Abstract
Synthetic lipoproteins represent a relevant tool for targeted delivery of biological/chemical agents (chemotherapeutics, siRNAs, photosensitizers and imaging contrast agents) into various cell types. These nanoparticles offer a number of advantages on drugs delivery over their native counterparts while retaining their natural characteristics and biological functions. Their ultra-small size (<30nm), high biocompatibility, favorable circulation half-life and natural ability to bind specific lipoprotein receptors i.e. low-density lipoprotein receptor (LDLR) and Scavenger receptor class B member 1 (SRB1) that are found in a number of pathological conditions (e.g. cancer, atherosclerosis), make them superior delivery strategies when compared to other nanoparticle systems. We review the various approaches that have been developed for the generation of synthetic lipoproteins and their respective applications in vitro and in vivo. More specifically, we summarize the way to address the limitation on use of reconstituted lipoproteins by means of natural or recombinant apolipoproteins, as well as apolipoprotein mimetic molecules. Finally, we provide an overview of the advantages and disadvantages of these approaches and discuss future perspectives for clinical translation of these nanoparticles.
Keywords: lipoprotein, nanoparticle, drug delivery, receptor-mediated, apolipoprotein, mimetic peptide, HDL, LDL
1. Introduction
Part of the great promise of nanomedicine has been its potential to enhance delivery and activity of bioactive/imaging agents into relevant cell types in vivo in a manner that minimizes toxicity to patients by enhancing specificity of activity in target cells [1]. Certainly, the use of liposomal nanoparticles as delivery vehicles represents a seminal technique in nanomedicine. Indeed ongoing efforts to further develop these and other nanoparticles as delivery platforms remain the mainstay in the field [2]. Despite FDA approval and their application in the clinic, liposomal particles suffer from a number of shortcomings including low encapsulation efficiency, fast burst release of drugs, poor storage stability and lack of tunable triggers for drug release and extracellular release of payload [3–5]. Significant efforts have been devoted to modify liposomal particles so as to address these shortcomings and to find additional delivery tools for pharmaceutical agents [4]. In this regard, the use of lipoprotein based nanoparticles is currently being investigated as a viable alternative for enhancing drug delivery.
Lipoproteins are assemblies of proteins and lipids, and constitute the endogenous carriers that naturally exist in the body and are responsible for the transportation of cholesterol in the circulation to maintain the homeostatic balance [6]. The typical structure of lipoproteins is composed of a neutral lipid core, an outer shell of phospholipids and amphipathic apolipoproteins that confer water solubility and precise size control [7]. The structure of lipoproteins such as LDL had been assumed to be spherical. However, using high performance gel filtration chromatography (HPGC), Teerlink et al (2004) suggested that LDL may be in fact discoidal. In this analysis, researchers examined 160 LDL preparations and showed that diameters calculated from volume correlate poorly with diameters determined by HPGC [8]. Overall, lipoproteins are typically divided into four groups (based on factors such as size, density, buoyancy and the type of major apolipoproteins (Table 1). The subclasses of lipoproteins include Chylomicron, VLDL (very low density liprotein), LDL (small low density lipoprotein) and HDL [9].
Table 1.
| CM | VLDL | LDL | HDL | |
|---|---|---|---|---|
| Diameter (nm)* | 75–1200 | 30–80 | 19–25 | 5–12 |
| Density (g/ml) | <0.96 | 0.96–1.006 | 1.019–1.063 | 1.063–1.210 |
| Mw (X 106 Da) | 400 | 10–80 | 2.3 | 0.17–0.36 |
| size | 75–1200 nm | 30–80 nm | 25–35 nm | 8–12 nm |
| source | Gut | liver | VLDL via ILDL | Gut, liver |
| Half-life§ | 5–13 min | 2–5 min | 15 hr | 11–12 hr |
| Lipid composition | ||||
| Triglyceride | 80–95 | 45–65 | 18–22 | 2–7 |
| Free cholesterol | 1–3 | 4–8 | 6–8 | 3–5 |
| Cholesterol ester | 2–4 | 6–22 | 45–50 | 5–20 |
| Phospholipid | 3–6 | 5–20 | 18–24 | 26–32 |
| Apolipoproteins | A-I, A-II, A-IV | A-I, A-II, A-IV | ||
| B48 | B100 | B100 | ||
| C-I, C-II, C-II | C-I, C-II, C-II | C-I, C-II, C-II | ||
| E | E | E |
Amphipathic apolipoproteins functions as structural components of lipoprotein particles. Apo B represents the structural apolipoprotein found in chylomicrons (Apo B-48), the atherogenic VLDL (ApoB-100) and LDL (ApoB-100). Apo B-48 (synthesized in gut) and Apo B-100 (synthesized in liver) are two isoforms of ApoB existed in the plasma. Either isoforms is responsible for the transport of lipids from the liver and/or gut to peripheral tissues. As each lipoprotein contains one ApoB molecule, it is possible to ascertain the total load of circulating atherogenic particles in the system [10]. Apo B-100 functions as a recognition signal for the cellular binding and internalization of LDL particles by the ApoB/E receptor [11]. The major apoliproprotein associated with anti-atherogenic HDL is primarily ApoA-I. Nevertheless other proteins (ApoA-II, ApoA-IV, ApoC (C-I, C-II, and C-III) and Apo E are also found associated with HDL [11] [12]. Further, HDL can be further subdivided into larger, less dense HDL2 or smaller, denser HDL3 according to particles’ density, but most HDL in the body is found in the form of HDL3 [13]. ApoA-I is responsible for the reverse cholesterol transport from peripheral tissues back to the liver for catabolism [14]. Thus HDLs play relevant roles with respect to anti-inflammatory, antioxidant, and profibrinolytic activities, as well as cardiovascular disease, cancer, Alzheimer’s and dementia [12, 15].
2. Rationale for the use of lipoproteins as delivery vehicles
2.1 Biocompatibility and favorable structure
Lipoproteins offer a number of advantages over other nanoparticles. First of all, lipoprotein nanoparticles are biocompatible and are thus both largely avoid detection by mononuclear phagocytic cells in the body’s defense system and can be readily broken down and recycled by cells [1]. The natural structure of lipoproteins (lipophilic surface and hydrophobic compartment) mediates their ability in functional delivery of hydrophobic triacylglycerols and cholesteryl esters[16], indicating their amiable potential for delivery of hydrophobic bioactive compounds (Figure). Their long blood circulation characteristics allow a favorable systemic drug delivery without the need of PEGylation [17]. The role of lipoproteins in drug delivery is further enhanced by their small size which allows superior penetration through interfibrillar openings in tumors.[5, 18, 19]. The size of these nanoparticles, however, is large enough to avoid rapid elimination by kidneys [20].
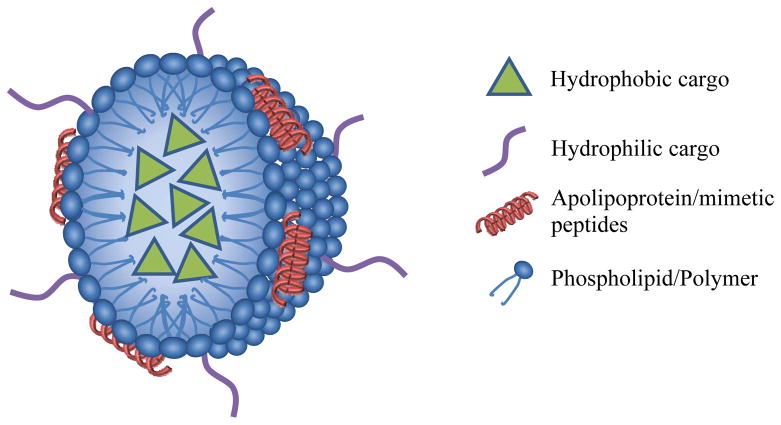
Figure.
The schematic (not drawn to scale) of a lipoprotein drug delivery vehicle. As depicted, it has the ability to carry both hydrophobic molecules through encapsulation in the core, and hydrophilic molecules through conjugation/insertion on its surface.
2.2 Receptor-mediated targeted delivery
The intracellular uptake of lipoproteins (either in the form of LDL and HDL) is governed by receptor-mediated interactions. The specificity of this uptake represents an additional advantage of lipoprotein based delivery vehicles by allowing targeted and discriminatory delivery of payloads to specific cell types of interest based on their expression of lipoprotein receptors. Indeed the presence of receptors for both LDL and HDL have been noted in a variety of cancer types [21–25], as well as in non-neoplastic diseases [26, 27]. For instance, Guo et al. (2011) has recently shown that the number of cells positive for LDLR expression in glioblastoma (GBM) tissue is significantly higher than that found in normal tissue (85% versus 29% for GBM and normal adjacent tissue respectively, P<0.0001). [28]. Similarly, using semi-quantitative RT-PCR, Chen and Hughes-Fulford (2001) showed that, unlike the case of normal fibroblasts, the levels of LDLR are not feedback regulated by addition of exogenous LDL in prostate cancer cells PC3 [25]. Similar use of this semi-quantitative RT-PCR approach has shown increased expression of LDLR in 7 out 11 tumor samples from colon cancer patients compared with paired normal colonic mucosa [29]. In the case of SR-BI, Leon et al. (2010) showed that increased expression in SR-BI (2 fold increase) in the progression in the progression to castration-resistance in the LNCaP xenograft model [30]. However, it should be noted that a number of normal tissues do show expression of lipoprotein receptors. This expression represents an important consideration as the use of LDL or HDL based nanoparticles may result in off-site toxicity.
The lipoprotein receptors can be functionally divided into two groups. Group 1 represents endocytic receptors such as LDL receptor (LDLR) and LDLR related proteins and scavenger receptor type A (SRAs) that bind lipid carrying lipoproteins and serve to facilitate lysosomal delivery. The second group is composed of receptors such as scavenger receptor type B member 1 (SR-BI; encoded by SCARB1 gene), scavenger receptor type B member 2 (SR-BII, encoded by SCARB2) and CD36 that mediate lipid exchange at the plasma membrane without cellular uptake of the protein component of the particle [31].
As the first lipoprotein receptor to be isolated and cloned [32], LDL receptor (LDLR) has served as the model to examine the role of intracellular trafficking, endocytosis and vesicle recycling [31]. In terms of structure, LDLR is closely related to the other members of the LDL receptor-related (LRP) family of proteins. These additional members include LRP1, LRP1b, megalin/LRP2, very low-density lipoprotein receptor, MEGF7/LRP4, and LRP8/apolipoprotein E receptor 2 [33]. The function of LDLR has been primarily ascribed to mediate the regulation of cholesterol homeostasis by endocytosis of lipoproteins by means of the clathrin-dependent pathway [34]. Indeed, in terms of its activity, the LDL receptor accounts for approximately two thirds of LDL clearance in the body [35]. A new perspective is slowly emerging that suggests that these receptors are further involved in a number of other physiological functions acting as signal transducers [33, 36]. Recent work by dos Santos et al (2014) suggested that LDL may also play a relevant role in breast cancer [37]. Their work showed that LDL-cholesterol but not HDL-cholesterol induces changes in gene expression and enhanced proliferation, migration and loss of adhesion in a number of breast cancer cell lines.
Acton et al. [38] were the first to provide evidence for SR-BI as a receptor for HDL. These studies identified the ability of this receptor to bind HDL and to selectively mediate cholesterol oleate uptake [38]. The SR-BI and HDL binding interaction is influenced by the relative content of ApoA-I versus ApoA-II in alpha HDL. In this regard, SR-BI shows greater affinity to ApoA-II-enriched particles but has reduced lipid uptake activity [39]. Beyond its binding to HDL, SR-BI can tightly bind phosphatidylserine (PS) and phosphatidylinositol (PI)-containing liposomes, but not phosphatidylcholine, phosphatidylethanolamine, or sphingomyelin liposomes [40]. Evidence provided by De La Llera-Moya et al. suggests that interaction between HDL and SR-BI serves to facilitate the bidirectional flux between HDL and plasma membrane by reorganization of lipids within cholesterol- and caveolae-rich domains within the plasma membrane [41]. Unlike SRAs, or LDLR which are located in clathrin-coated pits and that target internalized lipoproteins to lysosomes; SR-BI is found primarily in calveolae. This difference allows SR-BI to target internalized lipids into non-endosomal and non-lysosomal compartments [42–44] and thus mediate a more specific uptake than that mediated by LDLR [5]. The lipid transfer activity ascribed to SR-BI is suggested to occur via the formation of a hydrophobic channel through which cholesterol diffuses from the HDL particle into the plasma membrane [45].
SR-BI is expressed in a number of normal tissues including liver, adrenal and ocular and steroidogenic tissue, where its expression can be affected by a number of factors [46, 47]. In the liver parenchymal cells, for example, SR-BI expression is down-regulated by cholesterol and estradiol and up-regulated by polyunsaturated fatty acids [14]. Equally, the expression of SR-BI has been shown to be regulated by various transcription factors including HIF2 [48] and MITF [49]. Disease conditions have been also implicated in alterations of SR-BI expression. Qiu et al. have recently noted the up-regulation of SR-BI as well as caveolin in non-alcoholic fatty disease liver [26]. In addition, growing evidence links the expression of SR-BI with various cancer types. For instance, recent evidence suggests that SR-BI is a potential biomarker for nasopharyngeal carcinoma [50]. The high levels of SR-BI expression have also been noted in prostate, breast, colorectal and ovarian cancers [21–23]. In all these conditions the increased levels of SR-BI may serve to address the putative heightened cholesterol requirements by these cancer cells [22], thus being a potential target for cancer theranostics.
3. Development of lipoproteins delivery vehicles
The use of chylomicrons-like particles as delivery vehicles has been noted previously (see Rensen et al. for review [51]). Despite the fact that these preparations have been used to deliver a variety of agents to hepatocytes (e.g. antiviral analogues DNA fragments and pro-drugs)[52–54], their applications in other conditions such as cancer remain limited [55]. The aforementioned expression of LDL and HDL receptors in various tumor types has driven more significant efforts to develop these lipoproteins as delivery vehicles for bioactive agents into cancer cells as well as other disease conditions.
As early as 1979, Krieger et al [56] successfully replaced the native core of LDL with cholesteryl linoleate suggesting that a similar approach could be used to incorporate hydrophobic compounds into LDL. Since then, a number of applications have made use of this lipoprotein both in vivo and in vitro (tables 2 and 3). While some studies have examined the use of LDL in atherosclerosis treatment (delivery of dexamethasone into macrophages to prevent transformation into foam cells [57]), the preponderance of efforts for LDL nanoparticles have been made with the aim of delivering agents into cancer cells (i.e. anticancer drugs; see table 2). However, a significant challenge in this regard is that the number of cancers that overexpress LDL receptors is limited.
Table 2.
Examples of synthetic LDL nanoparticles
| Name | Lipid component | Lipoprotein component | Cargo | Target | Ref |
|---|---|---|---|---|---|
| Nano-LDL | PC Triolein |
ApoB mimetic peptide | Paclitaxel oleate | Glioblastoma multiforme cells | [81] |
| sLDL | Egg yolk PC Triolein Cholesteryl Oleate |
ApoB mimetic peptides | Lymphoma cell line | [111] | |
| rLDL | Extracted LDL Trolein |
ApoB | Bacteriochlorin e6 bisoleate | PDT in human liver subcutaneous tumor xenografts | [84] |
| (rITG) LDL | Extracted LDL Trolein |
ApoB | Poly-iodinated triglyceride | Liver cancer cells | [134] |
| LDL/dextran | Extracted LDL Dextran | ApoB | Hypericin | Human glioma cell line | [135] |
| Au-LDL | LDL | ApoB | Au-MHPC | CT and optical imaging in subcutaneous tumor xenografts | [87] |
Table 3.
Recent studies that utilized synthetic HDL nanoparticles for the delivery of chemotherapeutic agents, siRNAs, nanoparticles, and imaging agents.
| Name | Lipid component | Protein component | Cargo | Target/model | References |
|---|---|---|---|---|---|
| HPPS | DMPC Cholesteryl oleate (CO) |
R4F peptide | Paclitaxel oleate DiR-BOA (optical imaging contrast agent) Cholesterol conjugated siRNA |
Human KB subcutaneous tumor xenograft Orthotopic prostate tumor xenograft |
[17, 60, 118, 119] |
| sHDL | POPC | L37pA and D37pA ApoA-I mimetic peptides |
CO | Human umbilical vein endothelial cells I/R injury rat heart model |
[136] |
| HDL-mimicking NP | DSPE-PEG-COOH PLGA CO |
4F peptide | Quantum dot conjugated to PLGA-b-PEG Cationic stearyl-triphenyl phosphonium |
Human adipose-derived MSCs Mouse monocyte/macrophage RAW 264.7 cells |
[121] |
| rHDL | Egg yolk PC, cholesterol, CO | ApoA-I | siRNA/oligolysine mixture Paclitaxel All-trans-retinoic acid Fenretinide Valrubicin |
Human orthotopic ovarian tumor xenografts Human cancer cell lines (breast, prostate, ovarian) Neuroblastoma cell lines Retinal pigment epithelial cells Prostate and ovarian cancer cell lines |
[71, 91–94] |
| HDL-NP | PDP PE DPPC |
ApoA-I | 5nm gold nanoparticles Antisense cholesterylated DNA |
Lymphoma cell lines Human cells |
[95–97] |
| V156K- rHDL | POPC Cholesterol |
ApoA-I | Rapamycin | Human monocyte cell line Human dermal fibroblasts Zebra fish model |
[104] |
| rHDL/Chol-siRNA | Soybean PC Cholesterol Cholesteryl ester |
ApoA-I | Cholesterol conjugated siRNA | Liver cancer cell line Human liver subcutaneous tumor Xenograft |
[137] |
| TA-rHDL | Glycerol trioleate Cholesterol, CO |
ApoA-I | Tanshinone IIA | Mouse macrophage cell line | [138, 139] |
| GBCA-HDL | ApoA-I Synthetic peptide |
GBCA (MR contrast agent) | Macrophages ApoE-deficient mouse model of atherosclerosis |
[140] | |
| rHDL nanodiscs | DMPC | ApoE3-NT | Curcumin | Construct bearing LDLR ligand binding domains | [141] |
| rHDL nanodiscs | DMPC | ApoA-I | Curcumin | Human hepatocellular carcinoma cell line Mantle cell lymphoma cells |
[98, 99] |
| rHDL nanodiscs | DMPC | ApoA-I ApoE |
Curcumin | glioblastoma multiforme cells | [100] |
| Cationic rHDL nanodiscs | DMPC Glycerophospholipid DMTAP (cationic) |
ApoA-I | siRNA | Hepatoma cells | [102] |
| rHDL nanodiscs | Pyropheophorbide-conjugated lipid | ApoA-I ApoE3 |
Pyro | SR-BI over-expressing cell line (photosensitization) | [101] |
| [S]-rHDL | Lyso PC DMPC |
ApoA-I | Simvastatin | atherosclerotic plaques in Apo-E knockout mice | [103] |
The suitability of HDL particles as delivery vehicles was investigated by Kader et al. [58]. These early efforts showed that 5-fluorouracil (5-FU), 5-iododeoxyuridine (IUdR), doxorubicin (Dox) and vindesine could be successfully incorporated into both LDL and HDL resulting in lower IC50 and that uptake could be modulated by regulating the levels of lipoprotein receptors. A number of studies have now shown the applicability of HDL-like nanoparticles to deliver anticancer drugs (table 3). The application of these particles could extend potentially to other diseases conditions including cardiovascular diseases, Alzheimer’s disease, Type II diabetes, stroke, as well as for antifungal and antiviral treatment [5]. In addition, the specific nature of cytosolic delivery mediated by the HDL receptor, SR-BI, may benefit the efficient delivery of siRNAs to enhance RNAi by circumventing the lysosomal pathway [59, 60]. The application of LDL nanoparticles for this purpose is more limited in that it requires the incorporation of endosomal disruption techniques in order to prevent siRNA degradation [61].
3.1 Native Lipoprotein
The use of native lipoproteins as part of nanoparticle delivery systems offers a number of significant advantages. Principally endogenous lipoproteins do not induce an immune response and are not recognized by the reticuloendothelial system (RES) [51]. Indeed, a number of reports show efficacy of drug loaded LDL particles in vivo and in vitro[62]. Others have shown loading of native LDL with, amongst others, photosenzitizers [63], nucleosides [64] and fluorescent imaging agents [65]. Despite these advantages, the use of native lipoproteins as components of delivery nanoparticles is hindered by a number of factors. In the case of LDL, for instance, it has been noted that its storage leads to natural aggregation of these molecules [66]. In addition, the use of native LDL or HDL is limited by difficulties in isolating procedures [66], loading of therapeutic agents [67] and concerns with respect to safety [68]. Similar concerns have been raised for the use of chylomicrons that are isolated from human lymph [69]. These challenges have limited the use of native chylomicrons, LDL and HDL lipoproteins as part of delivery nanoparticles [16, 58]. As a result, the focus of lipoprotein nanoparticles development has shifted towards the use of synthetic lipoproteins [70].
3.2 Synthetic reconstituted lipoproteins - an extension of biological approaches
Reconstituted lipoproteins are the most widely studied class of synthetic lipoproteins. Contrary to their native counterparts, which are directly isolated from human donors and applied as is, reconstituted lipoproteins are formed using isolated apolipoproteins (recombinant or naturally derived) combined with various lipids, natural or synthetic [5]. In general, a reconstituted lipoprotein contains one single type of apolipoprotein and one or a few defined lipid components. This offers several advantages over the native lipoprotein. First of all, since all components of the lipoprotein nanoparticle are known, they can be characterized individually to better understand the overall formulation. In addition, adjusting these individual components, such as lipid/protein stoichiometry, lipid type, and apolipoprotein choice, can control the nanoparticles to exhibit uniform physiochemical properties such as uniform size, zeta potential, core and surface loading, etc. Finally, these reconstituted lipoproteins possess and take advantage of the known functions ascribed to native lipoproteins, which includes natural targeting, enzyme/pathway activation, cellular uptake and lipid transfer.
However, the development of reconstituted lipoprotein-based formulations has been hindered by limited availability of apoliproteins (Apo B-100, ApoA-I and Apo E). This imposes significant challenges to the scalability of the manufacture of these particles [71]. The development of recombinant apolipoproteins has been one approach that has been pursued to address this limitation. This approach has been undertaken based on the close similarity between recombinant molecules and their native counterparts [51]. Law et al. successfully cloned human ApoB-100 into an expression vector thus resulting in the 560 amino acid sequence for this protein [72]. These and other efforts have utilized recombinant ApoB-100 to examine defining elements of its biology. For instance, Boren et al. [73] used recombinant wild-type and mutant ApoB-100 molecules to identify specific sequences that are relevant for its interaction with the LDL receptor. However, the use of recombinant ApoB-100 for the generation of LDL is limited owing to the large size of this molecule (over 4500 amino acids), as well as the poor water solubility of this molecule [70].
The use of recombinant Apo-AI, which is produced by means of bacterial, plant and yeast systems, has provided a partial solution to the limitations imposed by isolation of native Apo-AI. This advancement has enhanced the viability of HDL-type delivery vehicles [71, 74–76].
3.2.1 Reconstituted LDL
Early work by Walsh et al. [77] described a method for the reconstitution of LDL using ApoB solubilized from human plasma and its recombination with phospholipid using sodium deoxy-cholate. Subsequently, Ginsburg et al [78] reported a process for solubilizing ApoB of LDL with phospholipid/cholesterol ester microemulsions to generate reassembled LDL particles which exhibited many of the structural properties of native LDL (e.g. competition with human 125I-LDL for binding to LDL receptor). Similar approaches have been described by Lundberg [79] which combined phosphatidylcholine, a hydrophobic drug (mustard carbamate) and Apo B-100 to successfully generate a 23-nm reconstituted LDL particle that was taken up by the LDL receptor of cultured fibroblasts. By the same token, Hirata et al. showed that the combination of LDL-like emulsions and detergent solubilized ApoB-100 displayed a fractional CO clearance rate similar to that of LDL derived cholesteryl ester [80]. Early work showed that reconstituted LDL could be effectively loaded with a variety of agents including fluorescent probes [56], chemotherapeutic agents/cytotoxic drugs [67, 81, 82] and siRNA [83]. More recent work has highlighted the viability of reconstituted LDL as a vehicle for delivery of photodynamic and imaging agents. For instance, reconstituted LDL which has been loaded with bacteriochlorin e6 bisoleate (Bchl-BOA) or napthalocyanine has shown efficacy as a photodynamic therapy agent delivery system [84, 85]. In the case of Marrotta et al. [84] Bchl-BOA-LDL was able to mediate a delay in tumor regrowth in xenografts of human hepatoblastoma G2 (HepG2) tumors. Further work by Corbin et al. [86] showed successful incorporation of amphiphilic gadolinium (Gd)-diethylenetriaminepentaacetic acid chelates into LDL thus generating a novel magnetic resonance imaging contrast agent that retained similar diameter/surface charge and selective uptake as the native LDL particle. Similarly, Allijn et al [87] showed that incorporation of fluorescently labeled gold nanocrystals into LDLs created a CT and optical contrast agent that maintained the biological functions of native LDL both in vitro and in vivo.
As the LDL receptor also recognizes ApoE, an alternative approach has been the use of ApoE for the generation of LDL [51]. Vogel et al. [88] showed that recombinant and natural plasma ApoE bind equally well to LDL receptor and that both were cleared at similar rates from circulation. The incorporation of recombinant Apo-E into nanoparticles may facilitate a more specific uptake than that mediated by LDL [51]. Recombinant ApoE has been used in the generation of ApoE enriched liposomes to mimic LDL targeting of tumors cells and delivery of biological agents [69].
In addition to these applications, Zheng et al. [89] have demonstrated that LDL nanoparticles can be modified so as to target different receptors and cell types. In this work, the conjugation of folate to ApoB-100 was able to reroute folate-LDL particle towards cells expressing folate receptor (KB cells) and away from those expressing LDL receptor (HepG2), thus expanding the horizon of lipoproteins for targeting delivery.
3.2.2 Reconstituted HDL
Reconstituted HDL (rHDL) is manufactured in the form of nanodiscs or spherical nanoparticles through the self-assembly of isolated or recombinant ApoA-I or ApoE with a phospholipid/cholesteryl ester emulsion [90]. Cargos of the nanoparticle, whether encapsulated in the core or conjugated on the surface, can be either added directly into the emulsion or after particle formation. The resultant nanoparticle retains both the physiochemical properties as well as the biological functions of the native HDL. As a versatile drug delivery vehicle, cargos carried by the rHDL nanoparticle include a diverse range of chemotherapeutics, siRNAs, photosensitizers and imaging agents (Table 3). Its small size (~10 nm), natural targeting ability (SR-BI receptor), unique delivery pathway, simple surface chemistry, and ability to solubilize hydrophobic molecules make rHDL a favorable alternative delivery strategy compared with some of the current nanoparticles.
A good example of a versatile rHDL is the formulation developed by McConathy et al in 2008 [71]. This rHDL formulation, made from egg yolk PC, cholesterol, cholesteryl oleate (CO) and recombinant ApoA-I, encapsulated paclitaxel (PTX) in its core. The resulting nanoparticle had flotation properties and size (11.4 nm) very similar to that of the native HDL. Studies demonstrated that rHDL delivery increased the effectiveness of PTX against ovarian, prostate and breast cancer cell lines in vitro and reduced the in vivo toxicity of PTX in C57/BL6 mice, where the weight loss of the animals did not reach 15% even with 100 mg/kg PTX dosage, compared to 15% weight loss reached with 30 mg/kg Taxol or 70 mg/kg Abraxane. In a follow up study in 2010 [91], Mooberry et al studied the biological mechanisms in which the rHDL delivered PTX is taken up into cancer cells. Through radiolabeling of the core contents (PTX and CO) of rHDL, in vitro cell uptake study showed that the CO and PTX had significantly higher cell uptake by SR-BI positive cells compared to SR-BI negative cells. In addition, competition studies demonstrated that 10-fold excess of rHDL or native HDL3 significantly inhibited the PTX uptake. These results validated that the rHDL delivery were indeed specifically mediated by the SR-BI protein. The authors also conjugated folic acid onto the rHDL surface and demonstrated the ability of the modified rHDL to target folic acid receptor. This shows that rerouting the rHDL nanoparticles to actively target a receptor other than SR-BI is entirely achievable. These rHDL were further explored in its potential to carry and deliver siRNAs for in vivo RNAi applications [92]. In this case, the siRNA was coupled to oligolysine peptides to achieve a neutralizing effect and was encapsulated in the core of the rHDL to achieve a robust loading of 4 mg siRNA/mL (>90% loading efficiency). More recently, this rHDL formulation has been extended to encapsulate retinoids for the treatment of neuroblastoma [93] and valrubicin to enhance its functionality in prostate and ovarian cancer cell lines [94].
Thaxton et al [95] developed an rHDL formulation that encapsulated 5 nm gold nanoparticles. The gold nanoparticles were first incubated with ApoA-I, after which disulfide and amine functionalized lipids (PDP PE and DPPC) were added to form the Au-rHDL. These nanoparticles mimicked the general size and structure of HDL, and were able to bind to cholesterol (Kd = 4 nM). A later study [96] showed that these Au-rHDLs were able to mimic the biological function of native HDLs, and delivered gold nanoparticles specifically through the SR-BI pathway, in which the cellular uptake of gold from Au-rHDL is three fold higher in SR-BI positive lymphoma cells (SUDHL-4) compared with SR-BI negative cells (Jurkat). These Au-rHDLs were also shown to be able to manipulate the cellular cholesterol flux in lymphoma cells. In addition, thesenanoparticles were found to adsorb antisense cholesterylated DNA and deliver them to human cells to regulate target gene expression [97].
rHDL nanodiscs, which are usually formed in the same manner as the spherical rHDLs but without cholesteryl ester, has also been studied for drug delivery. In 2011, Ghosh et al [98] developed an ApoA-I driven rHDL nanodisc formulation that incorporated and delivered curcumin, a bioactive molecule with poor absorption and low bioavailability when taken orally. The nanodiscs helped solubilize curcumin and enhanced its therapeutic effect through delivery [98, 99]. Specifically, in mantle cell lymphoma (MCL) cells, curcumin nanodiscs induced ~70% cell apoptosis, much higher compared to free curcumin (<20%) and empty nanodiscs (<10%). In addition, by using ApoE instead of ApoA-I on these nanodiscs, the delivery of curcumin to glioblastoma multiforme cells was significantly enhanced, as evidenced by an over two fold increased curcumin internalization of these cells compared with ApoA-I nanodiscs and free curcumin [100]. Modification of the lipid component of the rHDL nanodisc can lead to some unique properties. Ng et al [101] has used porphyrin-lipid as the sole lipid component along with ApoA-I, ApoE3 or membrane scaffold protein 1E3D1 to develop an rHDL nanodisc formulation (10–30 nm in size). The resultant porphyrin nanodiscs have porphyrin’s photoactivities highly quenched in the intact particles, but could efficiently restore the fluorescence and singlet oxygen generation when the nanostructure is disrupted. Therefore, porphyrin nanodiscs can be used not only as an activatable fluorescent imaging probe, but also as a smart photosensitizer in phototherapeutic applications. Recent work by Ghosh et al [102] demonstrated that by incorporating a synthetic cationic lipid (DMTAP) into the rHDL lipid component, researchers enabled the rHDL nanodisc (20–50 nm in size) to incorporate oligonucleotides (dsOligo) and siRNAs. . It was found that at 1:1 dsOligo:DMTAP charge ratio the dsOligo was completely loaded onto the nanopdiscs as determined by electrophoresis and ultracentrifugation. In vitro experiments showed that the nanodiscs carrying GAPDH specific siRNAs were able to reduce the GAPDH enzyme activity by 60% in HepG2 cells. Recently, Duivenvoorden et al [103] developed a rHDL disc formulation that incorporated simvastatin and delivered them to atherosclerotic plaques, one of the natural accumulation sites of HDL. MRI and optical imaging were used to determine the pharmacokinetics and biodistribution of the rHDL, achieved through co-loading of gadolinium and fluorescent dye labeled statin. Flow cytometry of the spleen cells demonstrated that the labeled rHDLs were mostly taken up by macrophages and Ly-6chi (Gr-1hi) monocytes, and no toxic effects were observed in liver, kidney or myocytes for the rHDLs at a dose of 60 mg/kg statin. In vivo study demonstrated that a 3-month low dose statin-rHDL (15 mg/kg statin, bi-weekly infusion) treatment inhibited plague inflammation progression (27% decrease in total plaque area and 37% decrease in plaque macrophage content compared to daily oral statin treatment at 15 mg/kg statin), while a 1-week higher dose regimen (60 mg/kg statin, four infusions over one week) improved the efficacy further (36% decrease in total plaque area and 77% decrease in macrophage positive area compared to the low dose treatment).
In addition to above examples, rHDL has been used to deliver therapeutic agents for various other diseases. These include the delivery of fungicides [104], antimicrobial agents [105], viruses [106] and antivirals [107] in various cell and animal models. One potentially useful application of rHDL delivery is in the treatment of Alzheimer’s disease (AD). It has been shown that low levels of HDL and impaired ApoE relates to increased risks of AD [108, 109]. To treat this problem, Song et al in 2014 [109] developed rHDL nanodiscs, formulated by phospholipid DMPC and ApoE3, that demonstrated high binding affinity to Amyloid-β (Aβ) (KD 5.88 × 10−9 M for binding to Aβ1-40 monomer, higher than ApoE3 alone, which was 3.06 × 10−9 M) as well as the ability to cross the blood-brain barrier (BBB) (0.4 %ID/g in the brain), thus resulting in the reduced memory loss in mice with AD. The authors concluded that the ability of the rHDL to cross the BBB is due to its small size (28 nm), which further validates the advantage of HDL delivery compared to larger size nanoparticles.
4. Lipoprotein-like nanoparticles using mimetic peptides
Although synthetic reconstituted lipoproteins extended the feasibility of lipoprotein delivery vehicles, the use of recombinant ApoB-100 or Apo-AI is still limited by the significant effort and time required for manufacturing relevant amounts of apolipoproteins, including the removal of the tag from the recombinant protein and performance of additional steps for the purification of the secreted protein (e.g. proApoA-I [76]). The other type of synthetic lipoproteins has been developed by employing peptides which mimic the functional properties of apolipoproteins to build lipoprotein-like nanoparticles. The major advantage of such synthetic lipoproteins is that they overcome several challenges faced by using apolipoproteins, such as their purity, quantity, length of process time, as well as their safety [5, 75]. As a result, the synthetic lipoproteins may significantly simplify the scaled-up manufacturing, as well as accelerate the clinical translation of the lipoprotein-based drug delivery. .
4.1 Synthetic-peptide-mimetic LDL-like nanoparticles
As discussed earlier, the use of ApoB-100 protein in synthetic LDL has encountered several difficulties owing to the size and complexity of the ApoB-100 protein [110]. Therefore, an alternative strategy for synthetic LDL is the use of ApoB-100 mimetic peptides. In 2002, Baillie et al [111] developed synthetic LDL (sLDL) formulations using lipid emulsions and four amphipathic peptides containing the ApoB receptor domain. In vitro cell proliferation assays using a lymphoma cell line demonstrated that the sLDLs behaved in a way that mimics the native LDL. It was observed that by utilizing different peptides, variable proliferation was achieved, which implied that the interaction with the LDLR can be controlled by varying peptide configurations.
The use of such synthetic LDLs as drug delivery vehicles was studied in 2007 by Nikanjam et al [112]. They developed a synthetic LDL formulation, termed nano-LDL, from microemulsions of phosphatidyl choline, triolein and cholesteryl oleate, as well as a 29-amino acid bifunctional peptide, which contained a lipid binding motif and the LDLRbinding domain of ApoB-100. This peptide is water soluble and readily binds to lipid emulsions in the manufacturing process. The nano-LDLs were sonicated and extruded to have a size of 10.5 nm. Through fluorescent labeling of the lipid and peptide moieties on the nano-LDL, it was shown through fluorescence microscopy that the nano-LDLs bound to the surface of glioblastoma multiforme (GBM) cells in a similar pattern as the plasma-derived LDLs. This binding was inhibited after the introduction of a LDL inhibitor, suramin, further confirming that the nano-LDLs bound specifically to LDLR. In addition, the peptide and the lipid components of these nano-LDLs were co-localized within the lysosome following particle internalization, which further validated the specific receptor mediated uptake of the nanoparticles. In a later study [81], paclitaxel oleate was incorporated into the core of these nano-LDLs (nLDL-PO). The optimal PO loading was found to be 6 % wt. of nano-LDL. In vitro GBM cell survival assay demonstrated high cell killing by nLDL-PO (90% cell death after 72 hours compared to less than 10% in PO alone at 10 uM PO dose), and was time, concentration, and cell line dependent. Again, the use of LDLR inhibitor, suramin, significantly decreased the cell killing by nLDL-PO, confirming that the naparticles specifically utilized the LDL pathway. Overall, although such synthetic LDL as delivery vehicles hold great promise, more in-depth studies regarding the physiochemical properties of the nanoparticles are still necessary. The key-limiting issue of LDL aggregation with long term storage is likely to extend to synthetic LDLs. In recent years, the research interest in synthetic LDLs has really diminished. Table 2 presents some of the examples of synthetic LDL that have been studied as drug delivery vehicles over the past 15 years.
4.2 Synthetic Peptide mimetic HDL-like nanoparticles
As noted above, although recombinant ApoA-I has been made using bacterial expression systems, a number of challenges arise relating to their purity and quantity [75]. As risks of pathogen contamination in human-derived samples also limit the safe application of ApoA-I. As a result, complex steps must be employed to ensure the isolated ApoA-I is pathogen free [113]. To overcome these challenges, synthetic peptide analogs are utilized in the formulation process. These peptides are largely based on the amphipathic helical repeating structure of ApoA-I. Although they do not directly reflect the specific amino acid sequence of ApoA-I, their physiochemical properties resemble ApoA-I and the family of exchangeable apolipoproteins. These peptides, such as the widely investigated 18 amino acid 4F peptide, have been shown to have anti-inflammatory, anti-oxidant, anti-atherogenic and anti-tumour effects, and have been reviewed elsewhere [114–116].
The Zheng lab has developed a synthetic HDL-like nanoparticle using a synthetic ApoA-I mimetic peptide [117]. This nanoparticle, termed synthetic HDL-mimicking peptide phopholipid scaffold (HPPS), was formulated through self-assembling interactions between the ApoA-I mimetic peptide, phospholipid, and cholesteryl ester. Same as HDL, with no addition of cholesteryl esters or other hydrophobic cargo, the HPPS took form of nanodiscs, while after cargo incorporation the HPPS were spherical nanoparticles of 10–15 nm in size. Through incorporation of a hydrophobic dye (DiR-BOA) in its core and fluorescently labeling the phospholipid or peptide, confocal microscopy demonstrated that the DiR-BOA fluorescence intensity was 55 fold higher in SR-BI positive cells versus SR-BI negative cells, and 98% of the intensity was inhibited by the introduction of native HDL. Furthermore, in SR-BI positive cells, the phospholipid and peptide were found to be retained on the cell surface while the DiR-BOA was mostly found in the cell cytosol and not in lysosomes, as evidenced by non-co-localization of DiR-BOA signal with that of LysoTracker, as well as a subcellular fractionation study. This demonstrated that the HPPS exhibits properties and a delivery mechanism that is SR-BI dependent, mimicking that of the native HDL. Additional in vivo study using mice bearing subcutaneous SR-BI positive (KB) and negative (HT1080) tumors demonstrated that the HPPS preferentially accumulated in the SR-BI positive tumors (3.8 fold higher fluorescence intensity in KB versus HT1080), and this accumulation was evident at 72 hours post injection. The delivery mechanism of HPPS were further investigated in depth in a recent study [59], which validated the HDL mimicking ability of the HPPS, and enhanced the understanding of HDL delivery pathway. In this study, through fluorescent labeling of the components of the HPPS, it was found that HPPS specifically recognized and bound to SR-BI receptor. Through interactions with SR-BI after binding, the fluorescent cargo carried inside the HPPS were transported directly into the cell cytosol without entire HPPS internalization. Such cytosolic transport pathway was also found to be independent of temperature and energy, and was significantly inhibited by disruption of lipid rafts using filipin or methyl-β-cyclodextrin. The authors concluded that this cytosolic delivery predominately mediated through a lipid rafts/caveolae-like pathway.
Based on their HDL-mimicking abilities and good stability, HPPS has been studied as an efficient delivery vehicle carrying chemotherapeutics, siRNAs, and imaging contrast agents to tumour sites and into tumour cells [17, 18, 59, 60, 117–119]. In an in vivo study [118], HPPS carrying paclitaxel-oleate were shown to be able to significantly reduce the toxicity of paclitaxel to non-malignant cells, thus resulting in selective cytotoxicity towards cancer cells. HPPS delivery of siRNA has also shown good efficacy in delivery siRNA into tumor cells to knock down the target oncogene in vivo [17, 60, 119]. In this case, the sense strand of the siRNA was conjugated with cholesterol, which was inserted into the phospholipid monolayer and served as an anchor to hold the siRNA on the HPPS surface. The cytosolic delivery of siRNA through HDL-mimicking provided a crucial alternative to conventional endocytosis, and prevented the endosomal degradation of the siRNAs [120]. Loading of siRNAs onto HPPS also improved its in vivo circulation half-life from 0.95 hour to 3.9 hours, and enhanced its distribution in tumours [119]. However, it should be noted that the half-life of the HPPS core load has been reported to be 15 hours [117], suggesting that the siRNAs likely dissociated from the HPPS in circulation. In vivo efficacy studies [17, 119] have demonstrated that HPPS delivered bcl2 specific siRNA achieved over 40% knock down of the bcl2 gene in a KB subcutaneous tumour model as well as an orthotopic PC3 prostate tumour model, which in turn resulted in significantly slower tumour growth (42% KB tumour volume increase versus 200–300% in controls) and higher cell apoptosis (40% in KB tumour versus 5–16% in controls).
Marrache et al [121] have also developed a synthetic HDL formulation to detect the vulnerable plaques by targeting the collapse of mitochondrial membrane potential that occurs during apoptosis. The synthetic HDL encapsulated diagnostically active quantum dots (QD) for optical imaging. These nanoparticles are much larger than native HDLs, with hydrodynamic diameters of 100–200 nm, in which the authors determined to be the optimum formulation for mitochondria targeting. The encapsulation efficiency of the QD was 70–80%. These nanoparticles exhibited a half-life of 72 hours in vivo, and showed good stability (stable in serum for 7 days), nontoxic (less than 10% cell killing in vitro), and nonimmunogenic (no secretion of IL-6 and TNF-α after incubation with macrophages) properties. Confocal microscopy and flow cytometry both demonstrated that the targeted nanoparticles were able to detect apoptotic macrophages (increase in nanoparticle fluorescent intensity inside apoptotic macrophages).
In addition to oncology, synthetic HDLs using mimetic peptides also have the potential to play a vital part in the treatment of Alzheimer’s disease. One study demonstrated that orally administered ApoA-I mimetic peptide D4F can bind amyloid-β (Aβ) in the brain and form aggregates to reduce the amyloid burden and improve cognitive function [122]. Therefore, using D4F synthetic HDLs, one could improve the bioavailability of the peptide for the CNS disease treatment.
Overall, synthetic HDLs present an attractive strategy in drug delivery vehicles. Some recent synthetic HDL drug delivery vehicles have been summarized in Table 3.
5. Future Perspective
The application of synthetic lipoproteins as delivery vehicles for various molecules and agents has been diverse, ranging from delivering chemotherapeutics, antiviral and antifungal agents, to siRNAs and imaging agents. These applications offer great potential in the translation of the synthetic lipoproteins into the clinical setting. Currently, the most widely used area for synthetic lipoproteins has been in oncology, where the development of these nanoparticles has taken advantage of the overexpression of receptors for these lipoproteins in cancer cells (e.g. LDLR and SR-BI). In a number of instances, the early stages of elucidating the efficacy of these particles has been performed with the aid of cancer cell lines that either show natural or genetically-induced overexpression of these receptors. A significant question that remains to be addressed, and which could influence the applicability of lipoproteins in cancer treatment, is whether these cell lines faithfully represent the levels of expression in the clinic. Efforts are now underway to pursue such studies using large clinical tissue microarrays from various tumour types. In this regard, the aim is to determine the extent of receptor overexpression, whether this is homogenous throughout the tumor, and whether levels of these receptors vary between different stages of cancer (e.g. primary tumor versus metastatic disease). This strategy provides a screening tool for the types of tumor that can be preferentially treated by lipoprotein-like nanoparticles. Cancer patients with these types of tumor can be further screened individually to determine the pretreatment expression level of these receptors, enabling the potential to maximize the treatment efficacy in high SR-BI expressing cancer patients.
Currently there is little research examining exchanges between artificial and endogenous lipoproteins. As the development of lipoprotein nanoparticles becomes more prevalent, it will be important to determine the extent to this potential exchange could affect biodistribution and efficacy of these particles.
An additional challenge is that LDLR and SR-BI are also highly expressed in normal tissues (e.g. SR-BI in liver and adrenal glands) such that delivery of cytotoxic agents by these nanoparticles may result in damage to these tissues. This undesired effect could be circumvented by the use of agents that specifically target relevant molecules in tumor cells. This could be achieved, for instance, by the delivery of siRNAs for mutant genes that play a significant role in tumor cell growth/survival (e.g. BRAF V600E in melanoma). Alternatively, additional targeting moieties can be added to the synthetic lipoproteins (21), rerouting them to preferentially reach specific tumor cells or diseased sites, and thereby personalizing the treatment. Lastly, in order to increase the relevance of LDL and HDL nanoparticles as a tool for treatment of cancer in the clinic, it is also essential to conduct extensive preclinical examination of their efficacy against advanced metastatic disease, which is the typical presentation of patients in clinical trials.
It is important to note that lipoproteins represent only one class of lipid-based nanoparticles in development. Liposomes, for instance, have shown significant advances both as delivery and diagnostic tools [123, 124] and indeed represent the nano-vehicles further along in clinical application [125, 126]. Additional advances have been achieved in the use of porphysomes (nanoparticles formed by self-assembly of porphyrin and lipid conjugates) as tools for novel therapeutic approaches [127, 128]. All experience gained from these nanoparticles development can be employed for developing advanced lipoprotein delivery system.
References
- 1.De Jong WH, Borm PJ. Drug delivery and nanoparticles:applications and hazards. International journal of nanomedicine. 2008;3(2):133–149. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobrovolskaia MA, Mcneil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2(8):469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 3.Laverman P, Carstens MG, Boerman OC, et al. Factors affecting the accelerated blood clearance of polyethylene glycol-liposomes upon repeated injection. J Pharmacol Exp Ther. 2001;298(2):607–612. [PubMed] [Google Scholar]
- 4.Bamrungsap S, Zhao Z, Chen T, et al. Nanotechnology in therapeutics: a focus on nanoparticles as a drug delivery system. Nanomedicine (Lond) 2012;7(8):1253–1271. doi: 10.2217/nnm.12.87. [DOI] [PubMed] [Google Scholar]
- 5.Sabnis N, Lacko AG. Drug delivery via lipoprotein-based carriers: answering the challenges in systemic therapeutics. Therapeutic delivery. 2012;3(5):599–608. doi: 10.4155/tde.12.41. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Song J, Cavigiolio G, et al. Morphology and structure of lipoproteins revealed by an optimized negative-staining protocol of electron microscopy. J Lipid Res. 2011;52(1):175–184. doi: 10.1194/jlr.D010959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva RA, Huang R, Morris J, et al. Structure of apolipoprotein A-I in spherical high density lipoproteins of different sizes. Proc Natl Acad Sci U S A. 2008;105(34):12176–12181. doi: 10.1073/pnas.0803626105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teerlink T, Scheffer PG, Bakker SJ, Heine RJ. Combined data from LDL composition and size measurement are compatible with a discoid particle shape. J Lipid Res. 2004;45(5):954–966. doi: 10.1194/jlr.M300521-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Frias JC, Williams KJ, Fisher EA, Fayad ZA. Recombinant HDL-like nanoparticles: a specific contrast agent for MRI of atherosclerotic plaques. J Am Chem Soc. 2004;126(50):16316–16317. doi: 10.1021/ja044911a. [DOI] [PubMed] [Google Scholar]
- 10.Davidson MH. Apolipoprotein measurements: is more widespread use clinically indicated? Clin Cardiol. 2009;32(9):482–486. doi: 10.1002/clc.20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barter P, Kastelein J, Nunn A, Hobbs R. High density lipoproteins (HDLs) and atherosclerosis; the unanswered questions. Atherosclerosis. 2003;168(2):195–211. doi: 10.1016/s0021-9150(03)00006-6. [DOI] [PubMed] [Google Scholar]
- 12.Mcgrowder D, Riley C, Morrison EY, Gordon L. The role of high-density lipoproteins in reducing the risk of vascular diseases, neurogenerative disorders, and cancer. Cholesterol. 2011;2011:496925. doi: 10.1155/2011/496925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mowat BF, Skinner ER, Wilson HM, Leng GC, Fowkes FG, Horrobin D. Alterations in plasma lipids, lipoproteins and high density lipoprotein subfractions in peripheral arterial disease. Atherosclerosis. 1997;131(2):161–166. doi: 10.1016/s0021-9150(97)06097-8. [DOI] [PubMed] [Google Scholar]
- 14.Krieger M. Charting the fate of the “good cholesterol”: identification and characterization of the high-density lipoprotein receptor SR-BI. Annual review of biochemistry. 1999;68:523–558. doi: 10.1146/annurev.biochem.68.1.523. [DOI] [PubMed] [Google Scholar]
- 15.Shah PK, Kaul S, Nilsson J, Cercek B. Exploiting the vascular protective effects of high-density lipoprotein and its apolipoproteins: an idea whose time for testing is coming, part II. Circulation. 2001;104(20):2498–2502. doi: 10.1161/hc4501.098468. [DOI] [PubMed] [Google Scholar]
- 16.Lacko AG, Nair M, Prokai L, Mcconathy WJ. Prospects and challenges of the development of lipoprotein-based formulations for anti-cancer drugs. Expert Opin Drug Deliv. 2007;4(6):665–675. doi: 10.1517/17425247.4.6.665. [DOI] [PubMed] [Google Scholar]
- 17.Lin Q, Jin CS, Huang H, et al. Nanoparticle-Enabled, Image-Guided Treatment Planning of Target Specific RNAi Therapeutics in an Orthotopic Prostate Cancer Model. Small. 2014 doi: 10.1002/smll.201303842. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z, Chen J, Ding L, et al. HDL-mimicking peptide-lipid nanoparticles with improved tumor targeting. Small. 2010;6(3):430–437. doi: 10.1002/smll.200901515. [DOI] [PubMed] [Google Scholar]
- 19.Pluen A, Boucher Y, Ramanujan S, et al. Role of tumor-host interactions in interstitial diffusion of macromolecules: cranial vs. subcutaneous tumors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(8):4628–4633. doi: 10.1073/pnas.081626898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 21.Danilo C, Gutierrez-Pajares JL, Mainieri MA, Mercier I, Lisanti MP, Frank PG. Scavenger receptor class B type I regulates cellular cholesterol metabolism and cell signaling associated with breast cancer development. Breast Cancer Res. 2013;15(5):R87. doi: 10.1186/bcr3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Twiddy AL, Cox ME, Wasan KM. Knockdown of scavenger receptor class B type I reduces prostate specific antigen secretion and viability of prostate cancer cells. Prostate. 2012;72(9):955–965. doi: 10.1002/pros.21499. [DOI] [PubMed] [Google Scholar]
- 23.Narasimhan K, Pillay S, Bin Ahmad NR, et al. Identification of a polyoxometalate inhibitor of the DNA binding activity of Sox2. ACS chemical biology. 2011;6(6):573–581. doi: 10.1021/cb100432x. [DOI] [PubMed] [Google Scholar]
- 24.Ho YK, Smith RG, Brown MS, Goldstein JL. Low-density lipoprotein (LDL) receptor activity in human acute myelogenous leukemia cells. Blood. 1978;52(6):1099–1114. [PubMed] [Google Scholar]
- 25.Chen Y, Hughes-Fulford M. Human prostate cancer cells lack feedback regulation of low-density lipoprotein receptor and its regulator, SREBP2. Int J Cancer. 2001;91(1):41–45. doi: 10.1002/1097-0215(20010101)91:1<41::aid-ijc1009>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Qiu Y, Liu S, Chen HT, et al. Upregulation of caveolin-1 and SR-B1 in mice with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2013;12(6):630–636. doi: 10.1016/s1499-3872(13)60099-5. [DOI] [PubMed] [Google Scholar]
- 27.Horiuchi S, Unno Y, Usui H, et al. Pathological roles of advanced glycation end product receptors SR-A and CD36. Ann N Y Acad Sci. 2005;1043:671–675. doi: 10.1196/annals.1333.076. [DOI] [PubMed] [Google Scholar]
- 28.Guo D, Reinitz F, Youssef M, et al. An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer Discov. 2011;1(5):442–456. doi: 10.1158/2159-8290.CD-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lum DF, Mcquaid KR, Gilbertson VL, Hughes-Fulford M. Coordinate up-regulation of low-density lipoprotein receptor and cyclo-oxygenase-2 gene expression in human colorectal cells and in colorectal adenocarcinoma biopsies. Int J Cancer. 1999;83(2):162–166. doi: 10.1002/(sici)1097-0215(19991008)83:2<162::aid-ijc3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 30.Leon CG, Locke JA, Adomat HH, et al. Alterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castration-resistant prostate cancer in a mouse xenograft model. Prostate. 2010;70(4):390–400. doi: 10.1002/pros.21072. [DOI] [PubMed] [Google Scholar]
- 31.Dieckmann M, Dietrich MF, Herz J. Lipoprotein receptors--an evolutionarily ancient multifunctional receptor family. Biol Chem. 2010;391(11):1341–1363. doi: 10.1515/BC.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Francke U, Brown MS, Goldstein JL. Assignment of the human gene for the low density lipoprotein receptor to chromosome 19: synteny of a receptor, a ligand, and a genetic disease. Proc Natl Acad Sci U S A. 1984;81(9):2826–2830. doi: 10.1073/pnas.81.9.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.May P, Woldt E, Matz RL, Boucher P. The LDL receptor-related protein (LRP) family: an old family of proteins with new physiological functions. Annals of medicine. 2007;39(3):219–228. doi: 10.1080/07853890701214881. [DOI] [PubMed] [Google Scholar]
- 34.Xu S, Olenyuk BZ, Okamoto CT, Hamm-Alvarez SF. Targeting receptor-mediated endocytotic pathways with nanoparticles: rationale and advances. Adv Drug Deliv Rev. 2013;65(1):121–138. doi: 10.1016/j.addr.2012.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Twisk J, Gillian-Daniel DL, Tebon A, Wang L, Barrett PH, Attie AD. The role of the LDL receptor in apolipoprotein B secretion. The Journal of clinical investigation. 2000;105(4):521–532. doi: 10.1172/JCI8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trommsdorff M, Gotthardt M, Hiesberger T, et al. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97(6):689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- 37.Dos Santos CR, Domingues G, Matias I, et al. LDL-cholesterol signaling induces breast cancer proliferation and invasion. Lipids Health Dis. 2014;13:16. doi: 10.1186/1476-511X-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fluiter K, Van Berkel TJ. Scavenger receptor B1 (SR-B1) substrates inhibit the selective uptake of high-density-lipoprotein cholesteryl esters by rat parenchymal liver cells. Biochem J. 1997;326 ( Pt 2):515–519. doi: 10.1042/bj3260515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pilon A, Martin G, Bultel-Brienne S, et al. Regulation of the scavenger receptor BI and the LDL receptor by activators of aldosterone production, angiotensin II and PMA, in the human NCI-H295R adrenocortical cell line. Biochim Biophys Acta. 2003;1631(3):218–228. doi: 10.1016/s1388-1981(03)00020-9. [DOI] [PubMed] [Google Scholar]
- 40.Rigotti A, Acton SL, Krieger M. The class B scavenger receptors SR-BI and CD36 are receptors for anionic phospholipids. The Journal of biological chemistry. 1995;270(27):16221–16224. doi: 10.1074/jbc.270.27.16221. [DOI] [PubMed] [Google Scholar]
- 41.De La Llera-Moya M, Connelly MA, Drazul D, et al. Scavenger receptor class B type I affects cholesterol homeostasis by magnifying cholesterol flux between cells and HDL. J Lipid Res. 2001;42(12):1969–1978. [PubMed] [Google Scholar]
- 42.Valacchi G, Sticozzi C, Lim Y, Pecorelli A. Scavenger receptor class B type I: a multifunctional receptor. Ann N Y Acad Sci. 2011;1229:E1–7. doi: 10.1111/j.1749-6632.2011.06205.x. [DOI] [PubMed] [Google Scholar]
- 43.Graf GA, Connell PM, Van Der Westhuyzen DR, Smart EJ. The class B, type I scavenger receptor promotes the selective uptake of high density lipoprotein cholesterol ethers into caveolae. J Biol Chem. 1999;274(17):12043–12048. doi: 10.1074/jbc.274.17.12043. [DOI] [PubMed] [Google Scholar]
- 44.Assmann G, Schulte H, Von Eckardstein A, Huang Y. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis. 1996;124 (Suppl):S11–20. doi: 10.1016/0021-9150(96)05852-2. [DOI] [PubMed] [Google Scholar]
- 45.Rodrigueza WV, Thuahnai ST, Temel RE, Lund-Katz S, Phillips MC, Williams DL. Mechanism of scavenger receptor class B type I-mediated selective uptake of cholesteryl esters from high density lipoprotein to adrenal cells. J Biol Chem. 1999;274(29):20344–20350. doi: 10.1074/jbc.274.29.20344. [DOI] [PubMed] [Google Scholar]
- 46.Fidge NH. High density lipoprotein receptors, binding proteins, and ligands. J Lipid Res. 1999;40(2):187–201. [PubMed] [Google Scholar]
- 47.Provost A, Pequignot M, Salle S, Sainton K, Abitbol M. Gene and Protein Expression of Srb1 (Scavenger receptor Class B, Type 1) a Cell Surface HDL Receptor of CD36 Superfamilly, in the Adult Rat Retina. Invest Ophthalmol Vis Sci. 2002;43(12):4562. [Google Scholar]
- 48.Halle C, Andersen E, Lando M, et al. Hypoxia-induced gene expression in chemoradioresistant cervical cancer revealed by dynamic contrast-enhanced MRI. Cancer Res. 2012;72(20):5285–5295. doi: 10.1158/0008-5472.CAN-12-1085. [DOI] [PubMed] [Google Scholar]
- 49.Hoek KS, Schlegel NC, Eichhoff OM, et al. Novel MITF targets identified using a two-step DNA microarray strategy. Pigment Cell Melanoma Res. 2008;21(6):665–676. doi: 10.1111/j.1755-148X.2008.00505.x. [DOI] [PubMed] [Google Scholar]
- 50.Zheng Y, Liu Y, Jin H, et al. Scavenger receptor B1 is a potential biomarker of human nasopharyngeal carcinoma and its growth is inhibited by HDL-mimetic nanoparticles. Theranostics. 2013;3(7):477–486. doi: 10.7150/thno.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rensen PC, De Vrueh RL, Kuiper J, Bijsterbosch MK, Biessen EA, Van Berkel TJ. Recombinant lipoproteins: lipoprotein-like lipid particles for drug targeting. Advanced drug delivery reviews. 2001;47(2–3):251–276. doi: 10.1016/s0169-409x(01)00109-0. [DOI] [PubMed] [Google Scholar]
- 52.Rensen PC, De Vrueh RL, Van Berkel TJ. Targeting hepatitis B therapy to the liver. Clinical pharmacokinetic considerations. Clin Pharmacokinet. 1996;31(2):131–155. doi: 10.2165/00003088-199631020-00005. [DOI] [PubMed] [Google Scholar]
- 53.Rensen PC, Van Dijk MC, Havenaar EC, Bijsterbosch MK, Kruijt JK, Van Berkel TJ. Selective liver targeting of antivirals by recombinant chylomicrons--a new therapeutic approach to hepatitis B. Nat Med. 1995;1(3):221–225. doi: 10.1038/nm0395-221. [DOI] [PubMed] [Google Scholar]
- 54.Maurer BJ, Kalous O, Yesair DW, et al. Improved oral delivery of N-(4-hydroxyphenyl) retinamide with a novel LYM-X-SORB organized lipid complex. Clin Cancer Res. 2007;13(10):3079–3086. doi: 10.1158/1078-0432.CCR-06-1889. [DOI] [PubMed] [Google Scholar]
- 55.Goncalves RP, Hungria VT, Chiattone CS, Pozzi DB, Maranhao RC. Metabolism of chylomicron-like emulsions in patients with Hodgkin’s and with non-Hodgkin’s lymphoma. Leuk Res. 2003;27(2):147–153. doi: 10.1016/s0145-2126(02)00087-5. [DOI] [PubMed] [Google Scholar]
- 56.Krieger M, Smith LC, Anderson RG, et al. Reconstituted low density lipoprotein: a vehicle for the delivery of hydrophobic fluorescent probes to cells. J Supramol Struct. 1979;10(4):467–478. doi: 10.1002/jss.400100409. [DOI] [PubMed] [Google Scholar]
- 57.Tauchi Y, Zushida L, Yokota M, et al. Inhibitory effect of dexamethasone palmitate-low density lipoprotein complex on low density lipoprotein-induced macrophage foam cell formation. Biol Pharm Bull. 2000;23(4):466–471. doi: 10.1248/bpb.23.466. [DOI] [PubMed] [Google Scholar]
- 58.Kader A, Pater A. Loading anticancer drugs into HDL as well as LDL has little affect on properties of complexes and enhances cytotoxicity to human carcinoma cells. J Control Release. 2002;80(1–3):29–44. doi: 10.1016/s0168-3659(01)00536-3. [DOI] [PubMed] [Google Scholar]
- 59.Lin Q, Chen J, Ng KK, Cao W, Zhang Z, Zheng G. Imaging the Cytosolic Drug Delivery Mechanism of HDL-Like Nanoparticles. Pharmaceutical research. 2013 doi: 10.1007/s11095-013-1046-z. [DOI] [PubMed] [Google Scholar]
- 60.Yang M, Jin H, Chen J, et al. Efficient cytosolic delivery of siRNA using HDL-mimicking nanoparticles. Small. 2011;7(5):568–573. doi: 10.1002/smll.201001589. [DOI] [PubMed] [Google Scholar]
- 61.Jin H, Lovell JF, Chen J, et al. Mechanistic insights into LDL nanoparticle-mediated siRNA delivery. Bioconjug Chem. 2012;23(1):33–41. doi: 10.1021/bc200233n. [DOI] [PubMed] [Google Scholar]
- 62.Vitols S, Soderberg-Reid K, Masquelier M, Sjostrom B, Peterson C. Low density lipoprotein for delivery of a water-insoluble alkylating agent to malignant cells. In vitro and in vivo studies of a drug-lipoprotein complex. Br J Cancer. 1990;62(5):724–729. doi: 10.1038/bjc.1990.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jori G, Reddi E. The role of lipoproteins in the delivery of tumour-targeting photosensitizers. Int J Biochem. 1993;25(10):1369–1375. doi: 10.1016/0020-711x(93)90684-7. [DOI] [PubMed] [Google Scholar]
- 64.Hammel M, Laggner P, Prassl R. Structural characterisation of nucleoside loaded low density lipoprotein as a main criterion for the applicability as drug delivery system. Chem Phys Lipids. 2003;123(2):193–207. doi: 10.1016/s0009-3084(03)00002-1. [DOI] [PubMed] [Google Scholar]
- 65.Li H, Gray BD, Corbin I, et al. MR and fluorescent imaging of low-density lipoprotein receptors. Acad Radiol. 2004;11(11):1251–1259. doi: 10.1016/j.acra.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 66.De Smidt PC, Van Berkel TJ. Prolonged serum half-life of antineoplastic drugs by incorporation into the low density lipoprotein. Cancer research. 1990;50(23):7476–7482. [PubMed] [Google Scholar]
- 67.Masquelier M, Vitols S, Peterson C. Low-density lipoprotein as a carrier of antitumoral drugs: in vivo fate of drug-human low-density lipoprotein complexes in mice. Cancer Res. 1986;46(8):3842–3847. [PubMed] [Google Scholar]
- 68.Almeida AJ, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Deliv Rev. 2007;59(6):478–490. doi: 10.1016/j.addr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 69.Rensen PC, Oosten M, Bilt E, Eck M, Kuiper J, Berkel TJ. Human recombinant apolipoprotein E redirects lipopolysaccharide from Kupffer cells to liver parenchymal cells in rats In vivo. J Clin Invest. 1997;99(10):2438–2445. doi: 10.1172/JCI119427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kostner SFaG., editor. Intechopen. 2012. Role of lipoproteins in carcinogenesis and in chemoprevention. [Google Scholar]
- 71.Mcconathy WJ, Nair MP, Paranjape S, Mooberry L, Lacko AG. Evaluation of synthetic/reconstituted high-density lipoproteins as delivery vehicles for paclitaxel. Anticancer Drugs. 2008;19(2):183–188. doi: 10.1097/CAD.0b013e3282f1da86. [DOI] [PubMed] [Google Scholar]
- 72.Law SW, Lackner KJ, Hospattankar AV, et al. Human apolipoprotein B-100: cloning, analysis of liver mRNA, and assignment of the gene to chromosome 2. Proc Natl Acad Sci U S A. 1985;82(24):8340–8344. doi: 10.1073/pnas.82.24.8340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boren J, Lee I, Zhu W, Arnold K, Taylor S, Innerarity TL. Identification of the low density lipoprotein receptor-binding site in apolipoprotein B100 and the modulation of its binding activity by the carboxyl terminus in familial defective apo-B100. J Clin Invest. 1998;101(5):1084–1093. doi: 10.1172/JCI1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yancey PG, Kawashiri MA, Moore R, et al. In vivo modulation of HDL phospholipid has opposing effects on SR-BI- and ABCA1-mediated cholesterol efflux. J Lipid Res. 2004;45(2):337–346. doi: 10.1194/jlr.M300231-JLR200. [DOI] [PubMed] [Google Scholar]
- 75.Nykiforuk CL, Shen Y, Murray EW, et al. Expression and recovery of biologically active recombinant Apolipoprotein AI (Milano) from transgenic safflower (Carthamus tinctorius) seeds. Plant Biotechnol J. 2011;9(2):250–263. doi: 10.1111/j.1467-7652.2010.00546.x. [DOI] [PubMed] [Google Scholar]
- 76.Tubb MR, Smith LE, Davidson WS. Purification of recombinant apolipoproteins A-I and A-IV and efficient affinity tag cleavage by tobacco etch virus protease. Journal of lipid research. 2009;50(7):1497–1504. doi: 10.1194/jlr.D900003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Walsh MT, Atkinson D. Solubilization of low-density lipoprotein with sodium deoxycholate and recombination of apoprotein B with dimyristoylphosphatidylcholine. Biochemistry. 1983;22(13):3170–3178. doi: 10.1021/bi00282a021. [DOI] [PubMed] [Google Scholar]
- 78.Ginsburg GS, Walsh MT, Small DM, Atkinson D. Reassembled plasma low density lipoproteins. Phospholipid-cholesterol ester-apoprotein B complexes. J Biol Chem. 1984;259(10):6667–6673. [PubMed] [Google Scholar]
- 79.Lundberg B. Preparation of drug-low density lipoprotein complexes for delivery of antitumoral drugs via the low density lipoprotein pathway. Cancer Res. 1987;47(15):4105–4108. [PubMed] [Google Scholar]
- 80.Hirata RD, Hirata MH, Mesquita CH, Cesar TB, Maranhao RC. Effects of apolipoprotein B-100 on the metabolism of a lipid microemulsion model in rats. Biochim Biophys Acta. 1999;1437(1):53–62. doi: 10.1016/s1388-1981(98)00004-3. [DOI] [PubMed] [Google Scholar]
- 81.Nikanjam M, Gibbs AR, Hunt CA, Budinger TF, Forte TM. Synthetic nano-LDL with paclitaxel oleate as a targeted drug delivery vehicle for glioblastoma multiforme. J Control Release. 2007;124(3):163–171. doi: 10.1016/j.jconrel.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 82.Firestone RA, Pisano JM, Falck JR, Mcphaul MM, Krieger M. Selective delivery of cytotoxic compounds to cells by the LDL pathway. J Med Chem. 1984;27(8):1037–1043. doi: 10.1021/jm00374a017. [DOI] [PubMed] [Google Scholar]
- 83.Kim HR, Kim IK, Bae KH, Lee SH, Lee Y, Park TG. Cationic solid lipid nanoparticles reconstituted from low density lipoprotein components for delivery of siRNA. Mol Pharm. 2008;5(4):622–631. doi: 10.1021/mp8000233. [DOI] [PubMed] [Google Scholar]
- 84.Marotta DE, Cao W, Wileyto EP, et al. Evaluation of bacteriochlorophyll-reconstituted low-density lipoprotein nanoparticles for photodynamic therapy efficacy in vivo. Nanomedicine (Lond) 2011;6(3):475–487. doi: 10.2217/nnm.11.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Song L, Li H, Sunar U, et al. Naphthalocyanine-reconstituted LDL nanoparticles for in vivo cancer imaging and treatment. Int J Nanomedicine. 2007;2(4):767–774. [PMC free article] [PubMed] [Google Scholar]
- 86.Corbin IR, Li H, Chen J, et al. Low-density lipoprotein nanoparticles as magnetic resonance imaging contrast agents. Neoplasia. 2006;8(6):488–498. doi: 10.1593/neo.05835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Allijn IE, Leong W, Tang J, et al. Gold nanocrystal labeling allows low-density lipoprotein imaging from the subcellular to macroscopic level. ACS Nano. 2013;7(11):9761–9770. doi: 10.1021/nn403258w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vogel T, Weisgraber KH, Zeevi MI, et al. Human apolipoprotein E expression in Escherichia coli: structural and functional identity of the bacterially produced protein with plasma apolipoprotein E. Proc Natl Acad Sci U S A. 1985;82(24):8696–8700. doi: 10.1073/pnas.82.24.8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zheng G, Chen J, Li H, Glickson JD. Rerouting lipoprotein nanoparticles to selected alternate receptors for the targeted delivery of cancer diagnostic and therapeutic agents. Proc Natl Acad Sci U S A. 2005;102(49):17757–17762. doi: 10.1073/pnas.0508677102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bricarello DA, Smilowitz JT, Zivkovic AM, German JB, Parikh AN. Reconstituted lipoprotein: a versatile class of biologically-inspired nanostructures. ACS Nano. 2011;5(1):42–57. doi: 10.1021/nn103098m. [DOI] [PubMed] [Google Scholar]
- 91.Mooberry LK, Nair M, Paranjape S, Mcconathy WJ, Lacko AG. Receptor mediated uptake of paclitaxel from a synthetic high density lipoprotein nanocarrier. J Drug Target. 2010;18(1):53–58. doi: 10.3109/10611860903156419. [DOI] [PubMed] [Google Scholar]
- 92.Shahzad MM, Mangala LS, Han HD, et al. Targeted delivery of small interfering RNA using reconstituted high-density lipoprotein nanoparticles. Neoplasia. 2011;13(4):309–319. doi: 10.1593/neo.101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sabnis N, Pratap S, Akopova I, Bowman PW, Lacko AG. Pre-Clinical Evaluation of rHDL Encapsulated Retinoids for the Treatment of Neuroblastoma. Front Pediatr. 2013;1:6. doi: 10.3389/fped.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sabnis N, Nair M, Israel M, Mcconathy WJ, Lacko AG. Enhanced solubility and functionality of valrubicin (AD-32) against cancer cells upon encapsulation into biocompatible nanoparticles. Int J Nanomedicine. 2012;7:975–983. doi: 10.2147/IJN.S28029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thaxton CS, Daniel WL, Giljohann DA, Thomas AD, Mirkin CA. Templated spherical high density lipoprotein nanoparticles. J Am Chem Soc. 2009;131(4):1384–1385. doi: 10.1021/ja808856z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang S, Damiano MG, Zhang H, et al. Biomimetic, synthetic HDL nanostructures for lymphoma. Proc Natl Acad Sci U S A. 2013;110(7):2511–2516. doi: 10.1073/pnas.1213657110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mcmahon KM, Mutharasan RK, Tripathy S, et al. Biomimetic high density lipoprotein nanoparticles for nucleic acid delivery. Nano Lett. 2011;11(3):1208–1214. doi: 10.1021/nl1041947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ghosh M, Singh AT, Xu W, Sulchek T, Gordon LI, Ryan RO. Curcumin nanodisks: formulation and characterization. Nanomedicine : nanotechnology, biology, and medicine. 2011;7(2):162–167. doi: 10.1016/j.nano.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Singh AT, Ghosh M, Forte TM, Ryan RO, Gordon LI. Curcumin nanodisk-induced apoptosis in mantle cell lymphoma. Leuk Lymphoma. 2011;52(8):1537–1543. doi: 10.3109/10428194.2011.584253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ghosh M, Ryan RO. ApoE enhances nanodisk-mediated curcumin delivery to glioblastoma multiforme cells. Nanomedicine (Lond) 2013 doi: 10.2217/nnm.13.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ng KK, Lovell JF, Vedadi A, Hajian T, Zheng G. Self-assembled porphyrin nanodiscs with structure-dependent activation for phototherapy and photodiagnostic applications. ACS Nano. 2013;7(4):3484–3490. doi: 10.1021/nn400418y. [DOI] [PubMed] [Google Scholar]
- 102.Ghosh M, Ren G, Simonsen JB, Ryan RO. Cationic lipid nanodisks as an siRNA delivery vehicle. Biochem Cell Biol. 2014;22:1–6. doi: 10.1139/bcb-2014-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Duivenvoorden R, Tang J, Cormode DP, et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat Commun. 2014;5:3065. doi: 10.1038/ncomms4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cho KH. Enhanced delivery of rapamycin by V156K-apoA-I high-density lipoprotein inhibits cellular proatherogenic effects and senescence and promotes tissue regeneration. J Gerontol A Biol Sci Med Sci. 2011;66(12):1274–1285. doi: 10.1093/gerona/glr169. [DOI] [PubMed] [Google Scholar]
- 105.Shiflett AM, Bishop JR, Pahwa A, Hajduk SL. Human high density lipoproteins are platforms for the assembly of multi-component innate immune complexes. J Biol Chem. 2005;280(38):32578–32585. doi: 10.1074/jbc.M503510200. [DOI] [PubMed] [Google Scholar]
- 106.Bhattacharya P, Grimme S, Ganesh B, et al. Nanodisc-incorporated hemagglutinin provides protective immunity against influenza virus infection. J Virol. 2010;84(1):361–371. doi: 10.1128/JVI.01355-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hu J, Liu H, Wang L. Enhanced delivery of AZT to macrophages via acetylated LDL. J Control Release. 2000;69(3):327–335. doi: 10.1016/s0168-3659(00)00319-9. [DOI] [PubMed] [Google Scholar]
- 108.Raygani AV, Rahimi Z, Kharazi H, Tavilani H, Pourmotabbed T. Association between apolipoprotein E polymorphism and serum lipid and apolipoprotein levels with Alzheimer’s disease. Neurosci Lett. 2006;408(1):68–72. doi: 10.1016/j.neulet.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 109.Song Q, Huang M, Yao L, et al. Lipoprotein-based nanoparticles rescue the memory loss of mice with Alzheimer’s disease by accelerating the clearance of amyloid-beta. ACS Nano. 2014;8(3):2345–2359. doi: 10.1021/nn4058215. [DOI] [PubMed] [Google Scholar]
- 110.Corbin IR, Zheng G. Mimicking nature’s nanocarrier: synthetic low-density lipoprotein-like nanoparticles for cancer-drug delivery. Nanomedicine (Lond) 2007;2(3):375–380. doi: 10.2217/17435889.2.3.375. [DOI] [PubMed] [Google Scholar]
- 111.Baillie G, Owens MD, Halbert GW. A synthetic low density lipoprotein particle capable of supporting U937 proliferation in vitro. J Lipid Res. 2002;43(1):69–73. [PubMed] [Google Scholar]
- 112.Nikanjam M, Blakely EA, Bjornstad KA, Shu X, Budinger TF, Forte TM. Synthetic nano-low density lipoprotein as targeted drug delivery vehicle for glioblastoma multiforme. Int J Pharm. 2007;328(1):86–94. doi: 10.1016/j.ijpharm.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 113.Lerch PG, Fortsch V, Hodler G, Bolli R. Production and characterization of a reconstituted high density lipoprotein for therapeutic applications. Vox Sang. 1996;71(3):155–164. doi: 10.1046/j.1423-0410.1996.7130155.x. [DOI] [PubMed] [Google Scholar]
- 114.Getz GS, Wool GD, Reardon CA. Biological properties of apolipoprotein a-I mimetic peptides. Curr Atheroscler Rep. 2010;12(2):96–104. doi: 10.1007/s11883-010-0097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hovingh GK, Bochem AE, Kastelein JJ. Apolipoprotein A-I mimetic peptides. Curr Opin Lipidol. 2010;21(6):481–486. doi: 10.1097/MOL.0b013e3283404507. [DOI] [PubMed] [Google Scholar]
- 116.Sherman CB, Peterson SJ, Frishman WH. Apolipoprotein A-I mimetic peptides: a potential new therapy for the prevention of atherosclerosis. Cardiol Rev. 2010;18(3):141–147. doi: 10.1097/CRD.0b013e3181c4b508. [DOI] [PubMed] [Google Scholar]
- 117.Zhang Z, Cao W, Jin H, et al. Biomimetic nanocarrier for direct cytosolic drug delivery. Angew Chem Int Ed Engl. 2009;48(48):9171–9175. doi: 10.1002/anie.200903112. [DOI] [PubMed] [Google Scholar]
- 118.Yang M, Chen J, Cao W, et al. Attenuation of nontargeted cell-kill using a high-density lipoprotein-mimicking peptide--phospholipid nanoscaffold. Nanomedicine (Lond) 2011;6(4):631–641. doi: 10.2217/nnm.11.10. [DOI] [PubMed] [Google Scholar]
- 119.Lin Q, Chen J, Jin H, et al. Efficient systemic delivery of siRNA by using high-density lipoprotein-mimicking peptide lipid nanoparticles. Nanomedicine (Lond) 2012;7(12):1813–1825. doi: 10.2217/nnm.12.73. [DOI] [PubMed] [Google Scholar]
- 120.Lin Q, Chen J, Zhang Z, Zheng G. Lipid-based nanoparticles in the systemic delivery of siRNA. Nanomedicine (Lond) 2014;9(1):105–120. doi: 10.2217/nnm.13.192. [DOI] [PubMed] [Google Scholar]
- 121.Marrache S, Dhar S. Biodegradable synthetic high-density lipoprotein nanoparticles for atherosclerosis. Proc Natl Acad Sci U S A. 2013;110(23):9445–9450. doi: 10.1073/pnas.1301929110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Handattu SP, Garber DW, Monroe CE, et al. Oral apolipoprotein A-I mimetic peptide improves cognitive function and reduces amyloid burden in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2009;34(3):525–534. doi: 10.1016/j.nbd.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Deshpande PP, Biswas S, Torchilin VP. Current trends in the use of liposomes for tumor targeting. :1748–6963. doi: 10.2217/nnm.13.118. (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Akbarzadeh A, Rezaei-Sadabady R, Davaran S, et al. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):102. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kshirsagar NA, Pandya SK, Kirodian GB, Sanath S. Liposomal drug delivery system from laboratory to clinic. J Postgrad Med. 2005;51 (Suppl 1):S5–15. [PubMed] [Google Scholar]
- 126.Sen K, Mandal M. Second generation liposomal cancer therapeutics: transition from laboratory to clinic. Int J Pharm. 2013;448(1):28–43. doi: 10.1016/j.ijpharm.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 127.Lovell JF, Jin CS, Huynh E, et al. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat Mater. 2011;10(4):324–332. doi: 10.1038/nmat2986. [DOI] [PubMed] [Google Scholar]
- 128.Huynh E, Zheng G. Porphysome nanotechnology: A paradigm shift in lipid-based supramolecular structures. Nano Today. 2014;9(2):212–222. [Google Scholar]
- 129.Ginsberg HN. Lipoprotein physiology. Endocrinol Metab Clin North Am. 1998;27(3):503–519. doi: 10.1016/s0889-8529(05)70023-2. [DOI] [PubMed] [Google Scholar]
- 130.Crook MA. Clinical Biochemistry and Metabolic Medicine. 8. Hodder Arnold; London: 2012. [Google Scholar]
- 131.Wakil SJ. Lipid metabolism. 1970 doi: 10.1146/annurev.bi.31.070162.002101. [DOI] [PubMed] [Google Scholar]
- 132.Faergeman O, Havel RJ. Metabolism of cholesteryl esters of rat very low density lipoproteins. J Clin Invest. 1975;55(6):1210–1218. doi: 10.1172/JCI108039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Eisenberg S, Windmueller HG, Levy RI. Metabolic fate of rat and human lipoprotein apoproteins in the rat. J Lipid Res. 1973;14(4):446–458. [PubMed] [Google Scholar]
- 134.Hill ML, Corbin IR, Levitin RB, et al. In vitro assessment of poly-iodinated triglyceride reconstituted low-density lipoprotein: initial steps toward CT molecular imaging. Acad Radiol. 2010;17(11):1359–1365. doi: 10.1016/j.acra.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 135.Huntosova V, Buzova D, Petrovajova D, et al. Development of a new LDL-based transport system for hydrophobic/amphiphilic drug delivery to cancer cells. Int J Pharm. 2012;436(1–2):463–471. doi: 10.1016/j.ijpharm.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 136.Gomaraschi M, Calabresi L, Rossoni G, et al. Anti-inflammatory and cardioprotective activities of synthetic high-density lipoprotein containing apolipoprotein A-I mimetic peptides. J Pharmacol Exp Ther. 2008;324(2):776–783. doi: 10.1124/jpet.107.129411. [DOI] [PubMed] [Google Scholar]
- 137.Ding Y, Wang W, Feng M, et al. A biomimetic nanovector-mediated targeted cholesterol-conjugated siRNA delivery for tumor gene therapy. Biomaterials. 2012;33(34):8893–8905. doi: 10.1016/j.biomaterials.2012.08.057. [DOI] [PubMed] [Google Scholar]
- 138.Zhang WL, Xiao Y, Liu JP, et al. Structure and remodeling behavior of drug-loaded high density lipoproteins and their atherosclerotic plaque targeting mechanism in foam cell model. Int J Pharm. 2011;419(1–2):314–321. doi: 10.1016/j.ijpharm.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 139.Zhang W, Li J, Liu J, Wu Z, Xu Y, Wang J. Tanshinone IIA-loaded reconstituted high density lipoproteins: Atherosclerotic plaque targeting mechanism in a foam cell model and pharmacokinetics in rabbits. Pharmazie. 2012;67(4):324–330. [PubMed] [Google Scholar]
- 140.Sigalov AB. Nature-inspired nanoformulations for contrast-enhanced in vivo MR imaging of macrophages. Contrast Media Mol Imaging. 2014 doi: 10.1002/cmmi.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Khumsupan P, Ramirez R, Khumsupan D, Narayanaswami V. Apolipoprotein E LDL receptor-binding domain-containing high-density lipoprotein: a nanovehicle to transport curcumin, an antioxidant and anti-amyloid bioflavonoid. Biochim Biophys Acta. 2011;1808(1):352–359. doi: 10.1016/j.bbamem.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]