Abstract
Background
Orthostatic hypotension (OH) is frequent in patients with Parkinson disease (PD) and can occur with or without symptoms. Pharmacological treatments are effective but often exacerbate supine hypertension. Guidelines exist for the diagnosis but not for the treatment of OH. We examined the relationship between blood pressure and symptoms in a cohort of PD patients with the goal of identifying a hemodynamic target to guide treatment.
Methods
We measured blood pressure supine and upright (tilt or active standing) and identified the presence or absence of symptomatic OH by using a validated patient-reported outcome questionnaire in 210 patients with PD. We evaluated the usefulness of the 20/10 and 30/15 mmHg diagnostic criteria (systolic/diastolic) to identify symptomatic OH.
Results
Fifty percent of the PD patient cohort met criteria for the 20/10 fall and 30% for the 30/15 blood pressure fall. Among the patients who met either OH criteria, the percentage of those with symptoms was small (33% of those with 20/10 and 44% of those with 30/15 mmHg; 16% and 13%, respectively overall). Symptomatic OH was associated with an upright mean blood pressure below 75 mmHg. A mean standing blood pressure <75 mmHg had a sensitivity of 97% and a specificity of 98% for detecting symptomatic OH.
Conclusions
Although the prevalence of OH in PD is high, not all patients have symptoms of organ hypoperfusion. A mean standing blood pressure below 75 mmHg appears to be a useful benchmark when deciding whether the benefits of initiating pharmacological treatment of OH outweigh the risks of exacerbating supine hypertension.
Keywords: Autonomic failure, Dysautonomia, Consensus Criteria, Supine hypertension, Standing blood pressure, Mean blood pressure
INTRODUCTION
An estimated 30-60% of patients with Parkinson disease have orthostatic hypotension (OH)1, which can occur with or without symptoms. Classic symptoms of OH include dizziness, lightheadedness and syncope. These symptoms occur when blood pressure (BP) falls on standing and cerebral perfusion pressure drops below a critical limit.2 OH increases the likelihood of falls and is an independent risk factor for mortality.3 Symptomatic burden can lead to immobility and physical deconditioning, which in turn further worsen the severity of OH4, 5. Randomized placebo-controlled clinical trials show that effective treatment of OH can decrease symptoms6-8, increase physical activity levels7, and improve functionality9 . This suggests that treatment of OH may help prevent this incapacitating downward spiral.
OH in PD is the result of defective sympathetic outflow and a failure to increase peripheral resistance when standing.10,11 Currently FDA-approved pharmacologic treatments for OH are pressor agents that work by raising peripheral vascular resistance 7, 12. The potential benefits of any pressor agent must be weighed against the risks of worsening supine hypertension, which is present in 20-70% of patients with PD.13, 14 Consensus guidelines exist for the diagnosis of OH and are based on measurements of BP in the supine and standing position. OH is defined as an orthostatic fall of at least 20 mmHg in systolic or 10 mmHg in diastolic BP within 3 minutes of standing.15 Although an orthostatic fall of 30/15 mmHg has been proposed in the setting of severe supine hypertension.16
Consensus guidelines for the treatment of OH are lacking. The decision to treat OH pharmacologically is geared towards lessening symptomatic burden. Relying on symptoms alone may not always be an accurate indicator of tissue hypoperfusion caused by OH particularly in patients with PD. Symptoms of OH in PD are many times non-specific (lethargy or difficulty concentrating) or atypical such as a frozen appearance that can mimic a levodopa “off” state. Patients may also have difficultly distinguishing unsteadiness when upright as a result of basal ganglia abnormalities17 from symptoms of cerebral hypoperfusion due to OH. Syncope can also occur with little or no premonitory symptoms in PD.
With these shortcomings in mind, we examined the association between the orthostatic fall, standing BP and symptoms of OH in a cohort of patients with PD from the US and Europe. Our goal was to identify a hemodynamic target that can be used when considering initiating pharmacological treatment of OH in PD in the clinic.
METHODS
Study design
We enrolled patients with idiopathic PD in a prospective observational study. Patients were consecutively recruited at the two participating medical centers in New York University (New York, USA) and Hospital de Cruces (Bilbao, Spain). Participants were studied in the morning after an overnight fast. Morning anti-hypotensive and anti-parkinsonian medications were withheld (allowing the patients to be studied in a ‘practical off’ situation). The diagnosis of PD was made using UK Parkinson Disease Brain Bank criteria18. Patients with cognitive impairment (Mini-Mental State Examination ≤ 25) were excluded. Eligible patients were assessed using the Hoehn-Yahr staging. Calculation of L-dopa equivalent dose (LED) was performed as previously described19. All procedures were approved by our local Institutional Review Boards and informed consent was obtained from all participants.
Blood pressure measurements
BP measurements were performed using an automated sphygmomanometer (Colin Press-Mate 8800, San Antonio, TX) placed at heart level on the left arm. After a minimum of 20 minutes supine rest, BP was taken twice and averaged to evaluate supine BP values. Patients were then tilted upright to a 60-degree angle or instructed to stand immobile for 3-minutes while BP was measured at one-minute intervals. Orthostatic stress testing was done as it was routinely performed at each center: 101 patients underwent passive head-up tilt in the US and 109 patients underwent active standing in Europe. The orthostatic fall was defined as the absolute difference between supine and BP values after 3-minutes of standing. In patients unable to tolerate 3 minutes of standing, the lowest captured BP value was used.
Symptom assessment
Symptoms of OH were assessed at three minutes of standing and measured by a trained physician using a patient-reported validated scale20. Patients were sub-divided based on the presence or absence of symptoms. Those that reported dizziness, lightheadedness, visual impairment (i.e., blurry vision, tunnel vision, diminished vision), or pain in the shoulders and back of the neck (coat hanger pain) i.e., items 1, 2 and 6 of the OH-questionnaire20, were included in the symptomatic group.
Criteria and definitions
In the absence of specific criteria to define supine blood pressure parameters in patients with OH, we defined supine hypertension according to the American Heart Association (seated) guidelines21 as: a SBP of ≥140 and/or DBP of ≥ 90 mmHg and/or MBP ≥110 mmHg. OH was defined using two criteria: 1) a fall of ≥20 mmHg systolic or ≥10 mmHg diastolic BP15; and 2) a fall of ≥30 mmHg systolic or ≥15 mmHg diastolic BP16; in both cases after 3 minutes of tilt or active standing.
Statistical analyses
Data were tested first for normality and parametric or non-parametric statistical tests were used as appropriate. Differences between groups were assessed using unpaired t-tests to compare continuous blood pressure variables. Patients were categorized according to the presence or absence of symptoms and data were analyzed by using Chi-squared test. Correlation analysis (Pearson's coefficient) was used to assess the relationship between blood pressure and patients’ characteristics. Sensitivity and specificity of the current OH criteria to predict orthostatic symptoms were assessed. We also calculated the sensitivity and specificity of systolic, diastolic, and mean BP after 3-min standing to predict orthostatic symptoms by using receiver-operating characteristic (ROC) curves assuming nonparametric conditions22. All tests were two-sided and significance was set at p<0.05. Analyses were performed with Prism 6 (GraphPad Software, USA).
RESULTS
Patients
The total sample consisted of 210 patients who met diagnostic criteria for PD (121 men and 89 women, mean age of 66±11 years). The majority of patients (93%) were receiving anti-parkinsonian medications (L-dopa, MAO-B inhibitors and dopamine agonists). At the time of testing, nine patients (4%) were receiving midodrine (average dose of 20 mg/day) and 22 (10%) were taking fludrocortisone (average dose 0.1 mg/day). The prevalence of supine hypertension was 21% (44 patients) and 25% (52 patients) were being treated with antihypertensive medications. None of the patients lost consciousness during the orthostatic challenge. Table 1 summarizes the demographics, disease characteristics, and orthostatic challenge results.
Table 1.
Sample characteristics and overall orthostatic testing results of PD patients
| Demographic features | |
|---|---|
| n | 210 |
| Age (years) | 66.3 ± 10.6 |
| Gender m:f | 121:89 |
| Disease duration (years) | 6.7 ± 5.6 |
| Hoehn-Yahr score | 2.2 ± 0.8 |
| L-dopa equivalent dose (mg) | 492 ± 449 |
| Treatments, n (%) | |
| None | 15 (7%) |
| L-dopa | 170 (81%) |
| Dopamine agonists | 67 (32%) |
| MAO-B inhibitors | 80 (38%) |
| COMT inhibitors | 34 (16%) |
| Amantadine | 8 (4%) |
| Antihypertensives | 52 (25%) |
| Midodrine | 9 (4%) |
| Fludrocortisone | 22 (10%) |
| Orthostatic testing results | |
| SBP supine (mmHg) | 141.7 ±21.4 |
| DBP supine (mmHg) | 78.9 ± 11.2 |
| MBP supine (mmHg) | 99.9 ± 13.3 |
| HR supine (bpm) | 73.8 ± 9.7 |
| Supine hypertension | 44 (21%) |
| SBP standing 3-min (mmHg) | 122.2 ± 24.2 |
| DBP standing 3-min (mmHg) | 71.1 ± 14.5 |
| MBP standing 3-min (mmHg) | 87.9 ± 17.1 |
| HR standing 3-min (bpm) | 77.6 ± 11.2 |
| OH according to 20/10 mmHg | 105 (50%) |
| OH according to 30/15 mmHg | 64 (30%) |
| Symptomatic OH 20/10 mmHg | 33 (16%) |
| Symptomatic OH 30/15 mmHg | 28 (13%) |
| OH – fall in 20 and 10 mmHg | 43 (20%) |
| OH – fall in 30 and 15 mmHg | 31 (15%) |
| No OH with either criteria | 105 (50%) |
| Δ SBP (mmHg) | −19.4 ± 24.3 |
| Δ DBP (mmHg) | −7.9 ± 12.2 |
| Δ MBP (mmHg) | −11.9 ± 15.7 |
| Δ HR (bpm) | 3.8 ± 7.1 |
SBP: systolic blood pressure; DBP: diastolic blood pressure; MBP: mean blood pressure; HR: heart rate; OH: orthostatic hypotension.
MBP after 3 minutes upright did not differ between head-up tilt and active standing neither in the patients that met the 20/10 nor in those that met the 30/15 OH criteria (78.5±20.2 mmHg vs. 84.4±12.4 mmHg, p=0.09; 74.5±21.2 mmHg vs. 80.1±12.1 mmHg, p=0.29). Here we present data from the whole cohort (i.e., from both tilt and active standing groups).
Prevalence of OH and symptomatic OH
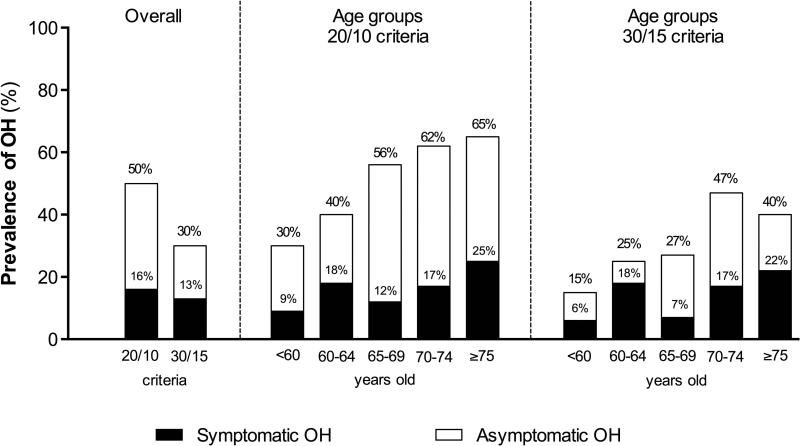
The overall prevalence of OH (with and without symptoms) in the PD cohort was 50% (105 of 210 subjects) when using the 20/10 criteria, and decreased to 30% (64 of 210 subjects) with the 30/15 mmHg criteria (p<0.001, Figure 1). The prevalence of symptomatic OH (i.e., patients who experienced OH and symptoms of hypoperfusion during the orthostatic challenge), however, was much lower: overall only 16% of patients met the 20/10 criteria and had symptoms and 13% met the 30/15 mmHg criteria and were symptomatic (Figure 1). In other words, only 31% of all patients that met the 20/10 OH criteria (33 out of 105) and 44% of those that met the 30/15 mmHg criteria (28 out of 64) had symptoms of hypoperfusion.
Figure 1. Prevalence of orthostatic hypotension.
Overall average shows the percentages of symptomatic and global OH according to each of the two criteria. According to age groups, there is a trend toward increased prevalence of global and symptomatic OH with increasing age regardless of the criteria used.
Blood pressure standing
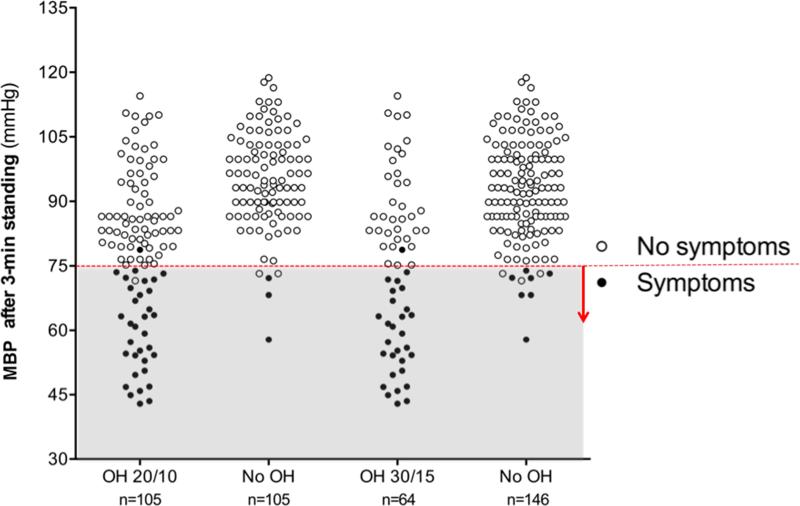
To explore the discrepancy between the high prevalence of OH and the low prevalence of symptoms of OH in PD patients, we evaluated the standing BP. As shown in Figure 2, categorizing patients according to the presence or absence of symptoms revealed a lower standing BP after 3-min standing in the symptomatic group (p<0.001). In the group of patients that met the 20/10 criteria, all but one (out of 33) had symptomatic OH associated with a MBP below 75 mmHg after 3-min upright. Conversely, all but one patient (out of 72) with asymptomatic OH had a MBP above 75 mmHg after 3-min standing. Similarly, in the 30/15 OH group all but one patient (out of 29) with symptomatic OH had a MBP below 75 mmHg after 3-min standing; while all except 3 patients (out of 35) with asymptomatic OH had a MBP after 3-min standing above 75 mmHg.
Figure 2. Mean Blood Pressure after 3-min standing in patients with and without orthostatic hypotension and with and without orthostatic symptoms.
Patients with symptoms had a significantly lower MBP standing than those with symptoms, regardless of the presence of OH (p<0.001). In the vast majority of patients, symptoms appeared when MBP<75 mmHg (denoted with a horizontal dashed line and shaded area). (60.1±10.5 mmHg vs. 89.5±12.3 mmHg in the 20/10 group; 58.4±10.1 mmHg vs. 90.3±11.1 mmHg in the 30/15 group).
Interestingly, we also identified 5 patients who did not have OH by either criteria but still had low MBP standing (i.e., MBP < 75 mmHg after 3-min standing). Of these 5 patients, 3 had orthostatic symptoms (Figure 2). Most of the patients with symptomatic OH after 3-min upright had a SBP below 100 mmHg and a DBP below 60 mmHg (Supplementary Figure 1).
Subjects with asymptomatic OH were more likely to have supine hypertension than those with symptomatic OH (p<0.001, Supplementary Figure 2). Subjects with symptomatic OH tended to have a more pronounced postural fall in mean, systolic and diastolic BP than those with asymptomatic OH (ΔMBP: −35.5±3.2 vs. −16.4±1.1; p<0.001; ΔSBP: −53.1±5.1 vs. −25.4±1.6; p<0.001; ΔDBP: −26.6±2.8 vs. −10.8±8.8; p<0.001). Twenty out of 25 patients taking anti-hypotensive medications (midodrine and/or fludrocortisone) had supine hypertension.
Relationship of OH with age, severity, disease duration and L-dopa dosage
Prevalence of OH and symptomatic OH, with both criteria, increased with age (Figure 1). Patients with OH were older (20/10: 68±10 vs. 64±11 and 30/15: 69±9 vs. 65±11 years old; both p=0.002), had higher MBP supine (20/10: 102±15 vs. 97±10 mmHg, p=0.003; 30/15: 105±16 vs. 97±7 mmHg, p<0.001), and tended to have longer disease duration (20/10: 7.5±0.6 vs. 5.9±0.4 years; p=0.049; 30/15: 7.3±0.8 vs. 5.9±0.5 years; p=0.11).
There were no significant differences in the Hoehn-Yahr stage when patients were divided according to the presence of OH (2.24±0.9 with OH vs. 2.53±0.7 without OH), and of orthostatic symptoms (2±0.9 symptomatic vs. 2±0.8 asymptomatic).
The magnitude of the fall in mean, systolic and diastolic BP was significantly correlated with age (age vs. ΔMBP p<0.001; r=-0.26, age vs. ΔSBP p<0.001; r=−0.30; age vs. ΔDBP p<0.001; r=−0.19). Supine BP also increased with age (age vs. supine MBP p=0.01; r=0.17; age vs. supine SBP p<0.001; r=0.28; age vs. supine DBP p=0.66; r=0.03) but standing MBP after 3-min standing did not (p=0.32). Therefore, the magnitude of the fall in BP increased with age. MBP after 3-min standing was not correlated with disease duration (p=0.67; r=−0.03), or LED (p=0.98; r=0.01).
Sensitivity and specificity to identify symptomatic OH
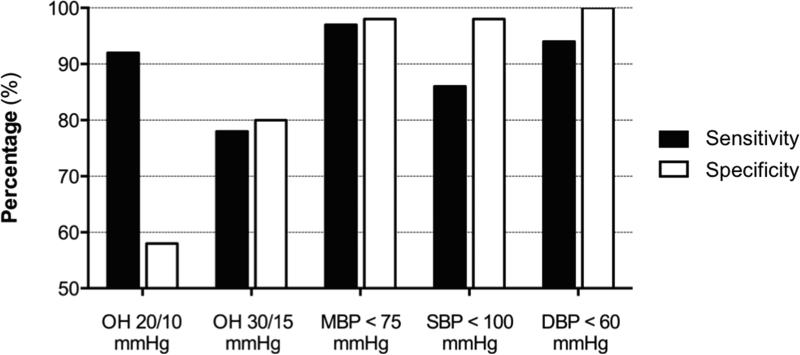
The 20/10 criteria had a sensitivity of 92% and a specificity of 58% to identify patients with orthostatic symptoms. When using the 30/15 criteria, the sensitivity decreased to 78% and the specificity increased to 80%. To calculate the value with the best sensitivity and specificity among systolic, diastolic, and mean BP after 3-min standing we used ROC curves. MBP after 3-min standing had the best-combined sensitivity and specificity (area under the ROC curve of 0.996) followed by DBP after 3-min standing (area under the ROC curve of 0.993) and SBP after 3-min standing (area under the ROC curve of 0.972). Within the MBP after 3-min values, the one with the highest combined sensitivity and specific was MBP < 75 mmHg (sensitivity: 97.2%; specificity: 98.3%). The use of SBP <100 mmHg after 3-min standing had a sensitivity of 86% and a specificity of 98%; When using DBP < 60 mmHg after 3-min standing sensitivity was 94% and specificity was 100%. Overall, the use of MBP < 75 mmHg after 3-min standing seems to provide the best sensitivity and specificity to identify symptomatic patients (Figure 3).
Figure 3. Sensitivity and specificity of different criteria to identify symptomatic orthostatic hypotension.
A MBP below 75 mmHg had the best-combined sensitivity (97.2%) and specificity (98.3%) to predict symptomatic orthostatic hypotension in patients with Parkinson disease.
DISCUSSION
OH diagnostic criteria were not intended as treatment guidelines. Indeed, as shown in this study and others23 current OH criteria overestimate the number of patients with symptomatic OH and therefore are not always useful for therapeutic decision-making. Our results suggest that a clinically useful reference level when considering pharmacologic treatment is a MBP of ~75 mmHg at heart level when upright. This BP is close to the lower limit of cerebral autoregulation,2, 24 and at this level, virtually all patients with PD experienced symptoms. Consistent with previously reported data, the prevalence of OH increased with age, but not significantly with disease duration or L-dopa equivalent dose1, 23, 25.
In line with our results, previous studies found that about one-third of patients with a SBP fall of 60 mmHg (or more) during tilt-table testing were asymptomatic. Although it was concluded that symptoms were not sensitive enough to predict OH,26, 27 these reports did not analyze the BP standing.28 A more likely explanation for their lack of orthostatic symptoms is that these patients, like the ones in our study, may have had a BP standing at or above normal range and therefore, not surprisingly, were asymptomatic, despite fulfilling criteria for OH.
In the upright normal subject, when MBP at heart level is 90-100 mmHg, cerebral MBP is around 60 mmHg, usually the lower level of cerebral autoregulation24. In patients with autonomic failure, including patients with PD29, it is common to observe an enhanced cerebral autoregulatory range30 and, therefore, the MBP required to elicit symptoms might be lower than in healthy individuals. In patients with autonomic failure and longstanding OH, prodromal symptoms are reported to occur when MBP at eye level decreases to 40 mmHg (i.e., MBP~70 mmHg at heart level); and unconsciousness appears at about cerebral MBP of 25 mmHg (i.e., MBP ~55 mmHg at heart level)2, 31. These results are in accordance with our findings as most of our subjects developed symptoms at MBP <75 mmHg at heart level.
Our results indicate that, for clinical decision-making, more relevant than the magnitude of the fall in BP from lying to standing is the actual MBP while standing, a value that determines cerebral perfusion levels and whether symptoms are present. In our sample of PD patients, a MBP of 75 mmHg when standing had a high sensitivity and specificity to identify symptomatic patients. This level, however, might not be exactly the same in all patients and, ideally, should be individually assessed by evaluating the presence of symptoms and if possible by using noninvasive techniques such as transcranial Doppler ultrasonography to measure cerebral blood flow. Ambulatory blood pressure monitoring has also proven valuable in identifying episodes of symptomatic OH that may not be apparent during an office visit and can be therefore useful to tailor treatment.32
Our study has important limitations. The US patient cohort was referred to an autonomic disorders center with suspected OH, and thus a selection bias is likely. In contrast, our European cohort was examined at a movement disorders clinic regardless of the presence of orthostatic intolerance. It is well known that L-dopa has a vasodepressor effect that can contribute to OH33-35. Virtually all of our patients were taking anti-parkinsonian medications and, although we measured OH during a ‘practical off’ situation, some residual effects might have contributed to worsen OH. Conversely, some patients were taking anti-hypotensive agents including midodrine and fludrocortisone, which might have contributed to supine hypertension. In fact, patients taking fludrocortisone had a significantly higher supine MBP compared to those not taking anti-hypotensive medications. Removing those on either treatment did not change the significance of our findings. Also, we did not evaluate delayed OH (i.e., OH after more than 3-minutes after standing), which is thought to be a consequence of milder adrenergic deficits36. The inclusion of patients with delayed OH in our sample would have certainly increased the proportion of OH27, and should be ascertained in further studies. Impaired cognition might be another reason that PD patients are unaware of OH symptoms37. However, this possibility seems unlikely as only patients with normal cognition were selected. Finally, we did not assess cerebral blood flow.
The decision to perform active standing or head-up tilt was based on geographical location. Active standing and passive tilt are not equivalent hemodynamic stressors. Only active standing engages the leg muscles (the “muscle pump”), which compresses capacitance vessels in the legs and aids venous return. Our study was not designed to address the differences between these two testing techniques, although this is an important issue that should be explored. During both active standing and HUT, however, most patients experienced symptoms at the <75 mmHg MBP cut off.
In conclusion, current OH criteria are useful for the diagnosis of autonomic failure but for therapeutic decision-making, it is more relevant how low the BP falls than the magnitude of the fall. Anti-hypotensive pressor agents should be avoided in patients with a normal or high standing BP. Likewise, treatment should be considered in a patient with a standing BP below 75 mmHg, even if the patient does not meet OH criteria. These findings have practical implications for clinical management in PD, and might be applicable to other autonomic synucleinopathies.
Supplementary Material
Supplementary Figure 1. Systolic (A) and diastolic (B) blood pressures after 3-min standing in patients with and without orthostatic hypotension and with and without orthostatic symptoms. In the majority of patients, symptoms appeared when SBP<100 mmHg and DBP<60 mmHg (denoted with horizontal dashed lines).
Supplementary Figure 2. Supine blood pressure in patients with and without orthostatic hypotension. Asymptomatic patients were more likely to have supine hypertension than patients with orthostatic symptoms.
Acknowledgments
Funding source for this work: National Institutes of Health and the Dysautonomia Foundation, Inc.
Footnotes
AUTHORS’ ROLES:
JAP: Project conception and design, data collection, data analysis, review and critique of subsequent drafts.
JCGE: Project conception and design, data collection, data analysis, writing of first draft, review and critique of subsequent drafts.
LNK: Project conception and design, data collection, data analysis, writing of first draft, review and critique of subsequent drafts.
JM: Data collection, data analysis, review and critique of subsequent drafts.
BT: Data collection, data analysis, review and critique of subsequent drafts.
KB: Data collection, data analysis, review and critique of subsequent drafts.
HK: Project conception and design, writing of the first draft, review and critique of subsequent drafts, study supervision.
FINANCIAL DISCLOSURES IN THE LAST 12 MONTHS:
JAP: receives research support from the Dysautonomia Foundation, Inc.
JCGE: None.
LNK: receives research support from the National Institutes of Health (U54NS065736) and the Dysautonomia Foundation, Inc.
JM: receives research support from the Dysautonomia Foundation, Inc.
BT: None.
KB: None.
HK: serves on a scientific advisory board for Lundbeck; serves as
Editor-in-Chief of Clinical Autonomic Research; receives research support from the National Institutes of Health (U54NS065736 [PI]), the FDA (FD-R-3731-01 [PI]), and the Dysautonomia Foundation, Inc.
REFERENCES
- 1.Velseboer DC, de Haan RJ, Wieling W, Goldstein DS, de Bie RM. Prevalence of orthostatic hypotension in Parkinson's disease: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2011;17(10):724–729. doi: 10.1016/j.parkreldis.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wieling W, Thijs RD, van Dijk N, Wilde AA, Benditt DG, van Dijk JG. Symptoms and signs of syncope: a review of the link between physiology and clinical clues. Brain. 2009;132(Pt 10):2630–2642. doi: 10.1093/brain/awp179. [DOI] [PubMed] [Google Scholar]
- 3.Masaki KH, Schatz IJ, Burchfiel CM, et al. Orthostatic hypotension predicts mortality in elderly men: the Honolulu Heart Program. Circulation. 1998;98(21):2290–2295. doi: 10.1161/01.cir.98.21.2290. [DOI] [PubMed] [Google Scholar]
- 4.Mtinangi BL, Hainsworth R. Effects of moderate exercise training on plasma volume, baroreceptor sensitivity and orthostatic tolerance in healthy subjects. Exp Physiol. 1999;84(1):121–130. doi: 10.1111/j.1469-445x.1999.tb00077.x. [DOI] [PubMed] [Google Scholar]
- 5.Bonnin P, Ben Driss A, Benessiano J, Maillet A, Pavy le Traon A, Levy BI. Enhanced flow-dependent vasodilatation after bed rest, a possible mechanism for orthostatic intolerance in humans. Eur J Appl Physiol. 2001;85(5):420–426. doi: 10.1007/s004210100483. [DOI] [PubMed] [Google Scholar]
- 6.Izcovich A, Gonzalez Malla C, Manzotti M, Catalano HN, Guyatt G. Midodrine for orthostatic hypotension and recurrent reflex syncope: A systematic review. Neurology. 2014 doi: 10.1212/WNL.0000000000000815. [DOI] [PubMed] [Google Scholar]
- 7.Kaufmann H, Freeman R, Biaggioni I, et al. Droxidopa for neurogenic orthostatic hypotension: A randomized, placebo-controlled, phase 3 trial. Neurology. 2014 doi: 10.1212/WNL.0000000000000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jankovic J, Gilden JL, Hiner BC, et al. Neurogenic orthostatic hypotension: a double-blind, placebo-controlled study with midodrine. Am J Med. 1993;95(1):38–48. doi: 10.1016/0002-9343(93)90230-m. [DOI] [PubMed] [Google Scholar]
- 9.Hohler AD, Amariei DE, Katz DI, et al. Treating orthostatic hypotension in patients with Parkinson's disease and atypical Parkinsonism improves function. J Parkinsons Dis. 2012;2(3):235–240. doi: 10.3233/JPD-2012-012101. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein DS, Holmes CS, Dendi R, Bruce SR, Li ST. Orthostatic hypotension from sympathetic denervation in Parkinson's disease. Neurology. 2002;58(8):1247–1255. doi: 10.1212/wnl.58.8.1247. [DOI] [PubMed] [Google Scholar]
- 11.Kaufmann H, Biaggioni I. Autonomic failure in neurodegenerative disorders. Semin Neurol. 2003;23(4):351–363. doi: 10.1055/s-2004-817719. [DOI] [PubMed] [Google Scholar]
- 12.Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. JAMA. 1997;277(13):1046–1051. [PubMed] [Google Scholar]
- 13.Mazza A, Ravenni R, Antonini A, Casiglia E, Rubello D, Pauletto P. Arterial hypertension, a tricky side of Parkinson's disease: physiopathology and therapeutic features. Neurol Sci. 2013;34(5):621–627. doi: 10.1007/s10072-012-1251-2. [DOI] [PubMed] [Google Scholar]
- 14.Berganzo K, Diez-Arrola B, Tijero B, et al. Nocturnal hypertension and dysautonomia in patients with Parkinson's disease: are they related? J Neurol. 2013;260(7):1752–1756. doi: 10.1007/s00415-013-6859-5. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res. 1996;6(2):125–126. doi: 10.1007/BF02291236. [DOI] [PubMed] [Google Scholar]
- 16.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21(2):69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 17.Leu-Semenescu S, Roze E, Vidailhet M, et al. Myoclonus or tremor in orthostatism: an under-recognized cause of unsteadiness in Parkinson's disease. Mov Disord. 2007;22(14):2063–2069. doi: 10.1002/mds.21651. [DOI] [PubMed] [Google Scholar]
- 18.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25(15):2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 20.Kaufmann H, Malamut R, Norcliffe-Kaufmann L, Rosa K, Freeman R. The Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scale. Clin Auton Res. 2012;22(2):79–90. doi: 10.1007/s10286-011-0146-2. [DOI] [PubMed] [Google Scholar]
- 21.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45(1):142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 22.Bewick V, Cheek L, Ball J. Statistics review 13: receiver operating characteristic curves. Crit Care. 2004;8(6):508–512. doi: 10.1186/cc3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ha AD, Brown CH, York MK, Jankovic J. The prevalence of symptomatic orthostatic hypotension in patients with Parkinson's disease and atypical parkinsonism. Parkinsonism Relat Disord. 2011;17(8):625–628. doi: 10.1016/j.parkreldis.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 24.Hainsworth R. Pathophysiology of syncope. Clin Auton Res. 2004;14(Suppl 1):18–24. doi: 10.1007/s10286-004-1004-2. [DOI] [PubMed] [Google Scholar]
- 25.Szewczyk-Krolikowski K, Tomlinson P, Nithi K, et al. The influence of age and gender on motor and non-motor features of early Parkinson's disease: Initial findings from the Oxford Parkinson Disease Center (OPDC) discovery cohort. Parkinsonism Relat Disord. 2014;20(1):99–105. doi: 10.1016/j.parkreldis.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 26.Arbogast SD, Alshekhlee A, Hussain Z, McNeeley K, Chelimsky TC. Hypotension unawareness in profound orthostatic hypotension. Am J Med. 2009;122(6):574–580. doi: 10.1016/j.amjmed.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 27.Jamnadas-Khoda J, Koshy S, Mathias CJ, Muthane UB, Ragothaman M, Dodaballapur SK. Are current recommendations to diagnose orthostatic hypotension in Parkinson's disease satisfactory? Mov Disord. 2009;24(12):1747–1751. doi: 10.1002/mds.22537. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein DS, Pechnik S, Holmes C, Eldadah B, Sharabi Y. Association between supine hypertension and orthostatic hypotension in autonomic failure. Hypertension. 2003;42(2):136–142. doi: 10.1161/01.HYP.0000081216.11623.C3. [DOI] [PubMed] [Google Scholar]
- 29.Niehaus L, Bockeler GC, Kupsch A, Meyer BU. Normal cerebral hemodynamic response to orthostasis in Parkinson's disease. Parkinsonism Relat Disord. 2002;8(4):255–259. doi: 10.1016/s1353-8020(01)00014-1. [DOI] [PubMed] [Google Scholar]
- 30.Horowitz DR, Kaufmann H. Autoregulatory cerebral vasodilation occurs during orthostatic hypotension in patients with primary autonomic failure. Clin Auton Res. 2001;11(6):363–367. doi: 10.1007/BF02292768. [DOI] [PubMed] [Google Scholar]
- 31.Henry JP, Gauer OH, Kety SS, Kramer K. Factors maintaining cerebral circulation during gravitational stress. J Clin Invest. 1951;30(3):292–300. doi: 10.1172/JCI102443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norcliffe-Kaufmann L, Kaufmann H. Is ambulatory blood pressure monitoring useful in patients with chronic autonomic failure? Clin Auton Res. 2014;24(4):189–192. doi: 10.1007/s10286-014-0229-y. [DOI] [PubMed] [Google Scholar]
- 33.Calne DB, Brennan J, Spiers AS, Stern GM. Hypotension caused by L-dopa. Br Med J. 1970;1(5694):474–475. doi: 10.1136/bmj.1.5694.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouhaddi M, Vuillier F, Fortrat JO, et al. Impaired cardiovascular autonomic control in newly and long-term-treated patients with Parkinson's disease: involvement of L-dopa therapy. Auton Neurosci. 2004;116(1-2):30–38. doi: 10.1016/j.autneu.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Wolf JP, Bouhaddi M, Louisy F, et al. Side-effects of L-dopa on venous tone in Parkinson's disease: a leg-weighing assessment. Clin Sci (Lond) 2006;110(3):369–377. doi: 10.1042/CS20050247. [DOI] [PubMed] [Google Scholar]
- 36.Gibbons CH, Freeman R. Delayed orthostatic hypotension: a frequent cause of orthostatic intolerance. Neurology. 2006;67(1):28–32. doi: 10.1212/01.wnl.0000223828.28215.0b. [DOI] [PubMed] [Google Scholar]
- 37.Freidenberg DL, Shaffer LE, Macalester S, Fannin EA. Orthostatic hypotension in patients with dementia: clinical features and response to treatment. Cogn Behav Neurol. 2013;26(3):105–120. doi: 10.1097/WNN.0000000000000003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Systolic (A) and diastolic (B) blood pressures after 3-min standing in patients with and without orthostatic hypotension and with and without orthostatic symptoms. In the majority of patients, symptoms appeared when SBP<100 mmHg and DBP<60 mmHg (denoted with horizontal dashed lines).
Supplementary Figure 2. Supine blood pressure in patients with and without orthostatic hypotension. Asymptomatic patients were more likely to have supine hypertension than patients with orthostatic symptoms.