Abstract
Attention problems are among the most impairing features associated with fragile X syndrome (FXS). However, few studies have examined behavioral development of inhibitory control in very young children with FXS. We examined attentional control in 3-6 year boys with FXS using both an experimental inhibitory control paradigm and parent-report of attention problems. Study 1 examined attentional control in FXS compared to comparison groups matched on chronological and mental age. To determine the stability of impairments over time in FXS, Study 2 examined patterns of developmental change in an expanded longitudinal sample. Across studies, males with FXS demonstrated persistent impairments in inhibitory control and parent-reported attention problems. Inhibitory control was related to, but not solely driven by, lower mental age. Although parent-rated attention problems remained stable across ages, inhibitory control improved with time. Children with more severe attention problems often displayed initially poorer inhibitory control. However, these trajectories also improved more rapidly with age. Our findings indicate that despite persistent deficits in attentional control in young children with FXS, multi-method assessment can be used to capture developmental growth that should be further supported through early, targeted intervention.
Keywords: Fragile X syndrome (FXS), attention, inhibitory control, longitudinal, snack delay
1. Introduction
Fragile X syndrome is the most common heritable form of intellectual disability, affecting 1:4,000 – 6,000 individuals. Fragile X is caused by a CGG repeat expansion on the promotor region of the FMR1 gene, resulting in reduced production of fragile X mental retardation protein necessary for mRNA transcription and synaptic plasticity. Among males with FXS, over 90% are diagnosed with comorbid conditions such as attention problems, anxiety, and autism symptomatology (Bailey, Raspa, Olmsted, & Holiday, 2008; Sullivan et al., 2006). In light of these high rates of behavior problems and the known genetic mechanisms of FXS, studying developmental psychopathology within FXS lends insight into complex interactions among genetics, experience, and behavior.
Attention problems are among the earliest and most impairing features associated with FXS, presenting in early infancy and toddlerhood (Cornish, Scerif, & Karmiloff-Smith, 2007; Roberts, Hatton, Long, Anello, & Colombo, 2011) and potentially “constraining” later neurocognitive development (Cornish, Cole, Longhi, Karmiloff-Smith, & Scerif, 2012). Attention involves multiple dimensions; including orienting, maintenance and regulation; which are reflected at both behavioral and neurocognitive levels. Behavioral indicators are often reported in terms of meeting criteria for an attention-related disorder such as attention deficit hyperactivity disorder (ADHD). In FXS, 54-74% of males meet behavioral ADHD diagnostic criteria and over 80% receive attention-related diagnoses or treatment (Backes et al., 2000; Bailey et al., 2012, 2008; Sullivan et al., 2006). Although children with FXS are reported to exhibit both inattentive and hyperactive symptoms (Bailey et al., 2008; Sullivan et al., 2006; Wheeler et al., 2014), within-group analyses suggest greater deficits in inattentive compared to hyperactive and oppositional symptoms in school-aged children (Cornish et al., 2012). As measured by behavior rating scales, attention symptoms in FXS are relatively stable in childhood (Cornish et al., 2012; Hatton et al., 2002) but appear to decrease into late adolescence and adulthood (Wheeler et al., 2014), consistent with findings of decreased attention-related medication use in adults versus children (Bailey et al., 2012).
Importantly, profiles of behavioral attention problems in school-aged children with FXS have been linked to early neurocognitive deficits within the FXS phenotype, with particular deficits noted in inhibitory control (Cornish et al., 2012; Scerif, Longhi, Cole, Karmiloff-Smith, & Cornish, 2012). Inhibitory control is defined as the ability to inhibit a prepotent or predominant response (Barkley, 1997), a skill that develops across childhood and into adulthood with maturation of ventral fronto-striatal circuitry (Durston et al., 2002). Although inhibitory control improves with age, inhibitory control abilities of children with FXS relative to same-aged peers remains relatively stable across childhood (Kochanska, Murray, & Coy, 1997), thus children with early impairments are likely to also experience continued difficulties later in life. Inhibitory control is central to a variety of daily tasks during the preschool period, such as taking turns during games and listening to a complete question before answering. Unsurprisingly, children who fail to inhibit predominant responses in these types of situations are vulnerable to poorer academic and socio-emotional experiences, as inhibition is critical to participation in both academic learning environments and healthy social networks (Blair & Razza, 2007; Kochanska et al., 1997).
The intersection of inhibitory control and both academic and socio-emotional functioning is particularly relevant for individuals with FXS who often exhibit cooccurring attention, learning, and socio-emotional challenges (Bailey et al., 2008). Previous attention studies suggest school-age children with FXS exhibit poor inhibition through greater errors on tasks that require sustaining or switching attention (Cornish et al., 2007; Scerif et al., 2012; Sullivan et al., 2007), even in comparison to children with Down syndrome (Cornish et al., 2007; Munir, Cornish, & Wilding, 2000), Williams syndrome (Cornish et al., 2007), and Prader Willi syndrome (Woodcock, Oliver, & Humphreys, 2009). These cross-syndrome comparisons indicate a relatively specific vulnerability to poor inhibitory control within the FXS phenotype contrasted to other neurodevelopmental disorders. These deficits likely emerge early in development, as toddlers with FXS often perseverate on previously selected items during visual search activities (Scerif, Cornish, Wilding, Driver, & Karmiloff-Smith, 2004, 2007) and show greater numbers of reflexive saccades on inhibitory visual saccade tasks (Cornish et al., 2007; Scerif et al., 2005). Given the pervasive nature and early emergence of these impairments, it is likely that poor inhibitory control contributes to cascading academic and socio-emotional challenges associated with FXS (Cornish et al., 2012).
A number of important advances have improved our understanding of the nature and timing of these inhibitory control deficits. First, contrasting attention in FXS to various comparison groups has informed understanding of the underlying mechanisms and specificity of attention deficits. Cross-sectional studies in young children have contrasted FXS groups to children without known genetic conditions, matched on both chronological and mental age (Roberts et al., 2011; Scerif et al., 2005, 2004, 2007), supporting conclusions that attentional control is atypical based on chronological age, yet impairments are not solely driven by lower intellectual abilities. In addition, cross-syndrome comparisons suggest that specific attention-related deficits appear unique to FXS, indicating mores severe inhibitory control impairments in FXS compared to other neurodevelopmental disorders (Cornish et al., 2007; Munir et al., 2000; Woodcock et al., 2009). In addition to the rich information afforded by multiple matching strategies, there has been increased emphasis on longitudinal characterization of attentional trajectories across childhood, consistent with evidence that disorders emerge from complex, nonlinear influences rather than global, static impairments (Karmiloff-Smith, 2009). Several recent studies in FXS have identified developmental changes in attentional control that were only detectable using longitudinal data (Cornish et al., 2012; Cornish, Cole, Longhi, Karmiloff-Smith, & Scerif, 2013; Scerif et al., 2012), highlighting the critical importance of capturing within-individual patterns of change over time.
It is increasingly recognized that early detection and treatment of attention problems holds promise for maximizing positive outcomes (Halperin, Bédard, & Curchack-Lichtin, 2012). Given the critical role of attention and inhibitory control in academic and socio-emotional development (Blair & Razza, 2007; Kochanska et al., 1997) and its known impairments in FXS (Cornish et al., 2007; Scerif et al., 2012; Sullivan et al., 2007), early interventions are likely to improve outcomes in young children with FXS. However, tailoring interventions to this population first requires understanding of the developmental course of attentional control during early childhood. Although several comprehensive, longitudinal studies of attention have been conducted in children with FXS (Cornish et al., 2012: FXS n=48, x̄ initial age=8.17 years; Cornish et al., 2013: FXS n=21, x̄ initial=8.75 years; Roberts et al., 2011: FXS n=13, initial= 9-12m; Scerif et al., 2012: FXS n=21, x̄ initial=8.6 years), studies focused in early childhood have predominantly examined attentional phenotype through cross-sectional comparisons, most commonly using computerized visual attention tasks rather than behavioral measures (Cornish et al., 2007; Scerif et al., 2005, 2004, 2007). To inform the timing and nature of early attentional deficits, as well as potential targets for early intervention, additional work is needed to characterize longitudinal changes in behavioral inhibitory control during the preschool period.
The present pair of studies addressed this need by employing two methods of attentional control measurement – performance on a behavioral inhibitory control task and parent-reported attention problems – in preschool-aged children with FXS. To inform whether potential impairments are driven by mental age, Study 1 examined whether young males with FXS display impaired attentional control compared to non-FXS controls matched on either chronological or mental age. To determine the stability of inhibitory control impairments over time, Study 2 examined patterns of change in an expanded longitudinal sample of males with FXS.
2. Study 1
Study 1 examined group differences in multiple indicators of attentional control in males with FXS and unaffected controls, matched on either chronological age (CA) or mental age (MA). We hypothesized that children with FXS would show impairments in attentional control across multiple measures and matching groups, indicating impairments in inhibitory control are not solely driven by intellectual impairment.
2.1 Methods
2.1.1 Participants
Participants included 14 males with FXS and two control groups matched on mean CA (n=14) and MA (n=14). Informed consent and study procedures were approved by the Institutional Review Board at XX. As exhibited in Table 1, the FXS group exhibited a wider range of CA and MAs than the respective matched groups. Within the CA-match group, 3 parents did not return attention problem questionnaires. Three males with FXS took medication on assessment day (1 stimulant, 1 sympathalytic and antipsychotic, 1 antipsychotic only). No TD controls were on medications.
Table 1.
Study 1 Demographic Data
| FXS | CA Match | MA Match | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | Median | SD | Min | Max | N | Mean | Median SD | Min | Max | N | Mean | Median | SD | Min | Max | ||
| Age in Years | 14 | 4.05 | 3.99 | 0.93 | 2.88 | 5.84 | 14 | 4.17 | 4.06 0.30 | 3.86 | 4.82 | 14 | 2.93 | 2.94 | 0.10 | 2.77 | 3.12 | |
| aMental Age | 14 | 2.49 | 2.32 | 0.51 | 2.04 | 3.79 | 14 | 4.42 | 4.43 0.63 | 3.35 | 5.50 | 14 | 2.77 | 2.76 | 0.18 | 2.44 | 3.02 | |
| bAttention Raw | 14 | 5.21 | 5 | 2.01 | 3 | 9 | 11 | 2.00 | 2 1.36 | 0 | 4 | 14 | 1.71 | 2 | 1.77 | 0 | 5 | |
| bAttention T | 14 | 62.43 | 62 | 8.22 | 53 | 77 | 11 | 52.18 | 51 2.71 | 50 | 57 | 14 | 52.36 | 51 | 3.69 | 50 | 62 | |
| cProportion Failed Trials | 14 | 0.27 | 0.17 | 0.30 | 0.00 | 0.67 | 14 | 0.00 | 0.00 0.00 | 0.00 | 0.00 | 14 | 0.05 | 0.00 | 0.18 | 0.00 | 0.67 | |
Mullen Scales of Early Learning (Mullen, 1995)
Child Behavior Checklist (Achenbach & Rescorla, 2000)
Proportion of failed trials within the snack delay task. Participants who passed all 6 trials “passed” the overall task.
2.1.2 Procedures
Across both studies, data were collected as part of a larger assessment battery. The inhibitory control task and developmental testing were conducted in participants’ homes, and parent rating scales were collected at the assessment.
2.1.3 Measures
Inhibitory control
The Snack Delay episode from the Laboratory Temperament Assessment Battery – Preschool Version (Lab-TAB; (Goldsmith & Rothbart, 1996) requires the participants to wait for a signal before eating a snack placed in front of them for six trials, with the “wait time” for the signal changing for each trial (5s, 10s, 0s, 20s, 0s, 30s). Coders rated three dependent variables off-line: proportion of failed trials, fidgeting, and distractibility. Interrater reliability over 20% of assessments was κ = .82. Due to low variability in scores within the TD group, proportion of failed trials were dichotomized as failing no trials (“passing” the task) versus failing any trials (“failing” the task) to facilitate group comparisons. Fidgeting and distractibility were rated for each trial then averaged across trials. The Lab-TAB has been previously used in clinical groups such as FXS (Roberts et al., 2011).
Broad Attention Problems
The Child Behavior Checklist 1.5-5 (CBCL (Achenbach & Rescorla, 2000) is a widely used 118 item parent rating scale used to assess child problem behavior. Items are answered using a 3-point Likert scale of “not true” (0), “somewhat or sometimes true” (1), and “very true” (2). Scores are aggregated into symptom-specific scales, composite scores, and DSM scales consistent with Diagnostic and Statistical Manual (DSM-IV) diagnoses. On the syndrome scales, T-scores between 65-69 indicate elevated risk, and T-scores > 70 are considered clinically significant. The present study focused on the Attention Problems subscale, although we also report item-level ratings on all 8 attention-related items on the CBCL. Consistent with publisher recommendations, analyses were conducted using raw scores to minimize floor effects.
Mental age
The Mullen Scales of Early Learning (MSEL; (Mullen, 1995) is a play-based developmental assessment that includes five domains, four of which were averaged to derive an overall age equivalent: fine motor, visual reception, receptive language, expressive language, gross motor (not included). The MSEL has excellent interrater reliability (>.90), and high correspondence to other developmental measures such as the Bayley's Mental Development Index (r = .70).
2.2. Analyses and Results
Due to the small sample size and non-normal distribution of scores, we used nonparametric methods. Fisher's exact test was used to compare groups on the inhibitory control task and to examine medication effects, and Wilcoxon two-sample exact tests were used to compare groups on fidgeting and distractibility, with effect sizes estimated as . We next examined parent reported attention problems at both the subscale and item levels. First, we used Wilcoxon two-sample exact tests to examine group differences parent-rated attention problems. Because the latent structure of CBCL subscales has not been examined in FXS, we also examined group differences in item-level ratings by employing a series of Fisher's Exact Tests with an adjusted α= 0.05/8=.006. Each analysis examined group differences in the proportion of participants with elevated (>1) versus non-elevated (0) scores, consistent with psychometric analyses conducted by the scale publishers (Achenbach & Rescorla, 2000).
2.2.1 Inhibitory Control
The FXS group failed the task at higher rates than both CA and MA controls (p <.001; CA Cramer's V=.63; MA V=.53), with overall task failure rates of 57% in the FXS group, 0% in CA controls, and 7% in MA controls. Proportion of failed trials within the task are listed in Table 1. The FXS group did not differ from CA controls in fidgeting (Z=0.16, p =.43, r =.03) or distractibility (Z=0.02, p=.48, r=.003). Compared to MA controls, the FXS group exhibited greater fidgeting behaviors (Z=1.98, p =.02, r =.37) but comparable distractibility (Z=.49, p=.31, r=.09).
Of the 3 participants with FXS who were on medication, task failure rates were similar to the broader sample (66%; V=.10; p=.62); in addition, there were no statistical differences in levels of fidgeting (Z=1.35, p=.10, r=.36) or distractibility (Z=0.07, p=.49, r=.02). Thus although the effect size of medication use on fidgeting was moderate, the effect was not statistically significant, and medication use and nonuse did not account for within-group variability in task performance.
2.2.2 Broad Attention Problems
Parents rated the FXS group as displaying greater attention problems compared to both CA (Z=3.84, p=<.001, r=.73) and MA controls (Z=3.70, p<.001, r=.70). No participants in the CA or MA groups were rated as having CBCL scores in the “at risk” or “clinically significant” ranges, whereas 5 participants with FXS (36%) received elevated scores (2 “at risk,” 3 “clinically significant”). Medication use was not associated with different levels of attention problems (Z=0.47, p=.32, r=.12). Table 2 lists tem rating frequencies and Fisher's exact test comparisons. Controlling for multiple comparisons, the FXS group was rated as having poorer concentration, greater clumsiness, and more difficulty sitting still compared to MA controls. These group differences produced large effects (.53<V<.66).
Table 2.
CBCL Item Analyses by Group
| FXS (n=14) | CA Match (n=11) | MA Match (n=14) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Abbreviated Item Description | DSM ADHD | Attn. Problems | % elev. | % elev | Fisher p | Cramer's V | % elev | Fisher p | Cramer's V |
| 5. Can't concentrate | X | X | 93 | 45 | .01 | .52 | 29 | <.001 | .66 |
| 6. Can't sit still | X | X | 93 | 55 | .04 | .44 | 36 | .002 | .60 |
| 8. Can't stand waiting | X | 100 | 80 | .16 | .36 | 71 | .05 | .41 | |
| 16. Demands must be met | X | 86 | 73 | .38 | .16 | 57 | .10 | .32 | |
| 36. Gets into things | X | 64 | 27 | .07 | .37 | 43 | .22 | .21 | |
| 56. Clumsy | X | 57 | 9 | .02 | .49 | 7 | .006 | .53 | |
| 59. Quickly shifts | X | X | 100 | 82 | .18 | .33 | 57 | .008 | .52 |
| 95. Wanders away | X | 36 | 0 | .04 | .44 | 29 | .50 | .08 | |
Note. One-tailed Fisher's exact tests were used to compare proportion of groups scoring 0 (“Not True”) versus 1 or 2 (“Somewhat/Sometimes, Very Often/Often True”). Results are compared to adjusted α = .006.
3. Study 2
To inform the developmental course of attentional control in FXS, Study 2 examined longitudinal changes in both experimental and parent-reported attentional control in an expanded sample of young males with FXS. This design permitted consideration of within- and between-individual patterns of change across 33-72 months (MAs 12-62m). We also examined interrelationships between these measures over time. Consistent with previous studies of attention in FXS, we hypothesized that although inhibitory control would improve across early childhood, parent-rated attention problems would remain stable.
3.1 Methods
3.1.1 Participants and Measures
Behavioral and parent-report data were collected from 41 participants with FXS on 1 to 3 occasions each, yielding 84 observations across participants (one observation n=14, 2 n=11, 3 n=16; Table 3). All participants from Study 1 were also included in Study 2, and procedures were identical across studies. Although Study 1 dichotomized Snack Delay performance as “pass” or “fail” due to low variability in scores in the TD group, Study 2 examined proportion of failed trials within the task to better capture nuanced change over time within FXS. Although medication use varied within participants over time, the proportion of assessments in which participants were on medication (20%) was similar to Study 1. Parent ratings of behavioral symptoms were available for 27 TD controls, 13 with two assessments. The average age of the FXS group was approximately 10 months older than the TD group (FXS range 33-7 2m, TD 33-58 m) to permit CA and MA comparisons in Study 1. Although the primary focus of Study 2 was to examine within-syndrome change in our FXS sample, we included data from TD controls in a subset of analyses as an approximated point of reference.
Table 3.
Study 2 Demographic Data across Assessments
| FXS (n=41, 84 observations) | Control (n=27, 37 observations) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | Median | SD | Min | Max | N | Mean | Median | SD | Min | Max | |
| Age in Years | 84 | 4.39 | 4.41 | 1.02 | 2.74 | 6.03 | 37 | 3.46 | 3.33 | 0.55 | 2.77 | 4.82 |
| aMental Age | 82 | 2.24 | 2.15 | 0.81 | 0.96 | 5.15 | 37 | 3.60 | 3.48 | 0.83 | 2.44 | 5.50 |
| bAttention Raw | 84 | 5.94 | 6 | 2.11 | 2 | 10 | 37 | 2.00 | 2 | 1.60 | 0 | 6 |
| bAttention T | 84 | 65.43 | 67 | 8.30 | 51 | 80 | 37 | 52.48 | 51 | 3.80 | 50 | 67 |
| cProportion Failed Trials | 84 | 0.60 | 0.6 | 0.07 | 0.50 | 0.76 | 37 | .02 | 0 | .01 | 0 | .67 |
Mullen Scales of Early Learning (Mullen, 1995)
Child Behavior Checklist (Achenbach & Rescorla, 2000)
Proportion of failed trials within the snack delay task. Participants who passed all 6 trials “passed” the overall task.
3.2 Analyses
We first examined behavioral and parent-report data in the FXS group only. Data were analyzed using multilevel modeling in SAS 9.3. Prior to analyses, behavioral and parent-report data were log transformed to reduce skew. Models using non-transformed data yielded similar results and are reported for interpretability. Across observations, the internal consistency for Attention Problems subscales was α=.81. Chronological and MA were centered at the mean of the FXS sample (52.66 months and 26.86 months, respectively). Variance and covariance matrices were set as unstructured, and degrees of freedom were calculated using the between/within method.
First, unconditional mean and growth models were used to estimate levels and change in proportion of failed trials across CA. We then added MA as a time-variant predictor of both mean levels and change in behavior. Effects of MA on change were signified by interaction between mental and CA. To examine whether parent-reported symptoms corresponded with inhibitory control mean levels and change over time, we next included parent-reported attention problems as level two time-variant predictors, controlling for MA. We did not examine longitudinal change in the TD group because only 1 participant across 40 observations failed any task trials.
We next examined mean levels and change in parent-reported symptoms of the FXS group over time, then examined the effects of MA on both levels and change parameters. Although not a primary focus of Study 2, parent-reported symptom data were also analyzed for the TD group. We estimated linear growth parameters of these participants, centered at the mean of the FXS group (53 months) as an approximated point of comparison to our FXS sample. Groups were not compared in the same model due to the differences in data structure across samples, as well as our primary theoretical focus on within-syndrome variability in FXS.
3.3 Results
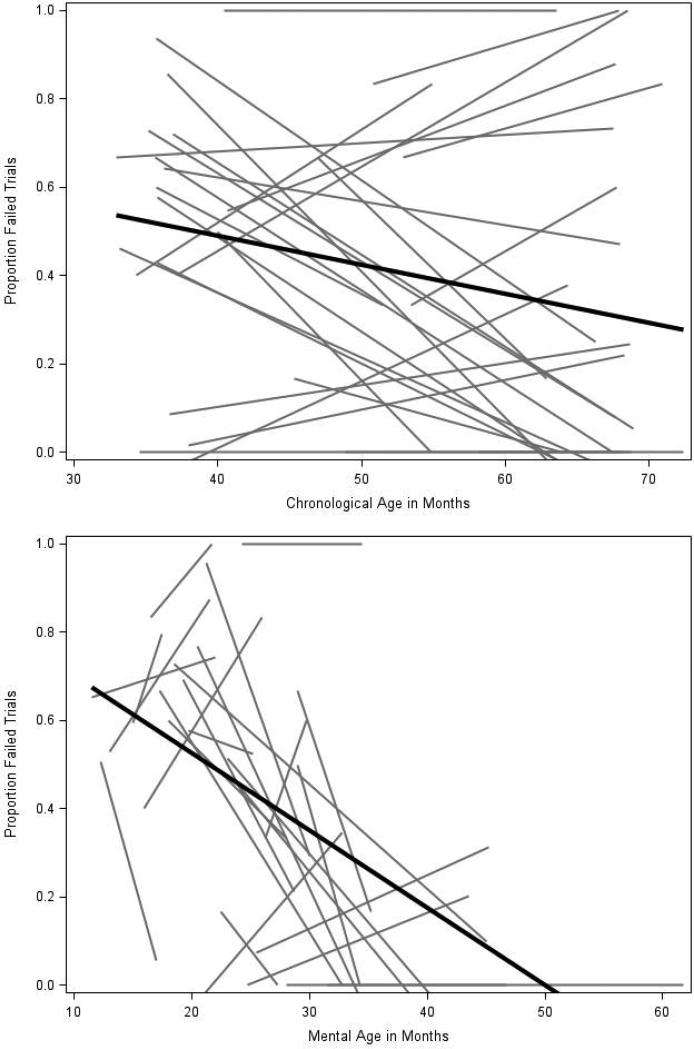
At the mean age of the sample, the average proportion of failed trials in the FXS group was 41% and decreased (improved) by .60% per month. When MA was added to the model, higher MA predicted lower proportion of failed trials across ages, and the main effect of CA was no longer significant. The effect of MA on task performance did not vary across CA. The inclusion of MA explained 42% of variability in mean proportion of failed trials and 8% of variability in slopes. Figure 1 depicts longitudinal changes in task performance across CA (1a) and MA (1b). Parent-reported attention problems differentially related to changes in behavioral performance over time. Specifically, participants who were rated as having less severe attention problems also demonstrated less change in behavioral performance over time, whereas those with greater attention problems exhibited initially poorer task performance that improved more rapidly with age. Compared to the unconditional model with MA intercept included, the added effects of Attention Problems scores accounted for 25% of variability in means and 34% of variability in slopes.
Figure 1.
Longitudinal changes in failed trials in FXS across chronological and mental age
We next examined parent-reported symptoms in the FXS group only. At the mean age of the sample, the average raw score on the Attention Problems subscale was 5.81 (out of 10) and did not change significantly over time (β=.02, p=.12). Mental age did not predict mean levels or change in scores. As a point of comparison, average raw score of the TD group was 2.03 and was stable over time (β=.04, p=.37).
4. Discussion
The present pair of studies used multiple methods to characterize the emergence and stability of attentional control in preschool males with FXS. The attention deficits we observed in our sample were striking in several ways. First, they emerged across multiple measures at the earliest age we assessed, with many participants displaying deficits by 3 years of age. Notably, 36% of boys with FXS received parent-rating scores in the “at risk” or “clinical” ranges, reflecting average problem scores that were nearly 3 times higher for FXS versus TD groups, and 93% were rated as unable to concentrate or to sit still. Second, the attention deficits were not accounted for by mental age or medication use. Third, behavioral inhibition showed a slight improvement over time, whereas global parent reported attention problems remained stable. These results suggest that preschool-aged children with FXS demonstrate early emerging, severe and persistent inhibitory control impairments and attention problems. Furthermore, our longitudinal analyses reveal patterns of developmental improvement that were not captured by cross-sectional comparisons, highlighting the importance of longitudinal designs. These findings are important to inform developmentally-sensitive treatment strategies in FXS, and to understand the intersection of individual differences and phenotypic stability in this population.
Our findings noting the high prevalence of parent reported attention problems in young boys with FXS are generally consistent with previous work using larger and older-aged samples. We report a prevalence rate of attention problems, as measured by the CBCL, in 36% in our sample which is, on average, 4 years old. Sullivan and colleagues (2006) and Hatton and colleagues (2002) reported prevalence rates of 54%-56% in males with average ages of 10 and 7 years, respectively. Although the higher prevalence of attention problems in the older aged samples suggests an increase in severity over time, both our and Hatton's longitudinal studies reported stability in ratings, with no increase noted within each respective sample. This pattern is likely due to the truncated age span and relatively small sample size in our current study. Alternatively, it is also possible that attention problems are sufficiently elevated by preschool, thus further elevations may be subtle yet still clinically meaningful. Additional longitudinal investigations that track the developmental trajectory of parent reported attention problems across a wider age-span are needed to address these questions.
Item-level analyses of CBCL data contextualized our scale-level analyses and suggested concentration, sitting still, and clumsiness as particular problem areas in our sample. These data provide new information about specific areas of concern in young males with FXS, as previous work focused on the subscale level (Hatton et al., 2002; Sullivan et al., 2006). It is possible that parent reports of clumsiness may relate to motor skill deficits related to the FXS phenotype rather than ADHD-related symptoms. However, clumsiness ratings did not appear to have solely driven effects observed in our data, as the FXS group received higher scores than MA controls on the majority of Attention Problems items. However given the variability of item-level performance in our sample, factor analysis could help determine whether latent structure of common problem behavior rating scales, such as the CBCL, are similar in FXS and non-FXS samples.
In addition to informing patterns of inhibitory control and parent-reported problem behaviors, we identified developmental associations between these constructs within the FXS sample. Specifically, children with less severe parent-rated attention problems displayed flatter trajectories of change in Snack Delay performance over time, whereas those with more severe parent-rated attention problems often displayed the poorest initial performance but improved more rapidly with age. One interpretation of this finding is that broader attention problems may reflect early compounding issues associated with poor attentional control, reflected by poorer initial performance on the inhibitory control task rather than moderately low task performance over time. For example, early attentional deficits may limit opportunities to learn from and engage in the environment, restricting developmental growth and increasing risk for further behavioral problems. . Indeed, previous studies have indicated similar age-related associations between early behavioral inhibition and anxiety-related problem behaviors in preschoolers with FXS (Tonnsen, Malone, Hatton, & Roberts, 2013), and attention problems have been posited to “constrain” later developmental trajectories in FXS (Cornish et al., 2012). Future studies may clarify the potential mechanisms and causal associations between early attentional control and broader attention-related behavior problems.
When structuring such surveillance and intervention efforts, it is critical to select measures sensitive enough to detect developmental growth. Relevant to this issue, only our experimental inhibitory control measure – not parent-reported attention symptoms – detected age-related changes in our sample. These contrasting patterns suggest that fine-grained analyses of experimentally controlled behavior may, in some instances, provide more nuanced information about developmental growth than behavioral screening tools in young children with FXS. The patterns we observed are consistent with previous studies contrasting experimental and rating-scale attentional measures in FXS (Cornish et al., 2012), as well as with findings that Attention Problem scores are stable over time in older children with FXS (Hatton et al., 2002). Thus, although rating scales may adequately capture broad group differences, experimental tasks may provide increased sensitivity to developmental growth critical to developmental monitoring.
Despite these studies contribute to our understanding of early attentional control in preschoolers with FXS, several limitations must be acknowledged, including relatively small sample sizes for cross-sectional analyses, the use of symptom-based versus diagnostic assessments, and no control sample for longitudinal data . Given the early and severe nature of deficits reported in our preschool sample, additional work is needed to clarify the early developmental course of attentional control during infancy and toddlerhood. The inclusion of both syndrome-specific (e.g., FMRP) and physiologically-relevant (e.g., cortisol, heart activity) biomarkers may inform the complex neurobiological systems that may be contributing to these impairments, particularly to address whether well-documented hyperarousal in FXS contributes to attentional deficits. Similarly, additional work is needed to examine whether early attention impairments relate to autism symptomatology given both high rates of autism symptomatology in this population (Bailey et al., 2008) and previously documented associations between experimental visual attention tasks and autism risk in infants with FXS (Roberts et al., 2011).
5. Conclusions
The present study documents persistent deficits in attentional control that are clearly present, even at very young ages, in 3-6 year old males with FXS. Although we identified small but stable improvements in inhibitory control over time, the early and severe nature of attentional deficits in FXS necessitates effective, early intervention to bolster developmental growth. Notably, attention-related diagnoses and treatments are often initiated around the age of school entry (Visser et al., 2014), thus the early-emerging attention problems observed in FXS may not be routinely addressed in preschool years. However, earlier identification and treatment is critical given the potential compounding effects of early attentional deficits on other developmental domains (Cornish et al., 2012) and the promising possibility of preventing symptoms by capitalizing on neural plasticity during the preschool period (Halperin et al., 2012). Our results also suggest that when monitoring early developmental growth, as in the context of intervention management, experimental behavioral measures may provide increased sensitivity to developmental changes compared to parent or teacher rating scales. Together, these findings indicate that despite persistent deficits in attentional control in young children with FXS, multi-method, developmental assessment can be used to capture developmental growth that should be further supported through early, targeted intervention.
Highlights.
○ Few studies have examined developmental course of attention in preschoolers with FXS.
○ Despite poorer attention overall, attentional control improved over time.
○ Early attentional control related to broad attention behavior problems.
○ Performance-based measures were most sensitive to developmental change.
○ Longitudinal surveillance is important to capturing attention development in FXS.
Table 4.
Fixed Effects of Proportion Failed Trials over Time within FXS
| Model | Est. | SE | df | t | p |
|---|---|---|---|---|---|
| Unconditional Model | |||||
| Intercept | 40.70 | 4.43 | 40 | 9.19 | <.001 |
| Slope | −.61 | .29 | 42 | −2.08 | .04 |
| Effects of Mental Age | |||||
| Intercept | 30.08 | 4.15 | 40 | 9.42 | <.001 |
| Slope | 0.12 | 0.38 | 38 | 0.32 | .75 |
| Mental Age * Intercept | −1.76 | 0.52 | 38 | −3.39 | .001 |
| Mental Age * Slope | 0.02 | 0.29 | 38 | 0.63 | 0.53 |
| Effects of Attention Problems | |||||
| Intercept | 73.55 | 39.75 | 40 | 1.85 | 0.07 |
| Slope | 9.10 | 3.63 | 37 | 2.50 | 0.02 |
| Mental Age * Intercept | −1.71 | 0.46 | 37 | −3.71 | 0.00 |
| Attention Problems * Intercept | −8.76 | 3.53 | 37 | −2.48 | 0.02 |
| Attention Problems * Slope | −31.22 | 38.44 | 37 | −0.81 | 0.42 |
Acknowledgements
This work was supported by the National Institute of Child Health and Human Development (P30-HD003110), the National Institute of Mental Health (R01-MH090194, F31-MH095318), and the office of Special Education Programs, U.S. Department of Education (H324C990042).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach T, Rescorla L. Manual for the ASEBA Preschool Forms & Profiles. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2000. [Google Scholar]
- Backes M, Genc B, Schreck J, Doerfler W, Lehmkuhl G, Von Gontard A. Cognitive and Behavioral Profile of Fragile X Boys: Correlations to Molecular Data. American Journal of Medical Genetics. 2000;156:150–156. [PubMed] [Google Scholar]
- Bailey DB, Raspa M, Bishop E, Olmsted M, Mallya UG, Berry-Kravis E. Medication utilization for targeted symptoms in children and adults with fragile X syndrome: US survey. Journal of Developmental and Behavioral Pediatrics. 2012;33(1):62–9. doi: 10.1097/DBP.0b013e318236c0e1. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. American Journal of Medical Genetics. Part A. 2008;146A:2060–9. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Blair C, Razza RP. Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Development. 2007;78:647–63. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Cole V, Longhi E, Karmiloff-Smith A, Scerif G. Does attention constrain developmental trajectories in fragile x syndrome? A 3-year prospective longitudinal study. American Journal on Intellectual and Developmental Disabilities. 2012;117:103–20. doi: 10.1352/1944-7558-117.2.103. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Cole V, Longhi E, Karmiloff-Smith A, Scerif G. Mapping developmental trajectories of attention and working memory in fragile X syndrome: developmental freeze or developmental change? Development and Psychopathology. 2013;25:365–76. doi: 10.1017/S0954579412001113. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Scerif G, Karmiloff-Smith A. Tracing Syndrome-Specific Trajectories of Attention Across the Lifespan. Cortex. 2007;43:672–685. doi: 10.1016/s0010-9452(08)70497-0. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Ulu AM, Zimmerman RD, Casey BJ. A neural basis for the development of inhibitory control. Developmental Science. 2002;5:9–16. [Google Scholar]
- Goldsmith HH, Rothbart M. The laboratory temperament assessment battery. University of Wisconsin; Madison: 1996. [Google Scholar]
- Halperin JM, Bédard A-CV, Curchack-Lichtin JT. Preventive interventions for ADHD: a neurodevelopmental perspective. Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics. 2012;9:531–41. doi: 10.1007/s13311-012-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton DD, Hooper SR, Bailey DB, Skinner ML, Sullivan KM, Wheeler A. Problem behavior in boys with fragile X syndrome. American Journal of Medical Genetics. 2002;108:105–16. doi: 10.1002/ajmg.10216. [DOI] [PubMed] [Google Scholar]
- Karmiloff-Smith A. Nativism versus neuroconstructivism: rethinking the study of developmental disorders. Developmental Psychology. 2009;45:56–63. doi: 10.1037/a0014506. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Murray K, Coy KC. Inhibitory control as a contributor to conscience in childhood: from toddler to early school age. Child Development. 1997;68:263–277. [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. Pearson; San Antonio, TX: 1995. [Google Scholar]
- Munir F, Cornish KM, Wilding J. A neuropsychological profile of attention deficits in young males with fragile X syndrome. Neuropsychologia. 2000;38:1261–70. doi: 10.1016/s0028-3932(00)00036-1. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Hatton DD, Long ACJ, Anello V, Colombo J. Visual attention and autistic behavior in infants with fragile X syndrome. Journal of Autism and Developmental Disorders. 2011;42:937–46. doi: 10.1007/s10803-011-1316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerif G, Cornish KM, Wilding J, Driver J, Karmiloff-Smith A. Visual search in typically developing toddlers and toddlers with Fragile X or Williams syndrome. Developmental Science. 2004;7:116–30. doi: 10.1111/j.1467-7687.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- Scerif G, Cornish KM, Wilding J, Driver J, Karmiloff-Smith A. Delineation of early attentional control difficulties in fragile X syndrome: focus on neurocomputational changes. Neuropsychologia. 2007;45:1889–98. doi: 10.1016/j.neuropsychologia.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerif G, Karmiloff-Smith A, Campos R, Elsabbagh M, Driver J, Cornish K. To look or not to look? Typical and atypical development of oculomotor control. Journal of Cognitive Neuroscience. 2005;17:591–604. doi: 10.1162/0898929053467523. [DOI] [PubMed] [Google Scholar]
- Scerif G, Longhi E, Cole V, Karmiloff-Smith A, Cornish K. Attention across modalities as a longitudinal predictor of early outcomes: the case of fragile X syndrome. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2012;53:641–50. doi: 10.1111/j.1469-7610.2011.02515.x. [DOI] [PubMed] [Google Scholar]
- Sullivan K, Hatton DD, Hammer J, Sideris J, Hooper S, Ornstein P. a, Bailey DB. Sustained attention and response inhibition in boys with fragile X syndrome: measures of continuous performance. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2007;144B:517–32. doi: 10.1002/ajmg.b.30504. [DOI] [PubMed] [Google Scholar]
- Sullivan K, Hatton D, Hammer J, Sideris J, Hooper S, Ornstein P, Jr DB. ADHD Symptoms in Children With FXS. American Journal of Medical Genetics Part A. 2006;140A:2275–2288. doi: 10.1002/ajmg.a.31388. [DOI] [PubMed] [Google Scholar]
- Tonnsen BL, Malone PS, Hatton DD, Roberts JE. Early negative affect predicts anxiety, not autism, in preschool boys with fragile X syndrome. Journal of Abnormal Child Psychology. 2013;41:267–80. doi: 10.1007/s10802-012-9671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, Blumberg SJ. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53:34–46. doi: 10.1016/j.jaac.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler A, Raspa M, Bann C, Bishop E, Hessl D, Sacco P, Bailey DB. Anxiety, attention problems, hyperactivity, and the Aberrant Behavior Checklist in fragile X syndrome. American Journal of Medical Genetics. Part A. 2014;164A:141–55. doi: 10.1002/ajmg.a.36232. [DOI] [PubMed] [Google Scholar]
- Woodcock K. a, Oliver C, Humphreys GW. Task-switching deficits and repetitive behaviour in genetic neurodevelopmental disorders: data from children with Prader-Willi syndrome chromosome 15 q11-q13 deletion and boys with Fragile X syndrome. Cognitive Neuropsychology. 2009;26:172–94. doi: 10.1080/02643290802685921. [DOI] [PubMed] [Google Scholar]