Abstract
Midbrain dopamine systems play important roles in Parkinson’s disease, schizophrenia, addiction, and depression. The participation of midbrain dopamine systems in diverse clinical contexts suggests these systems are highly complex. Midbrain dopamine regions contain at least three neuronal phenotypes: dopaminergic, GABAergic, and glutamatergic. Here, we review the locations, subtypes, and functions of glutamatergic neurons within midbrain dopamine regions. Vesicular glutamate transporter 2 (VGluT2) mRNA-expressing neurons are observed within each midbrain dopamine system. Within rat retrorubral field (RRF), large populations of VGluT2 neurons are observed throughout its anteroposterior extent. Within rat substantia nigra pars compacta (SNC), VGluT2 neurons are observed centrally and caudally, and are most dense within the laterodorsal subdivision. RRF and SNC rat VGluT2 neurons lack tyrosine hydroxylase (TH), making them an entirely distinct population of neurons from dopaminergic neurons. The rat ventral tegmental area (VTA) contains the most heterogeneous populations of VGluT2 neurons. VGluT2 neurons are found in each VTA subnucleus but are most dense within the anterior midline subnuclei. Some subpopulations of rat VGluT2 neurons co-express TH or glutamic acid decarboxylase (GAD), but most of the VGluT2 neurons lack TH or GAD. Different subsets of rat VGluT2-TH neurons exist based on the presence or absence of vesicular monoamine transporter 2, dopamine transporter, or D2 dopamine receptor. Thus, the capacity by which VGluT2-TH neurons may release dopamine will differ based on their capacity to accumulate vesicular dopamine, uptake extracellular dopamine, or be autoregulated by dopamine. Rat VTA VGluT2 neurons exhibit intrinsic VTA projections and extrinsic projections to the accumbens and to the prefrontal cortex. Mouse VTA VGluT2 neurons project to accumbens shell, prefrontal cortex, ventral pallidum, amygdala, and lateral habenula. Given their molecular diversity and participation in circuits involved in addiction, we hypothesize that individual VGluT2 subpopulations of neurons play unique roles in addiction and other disorders.
Midbrain dopamine (DA) neurons are hypothesized to play roles in reward-based behavior and addiction (Wise, 1978, 2008), reward prediction and learning by error detection (Schultz and Dickinson, 2000), effort-based decision making (Salamone and Correa, 2002), flexible reward-directed behaviors (Ikemoto and Panksepp, 1999; Nicola, 2010), incentive salience (Berridge, 2007), stimulus salience (e. g., prediction of rewarding and aversive events; Young et al., 2005), aversion (Lammel et al., 2014; Volman et al., 2014), depression (Nestler and Carlezon Jr, 2006; Yadid and Friedman, 2008), and fear (Pezze and Feldon, 2004). The extensive, divergent behavioral roles of midbrain dopamine neurons, predominantly from the VTA, indicate that this system is highly heterogeneous. This heterogeneity may be reflected in part by the diverse phenotypic characteristics among DAergic neurons and their interactive brain structures (Yetnikoff et al. 2014; Ford et al. 2014; Overton et al. 2014; Lammel et al., 2014; Morales and Pickel, 2012: Li et al., 2013; Margolis et al., 2006a,b; 2008; Ford et al., 2006).
The midbrain DAergic neurons are interspersed with GABAergic neurons and with glutamatergic neurons (Kawano et al., 2006; Yamaguchi et al., 2007; 2011; 2013; Nair-Roberts et al., 2008). Based on electrophysiological and pharmacological properties, ex vivo electrophysiological recordings from midbrain neurons have provided evidence for three subpopulations of neurons (primary, secondary and tertiary neurons) (Grace & Onn, 1989; Johnson & North, 1992; Cameron et al., 1997; Ungless et al., 2004). The subpopulation of primary neurons has been recognized as DAergic neurons that in their majority have long duration action potentials and hyperpolarization-activated cation current (Ih) (Grace & Onn, 1989). In contrast, the subpopulation of secondary neurons has been recognized as GABAergic neurons with short action potential durations and without Ih (Johnson & North, 1992). The third subpopulation of neurons lacks the electrophysiological properties associated with DAergic or GABAergic neurons, and it has been suggested to use glutamate as a signaling molecule (Ungless et al., 2004).
The presence of glutamatergic neurons within the midbrain DA regions was initially suggested from in vivo (Wilson et al., 1982; Mercuri et al., 1985; Ungless et al., 2004; Lavin et al., 2005; Chuhma et al., 2009), and in vitro electrophysiological findings (Sulzer et al., 1998; Joyce and Rayport, 2000; Chuhma et al., 2004). In vivo studies have shown that electrical stimulation of the substantia nigra pars compacta (SNC) evokes excitatory postsynaptic currents (EPSCs) in dorsal striatal neurons (Wilson et al., 1982). The presence of a nigrostrial glutamatergic pathway has been further supported by ultrastructural analysis of anterograde labeled material showing axon terminals originating from the SNC lacking Tyrosine Hydroxylase (TH, marker of midbrain DA neurons), and making putative excitatory asymmetric synapses in the dorsal striatum (Hattori et al., 1991). Electrical stimulation of the neighboring VTA also evokes EPSCs in neurons within the medial prefrontal cortex (mPFC, Mercuri et al., 1985; Lavin et al., 2005), and within the nucleus accumbens (nAcc, Chuhma et al., 2009).
The earliest electrophysiological in vivo studies reporting EPSCs evoked by midbrain electrical stimulation did not ascribe these excitatory responses to the release of glutamate from DA neurons (Wilson et al., 1982; Mercuri et al., 1985). However, later studies proposed release of glutamate from DA neurons as a mechanism to evoke EPSCs from VTA efferents (Lavin et al., 2005; Chuhma et al., 2009). The idea that glutamate is released by DAergic neurons was initially proposed by Kaneko et al., (1990) based on the observation that antibodies against glutaminase were able to immunolabel all catecholaminergic neurons. However, glutaminase is an enzyme necessary for the production of metabolic glutamate, as such is present in many non-glutamatergic neurons (Laake et al., 1999), thus glutaminase is not a selective marker for glutamate signaling neurons. Nevertheless, in vitro electrophysiological studies have shown glutamatergic signaling by midbrain cultured DA neurons (Sulzer et al., 1998; Joyce and Rayport, 2000; Bourque and Trudeau, 2000; Sulzer and Rayport, 2000) and midbrain slices (Chuhma et al., 2004, 2009).
Anatomical identification of midbrain glutamatergic neurons
The analysis of glutamatergic neurons has been greatly advanced in the last decade due to the cloning of three distinct vesicular glutamate transporters (VGluT1, VGluT2 and VGluT3), which accumulate glutamate into vesicles for its synaptic release (Bellocchio et al., 1998; Takamori et al., 2000; Bai et al., 2001; Fremeau et al., 2001; Fujiyama et al., 2001; Hayashi et al., 2001; Herzog et al., 2001; Varoqui et al., 2002). VGluT1 and VGluT2 are restricted to known glutamatergic neurons, and their presence has become a reliable molecular marker to identify the distribution and synaptic connectivity of glutamatergic neurons within different brain regions. While VGluT1 and VGluT2 are highly concentrated in synaptic vesicles in axonal terminals of glutamatergic neurons (Fremeau et al., 2001; Fujiyama et al., 2001; Herzog et al., 2001), they are undetectable in other neuronal compartments, such as cell bodies, dendrites, and axons. Thus, cellular detection of mRNA transcripts encoding VGluT1 or VGluT2 is so far the only available and reliable method to identify cell bodies of glutamatergic neurons in non-transgenic animals. To detect glutamatergic neurons within the rat DA midbrain regions, in situ hybridization methods have been applied with the use of radioactive and non-radioactive probes (Kawano et al., 2006; Yamaguchi et al., 2007; 2011; 2013; Nair-Roberts et al., 2008; Berube-Carriere et al., 2009). These in situ hybridization studies have shown the following major findings: first, that in the adult rat there are neurons expressing VGluT2 mRNA, but not VGluT1 nor VGluT3, in the VTA (Kawano et al., 2006; Yamaguchi et al., 2007), in the SNC (Yamaguchi et al., 2013), and in the retrorubral field (RRF; Yamaguchi et al., 2013). Second, that different experimental conditions used for detection of VGluT2 mRNA in the adult rat may explain discrepancies in the number of VGluT2-expressing neurons detected in the VTA (Kawano et al., 2006; Berube-Carriere et al., 2009; Yamaguchi et al., 2007, 2011), SNC and RFF (Nair-Roberts et al., 2008;Yamaguchi et al., 2013). Third, that the VGluT2 neurons within the RRF, SNC and neighboring lateral aspects of the VTA [lateral aspects of parabrachial pigmental (PBP) and paranigral (PN) nuclei] appear to be similar to each other, but different from those present in the midline nuclei of the A10 region (medial aspects of both PBP and PN; rostral linear nucleus, RLi; interfascicular nucleus, IF and caudal linear nucleus, CLi). Four, that although some VGluT2 neurons in the midline nuclei of the VTA co-express TH, the vast majority of VGluT2 neurons lack TH within the mature rat RRF, SNc, and lateral VTA (Yamaguchi et al., 2011, 2013; Li et al., 2013). These four major findings will be further detailed in this review.
Glutamatergic neurons within the RRF and the SNC
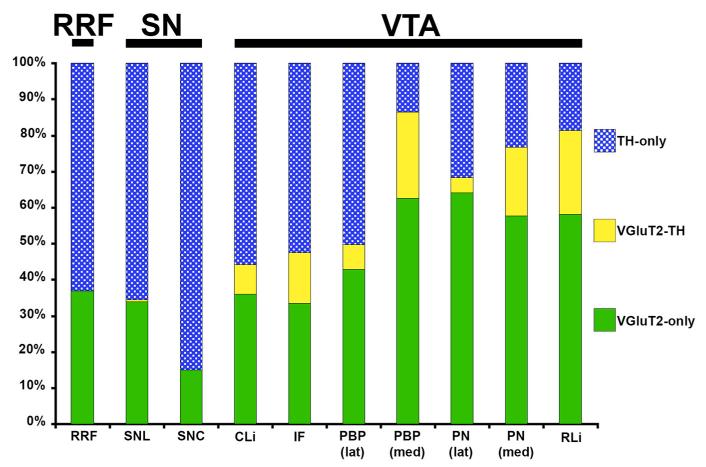
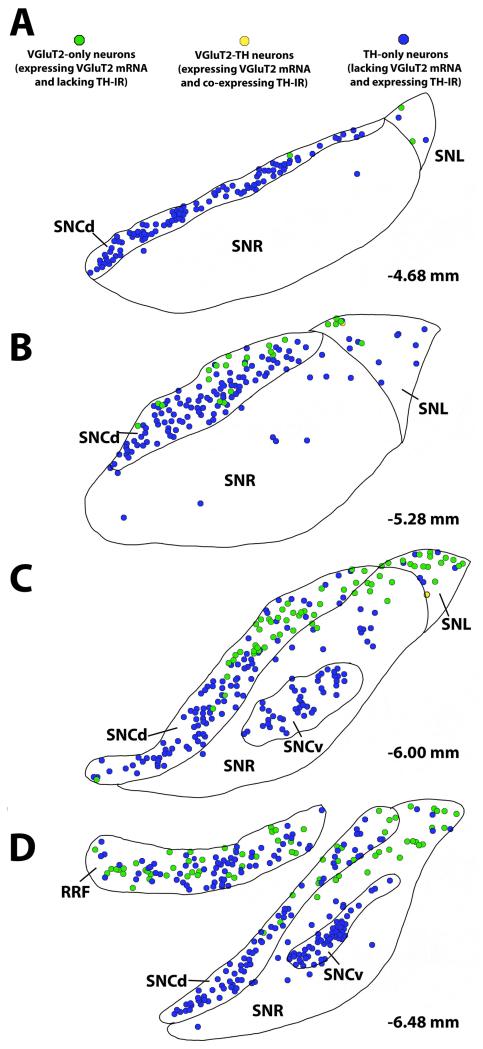
By applying radioactive in situ hybridization in combination with TH immunolabeling, we have found that the vast majority of VGluT2-expressing neurons do not co-express TH within the RRF, SNC (Yamaguchi et al., 2013), or lateral PBP and lateral PN of the VTA (Yamaguchi et al., 2007, 2011; Li et al., 2013) (Figures 1-2). In contrast, some of the VGluT2 neurons located in the midline nuclei of the VTA (medial PBP, medial PN, RLi, IF, and CLi) co-express TH (VGluT2-TH neurons; Figures 1-2). Quantitative analysis of the relative frequency of VGluT2, VGluT2-TH and TH neurons indicates that their prevalence is not constant throughout the RRF, SNC and lateral and medial nuclei of the VTA. VGluT2-TH neurons are not found in the RRF or the SNC. Within the RRF, the number of VGluT2 neurons increases from rostral to caudal levels, but the proportion of VGluT2 neurons in relation to those expressing TH is constant throughout its rostro-caudal levels. On average, the RRF neurons are in a ratio of 1 VGluT2 cell per every 1.7 TH cells. Within the SNC, VGluT2 neurons are often observed within its caudal laterodorsal portion and rarely observed in the rostral SNC. On average, the SNC VGluT2 cells are in a ratio of 1 VGluT2 cell per every 4.4 TH cells. Because the rat VGluT2 neurons located in the SNC lack TH, we concluded that glutamatergic neurons and DAergic neurons are two distinct subpopulations of neurons in the SNC and proposed that a single nigrostriatal dopaminergic neuron is unlikely to form two chemically distinct synaptic classes, a dopaminergic and an excitatory (Yamaguchi et al., 2013), as previously proposed by Hattori et al., (1991).
Figure 1.
Distribution of glutamatergic and dopaminergic neurons of the rat RRF, SNc, and VTA. VGluT2-only neurons express VGluT2 mRNA and lack TH-immunoreactivity. VGluT2-TH neurons co-express VGluT2 mRNA and TH-immunoreactivity. TH-only neurons lack VGluT2 mRNA but express TH-immunoreactivity. RRF - retrorubral field; SNL - lateral division of the substantia nigra pars compacta; SNC - substantia nigra pars compacta; CLi - caudal linear nucleus; PBP(lat) - lateral parabrachial pigmentosis nucleus; PBP(med) - medial parabrachial pigmentosis nucleus; PN(lat) - lateral paranigral nucleus; PN(med) - medial paranigral nucleus; RLi - rostral linear nucleus. Data from Yamaguchi et al., 2011.
Figure 2.
Distribution map of glutamatergic and dopaminergic neurons of the rat SNC across four anteroposterior planes and rat RRF in a single plane. SNCd – substantia nigra pars compacta dorsal tier; SNCv – substantia nigra pars compacta ventral tier; SNL – substantia nigra pars lateralis; SNR – substantia nigra pars reticulate; RRF – retrorubral field. Data from Yamaguchi et al., 2013.
VGluT2 neurons within the VTA
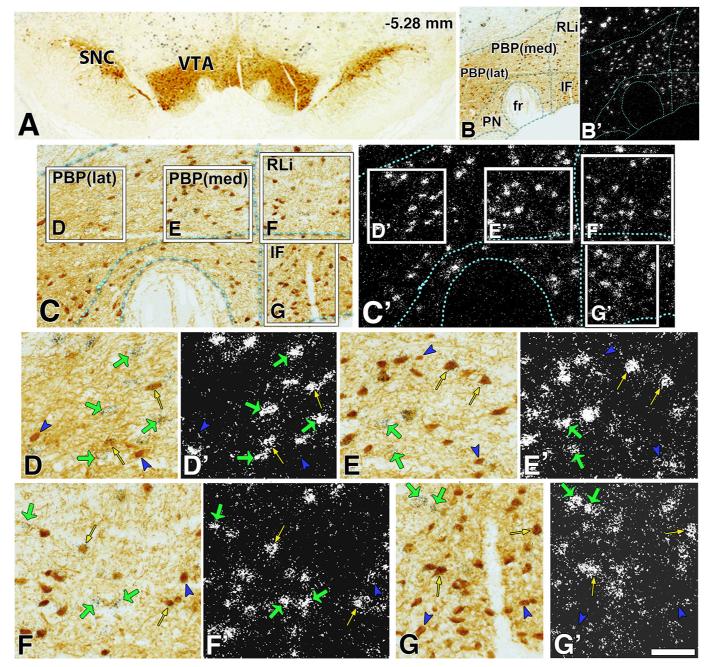
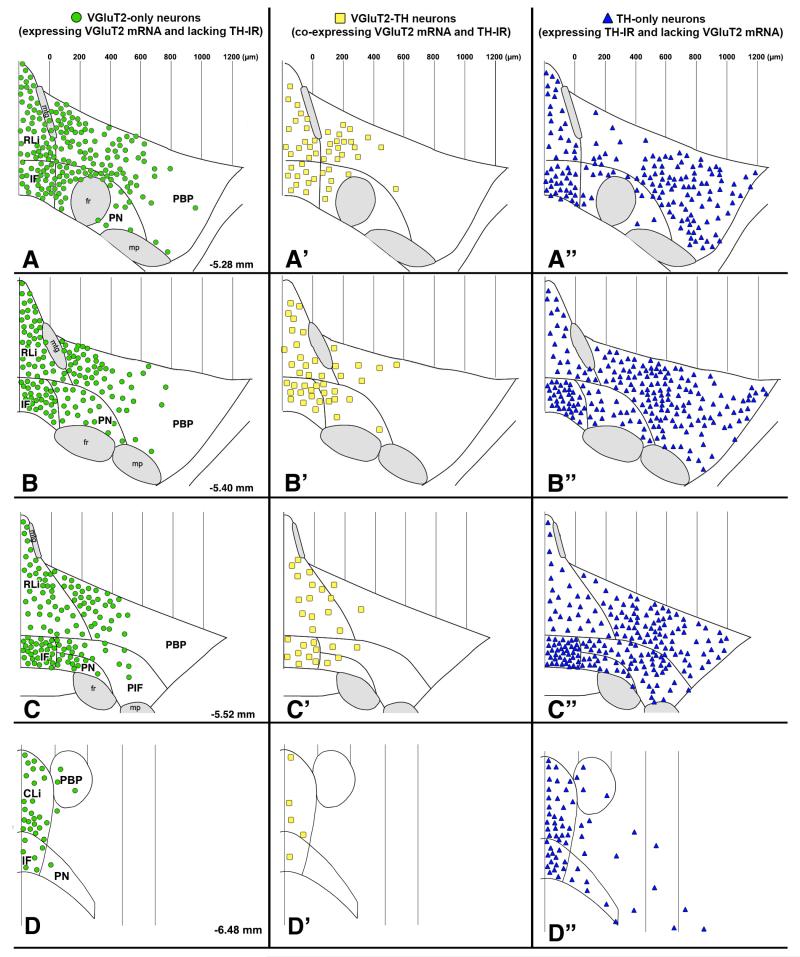
In contrast to the apparent uniformity among the VGluT2 neurons within the RRF and the SNC, the VGluT2 neurons within the VTA are heterogeneous in their concentration, distribution and composition (Yamaguchi et al., 2007, 2011; Li et al., 2013; Root et al., 2013). Although some VGluT2 neurons expressing either TH or GABAergic markers are present within the medial aspects of the VTA (Yamaguchi et al., 2007; Root et al, 2013), most VGluT2 neurons lack both TH and GABAergic markers. These findings lead us to propose that the VGluT2 neurons constitute a third type of neuron within the VTA (Yamaguchi et al., 2007). The VGluT2-TH neurons are present in a zone initiated at the border between the PBP and the RLi and expanding 400 μm laterally (medial PBP) (Figures 3-4), and in a zone initiated at the border between the PN and the IF and expanding 200 μm laterally (medial PN) (Figures 3-4). While the majority of VGluT2 neurons lack TH (72–79%) within these medial aspects of the PBP and PN, the subpopulation of dual VGluT2-TH neurons makes about half of the total population of TH neurons. Within the midline nuclei (RLi, IF, and CLi) of the VTA, most of the VGluT2 neurons lack TH (63–89%; Figure 1), and a few co-express TH (12–37%). These VGluT2-TH neurons account for roughly half of the neurons containing TH in the rostral aspects of both the RLi and the IF nuclei, and less than 17% of those neurons containing TH within the caudal levels of the IF and CLi. The differential distributions of VGluT2-only, VGluT2-TH, and TH-only neurons within each of the subdivisions of the VTA (Figure 1) underscore the cellular difference between the lateral (PBP and PN) and medial nuclei of the VTA.
Figure 3.
Glutamatergic and dopaminergic neurons of the rat VTA. A. TH-immunoreactivity (brown) showing the SNc (left) and VTA (middle). B-C. Higher power images of VTA. TH-immunoreactivity (B,C) and VGluT2 mRNA (B’,C’) are displayed as brown reaction product (TH-immunoreactivity under bright field) and aggregates of silver grains (white grains under dark field or black grains under darkfield). D. High power image of selected PBP(lat) region in C. E. High power image of selected PBP(med) region in C. F. High power image of selected RLi region in C. High power image of selected IF region in C. Green arrows indicate VGluT2-only neuron. Yellow arrows indicate VGluT2-TH neuron. Blue arrowheads indicate TH-only neuron. fr - fasciculus retroflexus. Figure modified from Yamaguchi et al., 2011.
Figure 4.
Distribution map of glutamatergic and dopaminergic neurons of the rat VTA across four anteroposterior planes. Figure modified from Yamaguchi et al., 2011.
Dual VGluT2-TH neurons
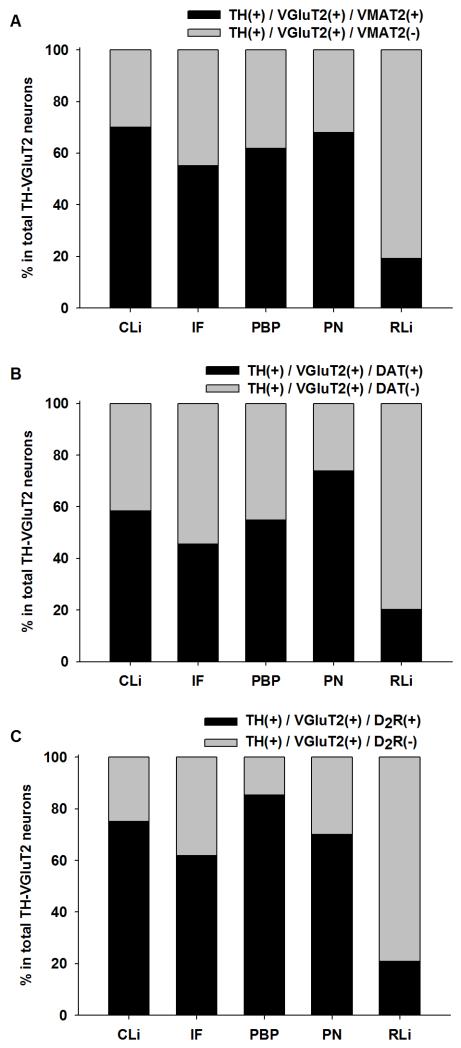
Evidence for the expression of VGluT2 mRNA in a subset of TH neurons observed by radioactive in situ hybridization procedures is supported by quantitative RT-PCR of individual laser micro-dissected VTA neurons (Yamaguchi et al., 2011; Li et al., 2013). These VGluT2-TH neurons express aromatic L-amino acid decarboxylase, and as such have the capability to synthesize DA. However, only a subset of VGluT2-TH neurons express vesicular monoamine transporter (VMAT2), dopamine transporter (DAT), or D2 receptor, indicating that although all VGluT2-TH neurons can produce DA, only some of them are capable of accumulating vesicular DA, capable of capturing extrasynaptic DA, or be autoregulated by dopamine (Figure 5). Interestingly, the relative amount of TH mRNA is similar between the TH-only and TH-VGluT2 neurons that contain both VMAT2 and DAT. By comparison, the amounts of both TH mRNA and DAT mRNA are lower in the TH-only and the TH-VGluT2 neurons that lack VMAT2. These findings indicate that the concentration of TH mRNA is not influenced by its coexistence with VGluT2, but rather by the absence of VMAT2.
Figure 5.
Subregional distribution of rat VTA VGluT2-TH neurons co-expressing VMAT2 mRNA, DAT mRNA, or D2 receptor mRNA (mean and SEM). Black bars in A, B, and C indicate VGluT2-TH neurons coexpressing VMAT2 mRNA, DAT mRNA, or D2 receptor mRNA respectively. Gray bars in A, B, or C indicate VGluT2-TH neurons lacking VMAT2 mRNA, DAT mRNA, or D2 receptor mRNA, respectively. Figure modified from Li et al., 2013
Corelease of glutamate and DA has been suggested from in vitro findings showing EPSCs in midbrain cultured DA neurons (Sulzer et al., 1998; Bourque and Trudeau, 2000; Joyce and Rayport, 2000; Sulzer and Rayport, 2000; Chuhma et al., 2004, 2009), and EPSCs in nAcc slices evoked by local light stimulation of VTA fibers expressing channelrhodopsin2 under the regulation of the TH- or DAT-promoters (Stuber et al., 2010; Tecuapetla et al., 2010). While these optogenetic studies provide evidence for the release of glutamate by mesoaccumbens putative DA neurons, the release of DA by midbrain glutamate neurons has not been directly demonstrated. Nevertheless, co-release of glutamate and DA from the same pool of vesicles has been suggested as a mechanism of neurotransmission by dual glutamate-DA neurons (Hnasko et al., 2010). This suggestion has been based on co-immunoprecipitation of VMAT2 and VGluT2 from ~6-week old rat ventral striatal (nAcc and olfactory tubercle) preparations as well as electrochemical findings from 3-week old rat ventral striatal preparations showing glutamate-mediated vesicular acidification that increases monoamine storage (Hnasko et al., 2010). It is unclear whether or not VGluT2 and VMAT2 are present within the same pool of vesicles after 6 weeks of age, and the extent to which VGluT2 participates in DA vesicular filling in vivo. However, ultrastructural analysis of the nAcc indicates that while VGluT2 and TH coexist in some nAcc axon terminals within 15 day old rats (Berube-Carrere et al., 2009), they do not co-exist in the adult rat (Berube-Carrere et al., 2009; Moss et al., 2011) or at any age in mice (Berube-Carrere et al., 2012). Despite the lack of support for subcellular co-existence of VGluT2 and TH in adult rodents, it has been suggested that VGluT2 facilitates vesicular DA filling in the nAcc of adult mice based on studies with conditioned knock-out mice depleted of VGluT2 in DAT neurons (cKO mice). In these studies, the cKO mice showed an attenuated drug-induced locomotor response following a single injection of cocaine or methamphetamine in comparison to the wild type (WT) mice (Birgner et al., 2010; Hnasko et al., 2010; Fortin et al., 2012). In addition, although cocaine conditioned place preference by cKO and WT mice are similar (Hnasko et al., 2010), cKO mice consume higher levels of both sugar and cocaine, and display a significant elevation in cue-induced drug seeking compared to WT mice (Alsiö et al., 2011). It has been suggested that these behavioral effects are due to the hypothesized lack of VGluT2 on DA vesicles, which would reduce vesicular DA levels (Alsiö et al., 2011). However, it has also been suggested that functional differences may result indirectly from developmental alterations within the DA system due to embryonic deletion of the VGluT2 gene, which has been shown to result in many morphological and functional alterations (Alsiö et al., 2011; Berube-Carriere et al., 2012). Further research is necessary to determine how VGluT2-TH neurons may corelease glutamate and dopamine.
Intrinsic and extrinsic inputs by VTA glutamatergic neurons
It is well known that VTA neurons receive extensive glutamatergic innervation (Geisler et al., 2007). A role for local VTA VGluT2 neurons in VTA neurotransmission has been suggested based on electrophysiological and anatomical findings showing that some VTA VGluT2 neurons establish local glutamatergic synapses on VTA DA and non-DA neurons (Dobi et al., 2010). These studies indicate that VTA VGluT2 neurons provide local glutamatergic neurotransmission. This novel model of local communication in the VTA expands the previously reported local GABAergic and DAergic signaling in this brain region (Johnson and North, 1992a,b; Cameron and Williams, 1993), raising the possibility that local glutamatergic neurons play a role in regulating VTA neuronal activity. Because functional μ-opioid receptors are present in VTA GABA neurons and μ-opioid receptor transcripts are detected in VTA VGluT2 neurons (Kudo et al., 2014), opioids may play an important role in the regulation of the local VTA neuronal network (Johnson and North, 1992 a,b).
In addition to the local projections of VTA VGluT2 neurons, subsets of VGluT2 neurons innervate the mPFC (Lavin et al., 2005; Hur and Zaborszky, 2005; Yamaguchi et al., 2011; Gorelova et al., 2012; Taylor et al. 2014), nAcc (Stuber et al., 2010; Tecuapetla et al., 2010; Yamaguchi et al., 2011; Adrover et al., 2014; Hnasko et al., 2012; Chuhma et al., 2009; 2014; Taylor et al. 2014), lateral habenula, amygdala, or ventral pallidum (Hnasko et al., 2012; Taylor et al. 2014). The degree to which VGluT2-only or dual VGluT2-TH neurons innervate the mPFC or nAcc has been determined by the implementation of a multistep procedure that combines in vivo injections of the retrograde tract tracer Fluoro-Gold in the mPFC or nAcc, and postmortem phenotypic characterization of the retrogradely labeled neurons by a combination of immunolabeling and radioactive in situ hybridization (Yamaguchi et al., 2011). By applying this experimental approach, it has been determined that near to 40% of the mesocortical neurons are VGluT2-only neurons, and about 10% have an unidentified phenotype (not VGluT2 or TH). Approximately 25% of mesocortical neurons are TH-only neurons, and the remaining 25% are VGluT2-TH neurons. These findings indicate that there is a major glutamatergic pathway from the midbrain to the mPFC (Yamaguchi et al., 2011). The identification of this mesocortical glutamatergic pathway is consistent with electrophysiological findings showing that stimulation of the VTA evokes EPSCs in mPFC neurons, which is eliminated by glutamate antagonists but not by DA antagonists (Lavin et al., 2005). In contrast to a major mesocortical glutamatergic pathway, TH is present in the vast majority (80%) of the VTA neurons that project to the nAcc. However, about one-third of these TH neurons co-express VGluT2 mRNA, despite the fact that VTA VGluT2-TH neurons represent only a small fraction of both the TH neurons and a small fraction of the VGluT2 neurons (Yamaguchi et al., 2011). Because VTA projections rarely collateralize (Fallon, 1981; Swanson, 1982; Albanese and Minciacchi, 1983), we suggest that the nAcc is a preferential target of the VGluT2-TH neurons. The identification of a mesoaccumbens pathway from VGluT2-TH neurons is consistent with electrophysiological results showing that optical stimulation of efferents from dopaminergic neurons expressing channelrhodopsin produces EPSCs in medium spiny neurons (Stuber et al., 2010; Tecuapetla et al., 2010; Adrover et al., 2014), and in cholinergic interneurons (Chuhma et al., 2014) of the nAcc.
Conclusions and future directions
All glutamatergic neurons within the major midbrain DA subdivisions (RRF, SNc, and VTA) contain VGluT2 mRNA, and they may provide fast non-DA excitatory signaling. The VGluT2 neurons within the RRF and SNc do not co-express TH, and as such they are incapable of DA co-release. The VTA contains two major classes of VGluT2 neurons: VGluT2 neurons lacking TH (VGluT2-only neurons), which are present in all subdivisions of the VTA, and VGluT2 neurons co-expressing TH (VGluT2-TH neurons) that are restricted to the medial portions. Dual VGluT2-TH neurons have the capability to produce DA, but not all have the ability to accumulate DA into synaptic vesicles or the capacity to take DA up from the extracellular space. While the release of glutamate from dual VGluT2-TH neurons has been established, it remains to be determined whether these neurons release DA. Both VGluT2-only and VGluT2-TH neurons innervate the mPFC and the nAcc; thus, in addition to the well-recognized mesocorticolimbic DA and GABA pathways, there exists a parallel mesocorticolimbic glutamatergic pathway from which some fibers may co-release glutamate and DA. The mechanism underlying this potential glutamate and DA co-release remains to be determined.
Even though the vast majority of VGluT2 neurons within the midbrain DA regions do not have the capacity to produce DA, studies of their role in brain function have been limited until recently in part due to the lack of reliable methods for targeting these neurons. Borgius et al., (2010) have recently developed VGluT2::cre transgenic mice that can be used for cre-dependent expression of selective molecules (opsins, neuronal tracers, etc.). However, the selective expression of cre within midbrain endogenous VGluT2 neurons remains to be determined in these mice. In addition, although great advances have been achieved in the analysis of the different neuronal phenotypes within the VTA, especially within its midline nuclei, several fundamental questions remain to be addressed. As we covered in this review, midbrain VGluT2 neurons, especially those within the VTA have considerable molecular heterogeneity. Most of midbrain VGluT2 neurons lack the capability of producing DA. Of the subset of VGluT2 neurons capable of producing DA, some may release DA by traditional vesicular mechanisms and others may either not release DA at all or may release it by a mechanism independent of vesicular accumulation of DA by VMAT2. Some VGluT2 neurons synthesize GABA and therefore may be capable of co-transmitting glutamate and GABA. The target areas and functions of each VGluT2 subtype remain to be determined. Given their molecular diversity and participation in circuits involved in addiction and depression, we hypothesize that individual VGluT2 subpopulations of neurons play unique roles in drug addiction and several motivated behaviors.
VGluT2-expressing glutamate neurons reside within RRF, SNC, and VTA
Some VTA VGluT2 neurons, but not RRF or SNC VGluT2 neurons, co-express tyrosine hydroxylase
Subsets of VGluT2-TH neurons co-express or lack VMAT2, DAT, or D2 receptor
VTA VGluT2 neurons exhibit local VTA projections as well as and extrinsic projections
Accumbens medial shell is a preferential target of VTA VGluT2-TH neurons
Acknowledgements
This research was supported by the NIDA Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adrover MF, Shin JH, Alverez VA. Glutamate and dopamine transmission from midbrain dopamine neurons share similar release properties but are differentially affected by cocaine. J Neurosci. 2014;34(9):3183–3192. doi: 10.1523/JNEUROSCI.4958-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanese A, Minciacchi D. Organization of the ascending projections from the ventral tegmental area: a multiple fluorescent retrograde tracer study in the rat. J Comp Neurol. 1983;216:406–420. doi: 10.1002/cne.902160406. [DOI] [PubMed] [Google Scholar]
- Alsiö J, Nordenankar K, Arvidsson E, Birgner C, Mahmoudi S, Halbout B, Smith C, Fortin GM, Olson L, Descarries L, Trudeau LÉ, Kullander K, Lévesque D, Wallén-Mackenzie A. Enhanced Sucrose and Cocaine Self-Administration and Cue-Induced Drug Seeking after Loss of VGLUT2 in Midbrain Dopamine Neurons in Mice. J Neurosci. 2011;31(35):12593–12603. doi: 10.1523/JNEUROSCI.2397-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Xu H, Collins JF, Ghishan FK. Molecular and functional analysis of a novel neuronal vesicular glutamate transporter. J Biol Chem. 2001;276(39):36764–9. doi: 10.1074/jbc.M104578200. [DOI] [PubMed] [Google Scholar]
- Bellocchio EE, Hu H, Pohorille A, Chan J, Pickel VM, Edwards RH. The localization of the brain-specific inorganic phosphate transporter suggests a specific presynaptic role in glutamatergic neurotransmission. J Neurosci. 1998;18:8638–8659. doi: 10.1523/JNEUROSCI.18-21-08648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Bérube-Carriére N, Riad M, Dal Bo G, Levesque D, Trudeau LE, Descarries L. The dual dopamine-glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain. J Comp Neurol. 2009;517:873–891. doi: 10.1002/cne.22194. [DOI] [PubMed] [Google Scholar]
- Bérube-Carriére N, Guay G, Fortin GM, Kullander K, Olson L, Wallén-Mackenzie A, Trudeau LE, Descarries L. Ultrastructural characterization of the mesostriatal dopamine innervation in mice, including two mouse lines of conditional VGLUT2 knockout in dopamine neurons. Eur J Neurosci. 2012;35:527–38. doi: 10.1111/j.1460-9568.2012.07992.x. [DOI] [PubMed] [Google Scholar]
- Birgner C, Nordenankar K, Lundblad M, Mendez JA, Smith C, le Greves M, Galter D, Olson L, Fredriksson A, Trudeau LE, Kullander K, Wallen-Mackenzie A. VGLUT2 in dopamine neurons is required for psychostimulant-induced behavioral activation. Proc Natl Acad SciU S A. 2010;107:389–394. doi: 10.1073/pnas.0910986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgius L, Restrepo CE, Leao RN, Saleh N, Kiehn O. A transgenic mouse line for molecular genetic analysis of excitatory glutamatergic neurons. Mol Cell Neurosci. 2010;45(3):245–57. doi: 10.1016/j.mcn.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Bourque MJ, Trudeau LE. GDNF enhances the synaptic efficacy of dopaminergic neurons in culture. Eur J Neurosci. 2000;12(9):3172–80. doi: 10.1046/j.1460-9568.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009;106(12):4894–9. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DL, Williams JT. Dopamine D1 receptors facilitate transmitter release. Nature. 1993;366(6453):344–7. doi: 10.1038/366344a0. [DOI] [PubMed] [Google Scholar]
- Chuhma N, Choi WY, Mingote S, Rayport S. Dopamine neuron glutamate cotransmission: frequency-dependent modulation in the mesoventromedial projection. Neuroscience. 2009;164:1068–1083. doi: 10.1016/j.neuroscience.2009.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Zhang H, Masson J, Zhuang X, Sulzer D, Hen R, Rayport S. Dopamine neurons mediate a fast excitatory signal via their glutamatergic synapses. J Neurosci. 2004;24:972–981. doi: 10.1523/JNEUROSCI.4317-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Bo G, Bérubé-Carrière N, Mendez JA, Leo D, Riad M, Descarries L, Lévesque D, Trudeau LE. Enhanced glutamatergic phenotype of mesencephalic dopamine neurons after neonatal 6-hydroxydopamine lesion. Neuroscience. 2008;156(1):59–70. doi: 10.1016/j.neuroscience.2008.07.032. [DOI] [PubMed] [Google Scholar]
- Dobi A, Margolis EB, Wang HL, Harvey BK, Morales M. Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J Neurosci. 2010;30:218–229. doi: 10.1523/JNEUROSCI.3884-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH. Collateralization of monoamine neurons: mesotelencephalic dopamine projections to caudate, septum, and frontal cortex. J Neurosci. 1981;1(12):1361–1368. doi: 10.1523/JNEUROSCI.01-12-01361.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.01.025. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci. 2006;26(10):2788–97. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin GM, Bourque MJ, Mendez JA, Leo D, Nordenankar K, Birgner C, Arvidsson E, Rymar VV, Bérubé-Carrière N, Claveau AM, Descarries L, Sadikot AF, Wallén-Mackenzie Å , Trudeau LÉ. Glutamate corelease promotes growth and survival of midbrain dopamine neurons. J Neurosci. 2012;32(48):17477–91. doi: 10.1523/JNEUROSCI.1939-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Furuta T, Kaneko T. Immunocytochemical localization of candidates for vesicular glutamate transporters in the rat cerebral cortex. J Comp Neurol. 2001;435:379–387. doi: 10.1002/cne.1037. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27(21):5730–43. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelova N, Mulholland PJ, Chandler LJ, Seamans JK. The glutamatergic component of the mesocortical pathway emanating from different subregions of the ventral midbrain. Cerebral Cortex. 2012;22(2):327–336. doi: 10.1093/cercor/bhr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T, Takada M, Moriizumi T, Van der Kooy D. Single dopaminergic nigrostriatal neurons form two chemically distinct synaptic types: possible transmitter segregation within neurons. J Comp Neurol. 1991;309(3):391–401. doi: 10.1002/cne.903090308. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Yamada H, Uehara S, Morimoto R, Muroyama A, Yatsushiro S, Takeda J, Yamamoto A, Moriyama Y. Secretory granule-mediated co-secretion of L-glutamate and glucagon triggers glutamatergic signal transmission in islets of Langerhans. J Biol Chem. 2003;278(3):1966–74. doi: 10.1074/jbc.M206758200. [DOI] [PubMed] [Google Scholar]
- Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21(22):RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S, Edwards RH. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–656. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Hjelmstad GO, Fields HL, Edwards RH. Ventral tegmental area glutamate neurons: electrophysiological properties and projections. J Neurosci. 2012;32(43):15076–85. doi: 10.1523/JNEUROSCI.3128-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur EE, Zaborszky L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization study [corrected] J Comp Neurol. 2005;483(3):351–73. doi: 10.1002/cne.20444. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31(1):6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Brain reward circuitry beyond the mesolimbic dopamine system: A neurobiological theory. Neurosci Biobehav Rev. 2010;35(2):129–150. doi: 10.1016/j.neubiorev.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992a;12(2):483–8. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992b;450:455–68. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce MP, Rayport S. Mesoaccumbens dopamine neuron synapses reconstructed in vitro are glutamatergic. Neuroscience. 2000;99:445–456. doi: 10.1016/s0306-4522(00)00219-0. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Akiyama H, Nagatsu I, Mizuno N. Immunohistochemical demonstration of glutaminase in catecholaminergic and serotoninergic neurons of rat brain. Brain Res. 1990;507:151–154. doi: 10.1016/0006-8993(90)90535-j. [DOI] [PubMed] [Google Scholar]
- Kawano M, Kawasaki A, Sakata-Haga H, Fukui Y, Kawano H, Nogami H, Hisano S. Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J Comp Neurol. 2006;498:581–592. doi: 10.1002/cne.21054. [DOI] [PubMed] [Google Scholar]
- Kudo T, Konno K, Uchigashima M, Yanagawa Y, Sora I, Minami M, Watanabe M. GABAergic neurons in the ventral tegmental area receive dual GABA/enkephalin-mediated inhibitory inputs from the bed nucleus of the stria terminalis. Eur J Neurosci. 2014:1–14. doi: 10.1111/ejn.12503. [DOI] [PubMed] [Google Scholar]
- Laake JH, Takumi Y, Eidet J, Torgner IA, Roberg B, Kvamme E, Ottersen OP. Postembedding immunogold labelling reveals subcellular localization and pathway-specific enrichment of phosphate activated glutaminase in rat cerebellum. Neuroscience. 1999;88(4):1137–1151. doi: 10.1016/s0306-4522(98)00298-x. [DOI] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2014;76(Pt B):351–9. doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin A, Nogueira L, Lapish CC, Wightman RM, Phillips PE, Seamans JK. Mesocortical dopamine neurons operate in distinct temporal domains using multimodal signaling. J Neurosci. 2005;25(20):5013–23. doi: 10.1523/JNEUROSCI.0557-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Qi J, Yamaguchi T, Wang HL, Morales M. Heterogenous composition of dopamine neurons of the rat A10 region: molecular evidence for diverse signaling properties. Brain Struct Funct. 2013;218:1159–1176. doi: 10.1007/s00429-012-0452-z. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci U S A. 2006a;103(8):2938–42. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006b;577(Pt 3):907–24. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. J Neurosci. 2008;28(36):8908–13. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri N, Calabresi P, Stanzione P, Bernardi G. Electrical stimulation of mesencephalic cell groups (A9-A10) produces monosynaptic excitatory potentials in rat frontal cortex. Brain Res. 1985;338(1):192–5. doi: 10.1016/0006-8993(85)90267-7. [DOI] [PubMed] [Google Scholar]
- Moss J, Ungless MA, Bolam JP. Dopaminergic axons in different divisions of the adult striatal complex do not express vesicular glutamate transporters. Eur J Neurosci. 2011;33:1205–1211. doi: 10.1111/j.1460-9568.2011.07594.x. [DOI] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152:1024–1031. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59(12):1151–9. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nicola SM. The flexible approach hypothesis: unification of effort and cue-responding hypotheses for the role of nucleus accumbens dopamine in the activation of reward-seeking behavior. J Neurosci. 2010;30(49):16585–600. doi: 10.1523/JNEUROSCI.3958-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton PG, Vautrelle N, Redgrave P. Sensory regulation of dopaminergic cell activity: phenomenology, circuitry, and function. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.01.023. in press. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004;74(5):301–20. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Root DH, Zhang S, Mejias-Aponte CA, Morales M. A novel glutamate-GABA neuronal subpopulation within the ventral tegmental area projects to the lateral habenula. Soc Neurosci Abstr. 2013;803:06. [Google Scholar]
- Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137(1-2):3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Joyce MP, Lin L, Geldwert D, Haber SN, Hattori T, Rayport S. Dopamine neurons make glutamatergic synapses in vitro. J Neurosci. 1998;18:4588–4602. doi: 10.1523/JNEUROSCI.18-12-04588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Rayport S. Dale’s principle and glutamate corelease from ventral midbrain dopamine neurons. Amino Acids. 2000;19:45–52. doi: 10.1007/s007260070032. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Taylor SR, Badurek S, DiLeone RJ, Nashmi R, Minichiello L, Picciotto MR. GABAergic and Glutamatergic Efferents of the Mouse Ventral Tegmental Area. J Comp Neurol. 2014 doi: 10.1002/cne.23603. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, Koos T. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303(5666):2040–2. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Volman SF, Lammel S, Margolis EB, Kim Y, Richard JM, Roitman MF, Lobo MK. New insights into the specificity and plasticity of reward and aversion encoding in the mesolimbic system. J Neurosci. 2013;33(45):17569–76. doi: 10.1523/JNEUROSCI.3250-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqui H, Schäfer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22(1):142–55. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Origins of postsynaptic potentials evoked in identified rat neostriatal neurons by stimulation in substantia nigra. Exp Brain Res. 1982;45(1-2):157–67. doi: 10.1007/BF00235775. [DOI] [PubMed] [Google Scholar]
- Wise RA. Catecholamine theories of reward: a critical review. Brain Res. 1978;152(2):215–47. doi: 10.1016/0006-8993(78)90253-6. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 2008;14(2-3):169–83. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadid G, Friedman A. Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog Brain Res. 2008;172:265–86. doi: 10.1016/S0079-6123(08)00913-8. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25:106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang HL, Li X, Ng TH, Morales M. Mesocorticolimbic glutamatergic pathway. J Neurosci. 2011;31:8476–8490. doi: 10.1523/JNEUROSCI.1598-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang HL, Morales M. Glutamatergic neurons in the substantia nigra compacta and retrorubral field. Eur J Neurosci. 2013;38:3602–3610. doi: 10.1111/ejn.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetnikoff L, Lavezzi HN, Reichard RA, Zahm DS. An update on the connections of the ventral mesencephalic dopaminergic complex. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.04.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AM, Moran PM, Joseph MH. The role of dopamine in conditioning and latent inhibition: what, when, where and how? Neurosci Biobehav Rev. 2005;29(6):963–76. doi: 10.1016/j.neubiorev.2005.02.004. [DOI] [PubMed] [Google Scholar]