Abstract
Mitochondria, the so-called “energy factory of cells” not only produce energy but also contribute immensely in cellular mortality management. Mitochondrial dysfunctions result in various diseases including but not limited to cancer, atherosclerosis, and neurodegenerative diseases. In the recent years, targeting mitochondria emerged as an attractive strategy to control mitochondrial dysfunction related diseases. Despite the desire to direct therapeutics to the mitochondria, the actual task is more difficult due to the highly complex nature of the mitochondria. The potential benefits of integrating nanomaterials with properties such as biodegradability, magnetization, fluorescence, and near-infrared absorption into a single object of nanoscale dimensions can lead to the development of hybrid nano-medical platforms for targeting therapeutics to the mitochondria. Only a handful of nanoparticles based on metal oxides, gold nanoparticles, dendrons, carbon nanotubes, and liposomes were recently engineered to target mitochondria. Most of these materials face tremendous challenges when administered in vivo due to their limited biocompatibility. Biodegradable polymeric nanoparticles emerged as eminent candidates for effective drug delivery. In this review we highlight the current advancements in the development of biodegradable nanoparticle platforms as effective targeting tools for mitochondrial medicine.
Introduction
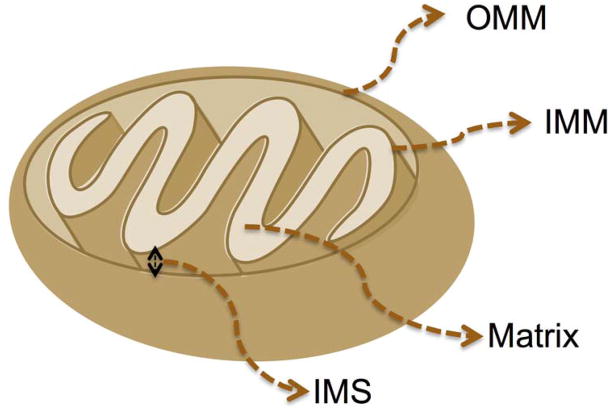
The mitochondria are complex organelles that can be found in most eukaryotic cells. They are essential to life and are associated with the production of adenosine triphosphate (ATP) (1). The introduction of mitochondria into cells occurred over a billion years ago when remnants of bacteria invaded prokaryotic cells (2, 3). This relationship proved to be synergistic and beneficial for both groups involved in that the remnants of and the host cells were provided with a new source of energy (4, 5). The mitochondria copy number per cell can vary from several hundred to several thousand depending on the cell type. Mitochondria participate in a number of functions including, but not limited to ATP production, amino acid biosynthesis, specific ion buffering, management of reactive oxygen species (ROS), and initiation of apoptotic pathways (6–8). Owing to these unique characteristic features, mitochondria play a central role in regulating cell death by systematic mechanism known as apoptosis (9). Since the first incidence of mitochondrial dysfunction related diseases (10), many pathological and toxicological problems were linked to the abnormalities in mitochondrial functions. Some of the acquired conditions associated with mitochondrial dysfunctions are presented in Table 1. Thus targeting mitochondria can be extremely advantageous to produce better therapeutic modalities for these diseases. Despite the desire to direct therapeutics to the mitochondria, the actual task is more difficult due to the highly complex nature of the mitochondria, which is composed of four parts: the outer mitochondrial membrane (OMM), the intermembrane space (IMS), the inner mitochondrial membrane (IMM), and the matrix (Figure 1).
Table 1.
Mitochondrial Dysfunction Related Diseases
Figure 1.
The structure of a mitochondrion. OMM: Outer mitochondrial membrane, IMS: intermembrane space, IMM: inner mitochondrial membrane.
Crossing the OMM is pure concentration driven in that molecules can freely pass back and forth through passive diffusion (34). The main hurdle, however, is that many of these therapeutics lack the structural components required to cross the complex mitochondrial membrane network to reach into the mitochondrial matrix. The presence of the unusual phospholipid cardiolipin (CL) and a strong negative membrane potential (Δψm) of ~-160 to-180 mV which exists across the membranes make it extremely difficult task for small molecules to cross the membranes. IMM has various proteins and selective ion transporters including variety of protein groups, which are involved in the electron transport chain (ETC) and ATP synthesis (35). Owing to its unique characteristic features, mitochondrion plays a central role in regulating cell death by a systematic apoptosis mechanism (36, 37). Therefore selective targeting of mitochondria will be of great advantage to produce better therapeutic modalities for various diseases. A handful of low molecular weight mitochondria-targeted small molecules are known in the literature (Table 2), but often these molecules demonstrate poor pharmacokinetic (pK) properties and unfavorable biodistribution (bioD) profiles when administered in vivo (38). In many instances, small molecules loose their efficacy with the attachment of new functionalities necessary for introduction of the mitochondria targeting ligands (39).
Table 2.
Mitochondria Targeting Ligands and Targeted Small Molecules for Mitochondrial Medicine
| Targeting Ligand | Small Molecule Therapeutic |
|---|---|
| Quaternary ammonium salt (Choline esters), pyridinium and alkyl rhodamine salts | GSH (MitoGSH) and N-acetyl cysteine (MitoNAC) (41, 42); MnSOD (43–46); Ceramides (47); 1,4-benzoquinone (SKQR1) (48) |
| Mitochondria penetrating peptides (MPPs) (49–52) | DOX (53, 54); Chlorambucil (55); Cisplatin (56); Mtx (51, 57) |
| Triphenylphosphonium cation | CoQ10 (58–60); Vit-E (61); Superoxide dismutase mimetic M40403 (62); Peroxidase (Ebselen) (63); TEMPO (64, 65); α-phenyl-N-tert-butylnitrone (α-PBN)(66); (2-hydroxyaminovinyl)-(HV)(67, 68); Oleic acid (OA)(69); dichloroacetic acid (DCA)(70); Chlorambucil (71); Dinitrophenol (DNP)(72); H2S donor GYY4137 (73) |
Nanotechnology made significant strides for the advancement of medicine by improving pK and bioD profiles of various therapeutics keeping their pristine form intact for therapeutic effects, however, there is a notable lack of progress in the development of mitochondria-targeted drug delivery systems and application of nanotechnology in mitochondrial medicine (Figure 2) (40).
Figure 2.
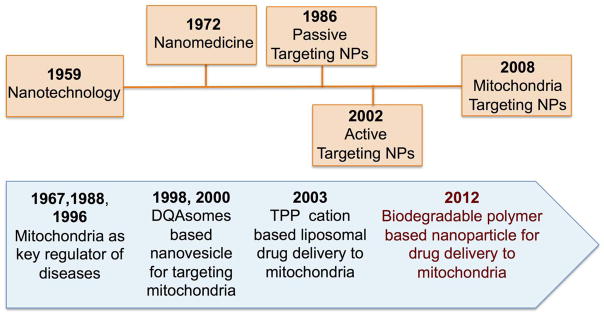
Evolution of nanomedicine (top) and nanotechnology approaches to mitochondrial medicine (bottom). DQA: dequalinium (1,1′-decamethylene bis (4-aminoquinaldiniumchloride), TPP cation: triphenylphosphonium cation.
The reasons behind this possibly include the notion that drugs targeted to the cell will eventually reach the mitochondrion by random interaction with subcellular components. Therefore, most drug delivery systems are designed to target only extracellular targets (74). However, drugs delivered to cell face tremendous challenges in their navigation to enter mitochondria. Nanotechnology based delivery vehicles provide numerous advantages to increase the therapeutic activities of small molecules (75, 76). Nano formulations also offer additional features such as delivering drug molecule in their pristine form, solubilizing hydrophobic drug, increasing the half-life, reducing side effects, and immunogenicity (77–79). Nano-delivery vehicles can be modified to target specific organelle for the effective delivery of drug cargo at its site of action. Thus site directed drug delivery with increased potency would circumvent the failure of current treatment modalities. To deliver payloads inside the mitochondrial matrix and to have spatio-temporal control over release of payloads in different mitochondrial compartments, nanoparticle (NP) based delivery vehicles need to be engineered with precise size, lipophilic surface, appropriate charge, and specific targeting moieties on the surface. The requirements of biocompatibility and biodegradability of the materials used for NPs for potential in vivo translation further impose additional factors. In this review, we will highlight the current advances of mitochondria targeted NP platforms with special emphasis on biodegradable polymeric scaffolds for mitochondrial delivery of therapeutics.
Mitochondria Targeted Micelles, Dendrimers, and Carbon Nanotubes
Several studies investigated the possibility of using increased mitochondrial accumulation and retention of delocalized lipophilic cations such as dequalinium (1,1′-decamethylene bis (4-aminoquinaldiniumchloride), DQA) (80). Given the amphiphilic nature of DQA, it forms positively charged liposome-like structures popularly known as DQAsomes which take advantage of the highly negative Δψm in cancer cells (81). Thus DQAsomes were extensively used as mitochondriotropic carriers to deliver cytotoxic drugs or DNA inside mitochondrial network (Figure 3) (81, 82).
Figure 3.
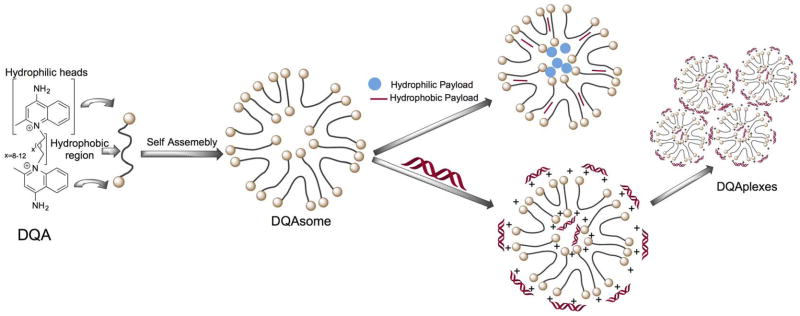
Chemical structure of DQA and its self-assembly into liposome-like vesicles. Redrawn based on Reference (80).
Suitably modified DQAsomes were used to deliver plasmid DNA (pDNA) in the form of ‘DQAplexes’, a hybrid structure made up of DQAsome and DNA, to the mitochondria (82). The utility of DQAsome was extended to deliver chemotherapeutic drugs selectively to the mitochondria. Taxol scaffold derived drug paclitaxel was used in the DQAsomes nanostructures to induce apoptosis followed by cell death (83). However, the lack of exact mechanism and versatility of these formulations limit their use as potential drug delivery systems.
Dendrimers are hyperbranched synthetic macromolecules with three components: central core, repeated branches, and controlled number of available groups on the surface to load multiple functionalities. The core and branched space can be used to entrap biomolecules and the functionalities on the surface can be used to incorporate various moieties, these properties make dendrimers versatile pharmaceutical nanocarriers (84). Dendrimers with a high generation number (G>5) and high net positive charge from lipophilic cationic molecules such as rhodamine and triphenylphosphonium (TPP) cation has the potential to promote endosomal escape and can participate in delivering chemotherapeutic drugs to the mitochondria. Poly(amidoamine) (PAMAM) dendrimer is extensively used as a scaffold to deliver drugs and genomic materials (85). Torchilin and coworkers developed PAMAM based G(5)-D-Ac-TPP where NH2 were acetylated for non specific binding and further coupled with TPP cation for mitochondria-targeted drug delivery (86) (Figure 4). These nanocarriers were further labeled with fluorescent dye to monitor intracellular localization of the dendrimers. Cytotoxicity of these carriers was found to be significantly less. The presence of mitochondriotropic TPP groups on the surface of PAMAM dendrimer demonstrated mitochondria-targeting property. Further investigations are needed to understand the ability of the mitochondria-targeted dendrimers to deliver therapeutic payloads to the mitochondria.
Figure 4.
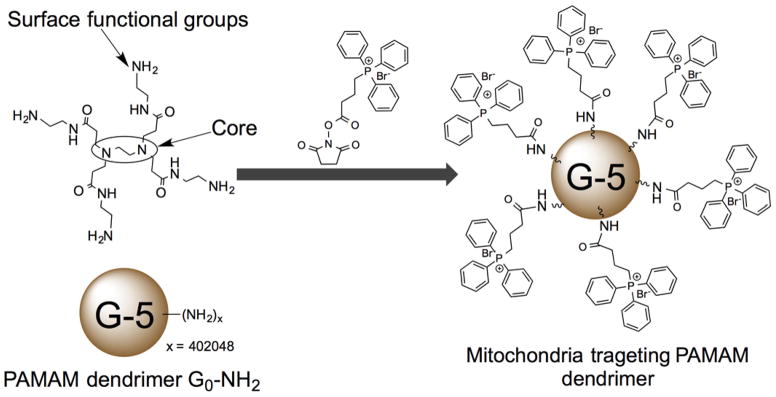
PAMAM dendrimers for mitochondria targeted delivery. Redrawn based on Reference (86).
A similar approach was used to deliver enhanced green fluorescent protein (EGFP) and luciferase gene to the HeLa and COS-7 cells utilizing mitochondria targeting TPP bearing PAMAM dendrimers (G5-TPP) (Figure 5) (87). These gene transfection dendrimers were found to be non-toxic under the transfection conditions. The transfection efficacy of G5-TPP was demonstrated to be similar to that of commercially available agent, Lipofectamine-2000 and even higher as compared to dendrimer-based gene transfection reagent-SuperFect and unmodified G5 dendrimer. G5-TPP dendrimer scaffold exhibited efficient DNA packing/unpacking, endosomal escape, and finally targeted the mitochondria demonstrating potential utility of this system in mitochondria targeted drug and genomic delivery.
Figure 5.
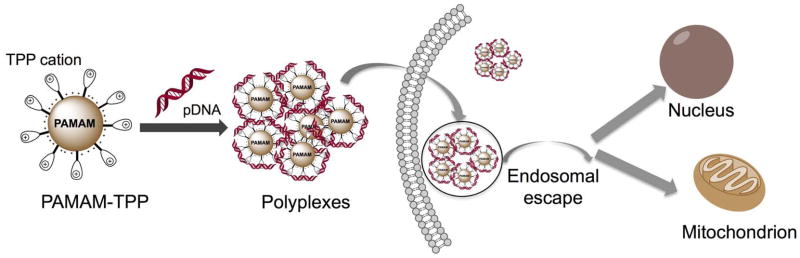
PAMAM dendrimers for mitochondria targeted gene delivery. Redrawn based on Reference 86.
Theodossiou et al., recently developed a lipophilic decyl-TPP coated poly(ethylene imine) (PEI-TPP) hyperbranched polymer nano-assembly of a diameter of ~100 nm to deliver encapsulated doxorubicin (DOX), a potent topoisomerase II inhibitor, to the mitochondria (88). This nano-formulation, PEI-TPP-DOX, was shown to localize in the mitochondria of human prostate carcinoma DU145 cells.
The use of multi-walled carbon nanotubes (MWCNTs) as scaffolds to deliver anticancer drugs upon appropriate surface functionalization with mitochondria targeted ligands was also recently explored (Figure 6 and 7).
Figure 6.
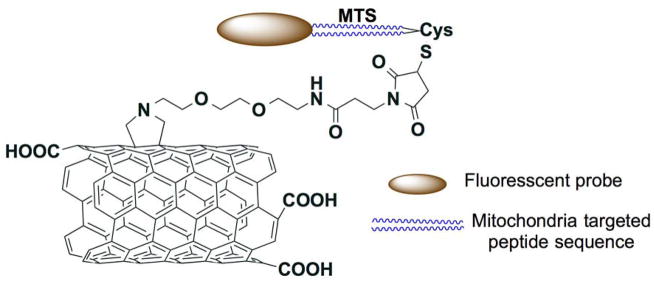
Multi-walled carbon nanotubes (MWCNTs) based mitochondria targeted drug delivery vehicle. Redrawn based on Reference 89.
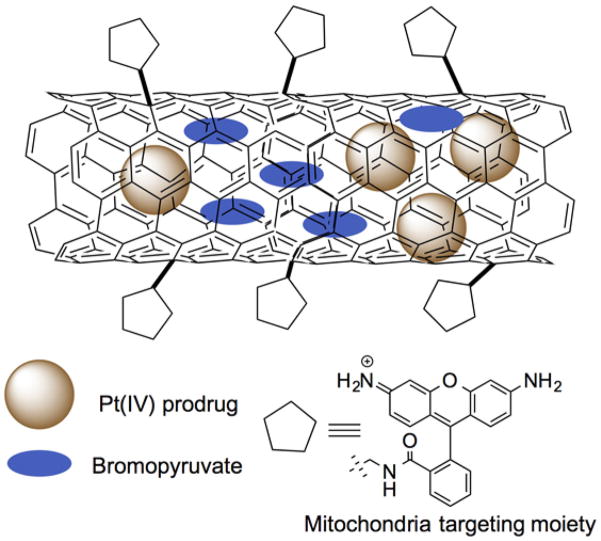
Figure 7.
Carbon nanotube based drug delivery to mitochondria. Redrawn based on Reference 90.
Mitochondria targeted peptide sequence (MTS) KMSVLTPLLLRGLTGSARRLPVPRAKC was installed on MWCNT surface to achieve effective mitochondria targeted vehicle for drug delivery (Figure 6). This extensively characterized nanosystem exhibited high levels of mitochondrial accumulation in macrophages and HeLa cells using confocal studies. Transmission electron microscopy (TEM) studies further confirmed the intracellular localization of the delivery vehicle inside the mitochondria. Interestingly these MTS decorated MWCNTs based nanosystems do not show any significant cytotoxicity which potentiate their use as effective delivery systems (89).
In another recent report cationic rhodamine-110 was used as a mitochondria targeting ligand (MWCNT-Rho) and fluorescein (MWCNT-Fluo) was used as a non-targeted control (Figure 7) (90). A platinum(IV) prodrug (PtBz) entrapped MWCNT-Rho showed enhanced potency with significantly higher mitochondrial localization as compared to the non-targeted construct. Interestingly, empty MWCNT-Rho neither showed cell toxicity nor modulated the Δψm. Further this platform was used to co-encapsulate PtBz and a chemosensitizer, 3-bromopyruvate (3-BP), to demonstrate synergistic effect in various cancer cell lines. Toxicity of this technology should be explored extensively to understand the potential of using carbon nanotubes to deliver therapeutics to the mitochondria.
Liposome Based Mitochondria Targeted Delivery Vehicles
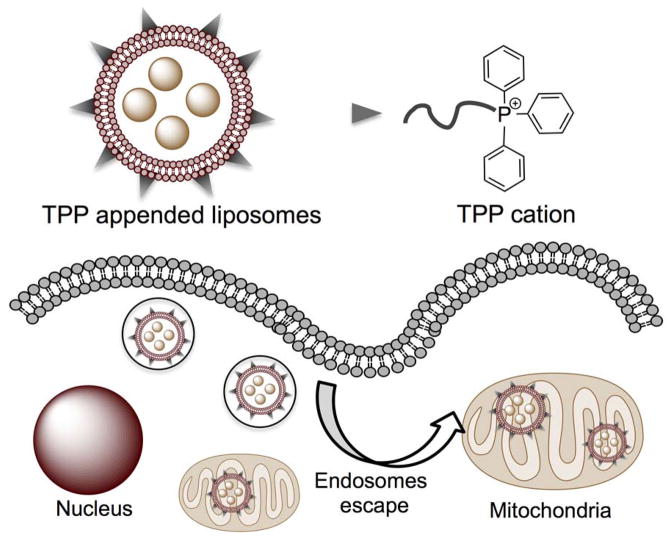
Among the numerous types of nanocarriers, liposomes emerged as promising delivery systems (75, 91). Several liposomal formulations are either food and drug administration (FDA) approved or are in the advanced stage of clinical trials (75, 92). Liposomes are lipid derived biodegradable nanomaterials that utilizes its unique structural features to entrap both hydrophobic and hydrophilic drugs. Liposomes are nano-or micro-particles which are essentially multilayer or bilayer vesicles that have an aqueous core surrounded by a lipid bilayer that can be anywhere from 50 to 5,000 nm in diameter. At the molecular level, a liposome is composed of amphiphilic lipid that upon hydration self-assembles into nanocarrier. Although, natural liposomes have the ability to fuse with cell membranes effectively releasing the cargo in the cytoplasm, such is not the case for synthetic lipids (93). Usually, synthetic lipids are taken up via endocytotic pathways which ultimately lead to their demise due to the fact that many times these are transported out of the cell (94). Appropriately surface functionalized liposomes with targeted moieties can provide potent therapeutic options for site directed drug delivery (95). A way to combat this is to have organelle-specific surface attached targeting moieties (96). This allows liposomes to escape endosomes and travel through the cytoplasm to its final destination (Figure 8).
Figure 8.
Mitochondria targeted liposomal nanocarriers for drug delivery. A generalized figure to indicate how the mitochondria targeted liposomes have the ability to escape endosomes to enter the mitochondria.
This concept was recently implemented where such a system was shown to undergo endosomal escape and target the mitochondria (97, 98). Using stearylTPP (STPP) bromide, a liposome was synthesized via film rehydration followed by ultrasonication. By encapsulating a rhodamine-PE dye, these liposomes were tracked in vitro in BT-20 human breast cancer cells. These liposome were found to colocalize with MitoTracker red in the mitochondria. However, size and polydispersity of the liposomes are the drawbacks of this technology. A smaller diameter and more monodispersed formulation are desired for effective mitochondria targeting.
Utilizing the mitochondria targeting characteristics of STPP, a recent study loaded DOX inside STPP derived liposomes and further surface functionalization with folic acid (FA) resulted in liposomes with dual targeting motif (99). These dual targeting liposomes were found to be more potent than the controls as a consequence of efficient delivery of DOX to the mitochondria. Efforts were also made to incorporate paclitaxel (PTX) in STPP modified liposomes for mitochondrial delivery (100). To further mimic the tumor environment, spheroids of PTX resistant Ovcar-3 cancer cells were devised and cytotoxicity of PTX containing STPP liposomes was evaluated. Targeting PTX to the mitochondria showed reduction in the IC50 values and greater PTX distribution in spheroids. However, the major contribution of cytotoxicity was found to be due to the cell-specific toxicity of STPP. Conjugation of DQA to 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol) (DSPE-PEG) at the PEG terminal resulted in DQA-PEG2000-DSPE as a mitochondria targeting phospholipid. Liposomes derived from this phospholipid were able to deliver resveratrol to the mitochondria and able to induce apoptosis in cisplatin resistant cancer cells (101).
In an approach to overcome the non-specific cytotoxicity of STPP derived liposomes, a polyethylene glycol-phosphatidylethanolamine (PEG-PE) was conjugated with TPP group to result in TPP-PEG-PE. This conjugate was incorporated into the liposomal lipid bilayer, and the modified liposomes were used in PTX delivery. The TPP-PEG-PE-modified liposomes (TPP-PEG-L) were found to be less cytotoxic compared to STPP-derived liposomes or PEGylated STPP liposomes. PTX-loaded TPP-PEG-liposomes demonstrated enhanced PTX-mediated cytotoxicity and anti-tumor efficacy both in vitro and in vivo compared to PTX-loaded unmodified plain liposomes (102). In another example, a liposomal delivery system functionalized with d-α-tocopheryl polyethylene glycol 1000 succinate-TPP cation as a targeting ligand (TPGS1000-TPP) was devised for delivery of PTX (103). Incorporation of TPP and TPGS1000 provided efficient delivery of paclitaxel in the mitochondria of drug-resistant cancer cells and induced apoptosis by releasing cytochrome c, and initiating a cascade of caspase 9 and caspase 3 events.
A nanocarrier based on oligolysine scaffold containing two TPP cations per oligomer was devised to deliver payloads inside the mitochondria of cells. The oligolysine-based nanocarrier failed to enter healthy cells, however, the TPP cation modified oligolysine carrier demonstrated mitochondrial specificity (104).
Polymeric NPs for Mitochondria Targeted Payload Delivery
Polymeric NPs from biocompatible and biodegradable polymers can serve as most promising delivery systems (76, 91, 105). By using an engineered composition of hydrophobic and hydrophilic blocks, these polymers can be used to encapsulate both water-soluble and water-insoluble therapeutics, through emulsion or nanoprecipitation, respectively (75, 79, 105, 106). Polymeric NPs have the ability to overcome many of the drawbacks associated with liposomes in that they possess higher stability and a more controlled payload release (75, 105, 107). Biocompatible and biodegradable polyesters such as poly(lactic acid) (PLA), poly(glyocolic acid) (PGA), their copolymer poly(lactic-co-glycolic acid) (PLGA), and polycaprolactone (PCL) are the most attractive choices as hydrophobic blocks (75, 108). The most widely used hydrophilic block is FDA approved PEG. The hydrophobic blocks typically provide added stability, PEG allows for longer retention time within the body, PEGylation also has the unique characteristic of allowing for further functionalization for the attachment of drugs or targeting moieties. These desirable qualities along with the ability to encapsulate numerous drugs efficiently with a precise control over size make polymeric NPs a prime candidate for mitochondria targeting.
One of the early examples of polymeric NPs as mitochondria targeted delivery vehicle included a PCL-PEG polymer modified with a linker containing TPP between PCL and PEG blocks (109). This polymer was used to encapsulate coenzyme Q10 (CoQ10) for its delivery to the mitochondria for efficient anti-oxidative effects (Figure 9). Fluorescence imaging demonstrated less efficient localization of the NPs inside the mitochondria. A major disadvantage of this technology lies at the design strategy, the poor mitochondria targeting might be due to the fact that TPP was incorporated in between PCL and PEG, thus the TPP moieties have the chance to be buried inside the hydrophobic PCL core. Although, NMR analysis of the NPs showed presence of aromatic peaks from TPP, however, some of the charge can be buried in the hydrophobic core, which would limit the system’s potential to cross the double barriers of mitochondria effectively.
Figure 9.
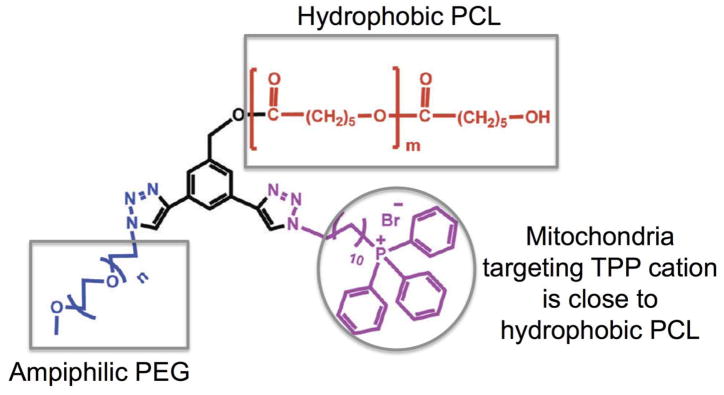
Mitochondria targeting polymer from PCL-PEG modified with a linker containing TPP between PCL and PEG. Drawn based on Reference (109).
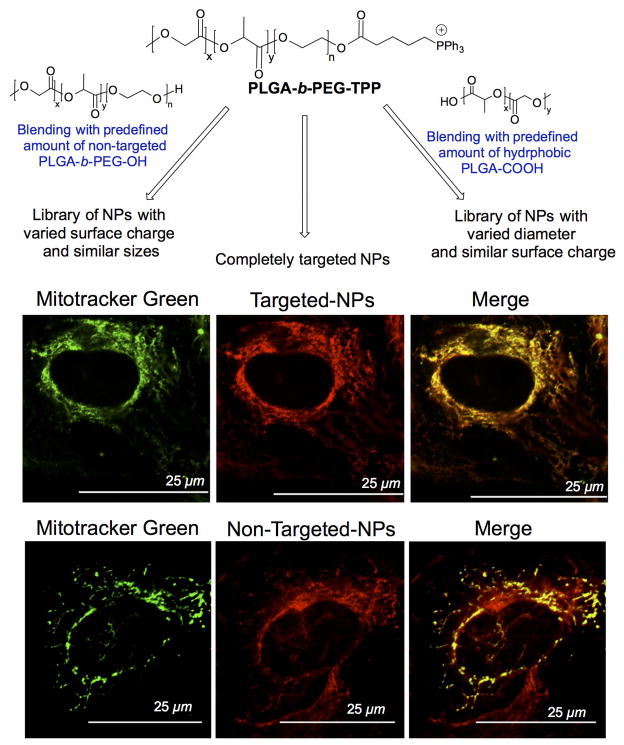
At the similar time, we developed a TPP terminated PLGA-PEG based block copolymer (79). By placing the mitochondria targeting TPP ligand on the PEG side of the polymer, we were able to take advantage of the Δψm for effective mitochondria targeting (Figure 10). We synthesized a TPP appended copolymer PLGA-b-PEG-TPP to take advantage of the TPP cation to cross into the mitochondrial matrix space (79) (110) (Figure 10). Our technology also included an engineer’s approach to the design in that we were able to create libraries of NPs with a range of sizes and zeta potentials by forming blended NPs through mixing of PLGA-b-PEG-TPP either with PLGA-b-PEG-OH or PLGA-COOH to fully understand the needs of mitochondrial targeting (Figure 10). We found that an optimum size of less than 100 nm and a positive zeta potential of greater than ~22 mV is needed for efficient mitochondrial uptake. The versatile nature of this system demonstrated possible uses for a variety of applications. The novelty of this system lies in its unique biological properties from its mechanism of uptake to non-toxic and non-immunogenic properties. The mass amount of lipophilic cations exposed on the NP surface allow for very unique endosomal escape after uptake. Once escaped from the endosomes, these NPs have the potential to efficiently maneuver through the cytoplasm to the mitochondria. Incorporation of quantum dot (QD) in targeted and non-targeted NPs was used to investigate the cellular distribution properties of these NPs in HeLa cells (Figure 10). Fluorescence imaging of targeted and non-targeted NP treated cells indicated significantly higher uptake of targeted NPs in the mitochondria of cells and the non-targeted NPs were distributed in the cytosol. The unique hydrophilic and delocalized surface charge from the TPP cation allows repelling of aqua water molecules. A combination of unique delocalized positive charge and steric encumbrance from the phenyl groups allow the NPs to avoid protein adsorption, and hence less aggregation and macrophage uptake was achieved. The inabilities to control aggregation, protein adsorption are the common pitfall for many positively charged systems. These properties make this system unique as a mitochondria targeted drug delivery vehicle.
Figure 10.
Precise engineering of polymeric NPs to control NP size and surface charge for effective mitochondria targeting properties (top) and mitochondrial localization targeted-NPs and cytosolic distribution of non-targeted NPs (bottom). Redrawn using original data from Reference 79.
The ability of these targeted NPs were explored to load a number of mitochondria-acting therapeutics (111) for possible use in a variety of mitochondrial dysfunction related disorders such as Alzheimer’s disease (AD) (112), obesity (113), and cancer chemotherapy (114) (Figure 11). An inhibitor of amyloid β-protein (Aβ), curcumin was incorporated in these NPs for AD (115, 116). By delivering curcumin to the mitochondria of IMR-32 neuroblastomas, the cytotoxicity induced by Aβ peptide was reduced to a minimum. It was also used to encapsulate lonidamine, a mitochondrial hexokinase inhibitor and α-tocopherol succinate, a mitochondrial respiratory chain complex I inhibitor (Figure 11). By directing these mitochondria-acting chemotherapeutics directly to the mitochondria, a significant reduction in IC50 values was observed in HeLa human cervical cancer cells compared to the free molecules or the non-targeted system (79). In order to show this system’s potential contribution to obesity, a protonophore 2,4-dinitrophenol (2,4-DNP) was encapsulated (Figure 11). 2,4-DNP has shown some success as an effective mitochondrial uncoupler (117, 118). However, the narrow therapeutic window of 2,4-DNP led to the abandonment of its use as a treatment for human obesity. Studies to deliver 2,4-DNP directly to the mitochondria by linking to TPP to improve the therapeutic window demonstrated that covalent modification of 2,4-DNP compromises its coupling efficacy (119). We therefore, incorporated our PLGA-b-PEG-TPP to entrap 2,4-DNP inside the hydrophobic polymeric core polymer to direct this uncoupler to the mitochondria of cells in its pristine form. We were able to successfully prevent the differentiation of 3T3-L1 preadipocytes to adipose tissue at a much lower concentration than that of free 2,4-DNP.
Figure 11.
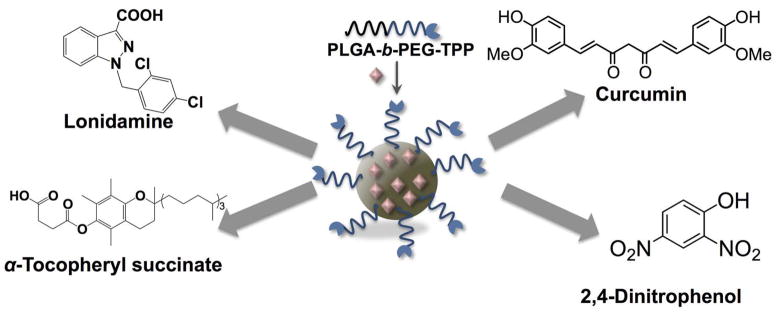
Mitochondria targeted NP system based on PLGA-b-PEG-TPP for entrapment of various mitochondria acting therapeutics. Redrawn based on Reference (79).
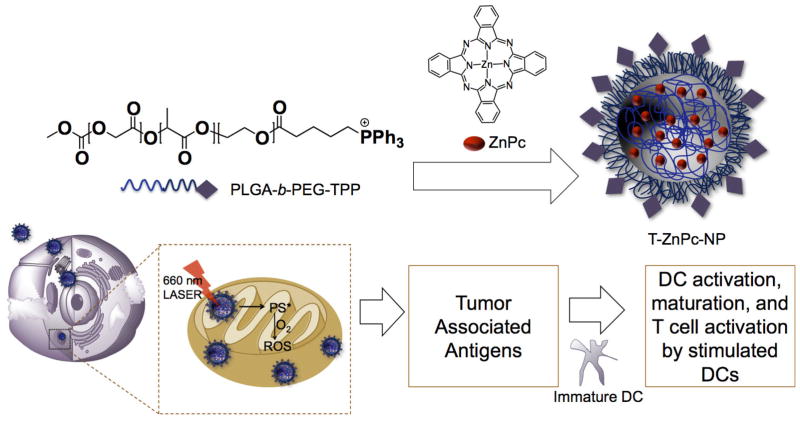
This system was also in mitochondria targeted photodynamic therapy (PDT) (Figure 12). PDT is an emerging field in cancer therapy that uses photosensitizer, light, and tissue oxygen (120, 121). Upon irradiation, the excited photosensitizer reacts with molecular oxygen to generate cytotoxic singlet oxygen to initiate mitochondrial apoptosis pathways. This photodynamic activity has the ability to boost the immune system. Zinc phthalocyanine (ZnPc), a mitochondria acting photosensitizer was encapsulated inside PLGA-b-PEG-TPP polymer (106). By targeting ZnPc to the mitochondria, singlet oxygen was locally produced inside the mitochondria to effectively initiate apoptosis (106). Not only did it significantly reduced the IC50 values in several cell lines, but it also was a much more potent immune system boosting compared to free ZnPc or when ZnPc was delivered to the cytosol using a non-targeted PLGA-b-PEG-OH NP system. By exposing breast cancer MCF-7 tumor associated antigens (TAAs) produced from mitochondria-targeted ZnPc-PLGA-b-PEG-TPP-NPs to dendritic cells (DC) ex vivo, we were able to stimulate DCs to produce significant levels of interferon-gamma (IFN-γ), an important cytokine considered as a product of T and NK cells. The remarkable ex vivo DC stimulation ability of the TAAs generated from mitochondria-targeted PDT opened up the possibility of using mitochondria-targeted-NP treated, light activated TAAs as possible vaccines.
Figure 12.
Mitochondria targeted NPs for PDT localized to mitochondria and mechanism of action for light triggered immune activation. Redrawn based on Reference (106)
The Outlook for the Future
Targeting the mitochondria of diseased cell provides a unique approach to selectively destroy vulnerable tissues over healthy mass. Mitochondria acting therapeutics faces tremendous challenges due to poor bioD, PK, and lack of appropriate structure for selective uptake by the mitochondria leading to their relative toxic nature. The multidimensional features of NPs to specifically target vulnerable cells and accumulate in particular organelle can revolutionize the therapeutic regimes for disease pathologies where mitochondrial dysfunction plays significant roles. NP formulations based on micelles, liposomes, dendrimers, and carbon nanotubes with specific targeting ingredient demonstrated their existence as delivery vehicles for shipment of cargos to the mitochondria. However, many studies are required to understand the safety of these vehicles and significant efforts should be put forward to study the actual mitochondrial location of these systems under in vitro and in vivo settings.
Engineered NPs from FDA approved polymers with lipophilic TPP cation as targeting moiety on the surface, successfully demonstrated the ability to deliver bioactive materials and drugs to the mitochondria to evade various diseases. In vitro studies suggested that these drug loaded NPs are non-toxic, non immunogenic, and behave as a platform for delivering various payloads for numerous diseases. Engineered NPs loaded with photosensitizer were demonstrated to generate specific antigens that stimulate dendritic cells ex vivo for cancer immunotherapy. These biodegradable and biocompatible NP formulations can serve as indispensable platforms to modulate drug delivery regime for better therapeutic options. Additionally these platforms can potentiate the personalized immune therapy with existing chemotherapy.
In summary, the mitochondria targeting approach potentiate therapeutic action and decrease side effects of particular drug, which are indeed an ideal goal for the future medicine. Despite the success of these nano-formulations for in vitro studies, systematic preclinical and clinical studies are required for their potential use in clinical setting. There is a lack of clear understanding about the potential safety aspects that may be unique to this type of nanomedicine. Thus safety of nanomedicine, which can target the powerhouse of cells, should be addressed through appropriate safeguards. There should be significant effort to understand whether nanomedicine is different from other new types of medical research. Current awareness should be enhanced to thoroughly characterize and understand physicochemical properties of nanomedicine that can be targeted to intracellular organelle such as mitochondria. There is an enormous effort globally to contribute to the development of targeted nanomedicine; however, unless full characterization and understanding about this unique subject is achieved, it will be extremely difficult to use these technologies, which have the potential to have immense impact on the future therapeutics.
Acknowledgments
We thank a start-up grant from the National Institutes of Health (P30 GM 092378) to UGA and by the Office of the Vice President for Research, UGA to S.D; Department of Defense Prostate Cancer Idea award (W81XWH-12–1–0406) to S.D; American Heart Association National Scientist Award to S.D. We thank Sean Marrache for his assistance during the preparation of this manuscript.
Footnotes
Financial Disclosure
S.D. discloses financial interest in Partikula LLC, a biotechnology company. Partikula did not support the aforementioned work.
References
- 1.Vander Heiden MGLJ, Swanson KD, Sharfi H, Heffron GJ. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 2010;329:1492–1499. doi: 10.1126/science.1188015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michelakis PDaED. Mitochondria in Vascular Health and Disease. Annu Rev Physiol. 2013;75:95–126. doi: 10.1146/annurev-physiol-030212-183804. [DOI] [PubMed] [Google Scholar]
- 3.Gray MW. Cold Spring Harbor Perspectives in Biology. 2012. Mitochondrial evolution; p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103:1283–1288. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiMauro S, Schon EA. Mitochondrial respiratory-chain diseases. N Engl J Med. 2003;348:2656–2668. doi: 10.1056/NEJMra022567. [DOI] [PubMed] [Google Scholar]
- 6.MRD Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. J Physiol. 1999;516:1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zamzami NKG. The mitochondrion in apoptosis: how Pandora’s box opens. Nat Rev Mol Cell Biol. 2001;2:67–71. doi: 10.1038/35048073. [DOI] [PubMed] [Google Scholar]
- 8.McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:R551–560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 10.Luft R, Ikkos D, Palmieri G, Ernster L, Afzelius B. A case of severe hypermetabolism of nonthyroid origin with a defect in the maintenance of mitochondrial respiratory control: a correlated clinical, biochemical, and morphological study. J Clin Invest. 1962;41:1776–1804. doi: 10.1172/JCI104637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fosslien E. Mitochondrial medicine--molecular pathology of defective oxidative phosphorylation. Ann Clin Lab Sci. 2001;31:25–67. [PubMed] [Google Scholar]
- 13.West IC. Radicals and oxidative stress in diabetes. Diabet Med. 2000;17:171–180. doi: 10.1046/j.1464-5491.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- 14.Koike K. Molecular basis of hepatitis C virus-associated hepatocarcinogenesis: lessons from animal model studies. Clin Gastroenterol Hepatol. 2005;3:S132–135. doi: 10.1016/s1542-3565(05)00700-7. [DOI] [PubMed] [Google Scholar]
- 15.Wallace DC. Mitochondria and cancer. Nat Rev Cancer. 2012;12:685–698. doi: 10.1038/nrc3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen EI. Mitochondrial dysfunction and cancer metastasis. J Bioenerg Biomembr. 2012;44:619–622. doi: 10.1007/s10863-012-9465-9. [DOI] [PubMed] [Google Scholar]
- 17.Boland ML, Chourasia AH, Macleod KF. Mitochondrial Dysfunction in Cancer. Front Oncol. 2013;3:292. doi: 10.3389/fonc.2013.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stavrovskaya IG, Kristal BS. The powerhouse takes control of the cell: is the mitochondrial permeability transition a viable therapeutic target against neuronal dysfunction and death? Free Radic Biol Med. 2005;38:687–697. doi: 10.1016/j.freeradbiomed.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 19.Puddu P, Puddu GM, Galletti L, Cravero E, Muscari A. Mitochondrial dysfunction as an initiating event in atherogenesis: a plausible hypothesis. Cardiology. 2005;103:137–141. doi: 10.1159/000083440. [DOI] [PubMed] [Google Scholar]
- 20.Ballinger SW. Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med. 2005;38:1278–1295. doi: 10.1016/j.freeradbiomed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia--reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001;33:1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- 22.Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007;100:460–473. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- 23.Mercer JR, Cheng KK, Figg N, Gorenne I, Mahmoudi M, Griffin J, et al. DNA damage links mitochondrial dysfunction to atherosclerosis and the metabolic syndrome. Circ Res. 2010;107:1021–1031. doi: 10.1161/CIRCRESAHA.110.218966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savitha S, Sivarajan K, Haripriya D, Kokilavani V, Panneerselvam C. Efficacy of levo carnitine and alpha lipoic acid in ameliorating the decline in mitochondrial enzymes during aging. Clin Nutr. 2005;24:794–800. doi: 10.1016/j.clnu.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Skulachev VP, Longo VD. Aging as a mitochondria-mediated atavistic program: can aging be switched off? Ann N Y Acad Sci. 2005;1057:145–164. doi: 10.1196/annals.1356.009. [DOI] [PubMed] [Google Scholar]
- 26.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corral-Debrinski M, Shoffner JM, Lott MT, Wallace DC. Association of mitochondrial DNA damage with aging and coronary atherosclerotic heart disease. Mutat Res. 1992;275:169–180. doi: 10.1016/0921-8734(92)90021-g. [DOI] [PubMed] [Google Scholar]
- 28.Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psychiatry. 2005;10:900–919. doi: 10.1038/sj.mp.4001711. [DOI] [PubMed] [Google Scholar]
- 29.Fattal O, Budur K, Vaughan AJ, Franco K. Review of the literature on major mental disorders in adult patients with mitochondrial diseases. Psychosomatics. 2006;47:1–7. doi: 10.1176/appi.psy.47.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Einat H, Yuan P, Manji HK. Increased anxiety-like behaviors and mitochondrial dysfunction in mice with targeted mutation of the Bcl-2 gene: further support for the involvement of mitochondrial function in anxiety disorders. Behav Brain Res. 2005;165:172–180. doi: 10.1016/j.bbr.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Fulle S, Mecocci P, Fano G, Vecchiet I, Vecchini A, Racciotti D, et al. Specific oxidative alterations in vastus lateralis muscle of patients with the diagnosis of chronic fatigue syndrome. Free Radic Biol Med. 2000;29:1252–1259. doi: 10.1016/s0891-5849(00)00419-6. [DOI] [PubMed] [Google Scholar]
- 32.Myhill S, Booth NE, McLaren-Howard J. Chronic fatigue syndrome and mitochondrial dysfunction. Int J Clin Exp Med. 2009;2:1–16. [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Wang W, Li L, Perry G, Lee HG, Zhu X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochimica et biophysica acta. 2014;1842:1240–1247. doi: 10.1016/j.bbadis.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross MF, GFK, Blaikie FH, James AM, Cochemé HM, Filipovska A, Da Ros T, Hurd TR, Smith RAJ, Murphy MP. Lipophilic Triphenylphosphonium Cations as Tools in Mitochondrial Bioenergetics and Free Radical Biology. Biochemistry (Moscow) 2005;70:273–283. doi: 10.1007/s10541-005-0104-5. [DOI] [PubMed] [Google Scholar]
- 35.Alberts BJA, Lewis J, et al. Molecular Biology of the Cell. 4. Garland Science; New York: 2002. [Google Scholar]
- 36.Nieminen AI, Eskelinen VM, Haikala HM, Tervonen TA, Yan Y, Partanen JI, et al. Myc-induced AMPK-phospho p53 pathway activates Bak to sensitize mitochondrial apoptosis. Proc Natl Acad Sci U S A. 2013;110:E1839–E1848. doi: 10.1073/pnas.1208530110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henry-Mowatt J, Dive C, Martinou J-C, James D. Role of mitochondrial membrane permeabilization in apoptosis and cancer. Oncogene. 2004;23:2850–2860. doi: 10.1038/sj.onc.1207534. [DOI] [PubMed] [Google Scholar]
- 38.Smith RA, Hartley RC, Murphy MP. Mitochondria-targeted small molecule therapeutics and probes. Antioxid Redox Signal. 2011;15:3021–3038. doi: 10.1089/ars.2011.3969. [DOI] [PubMed] [Google Scholar]
- 39.Susan Chalmers STC, Quin Caroline, Prime Tracy A, James Andrew M, Cairns Andrew G, Murphy Michael P, McCarron John G, Hartley Richard C. Selective Uncoupling of Individual Mitochondria within a Cell Using a Mitochondria-Targeted Photoactivated Protonophore. J Am Chem Soc. 2012;134:758–761. doi: 10.1021/ja2077922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rin Jean S, Tulumello DV, Wisnovsky SP, Lei EK, Pereira MP, Kelley SO. Molecular vehicles for mitochondrial chemical biology and drug delivery. ACS chemical biology. 2014;9:323–333. doi: 10.1021/cb400821p. [DOI] [PubMed] [Google Scholar]
- 41.Sheu SS, Anders MW, Xu L, Sharma VK. N-Acetyl L-Cysteine and L-Cysteine Choline Esters, and (R)-[2-(2,2-Dimethyl-thiazolidine-4-carbonyloxy)-ethyl]trimethyl-ammonium chloride, for example; compounds may be used to inhibit oxidative stress-induced cell injury or death both in vivo and ex vivo. Google Patents 2009
- 42.Sheu SS, Nauduri D, Anders MW. Targeting antioxidants to mitochondria: a new therapeutic direction. Biochimica et biophysica acta. 2006;1762:256–265. doi: 10.1016/j.bbadis.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Miriyala S, Spasojevic I, Tovmasyan A, Salvemini D, Vujaskovic Z, St Clair D, et al. Manganese superoxide dismutase, MnSOD and its mimics. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2012;1822:794–814. doi: 10.1016/j.bbadis.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ali DK, Oriowo M, Tovmasyan A, Batinic-Haberle I, Benov L. Late administration of Mn porphyrin-based SOD mimic enhances diabetic complications. Redox Biol. 2013;1:457–466. doi: 10.1016/j.redox.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keir ST, Dewhirst MW, Kirkpatrick JP, Bigner DD, Batinic-Haberle I. Cellular redox modulator, ortho Mn(III) meso-tetrakis(N-n-hexylpyridinium-2-yl)porphyrin, MnTnHex-2-PyP(5+) in the treatment of brain tumors. Anti-Cancer Agents In Medicinal Chemistry. 2011;11:202–212. doi: 10.2174/187152011795255957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheng H, Spasojevic I, Tse HM, Jung JY, Hong J, Zhang Z, et al. Neuroprotective Efficacy from a Lipophilic Redox-Modulating Mn(III) N-Hexylpyridylporphyrin, MnTnHex-2-PyP: Rodent Models of Ischemic Stroke and Subarachnoid Hemorrhage. Journal of Pharmacology and Experimental Therapeutics. 2011;338:906–916. doi: 10.1124/jpet.110.176701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szulc ZM, Bielawski J, Gracz H, Gustilo M, Mayroo N, Hannun YA, et al. Tailoring structure–function and targeting properties of ceramides by site-specific cationization. Bioorganic & Medicinal Chemistry. 2006;14:7083–7104. doi: 10.1016/j.bmc.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 48.Rokitskaya TI, Sumbatyan NV, Tashlitsky VN, Korshunova GA, Antonenko YN, Skulachev VP. Mitochondria-targeted penetrating cations as carriers of hydrophobic anions through lipid membranes. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2010;1798:1698–1706. doi: 10.1016/j.bbamem.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 49.Horton KL, Stewart KM, Fonseca SB, Guo Q, Kelley SO. Mitochondria-penetrating peptides. Chem Biol. 2008;15:375–382. doi: 10.1016/j.chembiol.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 50.Horton KL, Kelley SO. Engineered Apoptosis-Inducing Peptides with Enhanced Mitochondrial Localization and Potency. Journal of Medicinal Chemistry. 2009;52:3293–3299. doi: 10.1021/jm900178n. [DOI] [PubMed] [Google Scholar]
- 51.Lei EK, Pereira MP, Kelley SO. Tuning the Intracellular Bacterial Targeting of Peptidic Vectors. Angewandte Chemie International Edition. 2013:9660–9663. doi: 10.1002/anie.201302265. [DOI] [PubMed] [Google Scholar]
- 52.Rin Jean S, Tulumello DV, Wisnovsky SP, Lei EK, Pereira MP, Kelley SO. Molecular Vehicles for Mitochondrial Chemical Biology and Drug Delivery. ACS Chem Biol. 2014;9:323–333. doi: 10.1021/cb400821p. [DOI] [PubMed] [Google Scholar]
- 53.Zhang P, Cheetham AG, Lock LL, Cui H. Cellular Uptake and Cytotoxicity of Drug–Peptide Conjugates Regulated by Conjugation Site. Bioconjugate Chemistry. 2013;24:604–613. doi: 10.1021/bc300585h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chamberlain GR, Tulumello DV, Kelley SO. Targeted Delivery of Doxorubicin to Mitochondria. ACS Chemical Biology. 2013;8:1389–1395. doi: 10.1021/cb400095v. [DOI] [PubMed] [Google Scholar]
- 55.Fonseca Sonali B, Pereira Mark P, Mourtada R, Gronda M, Horton Kristin L, Hurren R, et al. Rerouting Chlorambucil to Mitochondria Combats Drug Deactivation and Resistance in Cancer Cells. Chem Biol. 2011;18:445–453. doi: 10.1016/j.chembiol.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 56.Wisnovsky Simon P, Wilson Justin J, Radford Robert J, Pereira Mark P, Chan Maria R, Laposa Rebecca R, et al. Targeting Mitochondrial DNA with a Platinum-Based Anticancer Agent. Chem Biol. 2013;20:1323–1328. doi: 10.1016/j.chembiol.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pereira MP, Kelley SO. Maximizing the Therapeutic Window of an Antimicrobial Drug by Imparting Mitochondrial Sequestration in Human Cells. Journal of the American Chemical Society. 2011;133:3260–3263. doi: 10.1021/ja110246u. [DOI] [PubMed] [Google Scholar]
- 58.Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, et al. Selective Targeting of a Redox-active Ubiquinone to Mitochondria within Cells: ANTIOXIDANT AND ANTIAPOPTOTIC PROPERTIES. Journal of Biological Chemistry. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 59.Jauslin ML, Meier T, Smith RAJ, Murphy MP. Mitochondria-targeted antioxidants protect Friedreich Ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. The FASEB Journal. 2003 doi: 10.1096/fj.03-0240fje. [DOI] [PubMed] [Google Scholar]
- 60.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RAJ, Murphy MP, et al. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. The FASEB Journal. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 61.Cheng G, Zielonka J, McAllister D, Mackinnon A, Joseph J, Dwinell M, et al. Mitochondria-targeted vitamin E analogs inhibit breast cancer cell energy metabolism and promote cell death. BMC Cancer. 2013;13:285. doi: 10.1186/1471-2407-13-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kelso GF, Maroz A, Cocheme HM, Logan A, Prime TA, Peskin AV, et al. A mitochondria-targeted macrocyclic Mn(II) superoxide dismutase mimetic. Chemistry & biology. 2012;19:1237–1246. doi: 10.1016/j.chembiol.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 63.Filipovska A, Kelso GF, Brown SE, Beer SM, Smith RA, Murphy MP. Synthesis and characterization of a triphenylphosphonium-conjugated peroxidase mimetic. Insights into the interaction of ebselen with mitochondria. J Biol Chem. 2005;280:24113–24126. doi: 10.1074/jbc.M501148200. [DOI] [PubMed] [Google Scholar]
- 64.Dessolin J, Schuler M, Quinart A, De Giorgi F, Ghosez L, Ichas F. Selective targeting of synthetic antioxidants to mitochondria: towards a mitochondrial medicine for neurodegenerative diseases? European journal of pharmacology. 2002;447:155–161. doi: 10.1016/s0014-2999(02)01839-3. [DOI] [PubMed] [Google Scholar]
- 65.Dhanasekaran A, Kotamraju S, Karunakaran C, Kalivendi SV, Thomas S, Joseph J, et al. Mitochondria superoxide dismutase mimetic inhibits peroxide-induced oxidative damage and apoptosis: role of mitochondrial superoxide. Free Radic Biol Med. 2005;39:567–583. doi: 10.1016/j.freeradbiomed.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 66.Murphy MP, Echtay KS, Blaikie FH, Asin-Cayuela J, Cocheme HM, Green K, et al. Superoxide activates uncoupling proteins by generating carbon-centered radicals and initiating lipid peroxidation: studies using a mitochondria-targeted spin trap derived from alpha-phenyl-N-tert-butylnitrone. J Biol Chem. 2003;278:48534–48545. doi: 10.1074/jbc.M308529200. [DOI] [PubMed] [Google Scholar]
- 67.Belikova NA, Jiang J, Stoyanovsky DA, Glumac A, Bayir H, Greenberger JS, et al. Mitochondria-targeted (2-hydroxyamino-vinyl)-triphenyl-phosphonium releases NO(.) and protects mouse embryonic cells against irradiation-induced apoptosis. FEBS Lett. 2009;583:1945–1950. doi: 10.1016/j.febslet.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stoyanovsky DA, Vlasova II, Belikova NA, Kapralov A, Tyurin V, Greenberger JS, et al. Activation of NO donors in mitochondria: peroxidase metabolism of (2-hydroxyamino-vinyl)-triphenyl-phosphonium by cytochrome c releases NO and protects cells against apoptosis. FEBS Lett. 2008;582:725–728. doi: 10.1016/j.febslet.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Birk AV, Chao WM, Bracken C, Warren JD, Szeto HH. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. British journal of pharmacology. 2014;171:2017–2028. doi: 10.1111/bph.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pathak RK, Marrache S, Harn DA, Dhar S. Mito-DCA: A Mitochondria Targeted Molecular Scaffold for Efficacious Delivery of Metabolic Modulator Dichloroacetate. ACS Chemical Biology. 2014 doi: 10.1021/cb400944y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Millard M, Gallagher JD, Olenyuk BZ, Neamati N. A Selective Mitochondrial-Targeted Chlorambucil with Remarkable Cytotoxicity in Breast and Pancreatic Cancers. Journal of Medicinal Chemistry. 2013;56:9170–9179. doi: 10.1021/jm4012438. [DOI] [PubMed] [Google Scholar]
- 72.Chalmers S, Caldwell ST, Quin C, Prime TA, James AM, Cairns AG, et al. Selective Uncoupling of Individual Mitochondria within a Cell Using a Mitochondria-Targeted Photoactivated Protonophore. Journal of the American Chemical Society. 2011;134:758–761. doi: 10.1021/ja2077922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Le Trionnaire S, Perry A, Szczesny B, Szabo C, Winyard PG, Whatmore JL, et al. The synthesis and functional evaluation of a mitochondria-targeted hydrogen sulfide donor, (10-oxo-10-(4-(3-thioxo-3H-1,2-dithiol-5-yl)phenoxy)decyl)triphenylphosphonium bromide (AP39) MedChemComm 2014 [Google Scholar]
- 74.Bao G, Mitragotri S, Tong S. Multifunctional nanoparticles for drug delivery and molecular imaging. Annu Rev Biomed Eng. 2013;15:253–282. doi: 10.1146/annurev-bioeng-071812-152409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marrache S, Pathak RK, Darley KL, Choi JH, Zaver D, Kolishetti N, et al. Nanocarriers for tracking and treating diseases. Current medicinal chemistry. 2013;20:3500–3514. doi: 10.2174/0929867311320280007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 77.Salvador-Morales C, Zhang L, Langer R, Farokhzad OC. Immunocompatibility properties of lipid-polymer hybrid nanoparticles with heterogeneous surface functional groups. Biomaterials. 2009;30:2231–2240. doi: 10.1016/j.biomaterials.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shi J, Votruba AR, Farokhzad OC, Langer R. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Letters. 2010;10:3223–3230. doi: 10.1021/nl102184c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marrache S, Dhar S. Engineering of blended nanoparticle platform for delivery of mitochondria-acting therapeutics. Proceedings of the National Academy of Sciences. 2012;109:16288–16293. doi: 10.1073/pnas.1210096109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weissig V, Lizano C, Torchilin VP. Micellar delivery system for dequalinium-A lipophilic cationic drug with anticarcinoma activity. Journal of Liposome Research. 1998;8:391–400. [Google Scholar]
- 81.Weissig V. From serendipity to mitochondria-targeted nanocarriers. Pharm Res. 2011;28:2657–2668. doi: 10.1007/s11095-011-0556-9. [DOI] [PubMed] [Google Scholar]
- 82.D’Souza GG, Rammohan R, Cheng SM, Torchilin VP, Weissig V. DQAsome-mediated delivery of plasmid DNA toward mitochondria in living cells. J Control Release. 2003;92:189–197. doi: 10.1016/s0168-3659(03)00297-9. [DOI] [PubMed] [Google Scholar]
- 83.Vaidya B, Paliwal R, Rai S, Khatri K, Goyal AK, Mishra N, et al. Cell-selective mitochondrial targeting: A new approach for cancer therapy. Cancer Therapy. 2009;7:141–148. [Google Scholar]
- 84.Tomalia DA, Reyna LA, Svenson S. Dendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imaging. Biochem Soc Trans. 2007;35:61–67. doi: 10.1042/BST0350061. [DOI] [PubMed] [Google Scholar]
- 85.Choi YS, Cho TS, Kim JM, Han SW, Kim SK. Amine terminated G-6 PAMAM dendrimer and its interaction with DNA probed by Hoechst 33258. Biophys Chem. 2006;121:142–149. doi: 10.1016/j.bpc.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 86.Biswas S, Dodwadkar NS, Piroyan A, Torchilin VP. Surface conjugation of triphenylphosphonium to target poly(amidoamine) dendrimers to mitochondria. Biomaterials. 2012;33:4773–4782. doi: 10.1016/j.biomaterials.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X, Shao N, Zhang Q, Cheng Y. Mitochondrial targeting dendrimer allows efficient and safe gene delivery. Journal of Materials Chemistry B. 2014;2:2546–2553. doi: 10.1039/c3tb21348j. [DOI] [PubMed] [Google Scholar]
- 88.Theodossiou T, Sideratou Z, Katsarou M, Tsiourvas D. Mitochondrial Delivery of Doxorubicin by Triphenylphosphonium-Functionalized Hyperbranched Nanocarriers Results in Rapid and Severe Cytotoxicity. Pharm Res. 2013;30:2832–2842. doi: 10.1007/s11095-013-1111-7. [DOI] [PubMed] [Google Scholar]
- 89.Battigelli A, Russier J, Venturelli E, Fabbro C, Petronilli V, Bernardi P, et al. Peptide-based carbon nanotubes for mitochondrial targeting. Nanoscale. 2013;5:9110–9117. doi: 10.1039/c3nr02694a. [DOI] [PubMed] [Google Scholar]
- 90.Yoong SL, Wong BS, Zhou QL, Chin CF, Li J, Venkatesan T, et al. Enhanced cytotoxicity to cancer cells by mitochondria-targeting MWCNTs containing platinum(IV) prodrug of cisplatin. Biomaterials. 2014;35:748–759. doi: 10.1016/j.biomaterials.2013.09.036. [DOI] [PubMed] [Google Scholar]
- 91.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 92.Chang HI, Yeh MK. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int J Nanomedicine. 2012;7:49–60. doi: 10.2147/IJN.S26766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kakudo T, Chaki S, Nakase I, Akaji K, Kawakami T, Maruyama K, et al. Transferrin-modified liposomes equipped with a pH-sensitive fusogenic peptide: an artificial viral-like delivery system. Biochemistry. 2004;43:5618–5628. doi: 10.1021/bi035802w. [DOI] [PubMed] [Google Scholar]
- 94.Huth U, Wieschollek A, Garini Y, Schubert R, Peschka-Suss R. Fourier transformed spectral bio-imaging for studying the intracellular fate of liposomes. Cytometry. 2004;57A:10–21. doi: 10.1002/cyto.a.10105. [DOI] [PubMed] [Google Scholar]
- 95.Yamada Y, Akita H, Kogure K, Kamiya H, Harashima H. Mitochondrial drug delivery and mitochondrial disease therapy – An approach to liposome-based delivery targeted to mitochondria. Mitochondrion. 2007;7:63–71. doi: 10.1016/j.mito.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 96.Yamada Y, Akita H, Kamiya H, Kogure K, Yamamoto T, Shinohara Y, et al. MITO-Porter: A liposome-based carrier system for delivery of macromolecules into mitochondria via membrane fusion. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2008;1778:423–432. doi: 10.1016/j.bbamem.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 97.Boddapati SV, D’Souza GGM, Erdogan S, Torchilin VP, Weissig V. Organelle-Targeted Nanocarriers: Specific Delivery of Liposomal Ceramide to Mitochondria Enhances Its Cytotoxicity in Vitro and in Vivo. Nano Letters. 2008;8:2559–2563. doi: 10.1021/nl801908y. [DOI] [PubMed] [Google Scholar]
- 98.Boddapati SV, Tongcharoensirikul P, Hanson RN, D’Souza GGM, Torchilin VP, Weissig V. Mitochondriotropic Liposomes. Journal of Liposome Research. 2005;15:49–58. doi: 10.1081/lpr-64958. [DOI] [PubMed] [Google Scholar]
- 99.Malhi SS, Budhiraja A, Arora S, Chaudhari KR, Nepali K, Kumar R, et al. Intracellular delivery of redox cycler-doxorubicin to the mitochondria of cancer cell by folate receptor targeted mitocancerotropic liposomes. International Journal of Pharmaceutics. 2012;432:63–74. doi: 10.1016/j.ijpharm.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 100.Solomon MA, Shah AA, D’Souza GGM. In Vitro assessment of the utility of stearyl triphenyl phosphonium modified liposomes in overcoming the resistance of ovarian carcinoma Ovcar-3 cells to paclitaxel. Mitochondrion. 2013;13:464–472. doi: 10.1016/j.mito.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 101.Wang X-X, Li Y-B, Yao H-J, Ju R-J, Zhang Y, Li R-J, et al. The use of mitochondrial targeting resveratrol liposomes modified with a dequalinium polyethylene glycol-distearoylphosphatidyl ethanolamine conjugate to induce apoptosis in resistant lung cancer cells. Biomaterials. 2011;32:5673–5687. doi: 10.1016/j.biomaterials.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 102.Biswas S, Dodwadkar NS, Deshpande PP, Torchilin VP. Liposomes loaded with paclitaxel and modified with novel triphenylphosphonium-PEG-PE conjugate possess low toxicity, target mitochondria and demonstrate enhanced antitumor effects in vitro and in vivo. Journal of Controlled Release. 2012;159:393–402. doi: 10.1016/j.jconrel.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou J, Zhao W-Y, Ma X, Ju R-J, Li X-Y, Li N, et al. The anticancer efficacy of paclitaxel liposomes modified with mitochondrial targeting conjugate in resistant lung cancer. Biomaterials. 2013;34:3626–3638. doi: 10.1016/j.biomaterials.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 104.Theodossiou TA, Sideratou Z, Tsiourvas D, Paleos CM. A novel mitotropic oligolysine nanocarrier: Targeted delivery of covalently bound D-Luciferin to cell mitochondria. Mitochondrion. 2011;11:982–986. doi: 10.1016/j.mito.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 105.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marrache S, Tundup S, Harn DA, Dhar S. Ex Vivo Programming of Dendritic Cells by Mitochondria-Targeted Nanoparticles to Produce Interferon-Gamma for Cancer Immunotherapy. ACS Nano. 2013;7:7392–7402. doi: 10.1021/nn403158n. [DOI] [PubMed] [Google Scholar]
- 107.Kolishetti N, Dhar S, Valencia PM, Lin LQ, Karnik R, Lippard SJ, et al. Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proc Natl Acad Sci USA. 2010;107:17939–17944. doi: 10.1073/pnas.1011368107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marin E, Briceno MI, Caballero-George C. Critical evaluation of biodegradable polymers used in nanodrugs. Int J Nanomedicine. 2013;8:3071–3090. doi: 10.2147/IJN.S47186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sharma A, Soliman GM, AI-Hajaj N, Sharma R, Maysinger D, Kakkar A. Design and Evaluation of Multifunctional Nanocarriers for Selective Delivery of Coenzyme Q10 to Mitochondria. Biomacromolecules. 2011;13:239–252. doi: 10.1021/bm201538j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smith RA, Porteous CM, Gane AM, Murphy MP. Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci U S A FIELD Full Journal Title:Proceedings of the National Academy of Sciences of the United States of America. 2003;100:5407–5412. doi: 10.1073/pnas.0931245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weissig V, D’Souza GGM. Mitochondria-targeted drug delivery. Targeted Delivery Small Macromol Drugs. 2010:255–273. [Google Scholar]
- 112.Blaikie FH, Brown SE, Samuelsson LM, Brand MD, Smith RAJ, Murphy MP. Targeting dinitrophenol to mitochondria: Limitations to the development of a self-limiting mitochondrial protonophore. Bioscience Reports. 2006;26:231–243. doi: 10.1007/s10540-006-9018-8. [DOI] [PubMed] [Google Scholar]
- 113.Liu JK, Shen WL, Zhao BL, Wang Y, Wertz K, Weber P, et al. Targeting mitochondrial biogenesis for preventing and treating insulin resistance in diabetes and obesity: Hope from natural mitochondrial nutrients. Advanced Drug Delivery Reviews. 2009;61:1343–1352. doi: 10.1016/j.addr.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 114.Wang F, Ogasawara MA, Huang P. Small mitochondria-targeting molecules as anti-cancer agents. Molecular Aspects of Medicine. 2010;31:75–92. doi: 10.1016/j.mam.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mulik RS, Monkkonen J, Juvonen RO, Mahadik KR, Paradkar AR. ApoE3 mediated poly(butyl) cyanoacrylate nanoparticles containing curcumin: Study of enhanced activity of curcumin against beta amyloid induced cytotoxicity using in vitro cell culture model. Mol Pharmaceutics. 2010;7:815–825. doi: 10.1021/mp900306x. [DOI] [PubMed] [Google Scholar]
- 116.Ono K, Hasegawa K, Naiki H, Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J Neurosci Res. 2004;75:742–750. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- 117.Blaikie FH, Brown SE, Samuelsson LM, Brand MD, Smith RA, Murphy MP. Targeting dinitrophenol to mitochondria: limitations to the development of a self-limiting mitochondrial protonophore. Biosci Rep. 2006;26:231–243. doi: 10.1007/s10540-006-9018-8. [DOI] [PubMed] [Google Scholar]
- 118.De Felice FG, Ferreira ST. Novel neuroprotective, neuritogenic and anti-amyloidogenic properties of 2,4-dinitrophenol: the gentle face of Janus. IUBMB Life. 2006;58:185–191. doi: 10.1080/15216540600702198. [DOI] [PubMed] [Google Scholar]
- 119.Chalmers S, Caldwell ST, Quin C, Prime TA, James AM, Cairns AG, et al. Selective uncoupling of individual mitochondria within a cell using a mitochondria-targeted photoactivated protonophore. J Am Chem Soc. 2012;134:758–761. doi: 10.1021/ja2077922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mroz P, Hashmi JT, Huang YY, Lange N, Hamblin MR. Stimulation of anti-tumor immunity by photodynamic therapy. Expert Rev Clin Immunol. 2011;7:75–91. doi: 10.1586/eci.10.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marrache S, Choi JH, Tundup S, Zaver D, Harn DA, Dhar S. Immune stimulating photoactive hybrid nanoparticles for metastatic breast cancer. Integr Biol (Camb) 2013;5:215–223. doi: 10.1039/c2ib20125a. [DOI] [PMC free article] [PubMed] [Google Scholar]