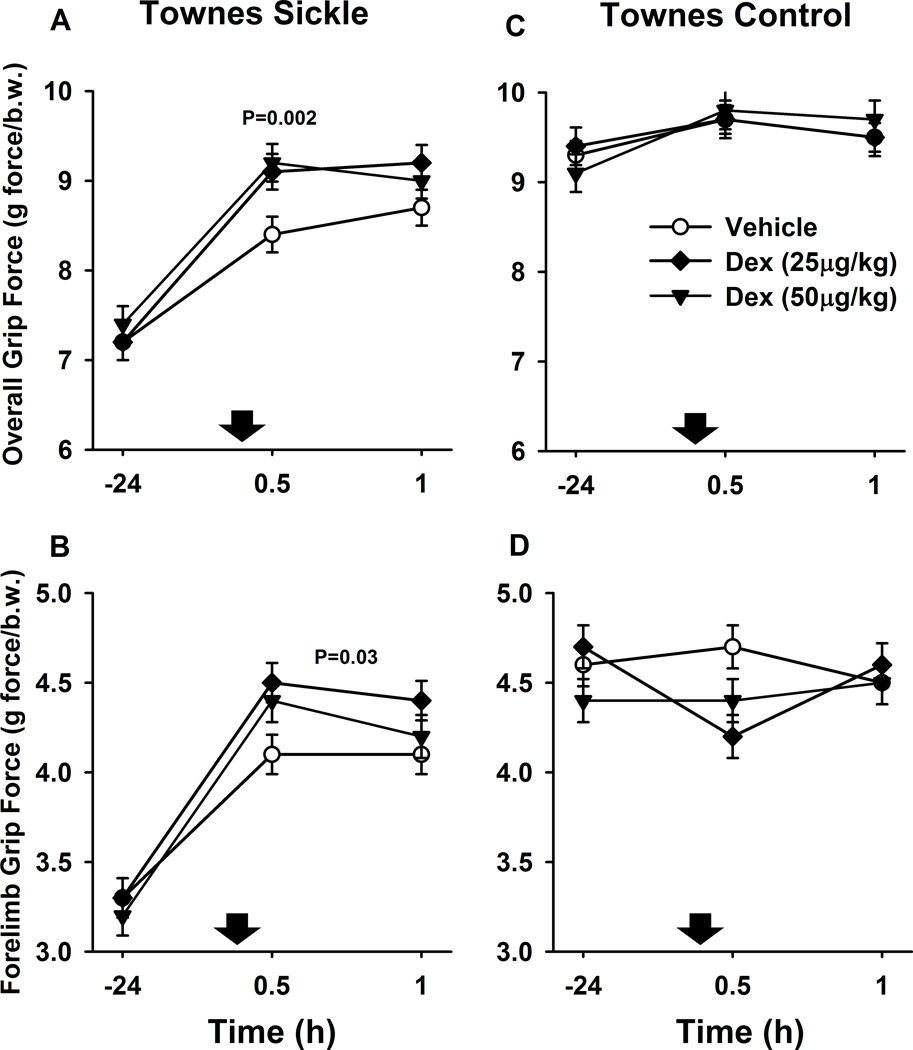
Grip force has been regarded as a surrogate measure of deep muscle pain in sickle cell mice (Kehl et al., 2000; Kohli et al., 2010). At baseline, Townes sickling mice had significantly lower overall (all four limbs) and forelimb grip forces compared to control mice (P<0.001, Table 2 and Fig. 3). Using a crossover design, we evaluated the effect of dexmedetomidine on grip force in a different cohort of Townes mice. After dexmedetomidine (25 and 50 µg/kg) injections, Townes sickling mice had significantly greater grip force at 30 min (+0.8±0.2 g force/body weight mean difference± S.E.M., P=0.002) and a trend towards higher grip force at 60 min (+0.5±0.2 g force/body weight, P=0.056) than after vehicle injections (Fig. 3A). Further, overall, Townes sickling mice had significantly greater forelimb grip force over 30 and 60 min after dexmedetomidine (25 and 50 µg/kg) than after vehicle injections [+0.2 (0.02, 0.3) effect of dexmedetomidine vs. vehicle (95% confidence interval) P=0.03, Fig. 3B). Similar to what was observed with quantitative sensory tests, over the dexmedetomidine dose-range studied (0, 25, and 50 µg/kg), there was a significant linear dose-effect relationship for overall grip force at 30 min (P=0.005) after subcutaneous injections in Townes mice.
Fig. 3.
Effect of dexmedetomidine (Dex) on grip force in Townes mice. Data are shown as means±S.E.M. and down arrows indicate administration of dexmedetomidine or vehicle. At baseline, Townes sickle mice had significantly lower overall (A and C, all four limbs) and forelimb grip force (B and D) compared to control mice. A. Townes sickle mice had significantly higher overall grip force at 30 min (P=0.002) after dexmedetomidine (25 and 50 µg/kg combined) compared to vehicle injections. B. Townes sickle mice also had significantly higher forelimb grip force over 30 and 60 min after dexmedetomidine (50 and 100 µg/kg combined) compared to vehicle injections (P=0.03, B). Contrary to the effects of dexmedetomidine in Townes sickle mice, in Townes control mice, there were no significant effects of dexmedetomidine on overall (C) or forelimb grip forces compared to vehicle injections (D, p≥0.3).
In Townes control mice, contrary to Townes sickling animals, dexmedetomidine had no significant effects on overall or forelimb grip forces compared to vehicle injections (p≥0.3). The lack of changes in grip force is Townes controls likely suggests absence of muscle hyperalgesia and that the maximum grip force has been reached.
3.4. Effect of dexmedetomidine on wakefulness score
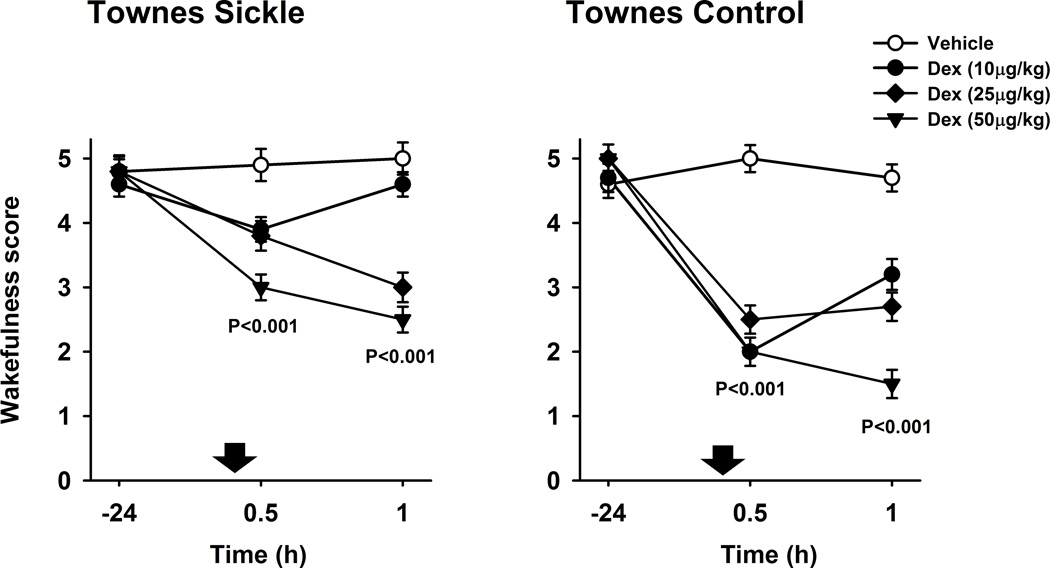
As dexmedetomidine is a known sedative and we observed that animals were sedated after its subcutaneous injections, we used a wakefulness scale in order to quantify the sedation effect in Townes mice (Fig. 4). At baseline, Townes sickling mice had similar wakefulness scores compared to control mice (P=0.7, Fig. 4). After dexmedetomidine (10, 25 and 50 µg/kg combined), Townes sickling mice had significantly lower wakefulness scores, i.e., were more sedated, both, at 30 (−1.3±0.3, effect of dexmedetomidine vs. vehicle ± S.E.M., P<0.001) and 60 min (−1.6±0.3, P<0.001) than after vehicle injections, Fig. 4A. In Townes sickling mice, over the dexmedetomidine dose-range studied (0, 10, 25, and 50 µg/kg), there was a significant linear dose-effect relationship for wakefulness score at 30 and 60 min after subcutaneous injections.
Fig. 4.
Effect of dexmedetomidine (Dex) on wakefulness score. Data are shown as means±S.E.M. and down arrows indicate administration of dexmedetomidine or vehicle. A. Townes sickle mice had significantly lower wakefulness scores, i.e., were more sedated, both, at 30 and 60 min after dexmedetomidine (10, 25 and 50 µg/kg combined) compared to vehicle injections (both, P<0.001). B. Townes control mice had significantly lower wakefulness scores i.e., were more sedated, both, at 30 and 60 min after dexmedetomidine (10, 25 and 50 µg/kg combined) compared with vehicle (both P<0.001).
After subcutaneous dexmedetomidine (10, 25 and 50 µg/kg combined) injections, Townes control mice had significantly lower wakefulness scores i.e., were more sedated, both, at 30 (−2.8±0.26, effect of dexmedetomidine vs. vehicle ± S.E.M., P<0.001) and 60 min (−2.3±0.26, P<0.001) than after vehicle injections (Fig. 4B). Noticeably, among Townes mice there were treatment*time*genotype interactions for the effect of dexmedetomidine on wakefulness scores. Specifically, dexmedetomidine injections, compared with vehicle, were associated with less decreases in wakefulness scores (less sedation) in Townes sickling than in Townes control mice, P=0.013 for treatment*time*genotype interaction (Fig. 4). Further, contrary to a linear dose-effect relationship observed in Townes sickling, in Townes control mice, over the dexmedetomidine dose-range studied (0, 10, 25, and 50 µg/kg), there was a significant quadratic (non-linear) dose-effect relationship for wakefulness score at 30 and 60 min after subcutaneous injections, both P<0.001 (Fig. 4B).
4. Discussion
We found that dexmedetomidine increased tolerance to noxious thermal stimuli, ameliorated deep tissue muscle hyperalgesia, and altered current threshold in SCD mice in a linear dose-effect relationship. As expected, dexmedetomidine also had a sedative effect in both sickling and control mice. Noticeably, in some nociception behavior assays, the effect of dexmedetomidine varied according to mouse genotype. Among Townes, the increases in hot plate latency and the decreases in wakefulness scores were of significantly lower magnitude in sickling than in control mice, thus suggesting that the beneficial effects of dexmedetomidine on those outcomes were attenuated in sickling mice. In BERKs, dexmedetomidine-related increases on hot plate latency were similar in sickling and in control mice. Thus, the findings that dexmedetomidine increased tolerance to thermal stimuli and improved grip force in sickling mice, support further testing of dexmedetomidine, possibly as an adjuvant to opioids, to treat pain in SCD patients.
The findings of differential effects of dexmedetomidine in control and sickling mice suggests that in some strains of SCD mice, the response to adrenoreceptor agonists, such as dexmedetomidine, may be altered. This possibility is supported by ex-vivo studies showing that aortas of SCD mice have enhanced contraction response to α1 agonists, such as phenylephrine and norepinephrine(Juncos et al., 2011). Further, human studies suggest that SCD patients might have autonomic nervous system alterations and resultant sympathetic/parasympathetic imbalances that correlate with clinical SCD severity(Connes and Coates, 2013; Hedreville et al., 2014; Pearson et al., 2005). Using heart rate variability analysis and microvascular perfusion measurements, researchers showed that transient hypoxia and sighs triggered greater sympathetic surges and parasympathetic withdrawal in SCD patients than in control subjects(Sangkatumvong et al., 2011). Taken together, these results support the hypothesis that in SCD, there might be autonomic nervous system imbalances, which might affect the response to adrenoreceptor agonists. Another possibility to explain the differential effects of dexmedetomidine between sickling and control mice relates to the altered nitric oxide biology known to occur in SCD(Kato et al., 2007). The effects of dexmedetomidine involve activation of nitric oxide synthase (NOS) as inhibition of endothelial NOS worsens the vasoconstrictive effects of dexmedetomidine(Snapir et al., 2009) and abolishes the beneficial effects of dexmedetomidine in models of visceral pain(Rangel et al., 2014). As SCD is associated with altered NOS function and decreased nitric oxide bioavailability, it is conceivable that the effects of dexmedetomidine were smaller in sickling than in control mice. Therefore, our findings of differential effects of dexmedetomidine in sickling and control mice and those of others support the hypothesis that the response to a2-agonists might be altered in SCD mice.
Others have shown that BERK mice have decreased grip force that is ameliorated by opioids and cannabinoid agonists(Kohli et al., 2010). These reductions in grip force are interpreted as deep tissue hyperalgesia and muscle pain(Kohli et al., 2010). In addition to increasing thermal stimuli tolerance, dexmedetomidine significantly increased grip force in a linear dose-response fashion in sickling mice. Interestingly, these increases in grip force in sickling mice occurred in the setting of increased sedation after dexmedetomidine. Contrary to these findings, others have shown that sedatives such as midazolam and diazepam actually decrease grip force in mice(Nevins et al., 1993). Thus, our findings and those of others suggest that the increases in grip force after dexmedetomidine injections are likely due to improvement in muscle hyperalgesia in SCD mice.
Might the nocifensive behavior changes observed in SCD mice result from the sedative effects of dexmedetomidine? We posit that while sedation could have contributed to changes in nocifensive behaviors, dexmedetomidine had independent analgesic effects. First, despite its sedative effects, dexmedetomidine increased grip force in sickling but not in control mice. This finding suggests an analgesic rather than a sedative effect as control mice were more sedated than sickling mice and yet had no changes in grip force. There is ample evidence suggesting that sedative and analgesic effects of systemic dexmedetomidine have different and independent mechanisms(Funai et al., 2014). Dexmedetomidine inhibition of neurons in the locus coeruleus underlies its sedative effects, whereas enhancement of inhibitory synaptic transmission of substancia gelatinosa neurons in the dorsal horn underlies its analgesic effects(Funai et al., 2014). Further, others have recently shown that the analgesic effect of dexmedetomidine is also associated with inhibition of hyperpolarization-activated cyclic nucleotide-gated channels current(Yang et al., 2014). However, despite the evidence suggesting that the sedative and analgesic effects of dexmedetomidine are independent, additional studies will determine the extent of the contribution of the sedative effect of dexmedetomidine to the reduced thermal sensitivity in sickle cell mice. Nevertheless, in sickle cell mice, dexmedetomidine appears to have sedative as well as analgesic effects that, on balance, lead to increased tolerance to thermal stimuli and improvement in muscle hyperalgesia.
The findings of decreases in 5Hz current threshold in Townes sickling and control mice are intriguing given that there were no changes in 2000 or 250 Hz thresholds. This finding is in contrast with those of others showing that α2 agonists, such and clonidine, inhibit C- fiber activation as they depress long term potentiation of C-fiber evoked potentials in the dorsal horn(Ge et al., 2006). These decreases in 5Hz thresholds are also in contrast with reports showing that α 2 agonists, such and clonidine and dexmedetomidine, inhibit capsaicin-evoked (C-fiber activation) glutamate release in spinal cord(Li and Eisenach, 2001). While one can postulate that methodological differences explain some of the discrepancies in results as we stimulate C-fibers (5Hz threshold) in awake animals, the mechanism and relevance of our findings are unclear. It is important to note that the decreases in 5Hz current threshold did not follow a linear dose-effect relationship and in normal C57BL/6J, dexmedetomidine was actually associated with increases in 5Hz current threshold. Further, in BERKs, dexmedetomidine was associated with significant increases in 250Hz threshold and no changes in 2000 or 5Hz thresholds. Therefore, while the mechanisms and relevance of decreases in 5Hz current threshold are unclear, the overall effect of dexmedetomidine on nocifensive behavior appear to be beneficial as dexmedetomidine increased hot plate latency, ameliorated grip force and in some strains of SCD mice it increased 250Hz (A5-fiber) thresholds.
Clinicians might argue that the use of a2-agonists in SCD patients could be associated with vasoconstriction resulting from activation of peripheral α2B adrenoceptors (Ebert et al., 2000; Link et al., 1996). In fact, in normal human volunteers, increasing doses of dexmedetomidine are associated with biphasic dose-response on mean arterial pressure and systemic vascular resistance. While at lower doses dexmedetomidine decreases mean arterial pressure and systemic vascular resistance, at higher doses, it increases these parameters (Ebert et al., 2000). Interestingly, this vasoconstrictive effect is accentuated by conditions associated with decreased sympathetic activity such a as general anesthesia or denervation of vascular bed with peripheral nerve block. During such settings, even lower doses of dexmedetomidine were associated with vasoconstriction, an effect not observed in awake volunteers(Talke et al., 2003). These data suggest that, given its sympatholytic effects, the vasoconstrictive effects of dexmedetomidine can vary according to the degree of existing sympathetic activity(Talke et al., 2003). While the concern of vasoconstrictive effects exists, it is unclear whether and at what dose-range dexmedetomidine would lead to clinically relevant vasoconstriction in SCD patients. Given the potential benefits of dexmedetomidine as an adjuvant to opioid therapy, these questions are worthy of further investigations.
There is evidence to suggest that complications of SCD and its inflammatory state are related to ischemia/reperfusion injury(Hebbel, 2014). Researchers suggest that endothelial dysfunction, acute chest syndrome, arterial vasculopathy, and pain associated vaso-occlusive phenomenon are examples of SCD-associated ischemia/reperfusion injury(Hebbel, 2014). Interestingly, dexmedetomidine has been shown to be protective in several models of organ ischemia/reperfusion injury. In a model of hind-limb ischemia, dexmedetomidine pre-treatment decreases plasma levels of inflammatory mediators and increases muscle levels of antioxidant enzymes(Dong et al., 2014). When administered prior to ischemia, dexmedetomidine preserves neurologic function and attenuates neuronal injury after aorta occlusion in mice(Bell et al., 2014; Bell et al., 2012), improves myocardial contractility and suppresses reperfusion-induced arrhythmias after coronary occlusion in pigs(Yoshitomi et al., 2012), and ameliorates liver injury after hepatic ischemia in rats(Sahin et al., 2013). Further, in rabbits, dexmedetomidine, administered during reperfusion following occlusion of the superior mesenteric artery, decreases the severity of damage to intestines and kidney, which is coupled with an improvement in total antioxidant tissue status(Kilic et al., 2012). Therefore, given that ischemia/reperfusion injury might underlie some SCD-related complications and that dexmedetomidine has beneficial effects in those settings, one could argue that further studies of dexmedetomidine in SCD are warranted.
Clinicians often use dexmedetomidine during the perioperative period(Blaudszun et al., 2012) and in intensive care units treating children and adults(Jakob et al., 2012). In the setting of acute pain, dexmedetomidine has been shown to decrease opioid consumption and pain intensity(Blaudszun et al., 2012). Therefore, given its favorable therapeutic index and the beneficial effects of dexmedetomidine on the nocifensive behavior of SCD mice described here, it seems reasonable to argue that studies to determine the safety, tolerated dose range, and efficacy of dexmedetomidine as an adjuvant to opioids during vaso-occlusive crises should be conducted in SCD patients.
Acknowledgments
The authors are grateful to LaShon Middleton, Sayuri Kamimura, MS, and Ji Sung Seo for expert technical support during experiments and to Caroline Lippold, BS for editorial assistance.
Funding: This work was supported by Award UL1RR031988 /UL1TR000075 from the National Institutes of Health, National Center for Advancing Translational Sciences (ZQ) and by an intramural grant from The Sheikh Zayed Institute for Pediatric Surgical Innovation, Children’s Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
Participated in research design: Li Wang, Luis E.F. Almeida, and Zenaide M.N. Quezado
Conducted experiments: Gabriela Calhoun, Nicholas Kenyon, Li Wang, and Nina Afsar
Contributed materials: Julia Finkel, and Zenaide Quezado
Performed data analysis: Mehdi Nouraie and Zenaide M.N. Quezado
Wrote the paper: Gabriela Calhoun and Zenaide M.N. Quezado
Reviewed the manuscript: Gabriela Calhoun, Li Wang, Nina Afsar, Luis E.F. Almeida, Mehdi Nouraie, Julia Finkel, and Zenaide M.N. Quezado
REFERENCES
- Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: a critical reappraisal. Blood. 2012;120:3647–3656. doi: 10.1182/blood-2012-04-383430. [DOI] [PubMed] [Google Scholar]
- Ballas SK, Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am J Hematol. 2005;79:17–25. doi: 10.1002/ajh.20336. [DOI] [PubMed] [Google Scholar]
- Bell MT, Puskas F, Bennett DT, Herson PS, Quillinan N, Fullerton DA, Reece TB. Dexmedetomidine, an alpha-2a adrenergic agonist, promotes ischemic tolerance in a murine model of spinal cord ischemia-reperfusion. J Thorac Cardiovasc Surg. 2014;147:500–506. doi: 10.1016/j.jtcvs.2013.07.043. [DOI] [PubMed] [Google Scholar]
- Bell MT, Puskas F, Smith PD, Agoston VA, Fullerton DA, Meng X, Weyant MJ, Reece TB. Attenuation of spinal cord ischemia-reperfusion injury by specific alpha-2a receptor activation with dexmedetomidine. J Vasc Surg. 2012;56:1398–1402. doi: 10.1016/j.jvs.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Blaudszun G, Lysakowski C, Elia N, Tramer MR. Effect of perioperative systemic alpha2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2012;116:1312–1322. doi: 10.1097/ALN.0b013e31825681cb. [DOI] [PubMed] [Google Scholar]
- Bol CJ, Vogelaar JP, Mandema JW. Anesthetic profile of dexmedetomidine identified by stimulus-response and continuous measurements in rats. J Pharmacol Exp Ther. 1999;291:153–160. [PubMed] [Google Scholar]
- Brandow AM, Farley RA, Panepinto JA. Neuropathic pain in patients with sickle cell disease. Pediatr Blood Cancer. 2014;61:512–517. doi: 10.1002/pbc.24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brousseau DC, Owens PL, Mosso AL, Panepinto JA, Steiner CA. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 2010;303:1288–1294. doi: 10.1001/jama.2010.378. [DOI] [PubMed] [Google Scholar]
- Brush DE. Complications of long-term opioid therapy for management of chronic pain: the paradox of opioid-induced hyperalgesia. J Med Toxicol. 2012;8:387–392. doi: 10.1007/s13181-012-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain DM, Vang D, Simone DA, Hebbel RP, Gupta K. Mouse models for studying pain in sickle disease: effects of strain, age, and acuteness. Br J Haematol. 2012;156:535–544. doi: 10.1111/j.1365-2141.2011.08977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuck TL, McLaughlin PJ, Arizzi-LaFrance MN, Salamone JD, Correa M. Comparison between multiple behavioral effects of peripheral ethanol administration in rats: sedation, ataxia, and bradykinesia. Life Sci. 2006;79:154–161. doi: 10.1016/j.lfs.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Connes P, Coates TD. Autonomic nervous system dysfunction: implication in sickle cell disease. C R Biol. 2013;336:142–147. doi: 10.1016/j.crvi.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Dong X, Xing Q, Li Y, Han X, Sun L. Dexmedetomidine protects against ischemia-reperfusion injury in rat skeletal muscle. J Surg Res. 2014;186:240–245. doi: 10.1016/j.jss.2013.07.052. [DOI] [PubMed] [Google Scholar]
- Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–394. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- Field JJ, Knight-Perry JE, Debaun MR. Acute pain in children and adults with sickle cell disease: management in the absence of evidence-based guidelines. Curr Opin Hematol. 2009;16:173–178. doi: 10.1097/MOH.0b013e328329e167. [DOI] [PubMed] [Google Scholar]
- Finkel J, Guptill V, Khaibullina A, Spornick N, Vasconcelos O, Liewehr DJ, Steinberg SM, Quezado ZM. The three isoforms of nitric oxide synthase distinctively affect mouse nocifensive behavior. Nitric Oxide. 2012;26:81–88. doi: 10.1016/j.niox.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel JC, Besch VG, Hergen A, Kakareka J, Pohida T, Melzer JM, Koziol D, Wesley R, Quezado ZM. Effects of aging on current vocalization threshold in mice measured by a novel nociception assay. Anesthesiology. 2006;105:360–369. doi: 10.1097/00000542-200608000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funai Y, Pickering AE, Uta D, Nishikawa K, Mori T, Asada A, Imoto K, Furue H. Systemic dexmedetomidine augments inhibitory synaptic transmission in the superficial dorsal horn through activation of descending noradrenergic control: an in vivo patch-clamp analysis of analgesic mechanisms. Pain. 2014;155:617–628. doi: 10.1016/j.pain.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison SR, Kramer AA, Gerges NZ, Hillery CA, Stucky CL. Sickle cell mice exhibit mechanical allodynia and enhanced responsiveness in light touch cutaneous mechanoreceptors. Mol Pain. 2012;8:62. doi: 10.1186/1744-8069-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge YX, Xin WJ, Hu NW, Zhang T, Xu JT, Liu XG. Clonidine depresses LTP of C-fiber evoked field potentials in spinal dorsal horn via NO-cGMP pathway. Brain Res. 2006;1118:58–65. doi: 10.1016/j.brainres.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM, Jaenisch R. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- Hebbel RP. Ischemia-reperfusion injury in sickle cell anemia: relationship to acute chest syndrome, endothelial dysfunction, arterial vasculopathy, and inflammatory pain. Hematol Oncol Clin North Am. 2014;28:181–198. doi: 10.1016/j.hoc.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Hedreville M, Charlot K, Waltz X, Sinnapah S, Lemonne N, Etienne-Julan M, Soter V, Hue O, Hardy-Dessources MD, Barthelemy JC, Connes P. Acute moderate exercise does not further alter the autonomic nervous system activity in patients with sickle cell anemia. PLoS One. 2014;9:e95563. doi: 10.1371/journal.pone.0095563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillery CA, Kerstein PC, Vilceanu D, Barabas ME, Retherford D, Brandow AM, Wandersee NJ, Stucky CL. Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood. 2011;118:3376–3383. doi: 10.1182/blood-2010-12-327429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, Bratty JR, Takala J. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012;307:1151–1160. doi: 10.1001/jama.2012.304. [DOI] [PubMed] [Google Scholar]
- Juncos R, Juncos L, Hebbel RP, Vercellotti GM, Katusic ZS, Nath KA. Selective enhancement of contractions to alpha 1-adrenergic receptor activation in the aorta of mice with sickle cell disease. J Cardiovasc Pharmacol. 2011;57:263–266. doi: 10.1097/FJC.0b013e318204bb34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamibayashi T, Maze M. Clinical uses of alpha2 -adrenergic agonists. Anesthesiology. 2000;93:1345–1349. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]
- Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21:37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl LJ, Trempe TM, Hargreaves KM. A new animal model for assessing mechanisms and management of muscle hyperalgesia. Pain. 2000;85:333–343. doi: 10.1016/S0304-3959(99)00282-1. [DOI] [PubMed] [Google Scholar]
- Kenyon N, Wang L, Spornick N, Khaibullina A, Almeida LE, Cheng Y, Wang J, Guptill V, Finkel JC, Quezado ZM. Sickle cell disease in mice is associated with sensitization of sensory nerve fibers. Exp Biol Med (Maywood) 2015;240:87–98. doi: 10.1177/1535370214544275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic K, Hanci V, Selek S, Sozmen M, Kilic N, Citil M, Yurtlu DA, Yurtlu BS. The effects of dexmedetomidine on mesenteric arterial occlusion-associated gut ischemia and reperfusion-induced gut and kidney injury in rabbits. J Surg Res. 2012;178:223–232. doi: 10.1016/j.jss.2012.03.073. [DOI] [PubMed] [Google Scholar]
- Koga K, Furue H, Rashid MH, Takaki A, Katafuchi T, Yoshimura M. Selective activation of primary afferent fibers evaluated by sine-wave electrical stimulation. Mol Pain. 2005;1:13. doi: 10.1186/1744-8069-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli DR, Li Y, Khasabov SG, Gupta P, Kehl LJ, Ericson ME, Nguyen J, Gupta V, Hebbel RP, Simone DA, Gupta K. Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: modulation by cannabinoids. Blood. 2010;116:456–465. doi: 10.1182/blood-2010-01-260372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, Crystal RG, Darnell RB, Ferrante RJ, Fillit H, Finkelstein R, Fisher M, Gendelman HE, Golub RM, Goudreau JL, Gross RA, Gubitz AK, Hesterlee SE, Howells DW, Huguenard J, Kelner K, Koroshetz W, Krainc D, Lazic SE, Levine MS, Macleod MR, McCall JM, Moxley RT, 3rd, Narasimhan K, Noble LJ, Perrin S, Porter JD, Steward O, Unger E, Utz U, Silberberg SD. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490:187–191. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- Li X, Eisenach JC. alpha2A–adrenoceptor stimulation reduces capsaicin-induced glutamate release from spinal cord synaptosomes. J Pharmacol Exp Ther. 2001;299:939–944. [PubMed] [Google Scholar]
- Link RE, Desai K, Hein L, Stevens ME, Chruscinski A, Bernstein D, Barsh GS, Kobilka BK. Cardiovascular regulation in mice lacking alpha2-adrenergic receptor subtypes b and c. Science. 1996;273:803–805. doi: 10.1126/science.273.5276.803. [DOI] [PubMed] [Google Scholar]
- Liu C, Cripe TP, Kim MO. Statistical issues in longitudinal data analysis for treatment efficacy studies in the biomedical sciences. Mol Ther. 2010;18:1724–1730. doi: 10.1038/mt.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicol ED, Midbari A, Eisenberg E. Opioids for neuropathic pain. Cochrane Database Syst Rev. 2013;8:CD006146. doi: 10.1002/14651858.CD006146.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins ME, Nash SA, Beardsley PM. Quantitative grip strength assessment as a means of evaluating muscle relaxation in mice. Psychopharmacology (Berl) 1993;110:92–96. doi: 10.1007/BF02246955. [DOI] [PubMed] [Google Scholar]
- Niscola P, Sorrentino F, Scaramucci L, de Fabritiis P, Cianciulli P. Pain syndromes in sickle cell disease: an update. Pain Med. 2009;10:470–480. doi: 10.1111/j.1526-4637.2009.00601.x. [DOI] [PubMed] [Google Scholar]
- Paszty C, Brion CM, Manci E, Witkowska HE, Stevens ME, Mohandas N, Rubin EM. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278:876–878. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- Pearson SR, Alkon A, Treadwell M, Wolff B, Quirolo K, Boyce WT. Autonomic reactivity and clinical severity in children with sickle cell disease. Clin Auton Res. 2005;15:400–407. doi: 10.1007/s10286-005-0300-9. [DOI] [PubMed] [Google Scholar]
- Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, Kinney TR. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325:11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- Rangel RA, Marinho BG, Fernandes PD, de Moura RS, Lessa MA. Pharmacological mechanisms involved in the antinociceptive effects of dexmedetomidine in mice. Fundam Clin Pharmacol. 2014;28:104–113. doi: 10.1111/j.1472-8206.2012.01068.x. [DOI] [PubMed] [Google Scholar]
- Sahin T, Begec Z, Toprak HI, Polat A, Vardi N, Yucel A, Durmus M, Ersoy MO. The effects of dexmedetomidine on liver ischemia-reperfusion injury in rats. J Surg Res. 2013;183:385–390. doi: 10.1016/j.jss.2012.11.034. [DOI] [PubMed] [Google Scholar]
- Sangkatumvong S, Khoo MC, Kato R, Detterich JA, Bush A, Keens TG, Meiselman HJ, Wood JC, Coates TD. Peripheral vasoconstriction and abnormal parasympathetic response to sighs and transient hypoxia in sickle cell disease. Am J Respir Crit Care Med. 2011;184:474–481. doi: 10.1164/rccm.201103-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WR, Penberthy LT, Bovbjerg VE, McClish DK, Roberts JD, Dahman B, Aisiku IP, Levenson JL, Roseff SD. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148:94–101. doi: 10.7326/0003-4819-148-2-200801150-00004. [DOI] [PubMed] [Google Scholar]
- Snapir A, Talke P, Posti J, Huiku M, Kentala E, Scheinin M. Effects of nitric oxide synthase inhibition on dexmedetomidine-induced vasoconstriction in healthy human volunteers. Br J Anaesth. 2009;102:38–46. doi: 10.1093/bja/aen316. [DOI] [PubMed] [Google Scholar]
- Spornick N, Guptill V, Koziol D, Wesley R, Finkel J, Quezado ZM. Mouse current vocalization threshold measured with a neurospecific nociception assay: the effect of sex, morphine, and isoflurane. J Neurosci Methods. 2011;201:390–398. doi: 10.1016/j.jneumeth.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner CA, Miller JL. Sickle Cell Disease Patients in U.S. Hospitals, 2004: Statistical Brief #21, Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Health Care Policy and Research (US) Rockville (MD) 2006 [Google Scholar]
- Talke P, Lobo E, Brown R. Systemically administered alpha2-agonist-induced peripheral vasoconstriction in humans. Anesthesiology. 2003;99:65–70. doi: 10.1097/00000542-200307000-00014. [DOI] [PubMed] [Google Scholar]
- Vincent L, Vang D, Nguyen J, Gupta M, Luk K, Ericson ME, Simone DA, Gupta K. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood. 2013;122:1853–1862. doi: 10.1182/blood-2013-04-498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright PE, Leatherdale ST, Dubin JA. Advantages of mixed effects models over traditional ANOVA models in developmental studies: a worked example in a mouse model of fetal alcohol syndrome. Dev Psychobiol. 2007;49:664–674. doi: 10.1002/dev.20245. [DOI] [PubMed] [Google Scholar]
- Wilkie DJ, Molokie R, Boyd-Seal D, Suarez ML, Kim YO, Zong S, Wittert H, Zhao Z, Saunthararajah Y, Wang ZJ. Patient-reported outcomes: descriptors of nociceptive and neuropathic pain and barriers to effective pain management in adult outpatients with sickle cell disease. J Natl Med Assoc. 2010;102:18–27. doi: 10.1016/s0027-9684(15)30471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LC, Sun CW, Ryan TM, Pawlik KM, Ren J, Townes TM. Correction of sickle cell disease by homologous recombination in embryonic stem cells. Blood. 2006;108:1183–1188. doi: 10.1182/blood-2006-02-004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JT, Zhao JY, Zhao X, Ligons D, Tiwari V, Atianjoh FE, Lee CY, Liang L, Zang W, Njoku D, Raja SN, Yaster M, Tao YX. Opioid receptor-triggered spinal mTORC1 activation contributes to morphine tolerance and hyperalgesia. J Clin Invest. 2014;124:592–603. doi: 10.1172/JCI70236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YC, Meng QT, Pan X, Xia ZY, Chen XD. Dexmedetomidine produced analgesic effect via inhibition of HCN currents. Eur J Pharmacol. 2014;740:560–564. doi: 10.1016/j.ejphar.2014.06.031. [DOI] [PubMed] [Google Scholar]
- Yoshitomi O, Cho S, Hara T, Shibata I, Maekawa T, Ureshino H, Sumikawa K. Direct protective effects of dexmedetomidine against myocardial ischemia-reperfusion injury in anesthetized pigs. Shock. 2012;38:92–97. doi: 10.1097/SHK.0b013e318254d3fb. [DOI] [PubMed] [Google Scholar]
- Yusuf HR, Atrash HK, Grosse SD, Parker CS, Grant AM. Emergency department visits made by patients with sickle cell disease: a descriptive study, 1999–2007. Am J Prev Med. 2010;38:S536–S541. doi: 10.1016/j.amepre.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]