Abstract
Not all memory processes are equally affected by aging. A widely accepted hypothesis is that older adults rely more on familiarity-based processing, typically linked with the perirhinal cortex (PRC), in the context of impaired recollection, linked with the hippocampus (HC). However, according to the dedifferentiation hypothesis, healthy aging reduces the specialization of MTL memory subregions so that they may mediate different memory processes than in young adults. Using fMRI, we tested this possibility using a conceptual fluency manipulation known to induce familiarity-related PRC activity. The study yielded two main findings. First, although fluency equivalently affected PRC in both young (18–28; N = 14) and older (62–80; N=15) adults, it also uniquely affected HC activity in older adults. Second, the fluency manipulation reduced functional connectivity between HC and PRC in young adults, but it increased it in older adults. Taken together, the results suggest that aging may result in reduced specialization of the HC for recollection, such that the HC may be recruited when fluency increases familiarity-based responding.
1. Introduction
There is wide agreement that subregions of the medial temporal lobe (MTL) make distinct contributions to memory retrieval. Although the processes and representations indexed by MTL regions continue to be debated, a predominant hypothesis is that the hippocampus (HC) contributes more to recollection, a rich, context-based form of retrieval, whereas the perirhinal cortex (PRC) contributes more to familiarity, a vague sense of memory in the absence of contextual detail (Brown & Aggleton, 2001; Daselaar, Fleck, Dobbins, Madden, & Cabeza, 2006; Eichenbaum, Yonelinas, & Ranganath, 2007; Yonelinas et al., 2007). Behavioral studies have shown that older adults rely more on preserved familiarity in contrast to impaired recollection (e.g., Anderson et al., 2008; Bastin & Van der Linden, 2003; Davidson & Glisky, 2002; Jacoby, 1999; Jennings & Jacoby, 1993; Naveh-Benjamin et al., 2009; Spencer & Raz, 1995; Yonelinas, 2002). Consistent with this behavioral data, there is volumetric MRI evidence that age-related atrophy is greater for HC than for PRC (Raz et al., 2005), and fMRI evidence that aging reduces recollection-related HC activity (Dennis, Kim, & Cabeza, 2008; Morcom, Li, & Rugg, 2007; but see Persson, Kalpouzos, Nilsson, Ryberg, & Nyberg, 2011), but enhances familiarity-related activity in PRC (Daselaar, Fleck, Dobbins et al., 2006).
Complicating the interpretation of these fMRI effects, however, is recent evidence of neural reorganization in aging. According to a dedifferentiation hypothesis, aging alters the basic organization of cognitive processes in the brain, so that a region that mediates a particular cognitive operation in young adults may mediate different or additional cognitive operations in older adults (for review, see Grady, 2012). This hypothesis has been used to explain findings in the visual recognition domain, where there is evidence that aging alters the organization of object knowledge (faces, houses, chairs, etc.) in ventral occipito-temporal regions (Park et al., 2004). For example, occipito-temporal regions responding to faces and houses tend to be more distinct in young adults and more overlapping in older adults (Park et al., 2012). This latter effect may reflect reduced sensitivity to preferred stimuli (e.g., less activity for faces in face-selective regions) or increased sensitivity to non-preferred stimuli (e.g., greater activity for houses in face-selective regions) (Park et al., 2012).
Recently we found evidence for age-related dedifferentiation in the memory domain. Consistent with the standard distinction between declarative and nondeclarative memory (e.g., Squire et al., 1990), Dennis and Cabeza (2011) found that young adults selectively recruited the MTL for explicit learning and the striatum for implicit learning. In contrast, older adults recruited not only the MTL but also the striatum for explicit learning, and they engaged not only the striatum but also the MTL for implicit learning. Moreover, whereas MTL and striatal activations were negatively correlated in young adults, they were positively correlated in older adults. In other words, these two memory systems seem to be in direct competition in young adults, but are instead co-activated in older adults. Thus, like ventral occipito-temporal cortex, memory systems may be also affected by a process of age-related dedifferentiation.
Although Dennis and Cabeza (2011) found evidence of age-related dedifferentiation between declarative and nondeclarative memory, they did not investigate the possibility of dedifferentiation within different forms of declarative memory, such as recollection vs. familiarity. Interestingly, there is evidence that recollection and familiarity processes become differentiated during childhood development. One study found that in younger children, HC contributes similarly to recollection and familiarity, and only becomes more specialized for recollection as children get older (Ghetti, DeMaster, Yonelinas, & Bunge, 2010; for review, see Ghetti & Bunge, 2012). Given that dedifferentiation processes during old age are assumed to mirror differentiation processes observed during childhood development, one possible explanation of spared familiarity in older adults is that in old age, familiarity is supported not only by PRC but also by HC.
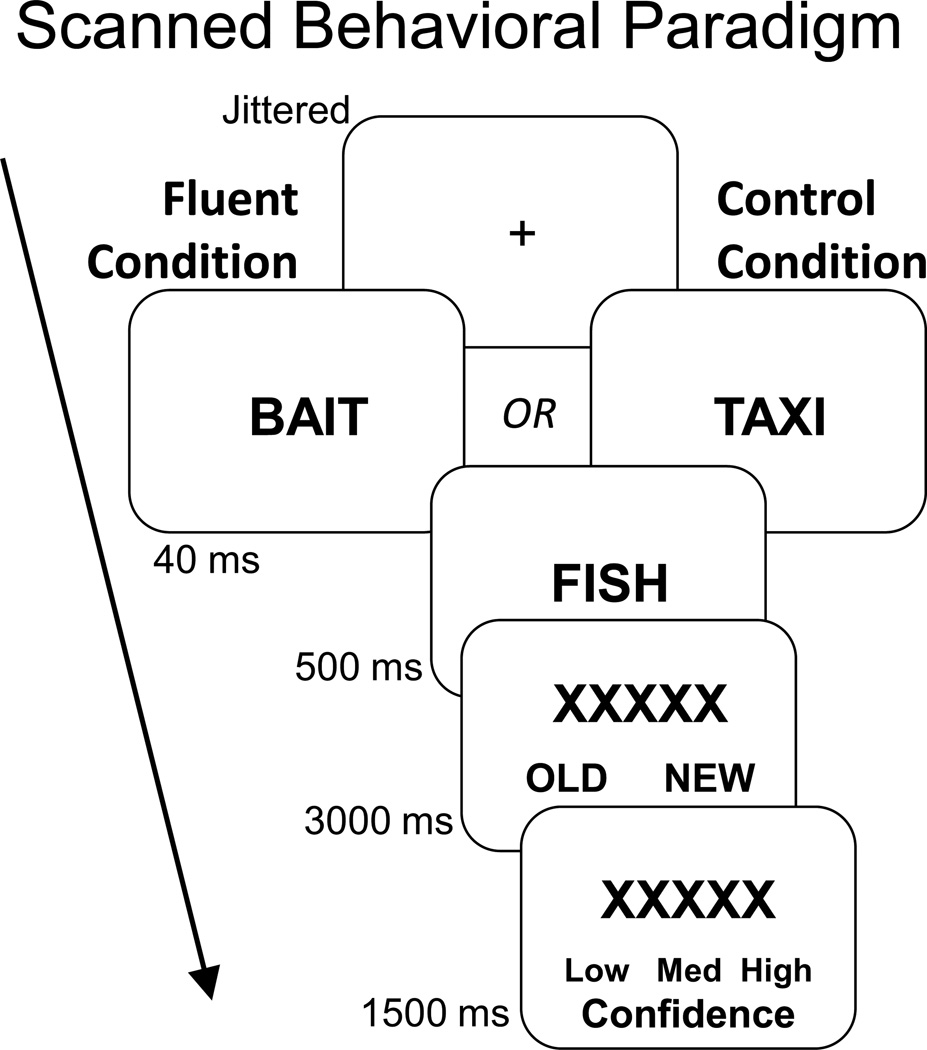
In the current study, we investigated this hypothesis using a variant of the masked priming paradigm (Jacoby & Whitehouse, 1989) (Figure 1). In this paradigm, items on a recognition test are preceded by a briefly presented (masked) prime that is either related or unrelated to its corresponding item. Items preceded by a related, relative to unrelated, prime are more likely to be judged “old.” Importantly, this increase in subjective oldness occurs regardless of whether the item is objectively old or new (Kurilla & Westerman, 2008; Rajaram & Geraci, 2000; Woollams, Taylor, Karayanidis, & Henson, 2008), and is thus a manipulation of perceived oldness rather than memory accuracy. The standard interpretation of this effect is that the unconscious prime increases the fluency of processing the recognition item; the increased fluency is then misattributed to the item having been previously studied (Jacoby & Dallas, 1981; Jacoby & Whitehouse, 1989; Johnston, Hawley, & Elliott, 1991; Kelley & Jacoby, 1998), which leads to increased responses based on familiarity, but not recollection (Ozubko & Yonelinas, 2014; Rajaram & Geraci, 2000; Woollams et al., 2008).
Figure 1.
Before scanning, participants incidentally encoded a list of words by making semantic judgments (living/nonliving). During scanning, participants viewed old and new words and responded to each one with an old/new decision followed by a confidence rating. The critical manipulation occurred before each recognition item when a 40-ms masked prime was presented. In the fluent condition, the masked prime was semantically related to the recognition item (e.g., bait-fish), making this item easier to process, whereas in the control condition, the masked prime was unrelated to the recognition item (e.g., taxi-fish).
In the current experiment, participants were presented with either unrelated or conceptually-related masked primes. This paradigm is well-suited for testing present goals, for several reasons. First, as mentioned above, masked priming reliably increases subjective familiarity, without affecting recollection (Ozubko & Yonelinas, 2014; Rajaram & Geraci, 2000; Woollams et al., 2008). Second, several behavioral studies have shown that the effect of fluency on recognition memory attributions is unaffected by aging (Gold, Marchant, Koutstaal, Schacter, & Budson, 2007; Thapar & Westerman, 2009; Wolk et al., 2005; Wolk, Gold, Signoff, & Budson, 2009). Third, we recently reported data in young adults using this paradigm (Dew & Cabeza, 2013) showing that the effect of fluency on oldness judgments is mediated by PRC. As described previously, the PRC has long been linked with familiarity-based processing (Eichenbaum et al., 2007) – often operationalized as the subjective oldness (i.e., familiarity strength) of both old and new items (Daselaar, Fleck & Cabeza, 2006; Montaldi, Spencer, Roberts, & Mayes, 2006; Wang, Ranganath, & Yonelinas, 2014) – and has shown preserved function in older adults (Daselaar, Fleck, Dobbins et al., 2006).
The main goal of the study was to investigate whether a fluency manipulation that reliably increases familiarity judgments would engage, in older adults, neural regions not otherwise associated with familiarity. Within this goal, we tested two related predictions. First, given recent evidence of dedifferentiation of the MTL memory system (Dennis & Cabeza, 2011), we predicted that, in older adults, a fluency manipulation that increases familiarity-based responses would engage not only the region associated with familiarity (i.e., PRC), but also the region associated with recollection, namely the HC (i.e., reduced task-selectivity). Second, we predicted that reduced task-selectivity in the MTL would be evidenced by age-related differences in not only activity, but also in functional connectivity. Previous research indicates that functional connectivity between HC and PRC decreases during item (i.e., familiarity-based) relative to source (i.e., recollection-based) recognition (Staresina, Fell, Do Lam, Axmacher, & Henson, 2012). Thus, we predicted that, during increased familiarity-based responding, functional integration between HC and PRC would be reduced in young adults. On the other hand, we expected the fluency manipulation to increase functional integration between HC and PRC in older adults, reflecting decreased task-selectivity of HC.
2. Results1
2.1. Behavioral Results
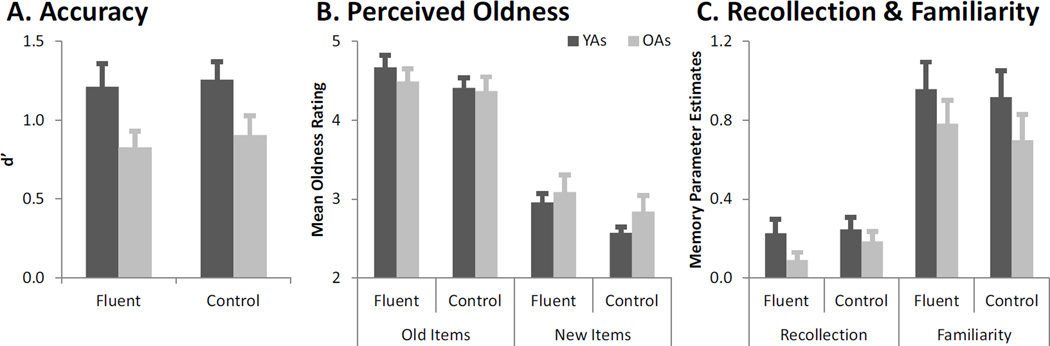
The masked priming paradigm tends to increase perceived oldness for both old and new items (Rajaram & Geraci, 2000; Kurilla & Westerman, 2008; Woollams et al., 2008), and hence, it is assumed to have no effect on recognition memory accuracy (i.e., sensitivity to the memory signal), as measured calculated by d’ (d-prime, the difference between z-transformed hits and FAs) (Macmillan & Creelman, 2005). To test this assumption, confidence ratings were combined with old/new responses to create a 1–6 oldness scale, from 1 = high confidence “new” to 6 = high confidence “old.” Mean oldness ratings were entered into a 2 × 2 × 2 ANOVA with factors of item status (old, new), fluency (fluent, control), and age (young, old). As would be expected, there was a main effect of item status, with higher mean oldness rating to true old trials relative to true new trials, (F[1, 27] = 155.93, p < .0001). There was also a main effect of fluency, (F[1, 27] = 27.87, p < .001), and no fluency by item status interaction, (F[1, 27] = 1.49, p =.23), indicating that fluent primes increased the likelihood that subjects would judge the recognition items as “old,” regardless of whether they were objectively old or new (Figure 2a). Importantly, neither fluency nor item status interacted with age (i.e., age did not participate in any significant two- or three-way interactions) (ps > .15). Thus, the fluency manipulation increased perceived oldness equivalently in young and older adults.
Figure 2.
Consistent with previous studies, the fluency manipulation – in both age groups – equivalently increased perceived oldness to both old and new trials (a), with no resulting effect on accuracy (b), or recollection or familiarity (c). Error bars indicate standard errors.
Next, to assess whether the fluency manipulation influenced memory accuracy, d’ scores were entered into a 2 × 2 ANOVA with factors of fluency (fluent, control) and age (young, old). As expected, there was neither a main effect of fluency (F[1, 27] = .59) nor a fluency by age interaction, (F[1, 27] = .04) indicating that the fluency manipulation did not influence accuracy in either age group. There was, however, a main effect of age (F[1, 27] = 5.58, p < .05). Consistent with the aging literature, independent samples t-tests indicated that d’ was higher in the young relative to old adults in both the fluent (t[27] = 2.08, p < .05) and control (t[27] = 2.14, p < .05) conditions (Figure 2b).
Importantly, masked conceptual priming has previously been shown to selectively increase judgments of familiarity and not recollection (e.g., Rajaram & Geraci, 2000), and previous reporting of the current young adults alone replicated this effect (Dew & Cabeza, 2013). However, it was possible that the fluency manipulation increased recollection in older adults, an effect that would be evidenced by a disproportionate increase in high- over lower-confidence hits in the fluent versus control condition. To determine whether the fluency manipulation interacted with age on the distribution of confidence judgments, proportions of “old” responses to old items (hits) were entered into a 2 × 2 × 3 ANOVA assessing age (young, old), fluency (fluent, control) and confidence (low, medium, high). As expected, there was a significant main effect of fluency (F[1, 27] = 8.34, p <.01), where the proportion of “old” responses was higher for fluent than control old trials. There was also a main effect of confidence level (F[1,27] = 72.20, p <.0001). Follow-up t-contrasts indicated that, collapsing across fluency trials, there were fewer overall low-confidence responses than medium (t[57] = −8.90, p<.0001), and fewer medium confidence responses than high (t[57) = −8.90, p<.0001). However, the 1) fluency × confidence, 2) fluency × age, 3) confidence × age, and 4) fluency × confidence × age interactions were non-significant (ps > .10). Thus, the fluency manipulation did not disproportionately increase high- over lower-confidence responses in either age group, a finding that argues against an influence of fluency on recollection in young or older adults.
Lastly, we sought to confirm that the fluency-related increase in familiarity-based responding to both old and new items did not influence recollection- and familiarity-based performance. To do this, we plotted receiver operating characteristics (ROCs) using the 6-point confidence responses described above. For each participant, hit and FA rates were plotted as a function of decreasing confidence, separately for fluent and control trials. Thus, the leftmost point reflects the proportion of old items receiving a “6” response (i.e., high confidence hits) against the proportion of new items receiving a “6” response (i.e., high confidence FAs). Each consecutive point reflects a more lax scoring criterion (i.e., in the second point, both “6”s and “5”s are treated as hits and FAs). The ROC for each participant was then fit with the dual-process signal-detection (DPSD) model using maximum likelihood estimation to derive the contributions of recollection, which approximates the y-intercept, and familiarity, which approximates the curvilinearity of the function (Yonelinas 1994; 1999). This allowed us to obtain recollection and familiarity parameters for the fluent and control conditions in both young and older adults. Goodness of fit measures (G), shown in Table 1, indicate that the model adequately fit the data in both fluent and control conditions in young and older adults (only one or two participants rejected in each condition).
Table 1.
Goodness of fit measures
| Young | Old | |||
|---|---|---|---|---|
| Fluent | Control | Fluent | Control | |
| Quartiles | ||||
| Lower | 2.29 | 1.40 | 1.92 | 1.33 |
| Median | 4.19 | 2.26 | 3.16 | 2.68 |
| Upper | 6.05 | 4.07 | 4.45 | 3.93 |
| % Rejected | 14% | 7% | 7% | 7% |
We assessed recollection in a 2 × 2 ANOVA of age (young, old) and fluency (fluent, control). There was no main effect of fluency (F[1, 27] = 1.13, p = .30), consistent with our expectation that the fluency manipulation would not change estimates of recollection (Figure 2c). The main effect of age was marginally significant, as recollection was worse in older relative to young adults (F[1, 27] = 3.16, p = .09). Importantly, the age × fluency interaction was not significant (F[1, 27] = .48), indicating that age did not differentially affect estimates of recollection in fluent or control trials. Although recollection was numerically lower in the fluent relative to the control condition in older adults, this difference was not statistically significant (t[14] = −1.47, p = .16). Next, we assessed familiarity in a similar analysis (Figure 2c). There was no effect of age (F[1, 27] = 1.83, p = .19), indicating that familiarity was not significantly impaired in older adults. Additionally, consistent with the d’ and recollection analyses, there was no main effect of fluency, and no age × fluency interaction, (ps > .58).
Together, these results are consistent with previous behavioral findings in the cognitive aging literature showing that fluency affects memory attributions, independently from accuracy, in older adults to the same extent as young (Gold et al., 2007; Thapar & Westerman, 2009; Wolk et al., 2005; Wolk et al., 2009).
2.2. fMRI results
2.2.1. Fluency-related activity reductions
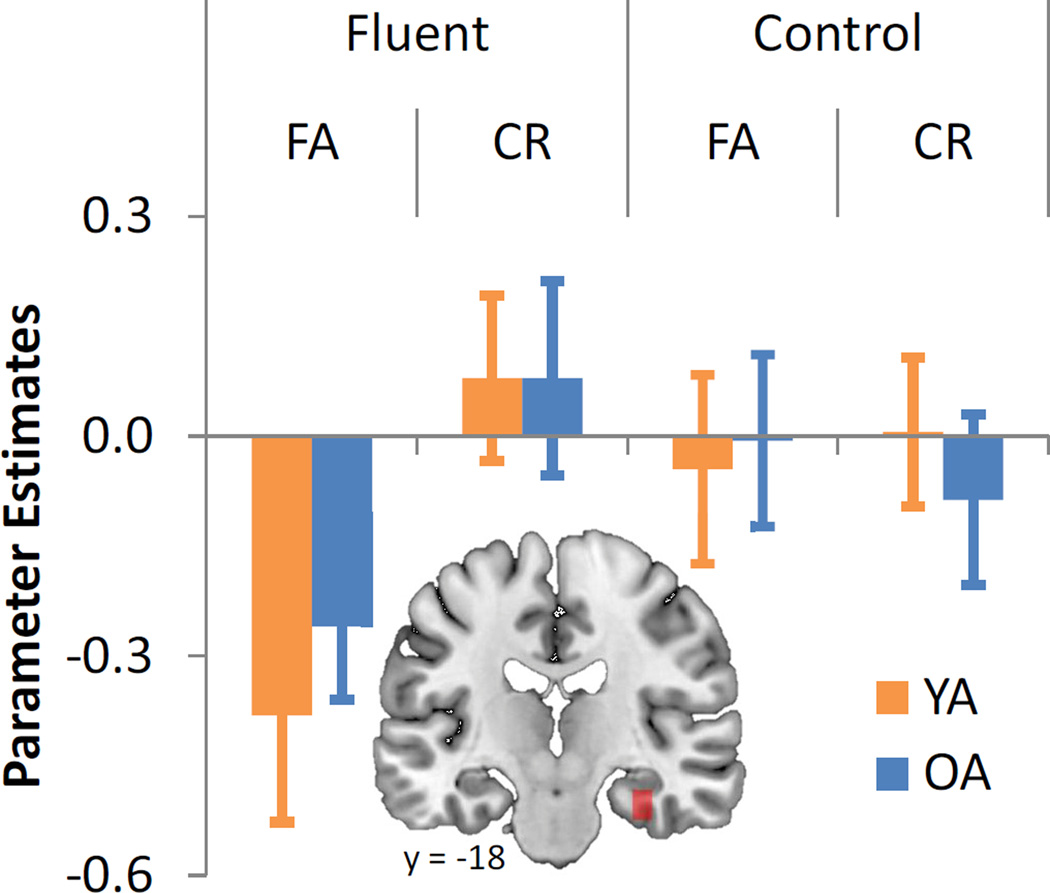
fMRI analyses began with verifying that the fluency effect previously reported in the young adult sample (Dew & Cabeza, 2013) would be similar in older adults. New (unstudied) trials were entered into a 2 × 2 × 2 ANOVA with factors of age (young, old), fluency (fluent, control), and response (FA, correct rejection [CR]). This analysis only assessed the new items, in order to identify regions linked with fluency, independently from an objective long-term memory signal. Within this ANOVA framework, we tested for regions within an MTL ROI showing a fluency by response interaction. Specifically, we examined whether any MTL regions exhibited disproportionately greater activity reductions for fluent FAs relative to fluent CRs than for control FAs relative to control CRs. This allowed us to replicate in the older adult sample the result previously observed in young adults and identify activity reductions associated with fluency-based memory misattributions. As illustrated in Figure 3, this analysis yielded a cluster in right PRC (xyz = 37, −18, −23; Table 2), corrected to p < .05 using Monte Carlo simulations (Ward, 2000), with no suprathreshold clusters exhibiting the opposite pattern.
Figure 3.
Average contrast value in right perirhinal cortex (PRC) in each age group, as a function of fluency, condition, and response. Bar graphs represent mean beta values (effect sizes) in each age group and experimental condition. Error bars indicate standard errors.
Table 2.
Fluency-related activations in MTL
| Region | x | y | z | CS | Z | |
|---|---|---|---|---|---|---|
| Fluency × response interaction (both age groups) | R PRC | 37 | −18 | −23 | 10 | 2.99 |
| Fluency × response interaction (older adults; SVC) | R PRC/HC | 33 | −18 | −20 | 5 | 3.15 |
| Age × fluency × response interaction | R HC | 37 | −26 | −12 | 12 | 3.08 |
To verify that PRC was engaged to the same extent in both age groups, mean activation estimates were extracted in each condition and submitted to a post-hoc mixed-effects ANOVA. Critically, there was no 3-way interaction (F[1, 27] = .002). Thus, this fluency by response interaction was driven by activity reductions to fluent FAs, which did not differ between age groups (t[27] = .69), and illustrates that PRC reductions during new trials in which perceived oldness is manipulated by fluency is unaffected in older adults, consistent with behavioral and patient-based studies showing preserved fluency operations in aging (Gold et al., 2007; Thapar & Westerman, 2009; Wolk et al., 2005; Wolk et al., 2009).
To confirm this result our older adult sample, we examined the same interaction contrast in older adults only within a 10mm sphere centered on the peak voxel reported in Dew & Cabeza (2013) (x = 37, y = −15, z = −27). As listed in Table 2, this yielded a significant right PRC/HC cluster (x = 33, y = −18, z = −20; small volume corrected to p < .05).
2.2.2. Age differences in fluency-related activity reductions
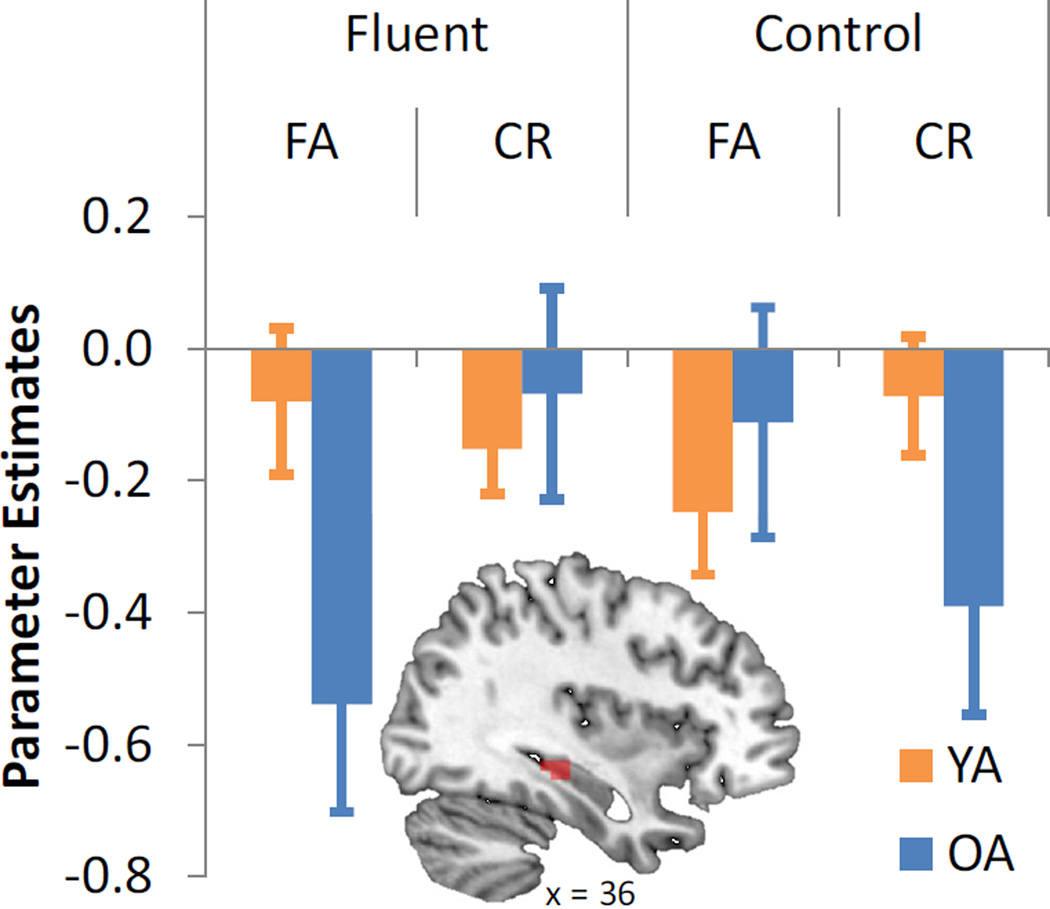
Next, analyses turned toward testing the first novel prediction, that older adults would exhibit fluency-related activity reductions in additional MTL regions. To examine this possibility, we tested for the presence of a 3-way interaction in the same ANOVA model wherein older adults exhibited the fluency effect observed in PRC to a greater extent than young adults. This analysis identified only one region within the MTL ROI: the right HC (xyz = 37, −26, −12; Table 2). As seen in Figure 4, this interaction was due to activity reductions during fluent FAs in older adults, with no differences in young adults. Additionally, a direct comparison of fluent FAs in the two age groups indicated that older adults exhibited significant activity reductions relative to young adults (t[27] = −2.28, p < .05). This finding is consistent with our prediction of age-related dedifferentiation within the MTL, whereby a fluency manipulation that induces familiarity-based responding may involve not only PRC in older adults, but also HC. No suprathreshold clusters showed the interaction in the opposite direction.
Figure 4.
The effects of age and fluency on hippocampal (HC) contributions to FAs and CRs. Bar graphs represent mean beta values (effect sizes) in each age group and experimental condition. Error bars indicate standard errors.
2.2.3. Age differences in fluency-related functional connectivity
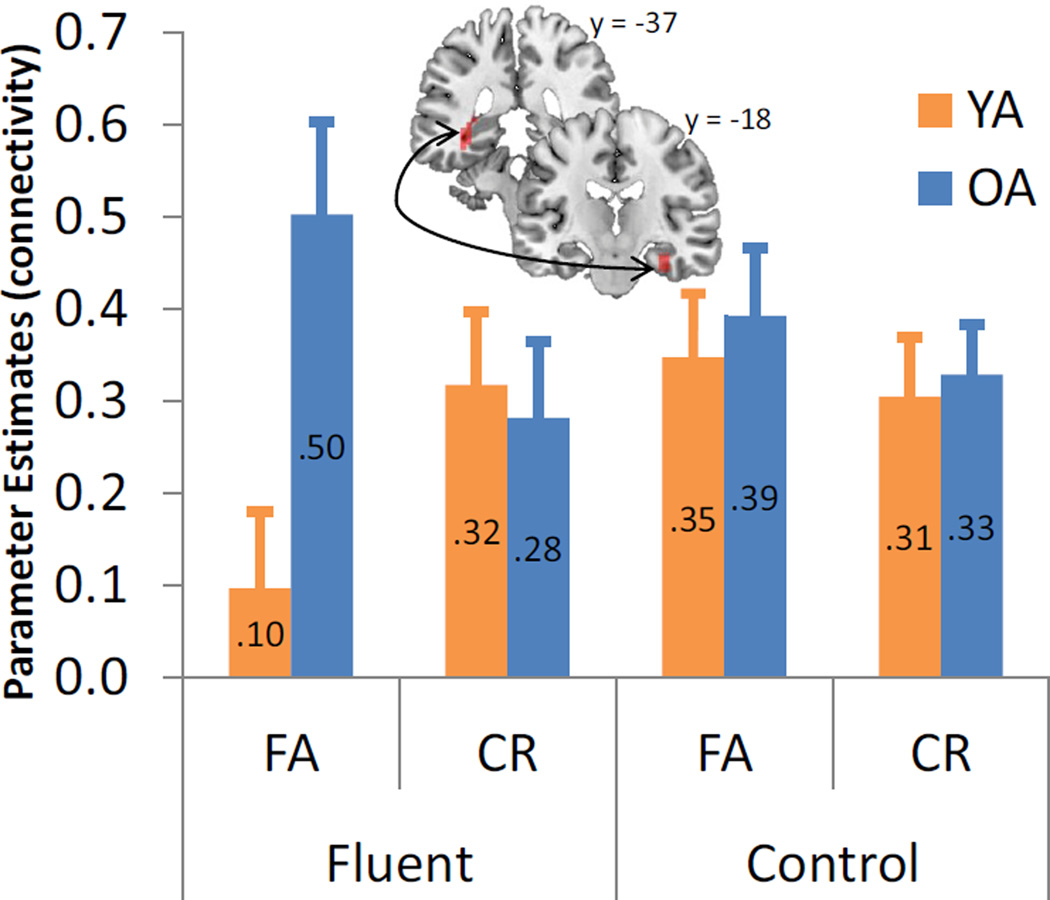
A functional connectivity analysis was then conducted to test the second prediction, that functional integration between HC and PRC would be differentially affected by the experimental manipulation in the two age groups. Using the PRC cluster associated with fluency in both age groups as the seed, we tested for regions showing an age by fluency by response interaction in connectivity with PRC. Specifically, we examined whether any MTL regions exhibited increased connectivity for fluent FAs than fluent CRs relative to control FAs and control CRs in older adults greater than young adults. This analysis yielded one cluster: the left HC (xyz = −35, −37, − 1). As shown in Figure 5, this interaction is driven by different patterns of connectivity in young and older adults. Specifically, we observed a connectivity reduction between HC and PRC in young adults during fluent FAs, which were not significantly different from zero (t[13] = 1.17, p = .26). In contrast to the young, older adults show increased functional integration between HC and PRC during fluent FA trials. A direct comparison showed that older adults exhibited significantly greater PRC-HC functional connectivity than young adults during fluent FAs (t[27] = 3.10, p < .005). No suprathreshold clusters showed the reverse interaction.
Figure 5.
Age × fluency × response interaction on hippocampal (HC) connectivity. Fluency reduced connectivity in young adults and increased connectivity in older adults between left HC and right perirhinal cortex (PRC) during fluent FAs. Bar graphs represent the mean fisher-transformed correlations over time in each age group and experimental condition. Error bars indicate standard errors.
3. Discussion
We investigated whether a fluency manipulation known to increase acontextual judgments of familiarity (Ozubko & Yonelinas, 2014; Rajaram & Geraci, 2000; Woollams et al., 2008) and PRC activity (Dew & Cabeza, 2013) would engage MTL regions in older adults not otherwise specialized for familiarity-based responses – specifically, the HC. The study yielded two main findings. First, although the fluency manipulation yielded PRC activity reductions related to fluency-driven memory misattributions equivalently in both age groups, fluency also uniquely increased HC activity reductions related to subjective familiarity in older adults. Second, we found that functional connectivity between HC and PRC was reduced during fluency-based familiarity memory misattributions in young adults. In contrast, older adults showed the opposite pattern wherein connectivity increased between HC and PRC during familiarity memory misattributions.
Regarding the first finding, the results extend recent evidence of age-related dedifferentiation in the neural mechanisms of memory. As described previously, a recent study found evidence of age-related dedifferentiation between declarative and nondeclarative memory (Dennis & Cabeza, 2011): whereas young adults recruited MTL for declarative memory and the striatum for nondeclarative memory, older adults recruited both regions for both forms of memory. The current study shows that age-related memory dedifferentiation can also occur between different forms of declarative memory, namely recollection- and familiarity-based responding. In particular, our results indicate that aging may result in reduced specialization of the HC for recollection, such that the HC may be engaged during increased familiarity-based processing. This finding mirrors the result of fMRI study in childhood development, in which HC contributed similarly to familiarity and recollection in younger children, and only become more specialized in recollection in older children (Ghetti et al., 2010). This result and the current finding fit with the common assumption in developmental psychology that during childhood, cognitive functions become more distinct and split from each other, whereas during old age, they become less distinct and fuse back together (Li & Sikstrom, 2002; Li, 2005; Li, Brehmer, Shing, Werkle-Bergner, & Lindenberger, 2006).
Familiarity-based d’ was unchanged by our fluency manipulation as fluent old and new items were both more likely to be endorsed as old due to a more liberal response criterion. We focused on fluent new items in the current study order to capture judgments of familiarity driven by a single, controlled manipulation: conceptual fluency. Judgments of oldness to both old and new items are often treated as indices of familiarity (e.g., Daselaar, Fleck & Cabeza, 2006; Montaldi et al., 2006; Wang et al., 2014), which is consistent with our interpretation that the results are driven by increased subjective familiarity to new items due to the fluency manipulation (Dew & Cabeza, 2013). Another intriguing alternative possibility is that our fMRI effects are related to increases in response bias (i.e., tendency to respond old), rather than increases in familiarity-based responding per se. However, consistent with our pattern of behavioral results, studies using the remember/know paradigm (e.g., Ozubko & Yonelinas, 2014; Rajaram & Geraci, 2000; Woollams et al., 2008) indicate that this response bias is restricted to familiarity-based responses, signifying that it is the unique interaction between response bias, familiarity, and fluency that drives our effects. In either case, our results do not necessarily mean that HC is sensitive to familiarity memory in older adults. Rather, our results indicate that in older adults, HC activity is associated with misattributions of familiarity based on increased conceptual fluency.
Although we interpret the HC finding as evidence that in older adults the HC becomes less specialized in recollection – an idea consistent with fMRI evidence dedifferentiation in older adults and differentiation in children – an alternative interpretation is that in older adults, the HC remains specialized in recollection but recollection becomes sensitive to fluency. This possibility is consistent with a few reports that masked priming can sometimes affect recollection estimates (Kurilla & Westerman, 2008; Taylor & Henson 2012; Taylor, Buratto, & Henson, 2013), but does not fit well with the current data. Given that recollection is associated with high-confidence responses (e.g., Koen & Yonelinas 2010; Smith, Wixted, & Squire, 2011; Yonelinas, 2001), this interpretation predicts that fluency effects in older adults should have occurred primarily for high-confidence responses. On the contrary, in both young and older adults, fluency increased perceived oldness ratings across all confidence levels. This pattern is more consistent with the interpretation that conceptual fluency primarily influences familiarity-based responses. This interpretation also accords with behavioral evidence that older adults use conceptual fluency as a heuristic for familiarity-based recognition (e.g., Wolk et al., 2005), and that conceptual fluency increases perceived oldness judgments in subjects with little or no recollection, including patients with Alzheimer’s disease (Budson, Daffner, Desikan, & Schacter, 2000; Gallo, Sullivan, Daffner, Schacter, & Budson, 2004; Wolk et al., 2005; Willems, Germain, Salmon, & Van der Linden, 2009) and MTL amnesics (Keane, Orlando, & Verfaellie, 2006).
To better understand interactions between fluency, familiarity, recollection, and response bias in relation to MTL regions such as PRC and HC, future studies should seek to separate these variables, such as examining increases in familiarity and/or response bias while controlling for fluency, examining other forms of fluency that influence familiarity such as perceptual fluency (Voss, Lucas, Paller, 2012; Yonelinas, 2002), testing the effects of fluency in memory retrieval tasks that rely on recollection (e.g., recall), or perhaps examining fluency in situations where it does not increase the tendency to respond old (e.g., Unkelbach, 2006). Our results would also benefit from future replication; one important caveat of the current study is the relatively small sample size. Thus it remains important for future studies to not only understand the role of fluency and familiarity in MTL regions, but to also confirm our findings in independent samples.
Taken together, the finding of fluency effects in HC in older, but not young, adults fits better with the idea that in older adults the HC becomes sensitive not only to recollection but also to familiarity-based responses (dedifferentiation hypothesis) than with alternative accounts. More generally, this finding is consistent with previous fMRI evidence of age-related increases in HC activity, which have been typically interpreted as reflecting inefficient HC processing (Duverne, Habibi, & Rugg, 2008; Morcom et al., 2007; Yassa et al., 2011). One final interpretation worth investigating further is that increased HC activity in older adults reflects a mechanism that compensates for impaired white-matter integrity. Consistent with this idea, we recently found that increased HC activity in low-performing older adults was negatively correlated with the integrity of white-matter tracts connecting HC (Daselaar et al., 2013). This hypothesis merits follow-up in future studies, as does more general attention in the field to the relationship between function and structure in aging.
Turning to our second main finding, we found that the fluency manipulation reduced functional connectivity between the HC and PRC in young adults but increased it in older adults. This finding is interesting because it speaks not only to aging effects but also adds independently to the literature on fluency-based recognition memory decisions in the young. The results of the new connectivity analysis here in young adults demonstrates that, during fluent FA trials, when familiarity-based responses are influenced by fluency in the absence of an objective memory signal, PRC disengages from HC. This is consistent with evidence that PRC and HC connectivity is decreased during item-based (i.e., familiarity) relative to source-based (i.e., recollection) recognition judgments (Staresina et al., 2012). Regarding the older adults, PRC and HC connectivity increased during fluent FA responses, consistent with our finding that older adults recruit HC during fluency-based familiarity memory misattributions. This pattern in the older group also fits with that reported by Dennis and Cabeza (2011), wherein negative correlations between MTL and striatum were found in young adults, but the competitive relationship between regions was attenuated in older adults. More generally, the results add to a growing literature showing that reduced task-selectivity in aging can be demonstrated both by altered univariate estimates of brain activity and by increased functional integration between regions that, in the young, may compete rather than cooperate.
One important question that remains from the current data is the cause and significance of HC dedifferentiation. What aspect of the fluency manipulation catalyzed the engagement of HC and how this might be related to abnormal HC function? There was no behavioral difference in memory accuracy between fluent versus control trials, and yet, fluent versus control trials differed in the recruitment of HC and its link with memory attributions in older adults. Note that the lack of a behavioral difference does not preclude a dedifferentiation explanation. For example, Dennis & Cabeza (2011) reported evidence of dedifferentiated memory systems in the absence of behavioral changes. This may reflect a compensatory mechanism as HC in older adults adopts processes that are unimpaired in aging. There is evidence of HC sensitivity during familiarity-based retrieval in young adults (Horner et al., 2012; Smith et al., 2011), as well as in both young and older adults (Duarte, Graham, & Henson, 2010), although its functional significance is debated. It has been proposed that familiarity-memory signals – which are induced by the fluency manipulation in the current paradigm – in HC may be a precursor to a contextual retrieval process such as recollection (e.g., Horner et al., 2012), and this process is initiated by item familiarity in PRC (Staresina et al., 2012). Thus, one possibility is that, in age-related dedifferentiation, HC is not only recruited to subserve objective recollective retrieval, but is also recruited by the fluency manipulation, which increased subjective familiarity. Another possible explanation is that the HC was recruited at least partly because of the semantic nature of the fluency manipulation. There is wide agreement that semantic processing is unaffected by aging (Light & Burke, 1988; Salthouse, 1982), and furthermore, older adults have been shown to rely on semantic processing to compensate for other forms of cognitive decline, including episodic retrieval (e.g., Dennis, Kim et al., 2008). There is some evidence that the HC can support semantic memory (Manns, Hopkins, & Squire, 2003), although other studies suggest that the link between HC and semantic memory only emerges when semantic retrieval tasks require episodic memory processes to achieve success (Sheldon & Moscovitch, 2012). Distinguishing between these possibilities will be an important direction for future research.
In summary, a fluency manipulation known to affect acontextual oldness judgments engaged the HC in older adults and contributed to memory misattributions. This result is consistent with evidence that older adults may rely more on familiarity or conceptual fluency for retrieval success in the context of deficient recollection and recollection-related neural activations. Additionally, the results are also indicative of reduced specialization of the HC for recollection in aging, such that the HC may be recruited for familiarity-based processing. This pattern is consistent with dedifferentiation of the MTL memory system in aging.
4. Experimental Procedure
4.1. Participants
A total of 23 young (M age = 22.22; M education = 15.00; 52% female) and 22 older (M age = 70.32; M education = 16.86; 55% female) participants provided informed consent in accordance with rules established by the Institutional Review Board of Duke University Medical Center. Older adults were prescreened for cognitive impairment based on age appropriate normative ranges on the Cambridge Neuropsychological Test Automated Battery (CANTAB), tested in a separate session. All participants were right-handed, native English speakers with no history of neurological or psychiatric disorders. The final sample included in the analyses is comprised of 14 young and 15 older adults. Demographic information for these young and older adults and MMSE and Shipley vocabulary (Shipley, 1940) scores for these older adults are listed in Table 3. MMSE scores were not available for 1 participant.
Table 3.
Participant demographics
| Young | Older | |
|---|---|---|
| N | 14 | 15 |
| % Female | 57% | 53% |
| Age | 22.00 (3.40) | 70.47 (5.96) |
| Education | 14.79 (1.85) | 16.93 (2.74) |
| MMSE | - | 28.86 (1.23) |
| Shipley Vocabulary | - | 37.13 (1.64) |
Nine young adults were excluded for the following reasons: 1 due to experimenter error, 3 due to MTL signal loss, 2 for failure to complete the experiment, 1 for falling asleep, 1 due to an insufficient number of behavioral responses to model in fMRI analyses, and 1 due to an outlying response bias score. Excluded young adults did not differ from our young adult sample in age, gender, or education (ps > .05). Seven older adults were excluded for the following reasons: 1 due to scanner noise, 1 due to an insufficient number of behavioral responses to model in fMRI analyses, 1 due to high blood pressure, 1 due to experimenter error, 1 for failure to complete the experiment, 1 due to poor vision, and 1 for misunderstanding the recognition memory test instructions. Excluded older adults had significantly lower Shipley vocabulary scores (p < .05) relative to our older adult sample, but did not differ in age, gender, education, or MMSE scores (ps > .05).
4.2. Design and Materials
We utilized the masked priming paradigm, a reliable technique from the behavioral memory literature for manipulating familiarity-based oldness judgments (Jacoby & Whitehouse, 1989; Rajaram & Geraci, 2000). Stimuli included 476 English words designated as items (e.g., fish; rope), (mean 3–11 letters, word frequency 74 (Kucera & Francis, 1967). For each item, a fluent prime was created such that it was semantically related to the item (e.g., bait; knot) according to free association norms (Nelson, McEvoy, & Schreiber, 2004) (mean forward association strength = .41, sd =.18). An additional 476 words were assigned as unrelated primes for the control condition (e.g., taxi; goal), with no apparent association and no listed forward- or backward- association as per the norms. Across all stimuli, no primes or items overlapped. Encoding included 238 items. Recognition included the 238 old items plus 238 new items, divided into 63 each of old and new fluent trials, and 112 each of old and new control trials, and another 63 old and new trials in a condition that is not relevant to the current goals. To control for item effects, stimuli were randomly ordered and assigned to condition uniquely for every subject. Lists were divided into seven, 8:14 min runs.
4.3. Behavioral procedure
Before scanning, participants incidentally encoded words via living/nonliving judgments (2000 ms each). During scanning, participants completed the old/new recognition test (see Figure 1). The critical manipulation occurred before each recognition item when a masked prime was presented. Each recognition trial began with the prime for 40ms, which was immediately backward masked by the recognition item for 500ms. The item was replaced by a row of Xs for 4500 ms. During this period, participants had 3000 ms for the old/new recognition judgment and 1500 ms for the low/medium/high confidence rating. These two phases were indicated by the response options, which were displayed at the bottom of the screen. A blind search algorithm determined the order of events within each run and the duration of each jittered fixation (average 2000ms).
4.4. fMRI methods and procedures
Images were collected using a 3T General Electric (Waukesha, WI) scanner with an 8-channel head coil. Anatomical image acquisition involved a T1-weighted 3-dimensional localizer series, with 96 axial slices parallel to the anterior-posterior commissures plane (FOV = 24, voxel dimensions .9 × .9 × 1.9). Functional images were acquired using a T2*-weighted gradient-recalled inward spiral pulse sequence (Glover & Law 2001) sensitive to the blood oxygenation level dependent (BOLD) signal (TR = 2000 ms, TE = 31 ms, FOV = 24, 34 oblique slices with voxel dimension 3.75 × 3.75 × 3.8). The first three volumes at the start of each run were discarded.
Preprocessing and statistical analyses were performed using Statistical Parametric Mapping software (SPM8; Wellcome Department of Cognitive Neurology). Images were corrected for offsets in the time of acquisition by resampling all slices to match the first slice, which was used as a reference slice of the volume. Images were realigned to correct for motion across runs and spatially normalized to an EPI template, using both a 12-parameter affine transformation and a nonlinear transformation using cosine basis functions. The images were spatially smoothed with an 8mm full-width half-maximum Gaussian kernel.
4.5. Statistical analysis
Volumes were examined as temporally correlated time series. Events were modeled as stick functions representing trial onsets, convolved with a canonical hemodynamic response function using the general linear model for event-related designs. Trial types from each condition were segregated based on accuracy and response (e.g., fluent FAs) and were used to model linear contrasts at the fixed effects level against implicit baseline, after which the second-level analysis used subjects as a random effect.
To identify MTL regions that were either activated by both young and older adults, or uniquely activated only by young adults or older adults, interactions were tested in an MTL ROI analysis. Due to the lower signal-to-noise ratio present in MTL (e.g., Schacter & Wagner, 1999), an uncorrected threshold of p < .005, k ≥ 10 was used. This uncorrected threshold has been used in other studies investigating MTL activity (e.g., Bader, Opitz, Reith, & Mecklinger, 2014; Diana, Yonelinas, & Ranganath, 2010; Meyer, Mecklinger, & Friederici, 2010; Wang et al., 2014), and using Monte Carlo simulations with the 3dClustSim program in the AFNI software package (NIMH), the selected voxel-wide and cluster-size thresholds allowed us to correct for multiple comparisons within the MTL ROI to p < .05. The MTL ROI was defined via the Harvard-Oxford cortical and subcortical atlases in the FSL software package (FMRIB), which have been used in studies of middle aged and older participants (e.g., Giorgio et al., 2010) as well as patient populations (e.g., Zarei et al., 2009), and included bilateral HC, and anterior (including PRC) and posterior parahippocampal gyrus. These atlases provide a probabilistic mask that was thresholded at 20 percent (i.e., 20 percent likelihood or greater of being within the prescribed regions) which allowed us to account for variability in brain anatomy due to aging.
An additional analysis was conducted on a 10mm sphere centered on the peak PRC voxel reported in Dew & Cabeza (2013) to examine the role of this PRC region in older adults. This analysis was corrected for multiple comparisons to p < .05 using SPM’s small volume correction function.
For the functional connectivity analysis, the PRC cluster exhibiting activity reductions during fluent FAs in both age groups was used as the seed region. Next, each trial was modeled as a separate event, yielding different beta values for each trial and each subject in the seed cluster of interest (e.g., Daselaar, Fleck, Dobbins et al., 2006; Daselaar, Fleck, Prince, & Cabeza, 2006; Dennis, Hayes et al., 2008; Rissman, Gazzaley, & D'Esposito, 2004). Correlation maps were created for each condition that displayed the correlation magnitude between every voxel and the HC seed region over time. These correlation maps were subsequently entered into SPM to identify brain regions showing differential connectivity as a function of experimental condition. To focus the analysis on the central predictions (i.e., the effects of aging on task-selectivity of MTL subregions), connectivity effects were assessed within the targeted anatomical MTL ROI described above, and as above, interaction effects within this mask were thresholded at p < .005 uncorrected, k ≥ 10.
Highlights.
-
-
We investigated the effects of aging on conceptual fluency during recognition.
-
-
Activity reductions were observed in both age groups in perirhinal cortex (PRC).
-
-
Reductions were also observed in hippocampus (HC) in older, but not young, adults.
-
-
Additionally, PRC-HC connectivity was increased in older relative to young adults.
-
-
Results indicate reduced specialization of HC in healthy aging.
Acknowledgements
We thank Maggie LaFalce, Jared Stokes, Kerry Townsend, Alex Stepanenko, and Alyssa Fowers for help with subject recruitment, stimuli creation, and data collection. We also thank Joshua Koen for assistance with calculating the goodness of fit statistic. This work was supported by the National Institute on Aging, Grants R01-AG23770 and R01-AG19731 (RC) and Fellowship F32-AG038298 (ID).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The fluency-related behavioral and PRC effects in the young adult sample were previously reported in Dew & Cabeza (2013). All other fMRI effects, the ROC analysis, and all comparisons with age are novel and have not been reported elsewhere.
References
- Anderson ND, Ebert PL, Jennings JM, Grady CL, Cabeza R, Graham SJ. Recollection-and familiarity-based memory in healthy aging and amnestic mild cognitive impairment. Neuropsychology. 2008;22:177–187. doi: 10.1037/0894-4105.22.2.177. [DOI] [PubMed] [Google Scholar]
- Bader R, Opitz B, Reith W, Mecklinger A. Is a novel conceptual unit more than the sum of its parts?: FMRI evidence from an associative recognition memory study. Neuropsychologia. 2014;61:123–134. doi: 10.1016/j.neuropsychologia.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Bastin C, Van der Linden M. The contribution of recollection and familiarity to recognition memory: a study of the effects of test format and aging. Neuropsychology. 2003;17:14–24. [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Budson AE, Daffner KR, Desikan R, Schacter DL. When false recognition is unopposed by true recognition: gist-based memory distortion in Alzheimer's disease. Neuropsychology. 2000;14:277–287. doi: 10.1037//0894-4105.14.2.277. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. Triple dissociation in the medial temporal lobes: recollection, familiarity, and novelty. J Neurophysiol. 2006;96:1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Iyengar V, Davis SW, Eklund K, Hayes SM, Cabeza RE. Less wiring, more firing: Low-performing older adults compensate for impaired white matter with greater neural activity. Cereb Cortex. 2013 doi: 10.1093/cercor/bht289. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Prince SE, Cabeza R. The medial temporal lobe distinguishes old from new independently of consciousness. J Neurosci. 2006;26:5835–5839. doi: 10.1523/JNEUROSCI.0258-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PS, Glisky EL. Neuropsychological correlates of recollection and familiarity in normal aging. Cogn Affect Behav Neurosci. 2002;2:174–186. doi: 10.3758/cabn.2.2.174. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R. Age-related dedifferentiation of learning systems: an fMRI study of implicit and explicit learning. Neurobiol Aging. 2011;32:2318.e17–2318.e30. doi: 10.1016/j.neurobiolaging.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettel SA, Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. J Exp Psychol Learn. 2008;34:791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Kim H, Cabeza R. Age-related differences in brain activity during true and false memory retrieval. J Cogn Neurosci. 2008;20:1390–1402. doi: 10.1162/jocn.2008.20096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dew IT, Cabeza R. A broader view of perirhinal function: from recognition memory to fluency-based decisions. J Neurosci. 2013;33:14466–14474. doi: 10.1523/JNEUROSCI.1413-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranaganath C. Medial temporal lobe activity during source retrieval reflects information type, not memory strength. J Cogn Neurosci. 2010;22:1808–1818. doi: 10.1162/jocn.2009.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Graham KS, Henson RN. Age-related changes in neural activity associated with familiarity, recollection and false recognition. Neurobiol Aging. 2010;31:1814–1830. doi: 10.1016/j.neurobiolaging.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Duverne S, Habibi A, Rugg MD. Regional specificity of age effects on the neural correlates of episodic retrieval. Neurobiol Aging. 2008;29:1902–1916. doi: 10.1016/j.neurobiolaging.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo DA, Sullivan AL, Daffner KR, Schacter DL, Budson AE. Associative recognition in Alzheimer's disease: evidence for impaired recall-to-reject. Neuropsychology. 2004;18:556–563. doi: 10.1037/0894-4105.18.3.556. [DOI] [PubMed] [Google Scholar]
- Ghetti S, Bunge SA. Neural changes underlying the development of episodic memory during middle childhood. Dev Cogn Neurosci. 2012;2:381–395. doi: 10.1016/j.dcn.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S, DeMaster DM, Yonelinas AP, Bunge SA. Developmental differences in medial temporal lobe function during memory encoding. J Neurosci. 2010;30:9548–9556. doi: 10.1523/JNEUROSCI.3500-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, Johansen-Berg H. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage. 51:943–951. doi: 10.1016/j.neuroimage.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Gold CA, Marchant NL, Koutstaal W, Schacter DL, Budson AE. Conceptual fluency at test shifts recognition response bias in Alzheimer's disease: Implications for increased false recognition. Neuropsychologia. 2007;45:2791–2801. doi: 10.1016/j.neuropsychologia.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL. The cognitive neuroscience of ageing. Nat Rev Neurosci. 2012;13:491–505. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner AJ, Gadian DG, Fuentemilla L, Jentschke S, Vargha-Khadem F, Duzel E. A rapid, hippocampus-dependent, item-memory signal that initiates context memory in humans. Curr Biol. 2012;22:2369–2374. doi: 10.1016/j.cub.2012.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby LL. Ironic effects of repetition: measuring age-related differences in memory. J Exp Psychol Learn. 1999;25:3–22. doi: 10.1037//0278-7393.25.1.3. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Dallas M. On the relationship between autobiographical memory and perceptual learning. J Exp Psychol Gen. 1981;110:306–340. doi: 10.1037//0096-3445.110.3.306. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Whitehouse K. An illusion of memory: False recognition influenced by unconscious perception. J Exp Psychol Gen. 1989;118:126–135. [Google Scholar]
- Jennings JM, Jacoby LL. Automatic versus intentional uses of memory: aging, attention, and control. Psychol Aging. 1993;8:283–293. doi: 10.1037//0882-7974.8.2.283. [DOI] [PubMed] [Google Scholar]
- Johnston WA, Hawley KJ, Elliott JM. Contribution of perceptual fluency to recognition judgments. J Exp Psychol Learn. 1991;17:210–223. doi: 10.1037//0278-7393.17.2.210. [DOI] [PubMed] [Google Scholar]
- Keane MM, Orlando F, Verfaellie M. Increasing the salience of fluency cues reduces the recognition memory impairment in amnesia. Neuropsychologia. 2006;44:834–839. doi: 10.1016/j.neuropsychologia.2005.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley CM, Jacoby LL. Subjective reports and process dissociation: Fluency, knowing, and feeling. Acta Psychol. 1998;98:127–140. [Google Scholar]
- Koen JD, Yonelinas AP. Memory variability is due to the contribution of recollection and familiarity, not to encoding variability. J Exp Psychol Learn Mem Cogn. 2010;36:1536–1542. doi: 10.1037/a0020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera H, Francis WN. Computational analysis of present-day American English. Providence, RI: Brown University Press; 1967. [Google Scholar]
- Kurilla BP, Westerman DL. Processing fluency affects subjective claims of recollection. Mem Cognit. 2008;36:82–92. doi: 10.3758/mc.36.1.82. [DOI] [PubMed] [Google Scholar]
- Li SC. Neurocomputational perspectives linking neuromodulation, processing noise, representational distinctiveness, and cognitive aging. In: Cabeza R, Nyberg L, Park D, editors. Cognitive neuroscience of aging: Linking cognitive and cerebral aging. New York: Oxford University Press; 2005. pp. 354–379. [Google Scholar]
- Li SC, Brehmer Y, Shing YL, Werkle-Bergner M, Lindenberger U. Neuromodulation of associative and organizational plasticity across the life span: empirical evidence and neurocomputational modeling. Neurosci Biobehav Rev. 2006;30:775–790. doi: 10.1016/j.neubiorev.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Li SC, Sikstrom S. Integrative neurocomputational perspectives on cognitive aging, neuromodulation, and representation. Neurosci Biobehav Rev. 2002;26:795–808. doi: 10.1016/s0149-7634(02)00066-0. [DOI] [PubMed] [Google Scholar]
- Light LL, Burke DM. Patterns of language and memory in old age. In: Light LL, Burke DM, editors. Language, memory and aging. New York: Cambridge University Press; 1988. pp. 244–271. [Google Scholar]
- Macmillan NA, Creelman CD. Detection Theory. New York: Lawrence Erlbaum Associates; 2005. [Google Scholar]
- Manns JR, Hopkins RO, Squire LR. Semantic memory and the human hippocampus. Neuron. 2003;38:127–133. doi: 10.1016/s0896-6273(03)00146-6. [DOI] [PubMed] [Google Scholar]
- Meyer P, Mecklinger A, Friederici AD. On the processing of semantic aspects of experience in the anterior medial temporal lobe: an event-related fMRI study. J Cogn Neurosci. 2010;22:590–601. doi: 10.1162/jocn.2009.21199. [DOI] [PubMed] [Google Scholar]
- Montaldi D, Spencer TJ, Roberts N, Mayes AR. The neural system that mediates familiarity memory. Hippocampus. 2006;16:504–520. doi: 10.1002/hipo.20178. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD. Age effects on the neural correlates of episodic retrieval: increased cortical recruitment with matched performance. Cereb Cortex. 2007;17:2491–2506. doi: 10.1093/cercor/bhl155. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Shing YL, Kilb A, Werkle-Bergner M, Lindenberger U, Li SC. Adult age differences in memory for name-face associations: The effects of intentional and incidental learning. Memory. 2009;17:220–232. doi: 10.1080/09658210802222183. [DOI] [PubMed] [Google Scholar]
- Nelson DL, McEvoy CL, Schreiber TA. The University of South Florida free association, rhyme, and word fragment norms. Behav Res Methods. 2004;36:402–407. doi: 10.3758/bf03195588. [DOI] [PubMed] [Google Scholar]
- Ozubko JD, Yonelinas AP. The disruptive effects of processing fluency on familiarity-based recognition in amnesia. Neuropsychologia. 54:59–67. doi: 10.1016/j.neuropsychologia.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proc Natl Acad Sci USA. 2004;101:13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Carp J, Kennedy KM, Rodrigue KM, Bischof GN, Huang CM, Rieck JR, Polk TA, Park DC. Neural broadening or neural attenuation? Investigating age-related dedifferentiation in the face network in a large lifespan sample. J Neurosci. 2012;32:2154–2158. doi: 10.1523/JNEUROSCI.4494-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Kalpouzos G, Nilsson LG, Ryberg M, Nyberg L. Preserved hippocampus activation in normal aging as revealed by fMRI. Hippocampus. 2011;21:753–766. doi: 10.1002/hipo.20794. [DOI] [PubMed] [Google Scholar]
- Rajaram S, Geraci L. Conceptual fluency selectively influences knowing. J Exp Psychol Learn. 2000;26:1070–1074. doi: 10.1037//0278-7393.26.4.1070. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Adult cognition: An experimental psychology of human aging. New York: Springer-Verlag; 1982. [Google Scholar]
- Sheldon S, Moscovitch M. The nature and time-course of medial temporal lobe contributions to semantic retrieval: an fMRI study on verbal fluency. Hippocampus. 2012;22:1451–1466. doi: 10.1002/hipo.20985. [DOI] [PubMed] [Google Scholar]
- Shipley WC. A self-administering scale for measuring intellectual impairment and deterioration. J Psychol. 1940;9:371–377. [Google Scholar]
- Smith CN, Wixted JT, Squire LR. The hippocampus supports both recollection and familiarity when memories are strong. J Neurosci. 2011;31:15693–15702. doi: 10.1523/JNEUROSCI.3438-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer WD, Raz N. Differential effects of aging on memory for content and context: a meta-analysis. Psychol Aging. 1995;10:527–539. doi: 10.1037//0882-7974.10.4.527. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S, Cave CB, Haist F, Musen G, Suzuki WA. Memory: organization of brain systems and cognition. Cold Spring Harb Symp Quant Biol. 1990;55:1007–1023. doi: 10.1101/sqb.1990.055.01.096. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Fell J, Do Lam AT, Axmacher N, Henson RN. Memory signals are temporally dissociated in and across human hippocampus and perirhinal cortex. Nat Neurosci. 2012;15:1167–1173. doi: 10.1038/nn.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Buratto LG, Henson RN. Behavioral and neural evidence for masked conceptual priming of recollection. Cortex. 2013;49:1511–1525. doi: 10.1016/j.cortex.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Henson RN. Could masked conceptual primes increase recollection? The subtleties of measuring recollection and familiarity in recognition memory. Neuropsychologia. 2012;50:3027–3040. doi: 10.1016/j.neuropsychologia.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Westerman DL. Aging and fluency-based illusions in recognition memory. Psychol Aging. 2009;24:595–603. doi: 10.1037/a0016575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- Unkelbach C. The learned interpretation of cognitive fluency. Psychol Sci. 2006;17:339–345. doi: 10.1111/j.1467-9280.2006.01708.x. [DOI] [PubMed] [Google Scholar]
- Voss JL, Lucas HD, Paller KA. More than a feeling: Pervasive influences of memory without awareness of retrieval. Cogn Neurosci. 2012;3:193–207. doi: 10.1080/17588928.2012.674935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Wang WC, Ranganath C, Yonelinas AP. Activity reductions in perirhinal cortex predict conceptual priming and familiarity-based recognition. Neuropsychologia. 2014;52:19–26. doi: 10.1016/j.neuropsychologia.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DB. Simultaneous Inference for fMRI Data. Biophysics Research Institute, Medical College of Wisconsin; 2000. [Google Scholar]
- Willems S, Germain S, Salmon E, Van der Linden M. Patients with Alzheimer's disease use metamemory to attenuate the Jacoby-Whitehouse illusion. Neuropsychologia. 2009;47:2672–2676. doi: 10.1016/j.neuropsychologia.2009.04.029. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Gold A, Signoff ED, Budson AE. Discrimination and reliance on conceptual fluency cues are inversely related in patients with mild Alzheimer's disease. Neuropsychologia. 2009;47:1865–1872. doi: 10.1016/j.neuropsychologia.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Schacter DL, Berman AR, Holcomb PJ, Daffner KR, Budson AE. Patients with mild Alzheimer's disease attribute conceptual fluency to prior experience. Neuropsychologia. 2005;43:1662–1672. doi: 10.1016/j.neuropsychologia.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Woollams AM, Taylor JR, Karayanidis F, Henson RN. Event-related potentials associated with masked priming of test cues reveal multiple potential contributions to recognition memory. J Cogn Neurosci. 2008;20:1114–1129. doi: 10.1162/jocn.2008.20076. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CE. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011;21:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. Receiver operating characteristics in recognition memory: Evidence for a dual process model. J Exp Psychol Learn. 1994;20:1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The contribution of recollection and familiarity to recognition and source memory: An analysis of receiver operating characteristics and a formal model. J Exp Psychol Learn. 1999;25:1415–1434. doi: 10.1037//0278-7393.25.6.1415. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Consciousness, control, and confidence: The 3 Cs of recognition memory. J Exp Psychol Gen. 2001;130:361–379. doi: 10.1037//0096-3445.130.3.361. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. J Mem Lang. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Widaman K, Mungas D, Reed B, Weiner MW, Chui HC. Memory in the aging brain: doubly dissociating the contribution of the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1134–1140. doi: 10.1002/hipo.20341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M, Damoiseaux JS, Morgese C, Beckmann CF, Smith SM, Matthews PM, Scheltens P, Rombouts SA, Barkhof F. Regional white matter integrity differentiates between vascular dementia and Alzheimer disease. Stroke. 2009;40:773–779. doi: 10.1161/STROKEAHA.108.530832. [DOI] [PubMed] [Google Scholar]