Abstract
Repeated social defeat (RSD) in mice causes myeloid cell trafficking to the brain that contributes to the development of prolonged anxiety-like behavior. Myeloid cell recruitment following RSD occurs in regions where neuronal and microglia activation is observed. Thus, we hypothesized that crosstalk between neurons, microglia, and endothelial cells contributes to brain-myeloid cell trafficking via chemokine signaling and vascular adhesion molecules. Here we show that social defeat caused an exposure- and brain region-dependent increase in several key adhesion molecules and chemokines involved in the recruitment of myeloid cells. For example, RSD induced distinct patterns of adhesion molecule expression that may explain brain region-dependent myeloid cell trafficking. VCAM-1 and ICAM-1 mRNA expression were increased in an exposure-dependent manner. Furthermore, RSD-induced VCAM-1 and ICAM-1 protein expression were localized to the vasculature of brain regions implicated in fear and anxiety responses, which spatially corresponded to previously reported patterns of myeloid cell trafficking. Next, mRNA expression of additional adhesion molecules (E- and P-selectin, PECAM-1) and chemokines (CXCL1, CXCL2, CXCL12, CCL2) were determined in the brain. Social defeat induced an exposure-dependent increase in mRNA levels of E-selectin, CXCL1, and CXCL2 that increased with additional days of social defeat. While CXCL12 was unaffected by RSD, CCL2 expression was increased by six days of social defeat. Last, comparison between enriched CD11b+ cells (microglia/macrophages) and enriched GLAST-1+/CD11b− cells (astrocytes) revealed RSD increased mRNA expression of IL-1β, CCL2, and CXCL2 in microglia/macrophages but not in astrocytes. Collectively, these data indicate that key mediators of leukocyte recruitment were increased in the brain vasculature following RSD in an exposure- and brain-region dependent manner.
Keywords: social defeat, neurovascular unit, reactive endothelium, adhesion molecules, chemokines, myeloid cell trafficking
Introduction
Psychosocial stress is associated with increased inflammation and higher prevalence of mental health disorders such as anxiety and depression (Miller et al., 2009). For example, psychological stress in humans increases the production of pro-inflammatory cytokines (Kiecolt-Glaser et al., 2003, O’Connor et al., 2009, Hansel et al., 2010) and promotes rapid leukocyte transmigration (Ottaway and Husband, 1994, Hong et al., 2005, Cole, 2008). Through the activation of neuroendocrine pathways, psychosocial stress promotes the release of glucocorticoids, catecholamines, and cytokines leading to significant physiological, immunological, and behavioral changes in both humans and rodents (Kiecolt-Glaser and Glaser, 2002, Kinsey et al., 2007, Cole et al., 2010, Wohleb et al., 2011). Repeated social defeat, a murine model of psychosocial stress, recapitulates many of the behavioral and immunological effects observed in humans (Miller et al., 2008, Cole et al., 2010). For example, RSD causes increased circulating cytokines (Brydon et al., 2005), myeloid cell trafficking (Engler et al., 2004), and prolonged anxiety-like behavior (Kinsey et al., 2007). Previous work in the RSD model has demonstrated an important role for neuroimmune signaling in the development of stress-induced changes in behavior. For example, the establishment, resolution, and recurrence of anxiety-like behavior, brain cytokine expression, microglia activation, and brain-myeloid trafficking were temporally associated (Wohleb et al., 2013, Wohleb et al., 2014a). Moreover, recent work showed that anxiety-like behavior and brain myeloid cell trafficking following RSD were absent in CCR2 and CX3CR1 deficient mice (Wohleb et al., 2013). Thus, mechanisms underlying brain myeloid cell trafficking may have important implications for the study of stress-induced changes in behavior.
It is known that chemokine receptor expression is necessary for brain myeloid cell trafficking following RSD (Wohleb et al., 2013), but the role of brain-derived chemokines and adhesion molecules has yet to be determined. Previous work may provide some clues regarding the regulation of brain-derived signals that promote brain myeloid adhesion and chemotaxis. For example, previous studies show that recruitment of GFP+ macrophages following RSD is region-specific (Wohleb et al., 2013) and spatially corresponds with fear/anxiety-related regions where neuronal C-Fos expression and microglia activation are observed (Martinez et al., 2002, Wohleb et al., 2011). Moreover, because myeloid cell trafficking is region-specific and occurs in the absence of classical inflammation and histopathology, it is likely that myeloid cell recruitment is orchestrated by neuronal activity. Because myeloid cell trafficking and microglia activation were spatially coupled to neuronal C-Fos expression following RSD (Wohleb et al., 2011), we hypothesized that neurovascular signaling resulted in region-specific myeloid cell recruitment via chemokine and adhesion molecule expression. The idea that local neuronal activity can be coupled to regulation of the cerebral vasculature strongly resembles the ‘neurovascular unit’ proposed in other studies. The concept of a ‘neurovascular unit’ is that local neuronal activity can be coupled to the regulation of cerebral vasculature (Mae et al., 2011). Thus, the concept of an immunological ‘neurovascular unit’ is helpful in understanding the mechanisms that contribute to RSD-induced brain myeloid cell trafficking.
In order for leukocytes to exit circulation and migrate into the CNS, they must extravasate through the endothelium (Wilson et al., 2010, Greenwood et al., 2011). The first stage of this process involves E- and P-selectin that bind to and initiate the rolling of leukocytes (Curry et al., 2010, Muller, 2014). Next, chemokines activate leukocytes and cause conformational changes to integrins that allow for firm adhesion of leukocytes onto the surface of the endothelium (Greenwood et al., 2011). The major integrin ligands responsible for regulating this step of leukocyte arrest are intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) (Nourshargh et al., 2010). Extravasation, or the penetration of leukocytes through the vascular wall, is regulated by cell adhesion molecules (e.g., platelet endothelial cell adhesion molecule-1: PECAM-1) and chemokine signaling processes to allow for successful transendothelial migration (Wilson et al., 2010, Muller, 2014). Thus, stress-induced expression of these adhesion molecules may play a role in the recruitment of myeloid cells to the brain in response to repeated social defeat.
In addition to adhesion molecules, chemokine release and ligand/receptor interactions play a significant role in mediating the recruitment of myeloid cells. For example, in LPS-injected mice, inflammatory CXC-chemokines CXCL1 (KC) and CXCL2 (MIP-2) induced myeloid cell recruitment through CXCR2, the mouse homolog of the IL-8 receptor (Hol et al., 2010). In addition, CXCL12 (SDF-1) binds to CXCR4 and functions to limit extensive leukocyte migration out of the perivascular space and into the brain parenchyma in models of CNS autoimmune disease (Cartier et al., 2005, McCandless et al., 2006). In response to activation by pro-inflammatory cytokines, nucleated cells will express CC-chemokine CCL2 (MCP-1), which binds CCR2 and facilitates Ly6Chi monocyte recruitment (Shi and Pamer, 2011). Moreover, RSD increases the percentage of Ly6Chi macrophages that traffic to the CNS (Wohleb et al., 2011). In studies with stress, RSD-induced myeloid cell recruitment corresponded with increased expression of CCL2 (Wohleb et al., 2013) and mice deficient in CCR2 failed to recruit macrophages to the brain (Prinz and Priller, 2010, Wohleb et al., 2013).
Therefore, the purpose of this study was to understand the role of adhesion molecules and chemokines in the recruitment of myeloid cells to the brain in response to repeated social defeat. Here we showed that RSD increased the mRNA expression of two crucial leukocyte adhesion molecules, VCAM-1 and ICAM-1, in the brain in an exposure-dependent manner. Furthermore, social defeat caused robust expression of VCAM-1 and ICAM-1 protein in specific brain regions implicated in fear and anxiety responses, including the amygdala and hypothalamus. Social defeat also enhanced the mRNA expression of E-selectin, CXCL1, and CXCL2 in the brain in an exposure-dependent manner. Last, RSD increased the mRNA levels of IL-1β, CCL2, and CXCL2 in enriched microglia/macrophages (CD11b+) but not in astrocytes.
Experimental Procedures
Mice
Male C57BL/6 (6–8 weeks old) and CD-1 (12 months, retired breeders) mice were purchased from Charles River Breeding Laboratories (Wilmington, MA). Mice were allowed to acclimate to their surroundings for 7–10 days prior to experiments. Resident C57BL/6 mice were housed in cohorts of 3 and aggressor CD-1 mice were singly housed. All mice were housed in polypropylene cage racks with ad libitum access to water and rodent chow. The rooms were maintained at 21°C under a 12-h light-dark cycle (lights on at 6 AM). Experimental and aggressor mice were routinely examined and showed no indications of illness or infection. All experiments were in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee.
Repeated Social Defeat (RSD)
Mice were subjected to RSD as previously reported (Wohleb et al., 2011, Wohleb et al., 2012). In brief, male aggressive intruder mice were introduced into cages of resident C57BL/6 mice from 17:00 to 19:00 (2 h) for one, three, or six consecutive nights. During each cycle, submissive behavior (e.g., standing upright, fleeing, and crouching) was observed by the resident mice, while aggressive behavior (e.g., back biting and tail rattling) was observed by the aggressor mice. A new intruder would replace the initial intruder if he did not initiate an attack on the resident mice within the first 5–10 minutes or if he was defeated by any of the resident mice. After the 2 h session, the intruder mouse was removed and the residents were left undisturbed until the next day when the paradigm was repeated. To avoid habituation, different intruders were used for each of the six consecutive cycles. The resident mice were carefully inspected for bite injuries after each cycle of RSD and any severely wounded mice were removed from the study. Consistent with our previous studies using RSD, less than 5% of the mice met the early removal criteria (Wohleb et al., 2011, Wohleb et al., 2013). Control mice (CON) were left undisturbed in their home cages and housed in a different room separate from the RSD cages.
Isolation of brain microglia and astrocytes
At 14 h after the last cycle of RSD, microglia and astrocytes were isolated from whole brain homogenates as previously reported (Norden et al., 2014). In brief, brains were homogenized in phosphate-buffered saline (PBS, pH 7.4) by passing through a 70 μm cell strainer. The resulting homogenates were centrifuged at 900 × g for 6 min. Supernatants were removed and cell pellets were re-suspended in 70% isotonic Percoll (GE-Healthcare). A discontinuous Percoll (GE-Healthcare) density gradient (50%, 35%, and 0%) was overlaid and centrifuged at 2000 × g for 20 min. Enriched microglia were collected from the interphase between the 70% and 50% Percoll layers. These cells were characterized as enriched brain CD11b+ cells, as previous studies have demonstrated that viable cells isolated by Percoll density gradient yields >90% CD11b+ cells (Wohleb et al., 2011, Wohleb et al., 2013). Enriched astrocytes were collected from the interphase between the 50% and 35% Percoll layers. As previously reported (Norden et al., 2014), 65–70% of the cells collected from this interphase were characterized as GLAST-1+ astrocytes.
RNA isolation and real time PCR
Total RNA was first extracted from homogenized brain regions using TRIzol (Life Technologies, Grand Island, NY). Next, RNA was reverse transcribed into cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems; Foster City, CA). RNA concentration was determined through spectrophotometry. For Percoll enriched microglia and astrocytes, the PrepEase kit (USB, CA) was used to isolate RNA according to manufacturer’s instructions. Quantitative PCR was performed using the Applied Biosystems’ TaqMan Gene expression assay protocol as previously reported (Godbout et al., 2005). The linear portion of amplification-curves for the housekeeping and genes of interest were parallel when plotted in excel (data not shown). Because the efficiency of primers was consistent between the housekeeping and genes of interest the ddct method was used (Livak and Schmittgen, 2001). In brief, experimental cDNA was amplified by real-time PCR where a target cDNA (i.e., VCAM-1, ICAM-1, E-Selectin, P-Selectin, PECAM-1, CXCL1, CXCL2, CXCL12, CCL2) and a reference cDNA (glyceraldehyde-3-phosphate dehydrogenase; GAPDH) were amplified using the Taqman Gene Expression assay that contains forward and reverse primers and a Taqman probe with a 3′ minor groove binder and a 5′ fluorescent reporter dye (6-FAM). Fluorescence was determined on an ABI PRISM 7300-sequence detection system (Applied Biosystems). Using Ct values, the ΔΔCt for each sample was calculated as follows: ΔCt = Cttarget gene − Ctcontrol gene. The values for each experimental mouse (RSD) were compared with the average value of the control mice (average CON): ΔΔCt = ΔCtRSD − Ctaverage CON gene. Finally, the fold change for each mouse was calculated as follows: fold change = 2−ΔΔCt. All results are expressed as fold change +/− SEM.
Immunohistochemistry
At 14 h after the last cycle of RSD, brains were collected from mice after carbon dioxide asphyxiation and transcardial perfusion with sterile phosphate-buffered saline (PBS, pH 7.4 with EDTA) and 4% formaldehyde. Brains were post-fixed in 4% formaldehyde for 24 h and incubated in 20% sucrose for an additional 48 h at 4°C. Fixed brains were frozen with isopentane (−80°C) and dry ice and sectioned (20 μm) using a Microm HM550 cryostat (Thermofisher). Brain regions were classified based on reference markers used in the stereotaxic mouse brain atlas (Paxinos and Franklin, 2008). To label for VCAM-1 (vascular cell adhesion molecule-1) and ICAM-1 (intercellular adhesion molecule-1), sections were placed free-floating in cryoprotectant until staining. Next, sections were washed in PBS with 1% bovine serum albumin, blocked with 3% normal donkey serum, and incubated with either goat anti-mouse VCAM-1 antibody (R&D Systems, CN AF643) or goat anti-mouse ICAM-1 antibody (R&D Systems, CN AF796). Sections were incubated in a donkey anti-goat secondary antibody (Alexa Fluor 488). Sections were mounted on slides and cover-slipped with Fluoromount and stored at −20°C. Fluorescent sections were visualized using an epi-fluorescent Leica DM5000B microscope. Images were captured using a Leica DFC300 FX camera and imaging software. For each image, a threshold for positive staining was determined that included all cell bodies while excluding background staining (ImageJ). All results are expressed as average percent area in the positive threshold for all representative images.
Statistical Analysis
Data were subjected to Shapiro-Wilk test using Statistical Analysis Systems (SAS) software. Observations more than three interquartile ranges from the first and third quartile were removed from analyses. To determine significant main effects and interactions between groups, data were analyzed through one-way (stress) or two-way (stress x region) ANOVA using the General Linear Model procedures of SAS. When appropriate, an F-protected t-test using the Least-Significant Difference procedure of SAS was used to determine differences between treatment means. All data are expressed as treatment means ± standard error of the mean (SEM).
Results
Social defeat increased mRNA expression of VCAM-1 and ICAM-1 in the caudal cortex, rostral cortex, hippocampus, and basal ganglia in an exposure-dependent manner
Previous studies indicate that six days of social defeat promoted trafficking of bone marrow (BM)-derived myeloid cells to specific brain regions associated with fear and threat appraisal, which contributed to the development of prolonged anxiety-like behavior (Wohleb et al., 2013, Wohleb et al., 2014a). Because microglia and neuronal activation after social defeat is region-specific (Wohleb et al., 2011, Wohleb et al., 2013), we hypothesize that components of the neurovascular unit increase adhesion molecule and chemokine expression to facilitate the recruitment of myeloid cells to the brain in response to the stressor. To understand how the brain may facilitate myeloid cell recruitment, VCAM-1 and ICAM-1 mRNA expression were determined in several general brain regions including the caudal cortex (CTX-C), rostral cortex (CTX-R), hippocampus (HPC), and basal ganglia (BG). These regions were collected by dissection of the whole brain and each area includes several different micro-anatomical brain regions.
In the first experiment, mice were exposed to one, three, or six days of social defeat and VCAM-1 and ICAM-1 mRNA levels were determined in several brain regions immediately following the final cycle of RSD. Table 1 shows that social defeat increased mRNA expression of VCAM-1 and ICAM-1 in the brain. For example, social defeat increased VCAM-1 mRNA expression in the CTX-C (F(3,22) = 25.88, p < 0.0001), CTX-R (F(3,22) = 51.05, p < 0.0001), HPC (F(3,21) = 3.44, p < 0.05), and BG (F(3,20) = 5.93, p < 0.005). The social defeat-induced increases in expression of VCAM-1 mRNA were dependent on exposure. For example, VCAM-1 mRNA levels were increased by either one or three days of social defeat in the CTX-R and HPC, and the highest levels of VCAM-1 mRNA in each brain region occurred after six days of social defeat (p < 0.05, for each region).
Table 1.
Social defeat increased mRNA expression of VCAM-1 and ICAM-1 in the CTX-C, CTX-R, HPC, and BG in an exposure-dependent manner
| Reg | mRNA | Control | Social Defeat | ||
|---|---|---|---|---|---|
| 1 d | 3 d | 6 d | |||
| CTX-C | VCAM-1 | 1.00 ± 0.15a | 1.10 ± 0.07a,b | 1.20 ± 0.06b | 1.70 ± 0.06c |
| ICAM-1 | 1.01 ± 0.04a | 2.01 ± 0.37b | 2.21 ± 0.28b | 4.69 ± 0.74c | |
|
| |||||
| CTX-R | VCAM-1 | 1.01 ± 0.05a | 1.32 ± 0.03b | 1.23 ± 0.05b | 1.90 ± 0.06c |
| ICAM-1 | 1.00 ± 0.04a | 2.39 ± 0.36b | 2.16 ± 0.19b | 5.47 ± 0.70c | |
|
| |||||
| HPC | VCAM-1 | 1.00 ± 0.02a | 1.14 ± 0.05b | 1.17 ± 0.07b | 1.40 ± 0.13b |
| ICAM-1 | 1.00 ± 0.03a | 2.08 ± 0.32b | 2.70 ± 0.43b,c | 4.68 ± 0.82c | |
|
| |||||
| BG | VCAM-1 | 1.00 ± 0.27a | 1.00 ± 0.09a | 1.08 ± 0.06a | 1.59 ± 0.17b |
| ICAM-1 | 1.00 ± 0.03a | 2.07 ± 0.48a,b | 2.11 ± 0.23b | 4.83 ± 1.07b | |
Male C57BL/6 mice were subjected to one, three, or six cycles of social defeat (RSD) or left undisturbed as controls (CON). Immediately following the final cycle of RSD, brains were removed, dissected, and frozen in liquid nitrogen. VCAM-1 and ICAM-1 mRNA levels were determined in the caudal cortex (CTX-C), rostral cortex (CTX-R), hippocampus (HPC), and basal ganglia (BG) (n = 6). Values represent average fold change compared to control. Means with different letters (a, b, or c) are significantly different (p < 0.05) from each other.
Social defeat also increased ICAM-1 mRNA expression in the CTX-C (main effect of stress, F(3,22) = 10.23, p < 0.001), CTX-R (F(3,21) = 16.86, p < 0.001), HPC (F(3,22) = 7.57, p < 0.001), and BG (F(3,20) = 5.08, p < 0.009). Increased expression of ICAM-1 mRNA was also dependent on repeated exposure to social defeat. For instance, one day of social defeat increased mRNA expression of ICAM-1 in the CTX-C (p < 0.05), CTX-R (p < 0.01), and HPC (p < 0.05). In addition, six days of social defeat caused a robust increase in ICAM-1 mRNA expression in the CTX-C (p < 0.005), CTX-R (p < 0.0005), HPC (p < 0.005) and BG (p < 0.05) as compared to the control. Furthermore, there was a marked induction in both VCAM-1 and ICAM-1 mRNA levels in the CTX-C and CTX-R after six days of social defeat compared to three days (p < 0.05, for each) and one day (p < 0.05, for each). Collectively, these data indicate that social defeat increased the mRNA expression of both VCAM-1 and ICAM-1 in the brain in an exposure-dependent manner.
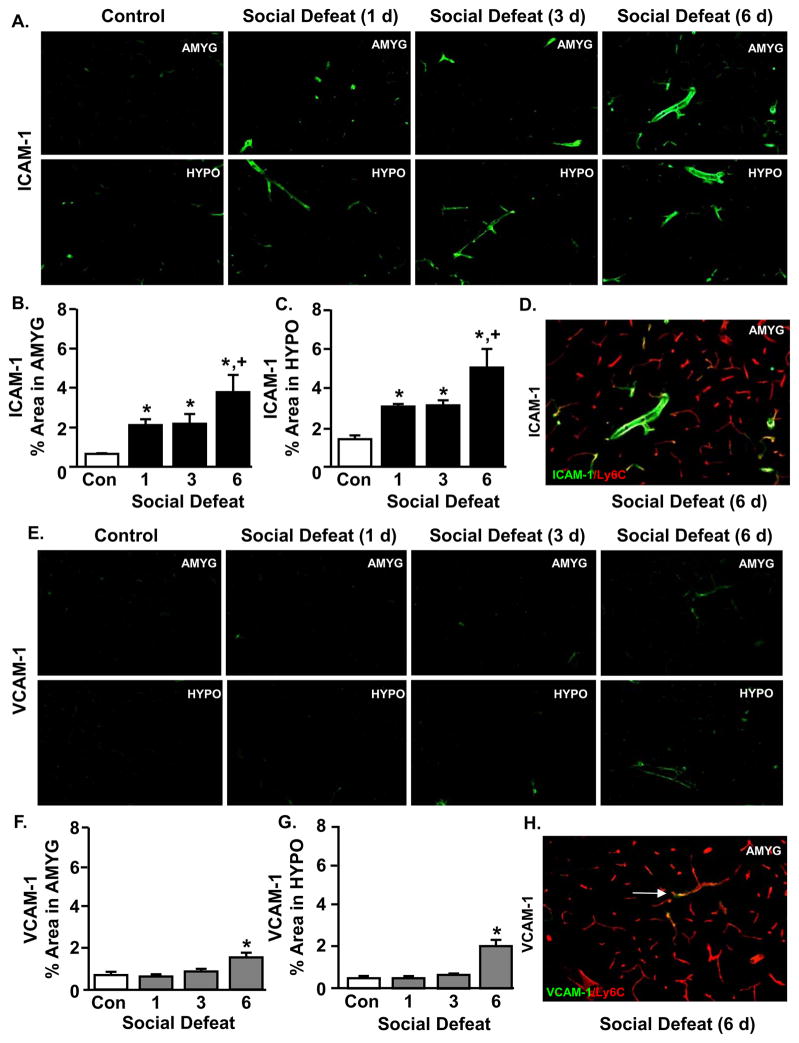
Repeated exposure to social defeat increased ICAM-1 expression on endothelial cells in the amygdala and hypothalamus in an exposure-dependent manner
Because mRNA expression of VCAM-1 and ICAM-1 were increased after RSD, we next sought to determine protein expression of these adhesion molecules within the brain. In these studies, VCAM-1 and ICAM-1 protein expression were determined in the amygdala (AMYG) and hypothalamus (HYPO) after one, three, or six days of social defeat. Figure 1A shows representative images of ICAM-1 labeling in the lumen of medium-sized blood vessels in the AMYG and HYPO after one, three, or six days of social defeat. These images are consistent with the pattern of mRNA results presented in Table 1 and show increased vascular labeling of ICAM-1 after one day of social defeat that was further increased by three and six days of social defeat.
Figure 1. Repeated exposure to social defeat increased ICAM-1 expression on endothelial cells in the amygdala and hypothalamus in an exposure-dependent manner.
Male C57BL/6 mice were subjected to one, three, or six cycles of social defeat (RSD) or left undisturbed as controls (CON). Brains were collected 14 h after the last cycle of RSD and VCAM-1 and ICAM-1 expression were determined by immunohistochemistry. A) Representative images of VCAM-1 labeling in the amygdala (AMYG) and hypothalamus (HYPO) are shown. The percent positive area for VCAM-1 labeling in the B) AMYG and C) HYPO was determined. D) Representative image of VCAM-1 and Ly6C co-labeling in the AMYG after RSD. Arrows depict co-localized labeling between VCAM-1 and Ly6C. E) Representative images of ICAM-1 labeling in the AMYG and HYPO are shown. The percent positive area for ICAM-1 labeling in the F) AMYG and G) HYPO was determined. H) Representative image of ICAM-1 and Ly6C co-labeling in the AMYG after RSD. Arrows depict co-localized labeling between ICAM-1 and Ly6C. Bars represent average ± SEM. Means with (*) are significantly different from respective CON (p ≤ 0.06). Means with (+) are significantly different from all other groups (p < 0.05).
Proportional area analysis revealed that social defeat increased ICAM-1 protein expression in the AMYG (Fig. 1B, (F(3,11) = 2.48, p = 0.1) and HYPO (Fig. 1C, F(3,12) = 3.99, p < 0.05). Consistent with the mRNA data, protein expression of ICAM-1 was robustly increased after one day of social defeat in the AMYG (p < 0.05) and the HYPO (p < 0.005). These increases were further enhanced after six days of social defeat compared to all other groups (p < 0.06, for each). To confirm that ICAM1 was increased on the brain vasculature, Ly6C labelling was used to label endothelial cells (Jutila et al., 1988). Fig. 1D confirms that RSD increased ICAM-1 protein expression on Ly6C+ endothelial cells of the brain vasculature. These findings indicate that social defeat increased ICAM-1 protein expression on brain vasculature after RSD in an exposure-dependent manner.
Representative images of VCAM-1 labeling in the AMYG and HYPO are shown in Figure 1E. Consistent with the pattern of mRNA results presented in Table 1, social defeat increased VCAM-1 protein expression in the AMYG (Fig. 1F, (F(3,14) = 5.31, p < 0.05)) and HYPO (Fig. 1G, (F(3,13) = 13.19, p < 0.001)). The protein expression of VCAM-1, however, was not increased after one or three days of social defeat. Following six days of social defeat, VCAM-1 protein expression was significantly increased in the AMYG (Fig. 1F, p < 0.05) and HYPO (Fig. 1G, p < 0 .01). As above, Ly6C labelling was used to label endothelial cells (Jutila et al., 1988). Fig. 1H confirms that RSD increased VCAM-1 protein expression on Ly6C+ endothelial cells of the brain vasculature. Taken together, these data indicate that VCAM-1 protein expression was increased on brain vasculature following exposure to social defeat.
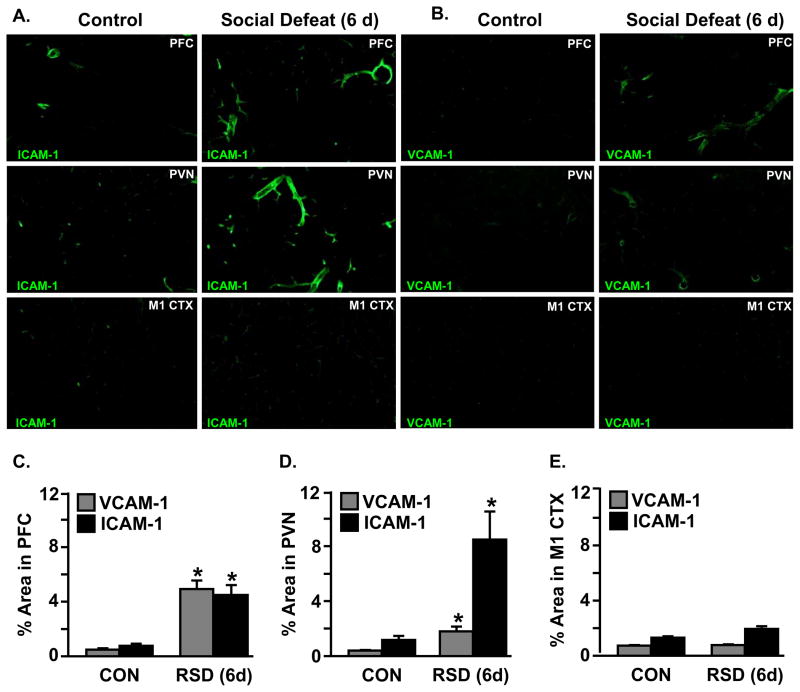
Social defeat induced ICAM-1 and VCAM-1 expression in specific brain regions associated with threat appraisal
Figure 1 shows that ICAM-1 and VCAM-1 protein expression were increased in the vasculature of the AMYG and HYPO following six days of social defeat. We have previously reported myeloid cell trafficking in BM-chimeric mice to brain regions associated with fear and anxiety responses including the PFC (prefrontal cortex) and PVN (paraventricular nucleus). Myeloid cell trafficking after RSD, however, was not detected in the M1 CTX (primary motor cortex) (Wohleb et al., 2013). These data indicate that myeloid cell trafficking in the brains of BM-chimeric mice occurred in a brain region-specific pattern.
ICAM-1 (Fig. 2A) and VCAM-1 (Fig. 2B) protein expression were determined in the PFC, PVN, and M1 CTX after six days of social defeat. Representative images for ICAM-1 (Fig. 2A) and VCAM-1 (Fig. 2B) are shown in each of the three selected regions. There were brain region-dependent increases in VCAM-1 (F(2,26) = 16.83, p < 0.0001) and ICAM-1 (F(2,28) = 3.74, p < 0.05) protein after six days of social defeat. For example, proportional area analysis confirmed that VCAM-1 and ICAM-1 levels were robustly increased after six days of social defeat in the vasculature of the PFC (Fig. 2C, p < 0.01, for both) and PVN (Fig. 2D, p < 0.02, for both). These increases, however, were not detected in the M1 CTX (Fig. 2E). Collectively, these data indicated that the induction of ICAM-1 and VCAM-1 protein expression by RSD was brain region-dependent.
Figure 2. Social defeat induced VCAM-1 and ICAM-1 expression in specific brain regions associated with fear and threat appraisal.
Male C57BL/6 mice were subjected to six cycles of social defeat (RSD) or left undisturbed as controls (CON). Brains were collected 14 h after RSD and positive VCAM-1 and ICAM-1 expression were determined. Representative images of A) VCAM-1 and B) ICAM-1 labeling in the prefrontal cortex (PFC), paraventricular nucleus (PVN), and primary motor cortex (M1 CTX) are shown. The percent positive area for VCAM-1 and ICAM-1 labeling in the C) PFC, D) PVN, and E) M1-CTX was determined. Bars represent average ± SEM. Means with an asterisk (*) are significantly different from respective CON (p < 0.05).
Social defeat increased E-selectin mRNA expression in the caudal cortex, rostral cortex, hippocampus, and basal ganglia after RSD in an exposure-dependent manner
Next, mRNA expression of several other adhesion molecules that are involved in regulating leukocyte trafficking was determined (Wilson et al., 2010, Greenwood et al., 2011). In this experiment, mice were exposed to one, three, or six days of social defeat and mRNA levels of E-selectin, P-selectin, and PECAM-1 were determined immediately after the final cycle of RSD in the caudal cortex, rostral cortex, hippocampus, and basal ganglia (Table 2). As with the data provided in Table 1, these general brain regions were collected by dissection of the whole brain. Table 2 shows that neither P-selectin nor PECAM-1 were increased by social defeat in any of the regions examined. The mRNA expression of E-selectin, however, was increased after RSD in the CTX-C (F(3,21) = 13.75, p < 0.0001), CTX-R (F(3,21) = 51.05, p < 0.0001), HPC (F(3,19) = 3.44, p < 0.01), and BG (F(3,18) = 6.97, p < 0.01). For each region examined, the mRNA levels of E-selectin were highest following six days of social defeat. For example, E-selectin mRNA levels were increased in the HPC after one day of social defeat (p < 0.05) and remained elevated at three days (p < 0.004), but reached the highest level after six days of social defeat (p < 0.05) compared to the control. Furthermore, in all regions examined, E-selectin mRNA levels were higher after six days of social defeat compared to one day (p < 0.05) and three days (p < 0.05). Collectively, these data indicate that social defeat increased E-selectin mRNA expression in the brain in an exposure-dependent manner.
Table 2.
Social defeat increased E-selectin mRNA expression in the CTX-C, CTX-R, HPC, and BG after RSD in an exposure-dependent manner
| Reg | mRNA | Control | Social Defeat | ||
|---|---|---|---|---|---|
| 1 d | 3 d | 6 d | |||
| CTX-C | E-Selectin | 1.01 ± 0.06a | 6.67 ± 2.58a,b | 8.52 ± 2.15b | 65.30 ± 13.73c |
| P-Selectin | 1.01 ± 0.04 | 1.09 ± 0.02 | 1.05 ± 0.04 | 1.10 ± 0.04 | |
| PECAM-1 | 1.00 ± 0.05 | 0.83 ± 0.04 | 1.35 ± 0.04 | 0.85 ± 0.04 | |
|
| |||||
| CTX-R | E-Selectin | 1.06 ± 0.16a | 3.68 ± 1.21b | 6.64 ± 1.12b | 53.03 ± 9.51c |
| P-Selectin | 1.00 ± 0.04 | 1.10 ± 0.04 | 1.05 ± 0.08 | 1.25 ± 0.10 | |
| PECAM-1 | 1.04 ± 0.15 | 0.97 ± 0.07 | 1.49 ± 0.14 | 1.27 ± 0.08 | |
|
| |||||
| HPC | E-Selectin | 1.07 ± 0.15a | 11.95 ± 4.67b | 36.95 ± 8.63b | 211.37 ± 63.77c |
| P-Selectin | 1.02 ± 0.07 | 1.17 ± 0.11 | 1.25 ± 0.11 | 0.97 ± 0.05 | |
| PECAM-1 | 1.00 ± 0.02 | 0.81 ± 0.10 | 1.44 ± 0.05 | 1.04 ± 0.04 | |
|
| |||||
| BG | E-Selectin | 1.04 ± 0.12a | 4.82 ± 1.95a,b | 5.72 ± 1.13b | 71.79 ± 20.86c |
| P-Selectin | 1.00 ± 0.02 | 1.14 ± 0.05 | 1.03 ± 0.06 | 1.05 ± 0.04 | |
| PECAM-1 | 1.00 ± 0.05 | 0.86 ± 0.07 | 1.44 ± 0.11 | 1.34 ± 0.15 | |
Male C57BL/6 mice were subjected to one, three, or six cycles of social defeat (RSD) or left undisturbed as controls (CON). Immediately following the final cycle of RSD, brains were removed, dissected, and frozen in liquid nitrogen. E-selectin, P-selectin, and PECAM-1 mRNA levels were determined in the CTX-C, CTX-R, HPC, and BG (n = 3–6). Values represent average fold change compared to control. Means with different letters (a, b, or c) are significantly different (p < 0.05) from each other.
Social defeat increased CXCL1, CXCL2 and CCL2 mRNA expression in the caudal cortex, rostral cortex, hippocampus, and basal ganglia
Because centrally-derived signals may aid in the recruitment of myeloid cells to the brain (Cartier et al., 2005, Takeshita and Ransohoff, 2012), we next determined the mRNA expression of several key chemokines after social defeat. In this experiment, mice were exposed to one, three, or six days of social defeat and the mRNA levels of CXCL1, CXCL2, CXCL12, and CCL2 were determined immediately after the final cycle of RSD in the caudal cortex, rostral cortex, hippocampus, and basal ganglia (Table 3). Table 3 shows that several of these key chemokines were increased by social defeat. For instance, CXCL1 expression was increased by social defeat in the CTX-C (F(3,20) = 5.81, p < 0.01), CTX-R (F(3,20) = 6.20, p < 0.005), HPC (F(3,19) = 5.60, p < 0.01), and BG (F(3,18) = 5.46, p < 0.01). Table 3 shows that CXCL1 mRNA levels were further enhanced in the CTX-C after six days of social defeat compared to three days (p < 0.05) or one day (p < 0.05). CXCL2 expression was also increased by social defeat in the CTX-C (main effect of stress, F(3,19) = 5.92, p < 0.01), CTX-R (F(3,20) = 3.46, p < 0.05), HPC (F(3,20) = 2.86, p < 0.07), and BG (F(3,19) = 3.18, p <0.06). For all these regions examined, the mRNA expression of CXCL2 was highest after six days of social defeat. For instance, the mRNA levels of CXCL2 were increased in the CTX-R after three days of social defeat (p < 0.01) and was the highest level by six days compared to all other groups (p < 0.05). It is important to note that CXCL12 was not increased after social defeat regardless of exposure or brain region. These data indicate that RSD increased the mRNA levels of CXCL1 and CXCL2 in an exposure-dependent manner in all brain regions examined.
Table 3.
Social defeat increased CXCL1, CXCL2, and CCL2 mRNA expression in the CTX-C, CTX-R, HPC, and BG
| Reg | mRNA | Control | Social Defeat | ||
|---|---|---|---|---|---|
| 1 d | 3 d | 6 d | |||
| CTX-C | CXCL1 | 1.02 ± 0.09a | 13.72 ± 4.86b | 25.93 ± 8.28b,c | 59.94 ± 16.61c |
| CXCL2 | 1.10 ± 0.18a | 7.18 ± 2.35b | 14.82 ± 6.30a,b,c | 47.98 ± 13.67c | |
| CXCL12 | 1.02 ± 0.11 | 1.09 ± 0.07 | 1.01 ± 0.09 | 0.78 ± 0.05 | |
| CCL2 | 1.01 ± 0.08 | 0.95 ± 0.11 | 0.90 ± 0.12 | 2.48 ± 0.48 | |
|
| |||||
| CTX-R | CXCL1 | 1.12 ± 0.25a | 17.53 ± 6.04b | 20.05 ± 7.08b,c | 43.49 ± 8.77c |
| CXCL2 | 1.03 ± 0.11a | 19.37 ± 8.57a,b | 8.29 ± 1.95b | 48.64 ± 18.59b | |
| CXCL12 | 1.00 ± 0.04 | 0.94 ± 0.02 | 1.14 ± 0.14 | 1.03 ± 0.04 | |
| CCL2 | 1.01 ± 0.06a | 1.03 ± 0.07a | 1.41 ± 0.16a,b | 2.83 ± 0.50b | |
|
| |||||
| HPC | CXCL1 | 1.01 ± 0.07a | 8.03 ± 3.08b | 9.94 ± 2.27b | 28.65 ± 8.16b |
| CXCL2 | 1.14 ± 0.21a | 22.03 ± 10.25a,b | 13.59 ± 4.88b | 48.45 ± 18.39b | |
| CXCL12 | 1.01 ± 0.06 | 0.96 ± 0.05 | 1.07 ± 0.15 | 0.80 ± 0.01 | |
| CCL2 | 1.01 ± 0.09 | 0.74 ± 0.08 | 0.84 ± 0.10 | 2.12 ± 0.38 | |
|
| |||||
| BG | CXCL1 | 1.00 ± 0.02a | 12.21 ± 4.91a,b,c | 14.84 ± 3.83b,c | 49.82 ± 14.16c |
| CXCL2 | 1.06 ± 0.17a | 18.30 ± 8.34a,b | 11.38 ± 3.98a,b | 48.13 ± 17.37b | |
| CXCL12 | 1.00 ± 0.03 | 0.83 ± 0.04 | 1.15 ± 0.07 | 0.78 ± 0.06 | |
| CCL2 | 1.02 ± 0.15 | 0.90 ± 0.06 | 1.01 ± 0.14 | 2.57 ± 0.73 | |
Male C57BL/6 mice were subjected to one, three, or six cycles of social defeat (RSD) or left undisturbed as controls (CON). Immediately following the final cycle of RSD, brains were removed, dissected, and frozen in liquid nitrogen. CXCL1, CXCL2, CXCL12, and CCL2 mRNA levels were determined in the CTX-C, CTX-R, HPC, and BG (n = 3–6). Values represent average fold change compared to control. Means with different letters (a, b, or c) are significantly different (p < 0.05) from each other.
Social defeat also increased CCL2 mRNA expression in the in the CTX-R (main effect of stress, F(3,10) = 6.12, p < 0.05), CTX-C (F(3,10) = 5.44, p < 0.05) and HPC (F(3,7) = 5.92, p < 0.05). The mRNA expression of CCL2 was not increased after one or three days of social defeat. CCL2 mRNA, however, was significantly increased by six days of social defeat in the CTX-R (p < 0.05) and tended to be increased in the CTX-C (p < 0.08) and HPC (p < 0.09). Taken together, social defeat increased CXCL1, CXCL2, and CCL2 mRNA expression in the brain.
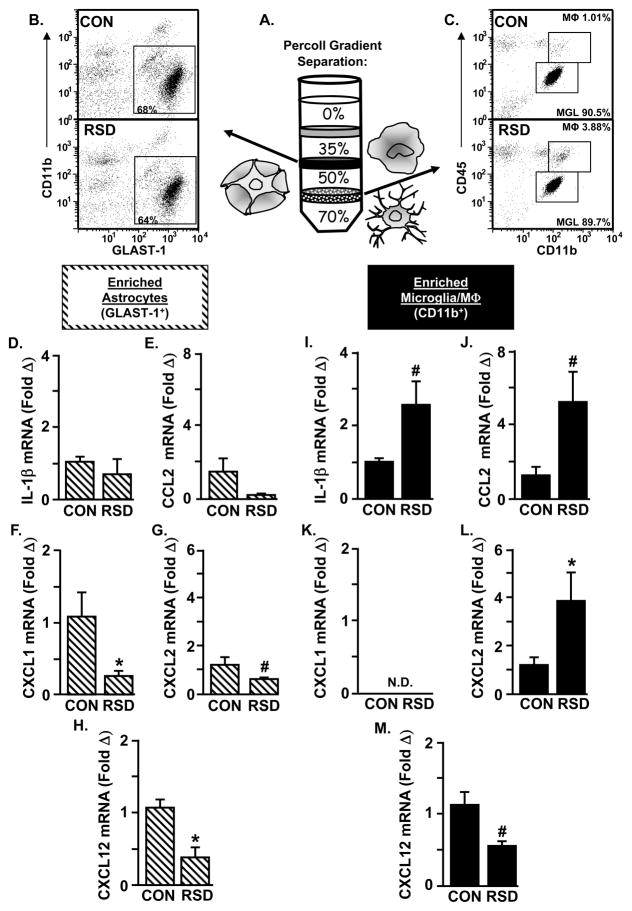
RSD increased IL-1β, CCL2, and CXCL2 expression in enriched microglia/macrophages, but not in astrocytes
To determine the cell type within the brain that was producing these chemokines, microglia/macrophages (CD11b+) and astrocytes (GLAST-1+) were enriched from the brain after RSD (six cycles of social defeat). The mRNA expression of several mediators of myeloid cell recruitment including IL-1β, CCL2, CXCL1, CXCL2, and CXCL12 was determined in each enriched cell population. As published previously (Norden et al., 2014), Figure 3A illustrates that enriched astrocytes were collected from the interphase between 35% and 50% Percoll and enriched microglia/macrophages were collected from the interphase between 50% and 70%. Figure 3B shows the representative dot plots of CD11b and GLAST-1 labeling of astrocytes. After Percoll enrichment, 65–70% of these cells were GLAST-1+. Figure 3C shows the representative dot plots of CD11b and CD45 labeling of microglia/macrophages. Although these enriched cells were primarily microglia (over 85% CD11b+/CD45low), Figure 3C shows that RSD increased the number of macrophages (CD11b+/CD45hi) from 1.01% to 3.88%. This is consistent with our previous work showing that the number of macrophages associated with the brain increases after RSD (Wohleb et al., 2013). Thus, using Percoll enrichment, astrocytes and microglia/macrophages can be obtained from the same mouse brain.
Figure 3. RSD increased IL-1β, CCL2, and CXCL2 expression in enriched microglia/macrophages, but not in astrocytes.
Male C57BL/6 mice were subjected to six cycles of social defeat (RSD) or left undisturbed as controls (CON). Enriched brain CD11b+ and GLAST-1+ cells were collected 14 h after the final cycle of RSD. A) Enriched brain CD11b+ and GLAST-1+ cells were collected using a Percoll density gradient. B) Representative bivariate dot plots of CD11b and GLAST-1 labeling of astrocytes and C) CD11b and CD45 labeling of microglia/macrophages are shown. The mRNA expression of D) IL-1β, E) CCL2, F) CXCL1, G) CXCL2, and H) CXCL12 was determined in enriched GLAST-1+ cells (n = 3–6). The mRNA expression of I) IL-1β, J) CCL2, K) CXCL1, L) CXCL2, and M) CXCL12 was determined in enriched CD11b+ cells (n = 3–6). Values represent average fold change compared to the respective control. Bars represent average ± SEM. Means with an asterisk (*) are significantly different from CON (p < 0.05) and means with a number sign (#) tended to be different from CON (p ≤ 0.1). (N.D., not detected).
In the enriched astrocyte population, RSD (6 cycles of social defeat) had no effect on the mRNA expression of either IL-1β (Fig. 3D) or CCL2 (Fig. 3E). Reduced mRNA expression of CXCL1 (Fig. 3F, p < 0.05) and CXCL12 (Fig. 3H, p < 0.01), however, was observed in astrocytes after RSD. CXCL2 mRNA levels also tended to be decreased in enriched astrocytes after RSD (Fig. 3G, p < 0.08). In the enriched CD11b+ cell population, RSD (6 cycles of social defeat) tended to increase both IL-1β (Fig. 3I, p = 0.1) and CCL2 (Fig. 3J, p = 0.1) mRNA expression. These data are consistent with our previous findings (Wohleb et al., 2011, Wohleb et al., 2013, Wohleb et al., 2014b). Moreover, CXCL1 (Fig. 3K) mRNA levels were undetectable in the enriched CD11b+ population, but the mRNA expression of CXCL2 (Fig. 3L, p < 0.05) was increased after RSD. Similar to astrocytes, CXCL12 (Fig. 3M, p = 0.1) mRNA levels were reduced after RSD. Collectively, these data indicated that RSD increased the mRNA of key chemokines in enriched microglia/macrophages, but not in astrocytes.
Discussion
Psychosocial stress activates a number of neuroendocrine pathways resulting in significant physiological, immunological, and behavioral changes that are associated with the development and recurrence of anxiety and depression (Kiecolt-Glaser et al., 2003, Kinsey et al., 2007, Miller et al., 2009, O’Connor et al., 2009, Hansel et al., 2010). Moreover, social defeat causes region-specific infiltration of peripheral myeloid cells into the brain parenchyma of BM-chimeric mice that is associated with the development of anxiety-like behavior (Wohleb et al., 2013, Wohleb et al., 2014b). It is important to note that the repeated social defeat model promotes myeloid cell trafficking to the brain in the absence of pathology usually associated with the recruitment of circulating myeloid cells to sites of tissue damage. Here we show that social defeat caused an exposure- and brain region-dependent increase in several key adhesion molecules and chemokines involved in the recruitment of myeloid cells. In support of previous reports showing that microglia and neuronal activation after social defeat is region-specific (Wohleb et al., 2011), our current study extends these findings to show that RSD induced VCAM-1 and ICAM-1 protein expression on endothelial cells in distinct brain regions associated with fear and threat appraisal. Furthermore, our data indicate that social defeat induced an exposure-dependent increase in the mRNA levels of E-selectin, CXCL1, and CXCL2, but not P-selectin, PECAM-1, or CXCL12. Last, we show that RSD increased IL-1β, CCL2, and CXCL2 expression in enriched microglia/macrophages, but not in astrocytes. Collectively, these findings provide novel evidence for the contribution of adhesion molecules and chemokines in facilitating the recruitment of myeloid cells to the brain in response to social stress.
Previous studies show that RSD increases production of pro-inflammatory cytokine IL-1β in the rostral cortex, hypothalamus, hippocampus, and basoganglia and also induces anxiety-like behavior that coincides with an exposure-dependent increase in the number of brain macrophages (Wohleb et al., 2013). Therefore, one important finding of the current study was that social defeat increased the mRNA expression of VCAM-1 and ICAM-1 in the caudal cortex, rostral cortex, hypothalamus, hippocampus, and basoganglia in an exposure-dependent manner. It is important to note that the mRNA analysis was completed from dissected brain samples and each area includes several different micro-anatomical brain regions. Nonetheless, the brain regions outlined above include areas associated with fear and threat appraisal. For example, the rostral cortex includes the prefrontal cortex and the basoganglia includes the amygdala which are regions affected by stress and anxiety disorders (Ressler and Mayberg, 2007). This is pertinent because RSD-induced anxiety-like behavior coincides with neuronal activation in brain regions associated with fear and threat appraisal (Wohleb et al., 2011). Furthermore, exposure to repeated social defeat increases levels of circulating IL-1β (Engler et al., 2008), IL-6 (Stark et al., 2002), and TNF-α (Avitsur et al., 2003), all of which facilitate myeloid cell trafficking by increasing the expression of adhesion molecules on endothelial cells (Petri et al., 2008, Greenwood et al., 2011). Additionally, stress-induced IL-1β production contributes to the neurobiological responses implicated in anxiety- and depressive-like behaviors (Goshen and Yirmiya, 2009). Furthermore, increased VCAM-1 and ICAM-1 mRNA expression corresponded with enhanced production of IL-1β in the brain after repeated exposure to stress. Therefore these data are interpreted to indicate that increased VCAM-1 and ICAM-1 mRNA expression after RSD were driven by elevated levels of pro-inflammatory cytokines in circulation and in the brain.
Another important aspect of this study was that social defeat induced ICAM-1 expression on endothelial cells of the amygdala and hypothalamus in an exposure-dependent manner. We confirmed that ICAM and VCAM labeling co-localized with Ly6C+ endothelial cells of the brain vasculature (Fig. 1D&H). Both the amygdala (Indovina et al., 2011, Sehlmeyer et al., 2011) and hypothalamus (Wilent et al., 2010, Canteras et al., 2012) are brain regions critical to the behavioral responses to fear and anxiety. Previous reports show that IL-1β mRNA levels in the hypothalamus tended to be increased after one day of social defeat and remained elevated at three and six days (Wohleb et al., 2013), which coincides with the pattern of ICAM-1 protein expression in the hypothalamus in our current study. Furthermore, IL-1β induces myeloid cell recruitment by upregulating ICAM-1 (Wang et al., 1995, del Zoppo et al., 2000, Curry et al., 2010). Our current findings extend these studies to suggest that increased IL-1β induces the expression of ICAM-1 on brain endothelial cells. Additionally, previous reports indicate that social stressors increase systemic levels of pro-inflammatory cytokine IL-6, which are further enhanced after LPS injection (Johnson et al., 2002, Stark et al., 2002). In the brain vasculature, neither ICAM nor VCAM is expressed at high level. In peripheral tissues including the lung, ICAM-1 is constitutively expressed on resting endothelium. ICAM-1 was further enhanced by cytokines associated with RSD (Curry et al., 2010). The higher induction of ICAM1 with RSD is a novel finding and is consistent other models where inflammatory signaling promotes the recruitment of immune cells into tissues. For example, IL-6 induced ICAM-1 expression in brain capillaries through post-transcriptional mechanisms that led to increased recruitment of leukocytes in a model of EAE (Roy et al., 2012). Therefore, these reports correspond with our current findings that show increased ICAM-1 protein expression in the amygdala and hypothalamus following one day of social defeat that remained elevated, while induced VCAM-1 protein in these regions was delayed until after six days of social defeat. Collectively, these findings indicate that elevated IL-1β levels observed in the amygdala and hypothalamus coincide with the pattern of increase in ICAM-1 protein expression, suggesting that ICAM-1 expression is being driven by inflammatory cytokine signaling. Furthermore, enhanced expression of ICAM-1 may be stimulated by RSD-induced increases in IL-6.
Previous studies show that RSD-induced recruitment of macrophages in BM-chimeric mice was detected in stress-responsive brain regions including the hippocampus, amygdala, and prefrontal cortex. There was no macrophage trafficking, however, detected in the motor cortex (Wohleb et al., 2013). A novel finding in the current study was that social defeat induced VCAM-1 and ICAM-1 expression in the same brain regions that our previous studies in BM-chimeric mice showed myeloid cell trafficking into the brain parenchyma. For example, RSD increased ICAM-1 and VCAM-1 expression in the hippocampus, amygdala, and prefrontal cortex but not in the motor cortex. Although all the current studies were performed in wild-type (not BM-chimeric) mice, it is important to note that the same results were seen when VCAM-1 and ICAM-1 protein expression was determined in the same brain regions using GFP+ BM-chimeric mice (data not shown). These data provide evidence for the interaction between RSD-induced macrophage recruitment and leukocyte adhesion mediators involved in myeloid cell recruitment. This is pertinent because RSD-induced macrophage recruitment to specific stress-responsive brain regions was critical for the development of anxiety-like behavior (Wohleb et al., 2013). Unlike pathological conditions that lead to a breakdown of the blood-brain barrier, the brain region-specific pattern of myeloid cell trafficking and expression of adhesion molecules following exposure to repeated social defeat was not associated with any neurological disease, trauma, or infection. Collectively, these findings provide compelling evidence that increased VCAM-1 and ICAM-1 expression on endothelial cells facilitates the recruitment of myeloid cells to the brain in response to repeated social stress in the absence of pathology.
Another key finding of this study was that social defeat selectively increased the mRNA expression of specific adhesion molecules involved in leukocyte extravasation. For example, social defeat caused an exposure-dependent increase in E-selectin mRNA expression, but did not affect P-selectin or PECAM-1. Both P- and E-selectin bind to Sialyl Lewis x present on leukocytes and are critical for mediating the early stage of recruitment through the stimulation of leukocyte rolling (Hidalgo et al., 2007). Although both selectins are expressed on vascular endothelial cells during inflammatory responses, P-selectin is constitutively found in cytoplasmic granules known as Wiebel-Palade bodies that allow it to be rapidly expressed on the external cell surface upon stimulation. E-selectin, on the other hand, is not expressed under resting conditions, but rather is induced by pro-inflammatory cytokines (Ley, 2003, Greenwood et al., 2011). Therefore, our current findings are consistent with previous reports indicating that social defeat does not affect P-selectin mRNA levels because the protein is not transcriptionally regulated (Curry et al., 2010). Furthermore, PECAM-1 is expressed on endothelial cells and leukocytes and is critical for regulating transendothelial cell migration (Nourshargh and Marelli-Berg, 2005). Unlike VCAM-1 and ICAM-1, PECAM-1 is not upregulated in response to pro-inflammatory cytokines (Greenwood et al., 2011). Therefore, the enhanced mRNA expression of E-selectin, but not P-selectin or PECAM-1, is consistent with previous studies showing increased production of pro-inflammatory cytokine IL-1β after RSD, suggesting that IL-1β signaling drives increased E-selectin expression (Wohleb et al., 2013). Additionally, these data suggest that neither P-Selectin nor PECAM-1 were affected by social defeat due to the fact that neither target’s mRNA changes were statistically significant. Collectively, these findings provide novel evidence for exposure-dependent increases in E-selectin mRNA expression after RSD that coincides with increased production of IL-1β in the brain.
Previous reports indicate that centrally-derived signals may facilitate myeloid cell trafficking to the brain in models of EAE (Shi and Pamer, 2011) and LPS-injected mice (Erickson and Banks, 2011). Furthermore, stress-induced myeloid cell recruitment coincides with neuroinflammatory mediators that promote trafficking of myeloid cells to the brain (Prinz and Priller, 2010). For example, CCR2 is critical for directing monocytes from the blood to the brain, while CX3CR1 is essential for their integration in the brain perivascular space and parenchyma (Mahad et al., 2006, Donnelly et al., 2011). This is pertinent because there was no prolonged anxiety-like behavior associated with repeated social defeat in models where myeloid cells could not traffic to the brain (i.e., IL-1R1, CCR2, and CX3CR1 deficiency) (Wohleb et al., 2011, Wohleb et al., 2013). Here we provide data showing that social defeat in the absence of pathology selectively increased the mRNA expression of specific chemokines critical for leukocyte trafficking. For instance, social defeat had no effect on CXCL12 expression, but increased CXCL1, CXCL2, and CCL2 mRNA levels in the brain. As indicated above, the increased mRNA levels of these chemokines were found in brain regions with enhanced production of IL-1β, suggesting that inflammatory cytokine signaling drives the expression of these chemokines (Wohleb et al., 2011, Wohleb et al., 2013). Previous studies indicate that CCR2, the receptor for CCL2, is critical for monocyte trafficking (Prinz and Priller, 2010). Furthermore, CCL2 stimulates the adherence of monocytes to vascular endothelium expressing E-selectin (Gerszten et al., 1999, Curry et al., 2010). Thus, we interpret these data to suggest that RSD-induced secretion of CCL2 enhances adhesion molecule expression to facilitate myeloid cell trafficking to the brain. Moreover, CXCL1 and CXCL2 also recruit myeloid cells in a P-selectin dependent manner (Zhang et al., 2001, Curry et al., 2010). Taken together, these data provide evidence for the interaction between cells that make up the neurovascular unit and RSD-induced cytokines, likely leading to increased adhesion molecule expression and elevated chemokine levels after exposure to social defeat.
Although social defeat induced the expression of several key cytokines and chemokines as indicated above, there is limited evidence that addresses the source of production of these molecules within the brain. Here we show that social defeat increased IL-1β, CCL2, and CXCL2 expression in enriched microglia/macrophages, but not in astrocytes. For instance, by using a Percoll density gradient to separate both enriched microglia/macrophages (CD11b+) and astrocytes (GLAST-1+), we show that microglia/macrophages collected from the brain increased or tended to increase the mRNA expression of IL-1β, CCL2, and CXCL2 after six days of social defeat. While there were apparent reductions in astrocyte expression of these inflammatory mediators, they were expressed at a low level basally in astrocytes so the biological significance of the mRNA reduction is unclear.
As we and other have previously reported, IL-1β and CCL2 are mediators associated with the recruitment of macrophages (Wohleb et al., 2013). CXCL2 is also a chemokine that is associated with myeloid cell recruitment through CXCR2, which is the mouse homolog of the IL-8 receptor (Hol et al., 2010). Others have reported that activated microglia release CXCL2 in models of P2X7 receptor stimulation (Shiratori et al., 2010) and LPS injection (Thomas et al., 2006). While CXCL1 was robustly increased by RSD (table 3), it did not show up as increased in either glia population (figure 3). Therefore, it is produced by either endothelial cells or neurons. In addition, the RSD-induced reduction of CXCL12 mRNA levels in enriched microglia/macrophages correspond with previous reports that indicate dysregulated CXCL12 expression leads to increased leukocyte infiltration (McCandless et al., 2008). However, the biological relevance of reduced CXCL2 and CXCL1 in astrocytes following stress is unclear. Collectively, these findings provide evidence for RSD-induced production of IL-1β, CCL2, and CXCL2 in enriched microglia/macrophages that contributes to the pro-inflammatory CNS profile after social defeat. These data have translational relevance because psychosocial stress in humans promotes a pro-inflammatory state within the CNS that may contribute to the development of anxiety and depressive-like behaviors (Suarez et al., 2003, Audet and Anisman, 2013, Iwata et al., 2013).
Conclusions
In summary, these results indicated that key mediators of leukocyte recruitment were increased in the brain vasculature after social defeat in an exposure- and brain-region dependent manner. Moreover, the bi-directional communication between cells that make up the neurovascular unit led to increased adhesion molecule and chemokine expression that was involved in the recruitment of circulating myeloid cells to the brain in response to social stress. In support of previous studies showing that microglia and neuronal activation after social defeat is region-specific, our current study extends these findings to show that RSD induced VCAM-1 and ICAM-1 protein expression on vascular endothelial cells in distinct brain regions associated with fear and threat appraisal. Furthermore, the repeated social defeat model promotes myeloid cell trafficking to the brain in the absence of pathology usually associated with the recruitment of circulating myeloid cells to sites of tissue damage. These findings are relevant because they begin to establish a mechanism by which the brain facilitates stress-induced myeloid cell recruitment that may underlie anxiety and mood disorders.
Highlights.
RSD increased adhesion molecule and chemokine expression in the brain in an exposure-dependent manner
Increased protein expression of adhesion molecules VCAM-1 and ICAM-1 was observed on brain vasculature after RSD
RSD-induced VCAM-1 and ICAM-1 protein expression were localized in brain regions implicated in fear and anxiety responses
IL-1β, CXCL2, and CCL2 expression were increased after RSD in enriched microglia/macrophages, but not in astrocytes
Increased adhesion molecule and chemokine expression facilitated myeloid cell recruitment to the brain with stress
Acknowledgments
This study was supported by National Institute of Mental Health (NIMH) grants R01-MH-093473 and R01-MH093472 to J.F.S.
CMS and DBM are supported by an NIDCR Training Grant T32-DE014320.
We thank Anzela Niraula, Daniel Shea, Dave Hammond, and Yufen Wang for their technical assistance.
Abbreviations
- RSD
repeated social defeat
- VCAM-1
vascular cell adhesion molecule-1
- ICAM-1
intercellular cell adhesion molecule-1
- PECAM-1
platelet endothelial cell adhesion molecule-1
- CXCL1
(C-X-C motif) ligand 1
- CXCL2
(C-X-C motif) ligand 2
- CXCL12
(C-X-C motif) ligand 12
- CCL2
(C-C motif) ligand 2
- IL-1
interleukin-1
- BM
bone marrow
- GFP
green fluorescent protein
- CTX-C
caudal cortex
- CTX-R
rostral cortex
- HPC
hippocampus
- BG
basal ganglia
- AMYG
amygdala
- HYPO
hypothalamus
- PFC
prefrontal cortex
- PVN
paraventricular nucleus
- M1 CTX
primary motor cortex
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Audet MC, Anisman H. Interplay between pro-inflammatory cytokines and growth factors in depressive illnesses. Front Cell Neurosci. 2013;7:68. doi: 10.3389/fncel.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avitsur R, Padgett DA, Dhabhar FS, Stark JL, Kramer KA, Engler H, Sheridan JF. Expression of glucocorticoid resistance following social stress requires a second signal. J Leukoc Biol. 2003;74:507–513. doi: 10.1189/jlb.0303090. [DOI] [PubMed] [Google Scholar]
- Brydon L, Edwards S, Jia H, Mohamed-Ali V, Zachary I, Martin JF, Steptoe A. Psychological stress activates interleukin-1beta gene expression in human mononuclear cells. Brain Behav Immun. 2005;19:540–546. doi: 10.1016/j.bbi.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Mota-Ortiz SR, Motta SC. What ethologically based models have taught us about the neural systems underlying fear and anxiety. Braz J Med Biol Res. 2012;45:321–327. doi: 10.1590/S0100-879X2012007500042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005;48:16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Cole SW. Social regulation of leukocyte homeostasis: the role of glucocorticoid sensitivity. Brain Behav Immun. 2008;22:1049–1055. doi: 10.1016/j.bbi.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Arevalo JM, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, Sheridan JF, Seeman TE. Computational identification of gene-social environment interaction at the human IL6 locus. Proc Natl Acad Sci U S A. 2010;107:5681–5686. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry JM, Hanke ML, Piper MG, Bailey MT, Bringardner BD, Sheridan JF, Marsh CB. Social disruption induces lung inflammation. Brain Behav Immun. 2010;24:394–402. doi: 10.1016/j.bbi.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol. 2000;10:95–112. doi: 10.1111/j.1750-3639.2000.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DJ, Longbrake EE, Shawler TM, Kigerl KA, Lai W, Tovar CA, Ransohoff RM, Popovich PG. Deficient CX3CR1 signaling promotes recovery after mouse spinal cord injury by limiting the recruitment and activation of Ly6Clo/iNOS+ macrophages. J Neurosci. 2011;31:9910–9922. doi: 10.1523/JNEUROSCI.2114-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler H, Bailey MT, Engler A, Stiner-Jones LM, Quan N, Sheridan JF. Interleukin-1 receptor type 1-deficient mice fail to develop social stress-associated glucocorticoid resistance in the spleen. Psychoneuroendocrinology. 2008;33:108–117. doi: 10.1016/j.psyneuen.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler H, Dawils L, Hoves S, Kurth S, Stevenson JR, Schauenstein K, Stefanski V. Effects of social stress on blood leukocyte distribution: the role of alpha- and beta-adrenergic mechanisms. J Neuroimmunol. 2004;156:153–162. doi: 10.1016/j.jneuroim.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Banks WA. Cytokine and chemokine responses in serum and brain after single and repeated injections of lipopolysaccharide: multiplex quantification with path analysis. Brain Behav Immun. 2011;25:1637–1648. doi: 10.1016/j.bbi.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Jr, Luster AD, Luscinskas FW, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Berg BM, Krzyszton C, Johnson RW. Alpha-tocopherol attenuates NFkappaB activation and pro-inflammatory cytokine production in brain and improves recovery from lipopolysaccharide-induced sickness behavior. J Neuroimmunol. 2005;169:97–105. doi: 10.1016/j.jneuroim.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Goshen I, Yirmiya R. Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol. 2009;30:30–45. doi: 10.1016/j.yfrne.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Greenwood J, Heasman SJ, Alvarez JI, Prat A, Lyck R, Engelhardt B. Review: leucocyte-endothelial cell crosstalk at the blood-brain barrier: a prerequisite for successful immune cell entry to the brain. Neuropathol Appl Neurobiol. 2011;37:24–39. doi: 10.1111/j.1365-2990.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- Hansel A, Hong S, Camara RJ, von Kanel R. Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neurosci Biobehav Rev. 2010;35:115–121. doi: 10.1016/j.neubiorev.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Peired AJ, Wild MK, Vestweber D, Frenette PS. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity. 2007;26:477–489. doi: 10.1016/j.immuni.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hol J, Wilhelmsen L, Haraldsen G. The murine IL-8 homologues KC, MIP-2, and LIX are found in endothelial cytoplasmic granules but not in Weibel-Palade bodies. J Leukoc Biol. 2010;87:501–508. doi: 10.1189/jlb.0809532. [DOI] [PubMed] [Google Scholar]
- Hong S, Johnson TA, Farag NH, Guy HJ, Matthews SC, Ziegler MG, Mills PJ. Attenuation of T-lymphocyte demargination and adhesion molecule expression in response to moderate exercise in physically fit individuals. J Appl Physiol (1985) 2005;98:1057–1063. doi: 10.1152/japplphysiol.00233.2004. [DOI] [PubMed] [Google Scholar]
- Indovina I, Robbins TW, Nunez-Elizalde AO, Dunn BD, Bishop SJ. Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron. 2011;69:563–571. doi: 10.1016/j.neuron.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun. 2013;31:105–114. doi: 10.1016/j.bbi.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Jutila MA, Kroese FG, Jutila KL, Stall AM, Fiering S, Herzenberg LA, Berg EL, Butcher EC. Ly-6C is a monocyte/macrophage and endothelial cell differentiation antigen regulated by interferon-gamma. Eur J Immunol. 1988;18:1819–1826. doi: 10.1002/eji.1830181125. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R. Depression and immune function: central pathways to morbidity and mortality. J Psychosom Res. 2002;53:873–876. doi: 10.1016/s0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Bailey MT, Sheridan JF, Padgett DA, Avitsur R. Repeated social defeat causes increased anxiety-like behavior and alters splenocyte function in C57BL/6 and CD-1 mice. Brain Behav Immun. 2007;21:458–466. doi: 10.1016/j.bbi.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003;9:263–268. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mae M, Armulik A, Betsholtz C. Getting to know the cast - cellular interactions and signaling at the neurovascular unit. Curr Pharm Des. 2011;17:2750–2754. doi: 10.2174/138161211797440113. [DOI] [PubMed] [Google Scholar]
- Mahad D, Callahan MK, Williams KA, Ubogu EE, Kivisakk P, Tucky B, Kidd G, Kingsbury GA, Chang A, Fox RJ, Mack M, Sniderman MB, Ravid R, Staugaitis SM, Stins MF, Ransohoff RM. Modulating CCR2 and CCL2 at the blood-brain barrier: relevance for multiple sclerosis pathogenesis. Brain. 2006;129:212–223. doi: 10.1093/brain/awh655. [DOI] [PubMed] [Google Scholar]
- Martinez M, Calvo-Torrent A, Herbert J. Mapping brain response to social stress in rodents with c-fos expression: a review. Stress. 2002;5:3–13. doi: 10.1080/102538902900012369. [DOI] [PubMed] [Google Scholar]
- McCandless EE, Piccio L, Woerner BM, Schmidt RE, Rubin JB, Cross AH, Klein RS. Pathological expression of CXCL12 at the blood-brain barrier correlates with severity of multiple sclerosis. Am J Pathol. 2008;172:799–808. doi: 10.2353/ajpath.2008.070918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandless EE, Wang Q, Woerner BM, Harper JM, Klein RS. CXCL12 limits inflammation by localizing mononuclear infiltrates to the perivascular space during experimental autoimmune encephalomyelitis. J Immunol. 2006;177:8053–8064. doi: 10.4049/jimmunol.177.11.8053. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, Ma R, Cole SW. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA. How endothelial cells regulate transmigration of leukocytes in the inflammatory response. Am J Pathol. 2014;184:886–896. doi: 10.1016/j.ajpath.2013.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Fenn AM, Dugan A, Godbout JP. TGFbeta produced by IL-10 redirected astrocytes attenuates microglial activation. Glia. 2014;62:881–895. doi: 10.1002/glia.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- Nourshargh S, Marelli-Berg FM. Transmigration through venular walls: a key regulator of leukocyte phenotype and function. Trends Immunol. 2005;26:157–165. doi: 10.1016/j.it.2005.01.006. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Andre C, Wang Y, Lawson MA, Szegedi SS, Lestage J, Castanon N, Kelley KW, Dantzer R. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci. 2009;29:4200–4209. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaway CA, Husband AJ. The influence of neuroendocrine pathways on lymphocyte migration. Immunol Today. 1994;15:511–517. doi: 10.1016/0167-5699(94)90206-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. 3 2008. [Google Scholar]
- Petri B, Phillipson M, Kubes P. The physiology of leukocyte recruitment: an in vivo perspective. J Immunol. 2008;180:6439–6446. doi: 10.4049/jimmunol.180.10.6439. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J. Tickets to the brain: role of CCR2 and CX3CR1 in myeloid cell entry in the CNS. J Neuroimmunol. 2010;224:80–84. doi: 10.1016/j.jneuroim.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Richard JF, Dumas A, Vallieres L. CXCL1 can be regulated by IL-6 and promotes granulocyte adhesion to brain capillaries during bacterial toxin exposure and encephalomyelitis. J Neuroinflammation. 2012;9:18. doi: 10.1186/1742-2094-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlmeyer C, Dannlowski U, Schoning S, Kugel H, Pyka M, Pfleiderer B, Zwitserlood P, Schiffbauer H, Heindel W, Arolt V, Konrad C. Neural correlates of trait anxiety in fear extinction. Psychol Med. 2011;41:789–798. doi: 10.1017/S0033291710001248. [DOI] [PubMed] [Google Scholar]
- Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratori M, Tozaki-Saitoh H, Yoshitake M, Tsuda M, Inoue K. P2X7 receptor activation induces CXCL2 production in microglia through NFAT and PKC/MAPK pathways. J Neurochem. 2010;114:810–819. doi: 10.1111/j.1471-4159.2010.06809.x. [DOI] [PubMed] [Google Scholar]
- Stark JL, Avitsur R, Hunzeker J, Padgett DA, Sheridan JF. Interleukin-6 and the development of social disruption-induced glucocorticoid resistance. J Neuroimmunol. 2002;124:9–15. doi: 10.1016/s0165-5728(02)00004-8. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Krishnan RR, Lewis JG. The relation of severity of depressive symptoms to monocyte-associated proinflammatory cytokines and chemokines in apparently healthy men. Psychosom Med. 2003;65:362–368. doi: 10.1097/01.psy.0000035719.79068.2b. [DOI] [PubMed] [Google Scholar]
- Takeshita Y, Ransohoff RM. Inflammatory cell trafficking across the blood-brain barrier: chemokine regulation and in vitro models. Immunol Rev. 2012;248:228–239. doi: 10.1111/j.1600-065X.2012.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. Gene expression profile of activated microglia under conditions associated with dopamine neuronal damage. FASEB J. 2006;20:515–517. doi: 10.1096/fj.05-4873fje. [DOI] [PubMed] [Google Scholar]
- Wang X, Feuerstein GZ, Gu JL, Lysko PG, Yue TL. Interleukin-1 beta induces expression of adhesion molecules in human vascular smooth muscle cells and enhances adhesion of leukocytes to smooth muscle cells. Atherosclerosis. 1995;115:89–98. doi: 10.1016/0021-9150(94)05503-b. [DOI] [PubMed] [Google Scholar]
- Wilent WB, Oh MY, Buetefisch CM, Bailes JE, Cantella D, Angle C, Whiting DM. Induction of panic attack by stimulation of the ventromedial hypothalamus. J Neurosurg. 2010;112:1295–1298. doi: 10.3171/2009.9.JNS09577. [DOI] [PubMed] [Google Scholar]
- Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J Clin Invest. 2010;120:1368–1379. doi: 10.1172/JCI41911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology. 2012;37:1491–1505. doi: 10.1016/j.psyneuen.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, Godbout JP. Re-establishment of Anxiety in Stress-Sensitized Mice Is Caused by Monocyte Trafficking from the Spleen to the Brain. Biol Psychiatry. 2014a doi: 10.1016/j.biopsych.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF. Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. J Neurosci. 2014b;34:2583–2591. doi: 10.1523/JNEUROSCI.3723-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33:13820–13833. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XW, Liu Q, Wang Y, Thorlacius H. CXC chemokines, MIP-2 and KC, induce P-selectin-dependent neutrophil rolling and extravascular migration in vivo. Br J Pharmacol. 2001;133:413–421. doi: 10.1038/sj.bjp.0704087. [DOI] [PMC free article] [PubMed] [Google Scholar]