Abstract
The principle of magnetic drug targeting, wherein therapy is attached to magnetically responsive carriers and magnetic fields are used to direct that therapy to disease locations, has been around for nearly two decades. Yet our ability to safely and effectively direct therapy to where it needs to go, for instance to deep tissue targets, remains limited. To date, magnetic targeting methods have not yet passed regulatory approval or reached clinical use. Below we outline key challenges to magnetic targeting, which include designing and selecting magnetic carriers for specific clinical indications, safely and effectively reaching targets behind tissue and anatomical barriers, real-time carrier imaging, and magnet design and control for deep and precise targeting. Addressing these challenges will require interactions across disciplines. Nanofabricators and chemists should work with biologists, mathematicians and engineers to better understand how carriers move through live tissues and how to optimize carrier and magnet designs to better direct therapy to disease targets. Clinicians should be involved early on and throughout the whole process to ensure the methods that are being developed meet a compelling clinical need and will be practical in a clinical setting. Our hope is that highlighting these challenges will help researchers translate magnetic drug targeting from a novel concept to a clinically-available treatment that can put therapy where it needs to go in human patients.
Introduction
Magnetic drug targeting refers to making therapy magnetically responsive, so that it can be manipulated inside the body by external magnets, and thus focused to disease locations such as deep tissue tumors. In the first human trials of magnetic drug targeting (1), the chemotherapy drug epidoxorubicin was attached to 100 nm diameter bio-compatible iron-core particles, these particles were administered systemically, and an external magnet was used to concentrate the therapy to inoperable but shallow tumors (Figure 1(a)). In these human safety trials, blood sample HPLC (high-performance liquid chromatography) and magnetic resonance imaging (MRI) measurements showed that the magnet removed about half of the particles from blood circulation and collected them to the vicinity of the tumor (1, 2). It was found that all patients tolerated the magnetic drug delivery procedure and that peak epidoxorubicin concentrations in blood plasma were much reduced for patients with magnetic drug targeting as compared to patients who received conventional systemic epidoxorubicin applications. Since a single magnet can only attract magnetic particles (3–5), these trials were restricted to treating tumors near the skin surface.
Figure 1.
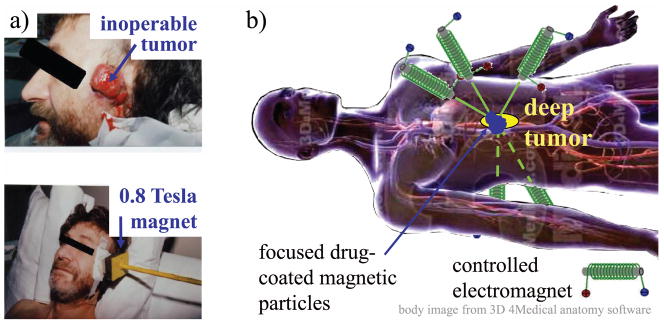
(a) The first human trials in magnetic drug targeting (1). Epidoxorubicin-coated magnetic nano-particles were administered systemically to advanced head and neck and breast cancer patients, and a single permanent magnet was held near inoperable but shallow tumors to concentrate the chemotherapy. (b) A goal in magnetic targeting is to use magnetic fields to focus therapy precisely to any desired target in the body, for example to a deep tumor as illustrated. Currently there are no magnetic systems that can achieve this kind of precise and deep focusing.
Today, 18 years later, although there have been significant advances in the field, we are still a long way from being able to magnetically direct therapy to wherever it needs to go in human patients – to deep targets (Figure 1(b)), to thousands of metastases, safely to targets in the brain, and to disease targets behind cellular and tissue barriers or anatomical obstructions. The majority of prior studies have been restricted to small animals, there have been only a small number of human clinical trials (1, 6–9), and the critical issue of scale-up to human dimensions is still open. Our goal in this article is to identify open challenges in magnetic drug targeting that must be solved so that it can safely and effectively target a broad range of human diseases.
Choosing Magnetic Carriers, for Specific Clinical Needs
A wide variety of magnetic carriers have been demonstrated and proposed in the literature. Drugs and gene therapy have been attached to magnetic nano-particles (10–16), bio-compatible microscopic or nano-scale capsules have been filled with both drugs and magnetic materials (17, 18), and live cells have been cultured in media that contains magnetic particles so that the cells injest the particles and can then be manipulated by magnetic fields (19, 20). Multiple excellent review articles are available that describe progress and challenges in developing safe and effective magnetic carriers (9–11, 21–24). These carriers differ in their properties (size, shape, flexibility, coatings, magnetic loading, drug loading), experience different size forces for the same applied magnetic field, and encounter different motion resistance for different bodily fluids, barriers, and tissue types. One carrier type does not fit all clinical needs, and thus there is a need to select, design, and implement carriers that are matched to specific clinical indications. The particles that are best for targeting of deep tumors after systemic administration are not necessarily best for crossing the blood-brain barrier (BBB) or for safely penetrating the window membranes to reach inner ear diseases.
For the situation where magnetic particles are administered systemically into the blood stream and external magnets are meant to capture and collect them to a desired target (as in the original Lübbe clinical trials (1), Figure 1(a)), a first question is whether the applied magnetic field is sufficient to hold particles against blood flow at the target region. We investigated this question in (25, 26). Based on physical first principles (particle diffusion and convection by blood, magnetic drift), we computed the distribution of particles in and around small and large blood vessels, located at any depth in the body, for the range of magnetic field strengths used/anticipated in magnetic drug targeting, and we compared our predictions to all then-available in-vitro and in-vivo experimental data. We collapsed the large magnetic delivery design space (particle size, magnet size, shape, and strength, blood vessel depth and flow velocity) to 3 essential non-dimensional parameters, and computed the parameter region where the applied magnetic field could hold particles against blood flow. This analysis answered which particles could or could not be captured by an applied magnet. It predicted in which blood vessels (with which diameter, depth, and blood velocity) which particles could be held magnetically against blood flow, and it matched till-then available experimental data. For example, we were able to predict the depth of particle focusing observed by MRI in the Lübbe clinical trials. Next questions for carrier behaviour in blood flow include expanding the analysis to cover non-spherical carriers (e.g. rods, wires, shells, cubes, triangles, etc, (27–29)), as well as extending the analysis to living cells (e.g. stem cells) loaded with magnetic materials.
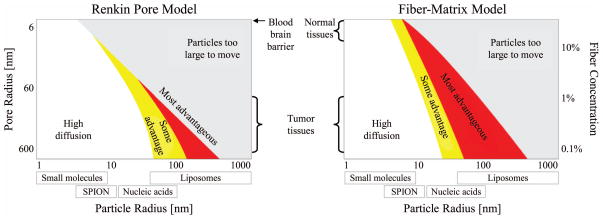
To optimize therapy delivery into tissues targets, the next steps are to better understand carrier transport and penetration under magnetic forces through vessel walls and into tissues (e.g. across the blood-brain barrier), through various tissue types (liver, muscle, fat, brain, etc.), and across anatomical barriers (skin, ear window membranes, eye sclera, etc.). In (30), based on breast-cancer patient autopsy data and numerical simulations, we predicted that magnetically shifting nano-particles would allow them to reach thousands of poorly-vascularized liver metastases, which otherwise would not be reached effectively by nano-therapy (Figure 2(a)). Normal and cancerous liver resistance to carrier motion was represented according to two commonly used mathematical models – the Renkin pore model (31) and the fiber-matrix model (32) – and we found an optimal particle size for shift effectiveness. Too small particles (< 10 nm diameter) would not experience sufficient magnetic force to move effectively through the liver (because magnetic forces scale with particle volume). However, if particles were too big (> 400 nm) they would encounter too much tissue resistance. Our optimal particle size prediction (see Figure 2(b) below) must now be tested against animal experiments, and to that end we have initiated a program to measure magnetically induced motion of different particle types in animals and freshly-excised tissue samples (33). In addition to size, our preliminary data indicates that particle surfaces and coatings are key parameters. For example, chitosan-coated particles move better than starch particles of the same size and magnetic loading through freshly excised rat liver tissue. Thus there is a need to select both carrier size and coatings to enable the most effective magnetic delivery of therapy to target tissues.
Figure 2.
The magnetic sweep concept to reach hundreds of poorly vascularised metastatic tumors. In human autopsy studies of breast cancer patients who died from their disease, we measured vascularisation in and around hundreds of micro-metastases (top middle panel: tumor marked by the black oval, blood vessels marked in gray). A magnet on either side of the patient could pull nano-scale magnetic carriers from the surrounding well-vascularized normal liver into each poorly-vascularized micro-metastasis. Our simulations indicate that there is an optimal nano-particle size: big enough to react to the applied magnet, small enough to move effectively through liver tissue. (See (30) for more details. Figure reproduced with permission from Copyright © 2011 Nacev et al, publisher and licensee Dove Medical Press Ltd.)
For directing magnetic nano-particles through tissue barriers, for example through the ear-drum to reach middle ear infections without ear-drum puncture or through the eye sclera to treat the retina (34, 35), it is also still an open question which particle sizes and coatings are best. To answer these type of questions, we believe there should be reproducible standardized experimental methods to characterize the transport of magnetic carriers through live tissue, to measure which carriers move most effectively yet safely through blood vessel walls, different tissue types, and across barriers.
The BBB is of particular interest as it can prevent or limit therapy from reaching brain tumors and other brain diseases (Figure 3(a)) (36–43). Unlike in other organs (e.g. liver, spleen, etc.), endothelial cells that separate the blood stream from brain tissue are tightly attached to each other, minimizing free passage of substances between blood and the brain (36, 37). Additionally, efflux transporters (e.g., P-glycoprotein) located in the membranes of these cells actively pump out most drugs that arrive (38). Thus, to deliver therapy to the brain requires strategies to safely and effectively bypass the BBB, as illustrated in Figure 3(b).
Figure 3.
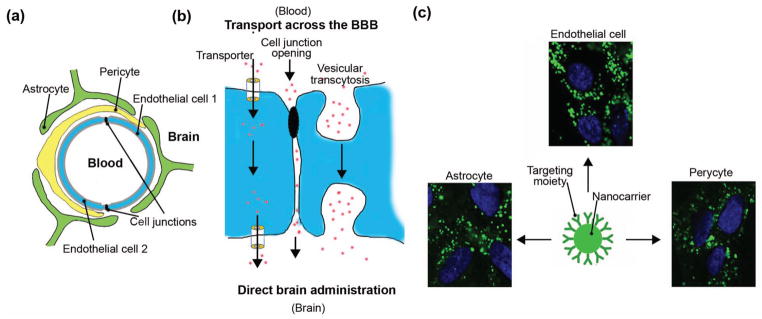
Transport across the blood-brain barrier (BBB). (a) Schematic representation of a blood capillary vessel in the brain. Endothelial cells surround the vessel lumen and seal the passage into the brain by tight cell-cell junctions. Pericytes and astrocytes surround the endothelial lining, further tightening the barrier. (b) Delivery of therapeutics into the brain can be achieved by direct administration through the skull, or by using therapeutics that will cross the BBB. The later involves temporary disruption of the BBB cell-cell junctions (paracellular route) or transport across the endothelial cell (transcellular route), including passage using transporter protein channels or vesicular transcytosis. (c) Nanoparticles coated with ligands which can bind to receptors of vesicular transcytosis (ICAM-1 is shown in this example) results in active uptake by cells of the BBB, including endothelial cells, astrocytes, and pericytes (42).
A potential approach to pass through the BBB is to mimic the active transport mechanisms by which natural body substances (e.g. nutrients) and natural body carriers (e.g. lipoproteins, exosomes) travel from the blood into the brain (36, 40). This “Trojan horse” approach leverages the presence of specific channels in cells of the blood-brain barrier (36, 37). Alternatively, transport can be mediated by binding to specific receptors on endothelial cells, which triggers uptake at the blood interface, transport across the barrier, and release at the brain interface (transcytosis (36, 41)). Magnetic forces can potentially help therapies cross the BBB. Recent studies in cell cultures and rodents have shown that magnetic nanoparticles of different types (silica, PAMAM dendrimers, liposomal, etc.) can traverse the BBB for gene transfection or drug delivery (44–49). Use of magnetic carriers for therapy delivery into the brain requires special attention to safety. Carriers that contain magnetic materials, and that also have protective, solubilizing, or molecular targeting coatings, can easily approach or exceed the size limitations of the natural transport pathways shown in Figure 3(b). For safe and effective delivery into the brain, carrier designs should be carefully adapted to physiological variables of blood flow, disease status, and brain tissue architecture (41).
After carriers have reached their disease target, in the body or in the brain, they must further safely and effectively release or provide their therapeutic payload to target tissues. Items that should be addressed include drug loading and drug release rates from the carriers under physiological conditions, as well as the resulting uptake and elimination of the drug and the body’s response to the drug (pharmacokinetics and pharmacodynamics)(9–11, 21–24, 50, 51). Magnetic carrier design should also avoid carrier agglomeration which can block blood vessels and must ensure adequate stability and shelf life to enable regulatory approval and subsequent clinical use.
To answer the type of magnetic drug delivery challenges posed above, as a research community we need to select and optimize magnetic carriers for specific clinical needs, and we must bring those carriers up to a level where they can pass regulatory scrutiny. Rather than continuing to implement new types of magnetic carriers because we can, we should take a step back and ask: which type of carrier is best for this clinical need? And what advances are needed for this carrier so that it can achieve FDA or EMA (European Medicines Agency) regulatory approval? Since different carriers travel differently through blood and tissue types, and since size, shape, and coatings can change magnetic forces and resistance to motion by orders of magnitude(26, 33, 52–54) as well as impact carrier drug release and safety, this is a choice that should be made with care. If only one or two parameters are considered (e.g. particle size and coating), it is conceivable to choose the best type of particle for a particular clinical need by live animal testing – one could imagine testing a 3 × 3 matrix of cases with 3 particle sizes and 3 coatings for a few animals in each group and selecting the best one. However, when we also consider carrier shape, flexibility, targeting coatings, the potential for agglomeration (which is influenced by magnetic field strength and concentration), drug release rates, and using living cells loaded with magnetic materials as carriers, then the design space becomes too large to search with animal studies alone. We need to begin to understand how magnetic carrier properties influence their motion and drug release in vivo, at least to the degree that we can begin to make sensible judgements about when to use which carriers. Then we need to select a few best candidates and carry out the extensive safety and efficacy animal testing that will enable human trials and regulatory approval.
Imaging of Carriers and Therapy, in Real-Time
To magnetically direct the therapy to the right place, in most cases it will be necessary to be able to visualize where the therapy is versus where the disease targets are located. Thus effective (real-time and deep) imaging of magnetic carriers is a key need, and is also a research challenge. In the Lübbe clinical trials, magnetic nano-particles were imaged immediately after patient treatment by magnetic resonance imaging (MRI). Accumulation of particles in and around the tumor disrupted the MR signal and was visible as an extinction phenomena (2). However, it was not possible to magnetically treat and image at the same time because the permanent magnet shown in Figure 1(a) would have interfered with MRI operation. Nor was it possible to quantify the amount of magnetic particles delivered to the tumor by the disruption of a MR signal.
Two emerging methods have the potential to image magnetic carriers deep in-vivo and in real time. Magnetic particle imaging (MPI) exploits the non-linear magnetic response of super-paramagnetic and paramagnetic nano-particles under strong (> 3 Tesla/meter) magnetic field gradients (55–59). It creates a magnetic field node point within the imaging location using two external coils, then additional driving coils apply a time-varying magnetization to which particles near the node point will respond. Finally, additional sensing coils interpret the particle magnetization response and infer the particle concentration at the node point. MPI was specifically designed for imaging of magnetic carriers, and has been shown to have sufficient spatial and temporal resolution to resolve particle concentrations in the beating heart of a mouse (59).
The spatial resolution of both MPI and MRI is limited by the strength of the spatial magnetic gradient that can be applied (60, 61). In prior studies, it was thought that peripheral nerve stimulation effects limited the allowable strength of the applied magnetic gradient times the pulse duration to be below a linear threshold (see Figure 6 in Glover 2009 (62)). Commercial MRI systems (with millisecond gradient rise times) are unable to achieve microsecond magnetic pulses. Based on Glover this limits their allowable magnetic spatial gradients to approximately 0.1 Tesla/meter. However, recent human trial data from Weinberg has shown that it is possible to eliminate nerve stimulation effects, even at higher field magnitudes, by using ultra-fast magnetic pulses (e.g., with rise times of less than 10 microseconds) (63). Such fast rise times require high voltages and currents that are not readily implemented with conventional MRI, but can be achieved with pulsed-power switching technology and custom high-voltage coils. Instead of the 0.040 – 0.080 T/m maximum gradients provided by human MRI systems (64), pulse-power enables ~ 1 T/m spatial gradients without peripheral nerve stimulation.
Experiments are now underway to interleave imaging and propulsive pulse sequences within the same pulsed-power platform so as to enable real-time image-guidance of magnetic targeting. Such a platform could measure the 3-dimensional anatomic distribution of magnetic carriers multiple times a second, and after each measurement could apply a precisely shaped magnetic field to modify the observed distribution. Using appropriate control algorithms (discussed next) the platform could thus be used to coincide the distribution of therapy with tumor margins while sparing healthy tissue. A reasonable first clinical target would be brain tumors, which are often difficult to treat due to irregular and indistinct margins, and the required extreme attention paid to reducing collateral damage to neighboring structures in healthy brain tissues.
Magnet Design and Control, to Reach Deep Targets
Overall, one of the biggest open challenges in magnetic delivery is precisely targeting deep tissue targets – there are as yet no imaging and actuation systems that can achieve the external-magnet deep-focusing shown in Figure 1(b). To achieve deep targeting requires solution of, at least, four major issues: i) sufficient magnetic fields/forces deep in the body, ii) real-time imaging, iii) sophisticated control algorithms, and iv) mathematical modelling of carrier motion in-vivo with at least enough fidelity to enable effective design of the imaging/actuation system and the control algorithms that will determine which magnets to turn on when and for how long. Imaging has already been discussed above, so we now turn to deep forces, mathematical modelling, and control design.
A first reason deep tissue magnetic targeting is difficult is because magnetic fields and forces fall off quickly with distance from external magnets (65, 66). There are two non-invasive ways to improve the situation: improve the external magnets to provide stronger and deeper magnetic gradients or optimizing the magnetic carriers to react more strongly to a magnetic gradient. Optimization of permanent and electro-magnets to increase the strength and depth of magnetic gradients has been reported in (67–73). In our own work, we showed that semi-definite optimization tools could be used to design and implement Halbach-array permanent magnets that provide improved pulling or pushing forces on magnetic nano-particles (74). Optimizing magnetic carriers, as discussed in the ‘Choosing Magnetic Carriers’ section, can potentially provide significant improvement in achievable targeting depth. Since magnetic forces are strongly dependent on carrier size, shape, magnetic material properties, and agglomeration, while bodily fluids and tissue resistance forces also depend on size and shape as well as on carrier coatings and potentially mechanical flexibility, it is likely there is significant design freedom to create much more responsive magnetic carriers. As a community, we need to understand enough about carrier motion in vivo to design more effective magnetic carriers, and enough about fabrication processes to make them.
To rationally select magnetic carrier designs, and also to implement magnet control algorithms that will drive magnetic carriers to their desired targets, requires understanding and mathematical models at the right level of fidelity and complexity. For magnetic carrier design, it is not feasible to search the large carrier design space by fabricating all-possible magnetic carriers and then testing each of them in animal experiments. Instead, we need to build up predictive-capabilities and mathematical models that will help guide us through the design space faster than animal or tissue experiment. Hence there is a need for simple but at least roughly-predictive models that can help tell us what kind of carriers to investigate experimentally.
Mathematical models are also required for dynamic control of magnets to precisely direct magnetic carriers to deep targets. To decide which magnets to turn on when, we need to know, again at least roughly, what each magnet will do to the magnetic carriers in vivo. Once a mathematical description is available, then there is a possibility that it can be inverted to decide how to actuate the external magnets to direct the carriers to where they need to go in the patient (22, 75, 76). Real-time imaging can greatly aid this process by providing real-time information on where the carriers are in the patient’s body, so that feedback control (discussed next) can shift the distribution of carriers from where they are observed to be towards where they should be at each control update time.
Precision feedback control of a single magnetic or magnetisable element has already been demonstrated in animals and in patients (77–83). However, focusing a collection (a ferrofluid) of magnetic carriers to a single deep location is more difficult than manipulating a single object because while one particle may be being driven towards its target, the same magnetic field may be driving another particle away from the target. A mathematics result from over 150 years ago summarizes a key challenge to deep tissue focusing. Samuel Earnshaw’s 1839 theorem (22, 84), when applied to Maxwell’s equations and the magnetic force acting on ferromagnetic nano-particles (85–87), shows that no arrangement of external magnets can create a static magnetic trap that will attract all particles to an interior target. To achieve deep targeting it is necessary to find ways to circumvent Earnshaw’s theorem. The most direct way is to exploit the dynamics of particle transport and feedback control to achieve deep focusing in some on-average manner.
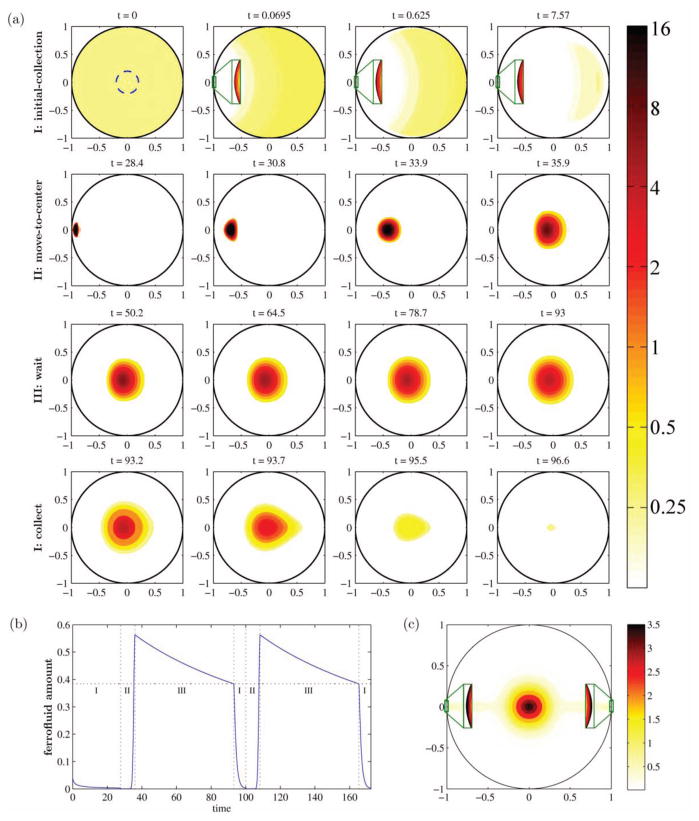
In (22) we showed, in simulations, that a collect-at-edge and move-to-center scheme could focus particles on average to the center of a 2-dimensional circular domain (Figure 4). This scheme exploited the edge of the domain to first collect the particles to a focused location. The control algorithm was provided with complete information on the distribution of particles at each moment in time (in other words, we assumed perfect real-time imaging of particle distributions) while it moved the ferrofluid optimally from edge to center. The decisions that the control algorithm made, which told it how to actuate the 8 magnets surrounding the circular domain, where based on a mathematical model of particle dynamics due to diffusion and magnetic forces (see (22) for details). This strategy successfully focused ferrofluid on average to a deep internal target (Figure 4). However, this control result was in idealized simulations, and is a long way from a practical system that can achieve the deep focusing shown in Figure 1(b). There may be other as yet undiscovered ways to bypass Earnshaw’s theorem to achieve deep tissue focusing. At present, precisely directing magnetic carriers to anywhere they need to go, including to deep tissue targets, remains a holy grail of magnetic drug targeting, and is an open challenge that will require imagination and collaboration.
Figure 4.
Focusing a ferrofluid to a central target on average in a computer simulation. (a) The concentration of the controlled ferrofluid over time. In phase I, the ferrofluid is collected to the left edge (zooms shown in green boxes). In phase II it is brought to the center with minimal spreading by dynamic control of 8 magnets outside the circular domain (magnets not shown). Then there is a wait step (phase III) and then collection repeats on the right side. (b) The amount of ferrofluid inside the center target at each time. (c) The average ferrofluid concentration. Control achieves a clear hot-spot in the center. (For further details, please see (22). (© 2012 IEEE. Reprinted with permission, from (22), doi: 10.1109/MCS.2012.2189052).
Conclusion
To advance magnetic drug targeting to the clinic requires solution of key remaining open challenges. There is first a need to develop methods to rationally select and design carriers for specific clinical indications. The magnetic carriers that will be best for targeting deep tumors will not be the same as those most appropriate for traversing the BBB or for non-invasively reaching eye diseases. Due to the large design space for magnetic carriers (e.g. size, shape, coatings), it is unlikely that carrier selection will be achieved through animal testing alone, instead animal experimentation will have to be combined with effective and predictive mathematical modelling to better search the design space in order to find the most appropriate carrier designs for different clinical indications.
Real-time imaging of magnetic carriers in vivo is a major need to enable precise magnetic targeting. In order for a magnetic system to direct therapy precisely to a disease target, the systems controller must be able to “see” where the therapy is so that correcting magnetic fields can be applied to move the therapy from where it is to where it should be. Without real-time imaging capabilities, magnetic manipulation will remain blind and inaccurate.
To reach deeper targets, in addition to carrier optimization, there is also a need to optimize the design and control of external magnets. Here also the design space is too large to search only experimentally. Mathematical models are necessary to predict how magnetic carriers will move through living tissue under the influence of magnetic fields that are being shaped in time and space. These mathematical models must be built at the right level of fidelity: rich enough to capture fundamental behaviour, but simple enough to be computationally tractable and useable for magnet design and control. Optimization and control tools must be implemented that exploit these models to design better magnets (with stronger and deeper forces) and to choose control algorithms for the magnets (to safely and effectively direct magnetic carriers to deep targets in live animals, and eventually in human patients).
Overall, there is a need to move beyond making the carriers we can make and testing them predominantly in cell cultures and small animals, to making the carriers we should make and creating magnetic systems that can precisely manipulate them in large animals, and then in human patients. To translate magnetic carriers from the lab to clinical use will also require regulatory approval, which means rigorous safety and toxicology testing in larger animals in addition to in rodents, before subsequent safety and efficacy trials in human patients. The regulatory framework for such testing is still uncertain, the FDA only recently issued industry guidance for recommended studies to establish the safety of nanomaterials in cosmetic products (88). To our best knowledge there is as yet no specific FDA guidance available for therapeutic magnetic carriers. Hence there are substantial challenges to translate magnetic targeting from lab demonstrations to a reality for patients. Overcoming these challenges will require significant effort and a genuine collaboration between engineers, mathematicians, chemists, biologists, nano-fabricators, and clinicians.
Acknowledgments
Funding from the National Institutes of Health (NIH, grant numbers 1R21CA140068-01, 1R41DC013534-01A1), the National Science Foundation (NSF, grant number NSF 1261938), FDA’s Pediatric Device Consortium, as well as the Technology Development Corporation (TEDCO), the BioMaryland Center, and the Maryland Industrial Partnerships (MIPS) agencies in the State of Maryland and Action on Hearing Loss in the United Kingdom, is gratefully acknowledged.
Footnotes
Dr. Shapiro has a financial stake in Otomagnetics, which is developing a minimally-invasive method to magnetically deliver drugs and other therapeutic payloads to ear compartments.
Dr. Nacev has a financial stake in Otomagnetics.
Dr. Weinberg has a financial stake in Otomagnetics.
Contributor Information
Dr. Benjamin Shapiro, Email: benshap@umd.edu, Fischell Department of Bioengineering and the Institute for Systems Research, University of Maryland at College Park
Sandip Kulkarni, Fischell Department of Bioengineering, University of Maryland at College Park.
Dr. Aleksander Nacev, Weinberg Medical Physics LLC, Bethesda MD
Silvia Muro, Institute for Bioscience and Biotechnology Research & Fischell Department of Bioengineering, University of Maryland at College Park.
Pavel Y. Stepanov, Weinberg Medical Physics LLC, Bethesda MD
Dr. Irving N. Weinberg, Weinberg Medical Physics LLC, Bethesda MD
References
- 1.Lubbe AS, Bergemann C, Riess H, Schriever F, Reichardt P, et al. Clinical experiences with magnetic drug targeting: a phase i study with 4′-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Research. 1996;56(20):4686–93. [PubMed] [Google Scholar]
- 2.Lemke AJ, von Pilsach MIS, Lubbe A, Bergemann C, Riess H, Felix R. Mri after magnetic drug targeting in patients with advanced solid malignant tumors. European Radiology. 2004;14(11):1949–55. doi: 10.1007/s00330-004-2445-7. [DOI] [PubMed] [Google Scholar]
- 3.Voltairas PA, Fotiadis DI, Michalis LK. Hydrodynamics of magnetic drug targeting. Journal of Biomechanics. 2002;35(6):813–21. doi: 10.1016/s0021-9290(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 4.Grief AD, Richardson G. Mathematical modeling of magnetically targeted drug delivery. Journal of Magnetism and Magnetic Materials - Proceedings of the Fifth International Conference on Scientific and Clinical Applications of Magnetic Carriers. 2005;293(1):455–63. [Google Scholar]
- 5.Shapiro B, Dormer K, Rutel IB. A two-magnet system to push therapeutic nanoparticles. AIP Conf Proc. 2010;1311(1):77–88. doi: 10.1063/1.3530064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson MW, Kerlan RK, Fidelman NA, Venook AP, LaBerge JM, et al. Hepatocellular carcinoma: regional therapy with a magnetic targeted carrier bound to doxorubicin in a dual mr imaging/conventional angiography suite–initial experience with four patients. Radiology. 2004;230(1):287–93. doi: 10.1148/radiol.2301021493. [DOI] [PubMed] [Google Scholar]
- 7.Koda J, Venook A, Walser E, Goodwin S. A multicenter, phase i/ii trial of hepatic intra-arterial delivery of doxorubicin hydrochloride adsorbed to magnetic targeted carriers in patients with hepatocellular carcinoma. European Journal of Cancer. 2002;38:S18–S18. [Google Scholar]
- 8.Johannsen M, Gneveckow U, Eckelt L, Feussner A, Waldöfner N, et al. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: presentation of a new interstitial technique. International Journal of Hyperthermia. 2005;21(7):637–47. doi: 10.1080/02656730500158360. [DOI] [PubMed] [Google Scholar]
- 9.Dobson J. Magnetic micro- and nano-particle-based targeting for drug and gene delivery. Nanomedicine. 2006;1(1):31–37. doi: 10.2217/17435889.1.1.31. [DOI] [PubMed] [Google Scholar]
- 10.Pankhurst QA, Connolly J, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedicine. J Phys D: Appl Phys. 2003;36(13):R167. [Google Scholar]
- 11.Pankhurst QA, Thanh NTK, Jones SK, Dobson J. Progress in applications of magnetic nanoparticles in biomedicine. J Phys D: Appl Phys. 2009;42(22):224001. [Google Scholar]
- 12.Sun C, Lee J, Zhang M. Magnetic nanoparticles in mr imaging and drug delivery. Advanced Drug Delivery Reviews. 2008;60(11):1252–65. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villemejane J, Mir LM. Physical methods of nucleic acid transfer: general concepts and applications. British Journal of Pharmacology. 2009;157(2):207–19. doi: 10.1111/j.1476-5381.2009.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry CC. Progress in functionalization of magnetic nanoparticles for applications in biomedicine. J Phys D: Appl Phys. 2009;42:224003–12. [Google Scholar]
- 15.Roca AG, Costo R, Rebolledo AF, Veintemillas-Verdaguer S, Tartaj P, et al. Progress in the preparation of magnetic nanoparticles for applications in biomedicine. Journal of Physics D: Applied Physics. 2009;42(22):224002. [Google Scholar]
- 16.McBain SC, Yiu HHP, Dobson J. Magnetic nanoparticles for gene and drug delivery. Int J Nanomedicine. 2008;3(2):169–80. doi: 10.2147/ijn.s1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veiseh O, Gunn JW, Zhang M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Advanced Drug Delivery Reviews. 2010;62(3):284–304. doi: 10.1016/j.addr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho K, Wang X, Nie S, Chen Z, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clinical Cancer Research. 2008;14(5):1310–16. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann U, Pilwat G. Organ specific application of drugs by means of cellular capsule systems. Zeitschrift fur Naturforschung Section C Biosciences. 1976;31(11–12):732–36. [PubMed] [Google Scholar]
- 20.Solanki A, Kim JD, Lee K-B. Nanotechnology for regenerative medicine: {n}anomaterials for stem cell imaging. Nanomedicine. 2008;3(4):567–78. doi: 10.2217/17435889.3.4.567. [DOI] [PubMed] [Google Scholar]
- 21.Lubbe AS, Alexiou C, Bergemann C. Clinical applications of magnetic drug targeting. Journal of Surgical Research. 2001;95(2):200–206. doi: 10.1006/jsre.2000.6030. [DOI] [PubMed] [Google Scholar]
- 22.Nacev A, Komaee A, Sarwar A, Probst R, Kim SH, et al. Towards control of magnetic fluids in patients: directing therapeutic nanoparticles to disease locations. IEEE Control Systems. 2012;32(3):32–74. [Google Scholar]
- 23.Polyak B, Friedman G. Magnetic targeting for site-specific drug delivery: applications and clinical potential. Expert Opinion on Drug Delivery. 2009;6(1):53–70. doi: 10.1517/17425240802662795. [DOI] [PubMed] [Google Scholar]
- 24.Bawa R. Nanoparticle-based therapeutics in humans: a survey. Nanotech Law & Bus. 2008;5:135. [Google Scholar]
- 25.Nacev A, Beni C, Bruno O, Shapiro B. Magnetic nanoparticle transport within flowing blood and into surrounding tissue. Nanomedicine. 2010;5(9):1459–66. doi: 10.2217/nnm.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nacev A, Beni C, Bruno O, Shapiro B. The behaviors of ferro-magnetic nano-particles in and around blood vessels under applied magnetic fields. Journal of Magnetism and Magnetic Materials. 2011;323(6):651–68. doi: 10.1016/j.jmmm.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colombo M, Carregal-Romero S, Casula MF, Gutiérrez L, Morales MP, et al. Biological applications of magnetic nanoparticles. Chemical Society reviews. 2012;41(11):4306–34. doi: 10.1039/c2cs15337h. [DOI] [PubMed] [Google Scholar]
- 28.Puntes VF, Krishnan KM, Alivisatos AP. Colloidal nanocrystal shape and size control: the case of cobalt. Science. 2001;291(5511):2115–17. doi: 10.1126/science.1057553. [DOI] [PubMed] [Google Scholar]
- 29.Cabot A, Puntes VF, Shevchenko E, Yin Y, Balcells L, et al. Vacancy coalescence during oxidation of iron nanoparticles. Journal of the American Chemical Society. 2007;129(34):10358–60. doi: 10.1021/ja072574a. [DOI] [PubMed] [Google Scholar]
- 30.Nacev A, Kim SH, Rodriguez-Canales J, Tangrea MA, Shapiro B, Emmert-Buck MR. A dynamic magnetic shift method to increase nanoparticle concentration in cancer metastases: a feasibility study using simulations on autopsy specimens. International Journal of Nanomedicine. 2011;6(1):2907–23. doi: 10.2147/IJN.S23724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renkin EM. Filtration, diffusion, and molecular sieving through porous cellulose membranes. Journal of General Physiology. 1954;38(2):225–43. [PMC free article] [PubMed] [Google Scholar]
- 32.Ogston AG, Preston BN, Wells JD. On the transport of compact particles through solutions of chain-polymers. Proceedings of the Royal Society of London. A. Mathematical and Physical Sciences. 1973;333(1594):297. [Google Scholar]
- 33.Kulkarni Sandip, Nacev Aleksander, Ramaswamy Bharath, Depireux Didier, Shapiro Benjamin. Understanding motion of magnetic nanoparticles in tissue. 2013. [Google Scholar]
- 34.Shapiro B, Depireux D, Sarwar A, Nacev A, Preciado D, Hausfeld J. Pre-clinical development of magnetic delivery of therapy to middle and inner ears. ENT & Audiology News. 2014;23(1):54–56. [Google Scholar]
- 35.Shapiro B, Kulkarni S, Nacev A, Sarwar A, Preciado D, Depireux DA. Shaping magnetic fields to direct therapy to ears and eyes. Annu Rev Biomed Eng. 2014 doi: 10.1146/annurev-bioeng-071813-105206. [DOI] [PubMed] [Google Scholar]
- 36.Pardridge WM. Biopharmaceutical drug targeting to the brain. J Drug Target. 2010;18(3):157–67. doi: 10.3109/10611860903548354. [DOI] [PubMed] [Google Scholar]
- 37.Banks WA. Blood-brain barrier as a regulatory interface. Forum Nutr. 2010;63:102–10. doi: 10.1159/000264398. [DOI] [PubMed] [Google Scholar]
- 38.Pardridge WM. Blood-brain barrier delivery. Drug Discov Today. 2007;12(1–2):54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 39.Groothuis DR. The blood-brain and blood-tumor barriers: a review of strategies for increasing drug delivery. Neuro Oncol. 2000;2(1):45–59. doi: 10.1093/neuonc/2.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lakhal S, Wood MJA. Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of rnai heralds new horizons for drug delivery across biological barriers. Bioessays. 2011;33(10):737–41. doi: 10.1002/bies.201100076. [DOI] [PubMed] [Google Scholar]
- 41.Muro S. Strategies for delivery of therapeutics into the central nervous system for treatment of lysosomal storage disorders. Drug Deliv Transl Res. 2012;2(3):169–86. doi: 10.1007/s13346-012-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu J, Rappaport J, Muro S. Specific binding, uptake, and transport of icam-1-targeted nanocarriers across endothelial and subendothelial cell components of the blood–brain barrier. Pharm Res. 2014:1–12. doi: 10.1007/s11095-013-1289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kreuter J. Drug delivery to the central nervous system by polymeric nanoparticles: what do we know? Advanced Drug Delivery Reviews. 2014;71:2–14. doi: 10.1016/j.addr.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Dilnawaz F, Singh A, Mohanty C, Sahoo SK. Dual drug loaded superparamagnetic iron oxide nanoparticles for targeted cancer therapy. Biomaterials. 2010;31(13):3694–3706. doi: 10.1016/j.biomaterials.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 45.Shubayev VI, Pisanic TR, 2nd, Jin S. Magnetic nanoparticles for theragnostics. Adv Drug Deliv Rev. 2009;61(6):467–77. doi: 10.1016/j.addr.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiao R, Jia Q, Hüwel S, Xia R, Liu T, et al. Receptor-mediated delivery of magnetic nanoparticles across the blood–brain barrier. ACS Nano. 2012;6(4):3304–10. doi: 10.1021/nn300240p. [DOI] [PubMed] [Google Scholar]
- 47.Saiyed ZM, Gandhi NH, Nair MP. Magnetic nanoformulation of azidothymidine 5′-triphosphate for targeted delivery across the blood-brain barrier. Int J Nanomedicine. 2010;5:157–66. doi: 10.2147/ijn.s8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han L, Zhang A, Wang H, Pu P, Kang C, Chang J. Construction of novel brain-targeting gene delivery system by natural magnetic nanoparticles. J Appl Polym Sci. 2011;121(6):3446–54. [Google Scholar]
- 49.Kong SD, Lee J, Ramachandran S, Eliceiri BP, Shubayev VI, et al. Magnetic targeting of nanoparticles across the intact blood–brain barrier. Journal of Controlled Release. 2012;164(1):49–57. doi: 10.1016/j.jconrel.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wahajuddin, Arora S. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomedicine. 2012;7:3445–71. doi: 10.2147/IJN.S30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei K-C, Yang H-W, Hua, Liu, Huang Potential of magnetic nanoparticles for targeted drug delivery. Nanotechnology, Science and Applications. 2012:73. doi: 10.2147/NSA.S35506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lai SK, O’Hanlon DE, Harrold S, Man ST, Wang Y-Y, et al. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. PNAS. 2007;104(5):1482–87. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lai SK, Wang Y-Y, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Advanced Drug Delivery Reviews. 2009;61(2):158–71. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalambur VS, Han B, Hammer BE, Shield TW, Bischof JC. In vitro characterization of movement, heating and visualization of magnetic nanoparticles for biomedical applications. Nanotechnology. 2005;16(8):1221. [Google Scholar]
- 55.Gleich B, Weizenecker J. Tomographic imaging using the nonlinear response of magnetic particles. Nature. 2005;435(7046):1214–17. doi: 10.1038/nature03808. [DOI] [PubMed] [Google Scholar]
- 56.Knopp T, Sattel TF, Biederer S, Rahmer J, Weizenecker J, et al. Model-based reconstruction for magnetic particle imaging. IEEE Transactions on Medical Imaging. 2010;29(1):12–18. doi: 10.1109/TMI.2009.2021612. [DOI] [PubMed] [Google Scholar]
- 57.Knopp T, Biederer S, Sattel TF, Rahmer J, Weizenecker J, et al. 2d model-based reconstruction for magnetic particle imaging. Med Phys. 2010;37(2):485–91. doi: 10.1118/1.3271258. [DOI] [PubMed] [Google Scholar]
- 58.Biederer S, Knopp T, Sattel TF, Lüdtke-Buzug K, Gleich B, et al. Magnetization response spectroscopy of superparamagnetic nanoparticles for magnetic particle imaging. J Phys D: Appl Phys. 2009;42(20):205007. [Google Scholar]
- 59.Weizenecker J, Gleich B, Rahmer J, Dahnke H, Borgert J. Three-dimensional real-time in vivo magnetic particle imaging. Physics in medicine and biology. 2009;54(5):L1–L10. doi: 10.1088/0031-9155/54/5/L01. [DOI] [PubMed] [Google Scholar]
- 60.Goodwill PW, Conolly SM. The x-space formulation of the magnetic particle imaging process: 1-d signal, resolution, bandwidth, snr, sar, and magnetostimulation. IEEE Trans Med Imaging. 2010;29(11):1851–59. doi: 10.1109/TMI.2010.2052284. [DOI] [PubMed] [Google Scholar]
- 61.Carmi E, Liu S, Alon N, Fiat A, Fiat D. Resolution enhancement in mri. Magnetic Resonance Imaging. 2006;24(2):133–54. doi: 10.1016/j.mri.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 62.Glover PM. Interaction of mri field gradients with the human body. Phys Med Biol. 2009;54(21):R99. doi: 10.1088/0031-9155/54/21/R01. [DOI] [PubMed] [Google Scholar]
- 63.Weinberg IN, Stepanov PY, Fricke ST, Probst R, Urdaneta M, et al. Increasing the oscillation frequency of strong magnetic fields above 101 khz significantly raises peripheral nerve excitation thresholds. Med Phys. 2012;39(5):2578–83. doi: 10.1118/1.3702775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alexander DC, Hubbard PL, Hall MG, Moore EA, Ptito M, et al. Orientationally invariant indices of axon diameter and density from diffusion mri. Neuro Image. 2010;52(4):1374–89. doi: 10.1016/j.neuroimage.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 65.Takeda S, Mishima F, Fujimoto S, Izumi Y, Nishijima S. Development of magnetically targeted drug delivery system using superconducting magnet. Journal of Magnetism and Magnetic Materials. 2007;311(1):367–71. [Google Scholar]
- 66.Rotariu O, Strachan NJC. Modelling magnetic carrier particle targeting in the tumor microvasculature for cancer treatment. Journal of Magnetism and Magnetic Materials. 2005;293(1):639–46. [Google Scholar]
- 67.Dames P, Gleich B, Flemmer A, Hajek K, Seidl N, et al. Targeted delivery of magnetic aerosol droplets to the lung. Nat Nano. 2007;2(8):495–99. doi: 10.1038/nnano.2007.217. [DOI] [PubMed] [Google Scholar]
- 68.Alexiou C, Diehl D, Henninger P, Iro H, Rockelein R, et al. A high field gradient magnet for magnetic drug targeting. IEEE Transactions on Applied Superconductivity. 2006;16(2):1527–30. [Google Scholar]
- 69.Yang Y, Jiang J-S, Du B, Gan Z-F, Qian M, Zhang P. Preparation and properties of a novel drug delivery system with both magnetic and biomolecular targeting. Journal of Materials Science: Materials in Medicine. 2009;20(1):301–7. doi: 10.1007/s10856-008-3577-0. [DOI] [PubMed] [Google Scholar]
- 70.Lohakan M, Junchaichanakun P, Boonsang S, Pintavirooj C. A computational model of magnetic drug targeting in blood vessel using finite element method. Industrial Electronics and Applications, 2007. ICIEA 2007. 2nd IEEE Conference on; 2007. pp. 231–34. [Google Scholar]
- 71.Slabu I, Röth A, Schmitz-Rode T, Baumann M. Optimization of magnetic drug targeting by mathematical modeling and simulation of magnetic fields. 4th European Conference of the International Federation for Medical and Biological Engineering; 2009. pp. 2309–12. [Google Scholar]
- 72.Creighton FM, Ritter RC, Werp P. Focused magnetic navigation using optimized magnets for medical therapies. Magnetics Conference, 2005. INTERMAG Asia 2005. Digests of the IEEE International; 2005. pp. 1253–54. [Google Scholar]
- 73.Creighton FM. Optimal distribution of magnetic material for catheter and guidewire cardiology therapies. Magnetics Conference, 2006. INTERMAG 2006. IEEE International; 2006. pp. 111–111. [Google Scholar]
- 74.Sarwar A, Nemirovski A, Shapiro B. Optimal halbach permanent magnet designs for maximally pulling and pushing nanoparticles. Journal of Magnetism and Magnetic Materials. 2012;324(5):742–54. doi: 10.1016/j.jmmm.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Komaee A, Kim SH, Nacev A, Probst R, Sarwar A, et al. Putting therapeutic nanoparticles where they need to go by magnet systems: design and control. In: Thanh NK, editor. Magnetic Nanoparticles: From Fabrication to Biomedical and Clinical Applications. CRC Press/Taylor and Francis; 2011. [Google Scholar]
- 76.Shapiro B. Towards dynamic control of magnetic fields to focus magnetic carriers to targets deep inside the body. Journal of Magnetism and Magnetic Materials. 2009;321(10):1594. doi: 10.1016/j.jmmm.2009.02.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yesin KB, Vollmers K, Nelson BJ. Modeling and control of untethered biomicrorobots in a fluidic environment using electromagnetic fields. The International Journal of Robotics Research. 2006;25(5–6):527–36. [Google Scholar]
- 78.Bergeles C, Kummer MP, Kratochvil BE, Framme C, Nelson BJ. Steerable intravitreal inserts for drug delivery: in vitro and ex vivo mobility experiments. In: Fichtinger G, Martel A, Peters T, editors. Medical Image Computing and Computer-Assisted Intervention – MICCAI 2011. Springer; Berlin Heidelberg: 2011. pp. 33–40. [DOI] [PubMed] [Google Scholar]
- 79.Kummer MP, Abbott JJ, Kratochvil BE, Borer R, Sengul A, Nelson BJ. Octomag: an electromagnetic system for 5-dof wireless micromanipulation. Robotics, IEEE Transactions on. 2010;26(6):1006–17. [Google Scholar]
- 80.Tamaz S, Gourdeau R, Chanu A, Mathieu J-B, Martel S. Real-time mri-based control of a ferromagnetic core for endovascular navigation. IEEE Transactions on Bio-Medical Engineering. 2008;55(7):1854–63. doi: 10.1109/TBME.2008.919720. [DOI] [PubMed] [Google Scholar]
- 81.Grady MS, Howard MA, 3rd, Molloy JA, Ritter RC, Quate EG, Gillies GT. Nonlinear magnetic stereotaxis: three-dimensional, in vivo remote magnetic manipulation of a small object in canine brain. Med Phys. 1990;17(3):405–15. doi: 10.1118/1.596520. [DOI] [PubMed] [Google Scholar]
- 82.Bauernfeind T, Akca F, Schwagten B, de Groot N, Van Belle Y, et al. The magnetic navigation system allows safety and high efficacy for ablation of arrhythmias. Europace. 2011;13(7):1015–21. doi: 10.1093/europace/eur073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ciuti G, Valdastri P, Menciassi A, Dario P. Robotic magnetic steering and locomotion of capsule endoscope for diagnostic and surgical endoluminal procedures. Robotica. 2010;28(Special Issue 02):199–207. [Google Scholar]
- 84.Earnshaw S. On the nature of the molecular forces which regulate the constitution of the luminiferous ether. Transactions of the Cambridge Philosophical Society. 1842;7:97–112. [Google Scholar]
- 85.Feynman RP, Leighton RB, Sands M. The Feynman Lectures on Physics. Addison-Wesley Publishing Company; 1964. [Google Scholar]
- 86.Fleisch DA. A Student’s Guide to Maxwell’s Equations. Cambridge, UK; New York: Cambridge University Press; 2008. [Google Scholar]
- 87.Iacob GH, Rotariu O, Chiriac H. A possibility for local targeting of magnetic carriers. Journal of Optoelectronics and Advanced Materials. 2004;6(2):713–17. [Google Scholar]
- 88.U.S. Department for Health and Human Services, Food and Drug Administration, Center for Food Safety and Applied Nutrition. Guidance for industry: safety of nanomaterials in cosmetic products. 2014. [Google Scholar]