Abstract
Objective
To assess the relation between socioeconomic status (SES) and inflammation during adolescence and determine whether daily affective and social experiences across a 15-day period mediate this relation.
Methods
Adolescents (n = 316) completed daily diary reports of positive affect, negative affect, and negative social interactions for 15 days and provided whole blood spot samples for the assessment of C-reactive protein (CRP). Parents provided information on SES, including the highest level of education they and their spouses completed and household income.
Results
Lower parent education was associated with higher levels of adolescent CRP, controlling for age, gender, ethnicity, and body mass index (β = −.12; p = .031). Mean daily positive affect, negative affect, and negative social interactions were examined as potential mediators of this association. In these models, parent education was no longer associated with adolescent CRP (β = −.09; p = .12), and only positive affect was related to CRP (β = −.12; p = .025). Bootstrapping confirmed the mediating role of positive affect (indirect effect = −.015, 95% CI = [−.038, −.002]).
Conclusions
Adolescents with less educated parents tended to have higher levels of CRP, which may be explained by their lower levels of positive affect. Findings suggest that a lack of positive affect may be a pathway by which SES confers early risk for poor health in adulthood. It is possible that adolescents who display positive affect during daily life in circumstances of relatively adverse socioeconomic circumstances may have better health outcomes related to lower inflammatory factors.
Keywords: social status, CRP, positive affect, adolescents
INTRODUCTION
The positive gradient between SES and physical health has been well-documented. Lower SES increases risk for premature death and numerous diseases, ranging from acute illness including upper respiratory infections and headaches, to chronic, life-threatening conditions, such as cardiovascular disease, diabetes, hypertension, obesity, and atherosclerosis (1–7). A growing number of studies have linked low SES to elevated levels of inflammation (8–10), a state implicated in several of the chronic diseases for which there are SES gradients (11–14). This suggests that increased inflammation may underlie, at least in part, some of the negative health effects of SES. However, underlying mechanisms linking SES to inflammation remain unclear. Moreover, this area of research has primarily focused on adults despite the fact that the SES- inflammation link may emerge during adolescence (15–17) to confer early risk for poor health later in life. Therefore, the purpose of the present study was to assess SES-inflammation associations and underlying socioemotional mechanisms in a sample of adolescents.
Negative social and affective experiences are hypothesized to mediate the link between SES and inflammation (18–20). Consistent with this notion, prior empirical studies of adults have shown that low SES individuals tend to have more negative social experiences, including interpersonal loss, social conflict, poor quality romantic relationships, disruptions in family relationships, exposure to harsh parenting, and hostile and unfriendly relationships (21–26). Low- SES individuals also have been shown to experience more depression, anxiety, and anger (27, 28). Separate literatures have shown that these negative social and affective experiences are, in turn, associated with greater inflammation (29–35). Taken together, these literatures suggest that negative social experiences and affect may explain the relation between SES and inflammation; however, only a handful of studies to date have empirically tested these pathways (36–38). To address this gap, the present study examined whether negative social experiences and negative affect underlie the link between SES and inflammation.
Most theoretical work and the few empirical studies assessing pathways have focused on negative experiences as mechanisms underlying the relation between SES and inflammation. Yet, the role of positive affect is important to examine given that positive and negative affect may represent separate dimensions of the affect system (39, 40). Positive and negative affect can be activated in an opposing manner, (e.g. increase in positive affect and decrease in negative affect), an uncoupled manner (e.g., increase in only positive affect) or in the same direction (e.g., increase in both positive and negative affect) (39, 41). As such, positive affect may affect inflammatory processes independent of negative affect.
Few studies have examined the relations among SES, positive affect, and inflammation. However, there is evidence that lower SES is related to lower positive affect (42), as well as lower happiness and subjective well-being (43–45). Research on positive affect and inflammation is relatively new, but several studies have found that greater positive affect, independent of negative affect, is associated with lower levels of circulating inflammatory markers (46, 47) and stimulated production of pro-inflammatory cytokines (48). Given the links among SES, positive affect, and inflammation, the current study assessed whether positive affect accounts for the association between SES and inflammation.
Much of the work on SES, social and affective experiences, and inflammation has focused on adult samples. It may be particularly important to examine these links in adolescence given that the teenage years are marked by significant changes in social and affective processes. Specifically, salience of social interactions, particularly those with peers, concern with social status, and emergence of romantic relationships all occur during adolescence (49). In addition, negative affect tends to increase while positive affect tends to decrease during adolescence, in part due to changes in neural networks underlying socioemotional processing and cognitive control (50–52). Because socioemotional development during adolescence lays the foundation for adult socioemotional functioning, and inflammatory processes are sensitive to social and affective factors, as described above, socioemotional experiences during adolescence can affect health in adulthood (53–56). Importantly, contextual factors, such as SES, can influence socioemotional experiences (42, 57, 58). Identifying underlying mechanisms linking SES and inflammation in adolescence, then, may help elucidate how SES contributes to early risk for chronic conditions in adulthood.
To better understand how SES affects health early in life and the mechanisms underlying this link, the current study examined whether daily social and affective experiences over a 15-day period mediated the link between SES and inflammation in a sample of adolescents. We focused on daily experiences because past studies have shown that SES affects daily experiences (42, 57, 58), and because daily experiences may accumulate over time to have a cumulative effect on inflammatory processes and health. For instance, negative social interactions and positive and negative affect averaged or aggregated across days have been shown to prospectively predict higher levels of inflammation, mortality, and chronic health conditions (29, 30, 59–61). Furthermore, in contrast to traditional questionnaire measures, daily diary methods do not ask respondents to generalize their experiences across time and as such, they are less susceptible to recall biases (62). On the basis of past research, we hypothesized that lower SES would be related to greater levels of CRP, more negative social interactions, more negative affect, and less positive affect. We further hypothesized that social experiences and affect would mediate the link between SES and CRP.
METHODS
Participants & Procedures
Participants were 316 adolescents (Mage = 16.4 years, SD = .74; 136 males and 180 females) recruited from the 10th and 11th grade classrooms of four high schools in the Los Angeles area. Adolescents were from European (29.1%), Latino (41.8%), Asian (23.1%), and other (6%) backgrounds.
Recruitment involved mailing fliers to students’ homes and presenting the study and distributing study fliers to students in their classrooms. Study staff phoned interested families to provide more details about the study, and those who provided verbal parental consent were scheduled for an initial visit in participants’ homes or a local field research center.
In the initial visit, study staff obtained written consent and primary caregivers reported on SES while adolescents completed a self-administered set of computer-assisted questionnaires. Whole blood spots were then collected for the assessment of CRP, after which participants were provided with instructions for the daily diary portion of the study.
After the initial visit, adolescents completed 4-page diary checklists of their social and emotional experiences each night for 15 consecutive days. The diary checklists took approximately 5–10 minutes to complete, and adolescents were instructed to complete diary checklists before going to bed each night. Diary checklists completed after noon the following day were considered non-compliant. To help ensure compliance, participants were provided with 15 stickers to seal the diary checklists, an electronic time stamper, and a stamping booklet. The date and time were pre-programmed to the current date and time, and participants were instructed to stamp the stamping booklet to indicate when they completed the diary checklists. A security code on the time stamper prevented participants from altering the correct date and time. At the end of the 15-day period, study staff returned to participants’ homes to collect completed materials. Adolescents received $50 for participating in the study. Participants who completed daily checklists correctly and on time also were mailed two movie passes. Approximately 94% of adolescents completed daily checklists for at least 14 days. Only 3.8% of adolescents completed the diary checklists for less than 7 days. Of the diaries that were completed, the vast majority (97.1–99.3%) were completed before noon on the following day, and were therefore judged to be compliant. More specifically, for any given day, only two to eight checklists were not completed on time. All study procedures were approved by the UCLA Institutional Review Board, and all data were collected between September 2011 and September 2012.
Measures
SES
Parental education and household income were used as indicators of SES, as these measures capture access to resources as well as prestige, and are widely used in SES research (20). Primary caregivers, typically biological mothers (89.5%), reported their own and their spouse’s highest level of education completed using an 11-point scale from (1 = some elementary school, 11 = graduated from medical, law, or graduate school). Highest levels of education were averaged across parents. Primary caregivers also reported specific dollar amount of total annual household income from all sources, including wages, salaries, government assistance, and dividends.
Daily Social Interactions
Each night for 15 consecutive nights, participants completed bedtime diary checklists that assessed whether they had encountered any of eight negative social interactions. Negative social interactions included argued with a parent, argued with another family member, argued with a friend, was punished by a parent, parents argued, something bad happened to a family member, had an argument or was punished by an adult at school, and was insulted, threatened, or made fun of by someone at school. These events were selected because they represent psychological stressors for adolescents across the domains of family, peers, and school (63, 64) and have been associated with CRP (30). A summary variable for negative social interactions was created by summing across the different types of interactions for a given day, creating a variable to indicate whether a negative social interaction occurred that day, and lastly, averaging across days. Thus, the summary variable indicates percentage of days on which any one of the negative events occurred (30) and represents an index of individual difference in the experience of negative social interactions. Bivariate correlational analyses indicated good week- to-week stability (r =.66, p < .001).
Daily Affect
Each night for 15 consecutive nights, participants indicated the extent to which they felt eleven negative and seven positive affect items using a 5-point scale (1 = not at all, 5 = extremely). Affect items were taken from the Profile of Mood States (65) and the Positive and Negative Affect Schedule (66). Negative emotions included sad, hopeless, discouraged, frightened, nervous, on edge, scared, threatened, uneasy, worried, unsafe, and positive emotions included calm, interested, cheerful, enthusiastic, excited, happy, joyful. Negative affect items and positive affect items were averaged each day and then averaged across days to create composite negative affect and positive affect variables. To assess reliability of the measures, items were first averaged over time. Alpha coefficients were then computed across the mean item scores. The alpha coefficient of both negative affect (.94) and positive affect (.94) indicated that the measures possessed good internal consistency as measures of individual difference. There was also good week-to-stability in the experience of negative affect (r = .75, p < .001) and positive affect (r = .84, p < .001).
Adolescents also completed the joviality subscale of the PANAS (67), allowing for examination of whether daily assessment of positive affect and a more global measure of a dimension of positive affect are similarly related to parent education and CRP. During the initial visit, adolescents indicated the extent to which they felt happy, joyful, delighted, cheerful, excited, enthusiastic, lively, and energetic in the past week, using a 5-point scale (1 = very slightly or not at all, 5 = extremely).
CRP
Dried blood spots (DBS), a well-validated, relatively non-invasive procedure, was used for the assessment of CRP (68). This method for CRP assessment is increasingly used in community and population-based studies, and validation studies indicate a high correlation between CRP from DBS and CRP from plasma and serum (r’s ≥ .96) (68–70). Blood spots were collected at baseline during the initial visit before assessment of daily experience. Trained study staff (medical personnel are not required for this procedure) first cleaned each participant’s finger with alcohol. Using a sterile, disposable microlancet commonly used by diabetics, study staff next punctured the participant’s finger. Five drops of capillary blood was then allowed to fall onto standardized filter paper. Blood spot samples were covered and allowed to dry overnight (cytokines are stable in DBS at room temperature for up to 14 days (68), after which they were stored at −80°C until shipped to the Laboratory for Human Biology Research at Northwestern University. Levels of CRP were assessed using high-sensitivity enzyme-linked immunosorbent assay, which has a lower detection limit of .030 mg/L. Seven samples fell under the lower detection of limit. All samples were run in duplicate, and intra- and inter-assay coefficients of variation were <6.4% and <9.3%, respectively.
Covariates
During the initial visit, trained staff assessed height and weight for body mass index (BMI) and adolescents reported on their age, gender, and ethnicity. Adolescents also reported on certain health behaviors, including smoking and alcohol consumption and sleep duration.
Cigarette use and alcohol consumption were assessed using items from the Youth Risk Behavior Survey (71). More specifically, adolescents reported the number of cigarettes smoked per day in the last 30 days and the number of days they had at least one alcoholic beverage in the last 30 days. Adolescents were assured that their responses to such questions would remain confidential and not be shared with anyone.
Sleep duration was assessed using daily checklists during the first seven days of the daily diary period. Each morning participants reported the time they turned off the lights to sleep, the number of minutes it took them to fall asleep, and the time they woke in the morning. Sleep duration for each night was based on the time between turning off the lights and waking in the morning minus the time it took to fall asleep. Average sleep duration was then computed by averaging sleep duration each night across the 7-day period.
Statistical Analysis
CRP levels were first examined for values greater than 10 mg/L given that such levels are indicative of an acute inflammatory response likely due to infection (72). Four participants had CRP levels greater than 10 mg/L. Self-reports of illness confirmed that these participants had an acute infection in the last 24 hours. Thus, these four participants were excluded from analyses including CRP as a variable of interest. CRP values were skewed and thus log transformed to correct for nonnormality.
To examine whether SES was associated with higher levels of inflammation, regression analyses predicting CRP from SES variables (i.e., parent education, income) were conducted. Any significant associations between the SES variables and CRP were then tested for mediation by psychosocial experience variables (i.e., positive affect, negative affect, negative social interactions) by adding psychosocial experience variables to models predicting CRP from SES. Given that indirect effects are not normally distributed, significance testing of any indirect effects was conducted using 95% bias-corrected bootstrap confidence intervals based on 5,000 bootstrap samples (73). Indirect SPSS macros (Preacher & Hayes, 2008) was used to conduct the bootstrapping analyses.
Except for the four participants whose CRP levels were above 10 mg/L, all available data, including those not completed on time, were included in analyses given the high rates of compliance. All analyses included age, gender, ethnicity, and BMI as covariates, as these variables have previously been related to inflammation (74).
RESULTS
Sample demographic, psychosocial, and biological characteristics are presented in Table 1. Participants were from middle-class backgrounds. On average, parents completed some vocational or trade school. Approximately 29% of participants had parents who completed high school or less, 42.4% had parents who completed some college, 16.8% had parents who completed college, and 11% had parents who at least completed some medical, law or graduate school. The median household income was $50,000.
Table 1.
Participant characteristics (n = 302–313).
| Variable | Mean | SD | Range |
|---|---|---|---|
| Parent education | 7.19 | 1.81 | 1.5–11 |
| Household income | $70,316.71 | $76,665.98 | 0–825,000 |
| Positive affect | 2.88 | .71 | 1.12–5 |
| Negative affect | 1.34 | .40 | 1–3.73 |
| Negative interactions | .29 | .25 | 0–1 |
| BMI (kg/m2) | 23.16 | 5.01 | 14.68–47.58 |
| Age (years) | 16.40 | .74 | 14.5–20.5 |
| CRP (mg/l) | .96 | 2.92 | .001–36.37 |
| CRP (mg/l) (excluding >10 mg/l) | .69 | 1.27 | .001–8.45 |
Note. Categories for parent education were 1=some elementary school, 2 =completed elementary school, 3=some junior high school, 4=completed junior high school, 5=some high school, 6=graduated high school, 7=trade or vocational school, 8=some college, 9=graduated from college, 10=some medical, law, or graduate school, and 11 = graduated from medical, law, or graduate school.
Negative social interactions were relatively uncommon, occurring on about 4.35 of the 15 days (29% of days). Arguing with a parent was the most common negative interaction, with 65.5% of adolescents reporting that it occurred at least once over the 15-day period. The next most common negative interaction was arguing with other family member (42.5%), followed by arguing with a friend (35.5%), and something bad happening to a family member (26.8%). The least common stressors were arguing with or punished by an adult at school and threatened or insulted by someone at school, with 11.5% of adolescents reporting their occurrences.
As shown in Table 1, average levels of CRP were somewhat low. According to clinical cutoff points used to assess risk for cardiovascular disease (75), 255 adolescents were in the low risk category (CRP < 1mg/L), 37 adolescents were in the moderate risk category (CRP = 1–3mg/L), and only 18 adolescents were in the high-risk category (CRP > 3mg/L).
Bivariate correlations among the study variables are shown in Table 2. Higher parent education was associated with higher household income. Higher parent education, but not household income, was associated with greater frequency of negative social interactions and higher levels of positive affect. Similarly, only higher parent education was associated with lower levels of CRP. Among the psychosocial experience variables, only higher levels of positive affect were related to lower levels of CRP.
Table 2.
Bivariate correlations between SES, daily experience, and CRP (n = 287–306).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. Parent education | ||||||||
| 2. Household income | .34** | |||||||
| 3. Negative interactions | .12* | −.04 | ||||||
| 4. Negative affect | .06 | .01 | .45** | |||||
| 5. Positive affect | .16** | .08 | −.04 | .00 | ||||
| 6. Age | −.16** | −.05 | .01 | .01 | .012 | |||
| 7. BMI | −.12* | −.17** | .05 | −.03 | −.07 | .08 | ||
| 8. lnCRP | −.18** | −.04 | .00 | −.10 | −.18** | .24** | .47** |
p < .05;
p < .01;
p < .001.
Hierarchical regression analyses first focused on the relation between the SES indicators and CRP, controlling for age, gender, ethnicity, and BMI (see Table 3, column 1). Higher levels of parent education were associated with lower levels of CRP (p = .031). Household income was not related to levels of CRP (b (SE) = .00 (.00); β = .04, p = .47). Given the shared variance among positive affect, negative affect, and negative social interactions, we then added these potential mediating variables to the model (Table 3, column 2). The association between parent education and CRP was attenuated (p = .12) and only the association between positive affect and CRP was significant (p = .025).
Table 3.
Regression analyses predicting CRP from parent education and daily experience variables.
|
|
||||
|---|---|---|---|---|
| 1 (n = 298) |
2 (n = 287) |
|||
|
|
||||
| b (SE) | β | b (SE) | β | |
|
|
||||
| Intercept | −9.48 (1.78)*** | −7.93 (1.75)*** | ||
| Age | .37 (.10)*** | .19 | .35 (.10)*** | .18 |
| Female | .15 (.15) | .05 | .16 (.15) | .06 |
| Latino | −.12 (.19) | −.04 | −.10 (.18) | .00 |
| Asian | −.40 (.22) | −.11 | −.43 (.21) | −.13 |
| Other | .01 (.33) | .00 | .05 (.33) | .01 |
| BMI | .12 (.02)*** | .43 | .11 (.02)*** | .41 |
| Parent education | −.10 (.05)* | −.12 | −.07 (.05) | −.09 |
| Positive affect | −.24 (.10)* | −.12 | ||
| Negative affect | −.39 (.20) | −.11 | ||
| Negative social interactions | −.01 (.33) | .00 | ||
Note. Gender was dummy coded. European Americans were coded as the reference group for ethnicity.
p < .05;
p < .01;
p < .001.
Given that the regression results suggested that positive affect mediated the relation between parent education and CRP, additional analyses were conducted to estimate this mediation. As shown in Figure 1, parental education predicted higher positive affect after controlling for age, gender, ethnicity, BMI, negative social interactions, and negative affect. Positive affect, in turn, predicted CRP with the same controls and parent education. Mediation of the initial parental education – CRP association by positive affect was confirmed by bootstrapping (indirect effect (SE)= −.015 (.01), 95% CI = [−.038, −.002]). Given that smoking, alcohol consumption, and sleep duration have been shown to affect inflammatory processes (74), we also conducted analyses with these additional health behavior covariates. Controlling for health behaviors did not alter results.
Figure 1.
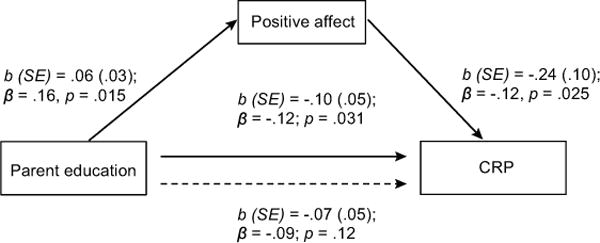
Mediation of the relation between parent education and CRP by positive affect.
We also examined single mediation models in which each psychosocial variable was added separately rather than simultaneously to the model predicting CRP from parent education due to potential suppressor effects of one psychosocial variable on another in a multiple mediation model. The single mediation models yielded similar results: only positive affect mediated the association between parent education and CRP.
Although we collapsed education across the two parents to obtain a measure of family SES, family SES may also be represented by the higher status of two parents. We conducted analyses in which parent education was coded as the greater amount of education completed of the two parents, which yielded similar results.
Because inflammation may induce sickness behaviors, including increases in negative affect and decreases in positive affect (76, 77), lower levels of CRP may account for the association between higher parent education and higher positive affect (rather than vice versa). As such, we examined a meditational model with CRP as the mediator and positive affect as the outcome. In this model, higher parent education was significantly related to higher levels positive affect (b (SE) = .06 (.03), β = .16, p = .015) and marginally related to lower levels of CRP (b (SE) = −.09 (.05), β = −.11, p = .057), controlling for age, gender, ethnicity, BMI, negative affect, and negative social interactions. When CRP was added to the model predicting positive affect from parent education, the association between parent education and positive affect was attenuated (b (SE) = .06 (.03), β = .14, p = .029) while the association between CRP and positive affect was significant (b (SE) = −.08 (.03), β = −.15, p = .025). Bootstrapping confirmed partial mediation of the parent education-positive affect relation by CRP (indirect effect (SE) = .007 (.01), 95% CI = [.001, .019]).
In light of increasing evidence indicating that compared to conventional global self- reports, psychosocial experiences assessed near real-time experience may be more strongly related to markers of biological processes (78), we examined whether the joviality subscale of the PANAS also mediated the parent education-CRP association. The PANAS measure and daily measure of positive affect were significantly correlated (r = .50, p < .01). However, unlike positive affect assessed using daily checklists, the PANAS measure of positive affect was not correlated with parent education (r = −.01, p = .85) and CRP (r = −.07, p = .25) and did not mediate the association between parent education and CRP levels (indirect effect (SE) = .001(.005), 95% CI = [−.007, .015]).
DISCUSSION
The current study examined the relationship between SES and inflammation and the mediating role of social interactions and affect in an ethnically diverse sample of adolescents. Consistent with our hypothesis, lower parent education was associated with higher levels of CRP. Negative social and affective experiences did not account for the relation between parent education and inflammation. However, we found evidence that the association between parent education and inflammation was mediated by daily positive affect, even among adolescents whose CRP levels are fairly low. This finding is consistent with the only other study that we are aware of examining SES and daily positive affect (42) and with past studies showing an inverse relationship between positive affect and inflammation (46, 47).
Curiously, only parent education, but not household income, was related to CRP. A possible explanation is that education is typically completed by early adulthood and therefore, parent education tends to remain relatively constant for most children and youth. By contrast, income tends to vary much more across time (79). As such, a single assessment of income may reflect current SES whereas a single of assessment of education may better capture SES across time. To the extent that experiences over time accumulate to affect inflammatory processes (80), and that that early childhood may be a sensitive period during which social context can program inflammatory processes (81), parent education, then, may be a better predictor of inflammation during adolescence. Consistent with this notion, a recent study that included household income and parental education in a combined model found that only parental education was inversely related to adolescent IL-6 whereas household income was not related to markers of inflammation (17).
To our knowledge, the present study is one of the first to examine positive affect as a pathway by which SES affects inflammatory processes in adolescents. Results indicate that adolescents with less educated parents tended to experience lower levels of positive affect independent of negative social interactions and negative affect. This suggests that the lower levels of positive affect among these adolescents do not reflect a presence of more negative experiences. Similarly, the finding that adolescents with lower levels of positive affect tended to have higher levels of CRP independent of parent education, negative affect, and negative social interactions suggests that to the extent that adolescents can maintain positive affect in the face of socioeconomic disadvantage and daily negative experiences, they may be able to maintain lower levels of inflammation.
The fact that we found evidence for associations between positive affect, but not negative experiences and inflammation is consistent with past studies demonstrating that positive affect is related to inflammatory processes independent of negative affect. Together, these studies suggest that a lack of positive affect, and not just the presence of negative experiences, can have harmful effects on inflammatory processes and health. The present finding extends the growing literature on the effects of positive affect on inflammatory processes by revealing that the link between positive affect and inflammation is evident relatively early in life and that contextual factors such as SES may decrease positive affect. More studies are needed to identify individual characteristics and other contextual factors that may increase vulnerability to a lack of positive affect.
Interestingly, in contrast to the daily measure of positive affect, the PANAS measure of joviality, a global assessment of a dimension of positive affect, was related neither to parent education nor CRP. This discrepancy is consistent with growing literature showing that biological markers may be more sensitive to more proximal assessments of psychosocial experiences than to global self-reports (78). The PANAS measure of positive affect required participants to generalize their feelings of positive emotions over the past week, which may result in responses reflecting semantic knowledge and memory (78). By contrast, daily assessment of positive affect was closer to real-time experience and thus may more accurately reflect actual experience (78). As such, positive affect assessed using daily checklists, but not by a global measure, may have mediated the association between parent education and CRP levels.
We also found no relation between SES and mean daily negative affect, which contrasts a body of work showing an inverse association between SES and negative affect such as depression, anxiety, and anger (27). Of note, these studies have primarily used conventional, global self-reports of negative affect. The lack of association between SES and mean daily negative affect found in the present study is consistent with prior daily diary studies that have also found nonsignificant associations between SES and daily measures of negative affect (26, 42). Together, these findings suggest that SES may be related to negative emotions assessed by conventional self-reports rather than to those assessed by more proximal measurers of actual experience.
Negative social interactions did not mediate the relation between parental education and adolescent inflammation. Contrary to our hypothesis, parent education was related to higher levels of negative social interactions. Although it is widely believed that SES is inversely related to stressful experiences, including negative social interactions, findings have been mixed, with studies showing no relationship between SES and stressful experiences and other studies showing a positive association (82). The discrepancy among findings may stem from the fact that stress is multi-faceted and therefore has been operationalized in various ways (82). Indeed, in a recent study, higher SES was related to greater chronic stress, but lower perceived stress and low-control daily stress, and was unrelated to daily demands (83). More studies are needed to identify which aspects of stress are related to higher SES and why.
The lack of association between the frequency of daily negative social experiences and CRP contrasts previous work demonstrating a positive association between frequency of daily interpersonal stress and inflammation among adolescents (84). Although the frequencies of negative social interactions were consistent across the two studies (M = .29 vs. M = .33), age and CRP levels differed between the two studies, which may contribute to the discrepant results. Specifically, inflammation is known to increase with age, and given that the current sample was younger (16.4 vs. 18.4 years of age) than the sample in the study by Fuligni and colleagues, they had lower levels of CRP (.69 mg/L vs. 1.26 mg/L). The effects of negative social experiences on inflammation at younger ages when inflammation is relatively low may be better observed when the system is challenged (85, 86). They may also depend on other factors such as coping (87) or genetic variation (88).
Limitations of the current study warrant caution in interpreting results. First, the nonexperimental design of the study precludes inferences of causality. Previous work indicates that higher levels of inflammation can lead to decreases in positive affect (76), and indeed additional analyses showed that higher levels of CRP may help explain the association between lower parent education and lower positive affect. Experimental studies manipulating subjective social status or positive affect and prospective, longitudinal studies examining the effects of upward or downward mobility in socioeconomic status on positive experiences and inflammation could help elucidate causal links among SES, positive affect and inflammation. Given the correlational nature of the study, a third variable may also be implicated. Analyses controlling for demographic characteristics, BMI, smoking, alcohol consumption, and sleep duration suggest that these factors do not account for the associations among parent education, positive affect, and CRP. However, the present study did not assess other variables that have been associated with SES, positive affect, and inflammation. For instance, higher SES is associated with more physical activity in adults and adolescents (89), and physical activity, in turn, is related to greater positive affect (90) and lower levels of CRP (91). Assessment or manipulation of physical activity and other variables related to SES, positive affect, and inflammation in future studies could help clarify underlying pathways linking SES, positive affect, and inflammation. Second, daily experience was assessed after blood spot collection for assessment of CRP. However, levels of CRP are relatively stable across an 18-month period (ρ = .67) (92). Furthermore, daily assessment techniques are based on sampling experience, and thus daily diaries provide glimpses into people’s typical experiences. Indeed, there was good week-to-week stability in the occurrence of social and affective experiences in the present sample (all r’s ≥ .66). Lastly, the health implications of the present findings are unclear, as we did not examine health outcomes. The low levels of CRP in our sample indicate that adolescents facing socioeconomic disadvantage are not currently at risk for poor health. Nevertheless, to the extent that these adolescents facing socioeconomic disadvantage continue to experience low levels of positive affect over extended periods of time, they may become at increased risk for particular chronic conditions later in life. This is an important direction for future research.
Despite these limitations, the present study nevertheless extends previous work on the mechanisms underlying the SES-health gradient. It is one of the few studies to empirically test potential psychosocial pathways linking SES to inflammation and is one the first to do so in a sample of adolescents. Past studies have shown that adiposity, chronic stress, and threat perception do not completely explain the association between SES and inflammation (17, 37). Results from the present study suggest that a lack of positive affect is another pathway by which SES affects inflammatory processes. A lack of positive affect, then, may be an additional mechanism that confers early risk of low SES for poor adult health.
Acknowledgments
Source of Funding: This research was supported by funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01-HD062547) to AJF, the UCLA California Center for Population Research, which is supported by the National Institute of Child Health and Human Development (R24-HD041022), the UCLA Older Americans Independence Center, which is supported by the National Institute of Aging (P30-AG028748) and a National Science Foundation Graduate Research Fellowship (DGE-1144087) to JJC. The content does not necessarily represent the official views of the National Institute of Child Health and Human Development, the National Institute of Aging, the National Institutes of Health, or the National Science Foundation. Supported by R01-AG034588; R01-AG026364; R01-CA160245-01; R01- CA119159; R01-HL095799; R01-DA032922-01; P30-AG028748 to MRI; and UCLA CTSI UL1TR000124, and the Cousins Center for Psychoneuroimmunology.
Glossary
- SES
socioeconomic status
- CRP
C-reactive protein
- BMI
body mass index
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
References
- 1.Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, Syme SL. Socioeconomic status and health: the challenge of the gradient. Am Psychol. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Everson SA, Maty SC, Lynch JW, Kaplan GA. Epidemiologic evidence for the relation between socioeconomic status and depression, obesity, and diabetes. J of Psychosom Res. 2002;53:591–895. doi: 10.1016/s0022-3999(02)00303-3. [DOI] [PubMed] [Google Scholar]
- 3.Grotto I, Huerta M, Sharabi Y. Hypertension and socioeconomic status. Curr Opin Cariodol. 2008;23:335–9. doi: 10.1097/HCO.0b013e3283021c70. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993;88:1973–93. doi: 10.1161/01.cir.88.4.1973. [DOI] [PubMed] [Google Scholar]
- 5.McLaren L. Socioeconomic status and obesity. Epidemiol Rev. 2007;29:29–48. doi: 10.1093/epirev/mxm001. [DOI] [PubMed] [Google Scholar]
- 6.Pollitt RA, Rose KM, Kaufman JS. Evaluating thee evidence for model of life course socioeconomic factors and cardiovacular outcomes: a systematic review. BMC Public Health. 2005:5. doi: 10.1186/1471-2458-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stringhini S, Batty GD, Shipley MJ, Marmot MG, Kumari M, Tabak AG, Kivimaki M. Association of lifecourse socioeconomic status with chronic inflammation and type 2 diabetes risk: The Whitehall II propsective cohort study. PLoS Med. 2013;10:e1001479. doi: 10.1371/journal.pmed.1001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aiello AE, Kaplan GA. Socioeconomic position and inflammatory and immune biomarkers of cardiovascular disease: applications to the panel study of income dynamics. Biodemography Soc Biol. 2009;55:178–205. doi: 10.1080/19485560903382304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman EM, Herd P. Income, education, and inflammation: differential associations in a national probability sample (the MIDUS study) Psychosom Med. 2010;72:290–300. doi: 10.1097/PSY.0b013e3181cfe4c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nazmi A, Victora CG. Socioeconomic and racial/ethnic differentials of C-reactive protein levels: a systematic review of population-based studies. BMC Public Health. 2007;7:212–24. doi: 10.1186/1471-2458-7-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aggarwal BB. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu Rev of Nutr. 2010;30:173–99. doi: 10.1146/annurev.nutr.012809.104755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;83:456S–60S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 13.Pauletto P, Rattazzi M. Inflammation and hypertension: the search for a link. Nephrology Dial Transplantation. 2006;21:850–3. doi: 10.1093/ndt/gfl019. [DOI] [PubMed] [Google Scholar]
- 14.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dowd JB, Zajacova A, Aiello AE. Predictors of inflammation in US children aged 3–16 years. Am J of Prev Med. 2010;39:314–20. doi: 10.1016/j.amepre.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howe LD, Galobardes B, Sattar N, Hingorani AD, Deanfield J, Ness AR, Davey-Smith G, Lawlor DA. Are there socioeconomic inequalities in cardiovascular risk factors in childhood, and are they mediated by adiposity? Findings from a prospective cohort study. Int J Obesity. 2010;34:1149–59. doi: 10.1038/ijo.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pietras SA, Goodman E. Socioeconomic status gradients in inflammation in adolescence. Psychosom Med. 2013;75:442–8. doi: 10.1097/PSY.0b013e31828b871a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adler NE, Ostrove JM. Socioeconomic status and health: what we know and what we don’t. Ann NY Acad Sci. 1999;896:3–15. doi: 10.1111/j.1749-6632.1999.tb08101.x. [DOI] [PubMed] [Google Scholar]
- 19.Chen E, Miller GE. Socioeconomic status and health: mediating and moderating factors. Ann Rev Clin Psychol. 2013;9:723–49. doi: 10.1146/annurev-clinpsy-050212-185634. [DOI] [PubMed] [Google Scholar]
- 20.Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Ann Rev Psychol. 2011;62:501–30. doi: 10.1146/annurev.psych.031809.130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conger RD, Conger KJ, Martin MJ. Socioeconomic status, family processes, and individual development. J Marriage Fam. 2010;72:685–704. doi: 10.1111/j.1741-3737.2010.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conger RD, Donnellan MB. An interactionist perspective on the socioeconomic context of human development. Ann Rev Psychol. 2007;28:175–99. doi: 10.1146/annurev.psych.58.110405.085551. [DOI] [PubMed] [Google Scholar]
- 23.Evans GW. The environment of childhood poverty. Am Psychol. 2004;59:77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- 24.Gallo LC, Smith TW, Cox CM. Socioeconomic status, psychosocial processes, and percevied health: An interpersonal perspective. Ann Behav Med. 2006;31:109–19. doi: 10.1207/s15324796abm3102_2. [DOI] [PubMed] [Google Scholar]
- 25.Lantz PM, House JS, Mero RP, Williams DR. Stress, life events, and socioeconomic disparities in health: Results from the Americans’ changing lives study. J Health Soc Behav. 2005;46:274–88. doi: 10.1177/002214650504600305. [DOI] [PubMed] [Google Scholar]
- 26.Matthews KA, Raikkonen K, Everson SA, Flory JD, Marco CA, Owens JF, Lloyd CE. Do the daily experiences of healthy men and women vary according to occupational prestige and work strain? Psychosom Med. 2000;62:346–53. doi: 10.1097/00006842-200005000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Gallo LC, Matthews KA. Understanding the association between socioeconomic status and physical health: do negative emotions play a role? Psychol Bull. 2003;129:10–51. doi: 10.1037/0033-2909.129.1.10. [DOI] [PubMed] [Google Scholar]
- 28.Lorant V, Deliège D, Eaton W, Robert A, Philippot P, Ansseau M. Socioeconomic inequalities in depression: A meta-analysis. Am J Epidemiol. 2003;157:98–112. doi: 10.1093/aje/kwf182. [DOI] [PubMed] [Google Scholar]
- 29.Chiang JJ, Eisenberger NI, Seeman TE, Taylor SE. Negative and competitive social interactions are related to heightened proinflammatory cytokine activity. Proc Natl Acad Sci. 2012;109:1878–82. doi: 10.1073/pnas.1120972109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuligni AJ, Telzer EH, Bower J, Cole SW, Kiang L, Irwin MR. A preliminary study of daily interpersonal stress and C-reactive protein levels among adolescents from Latin American and European backgrounds. Psychosom Med. 2009;71:329–33. doi: 10.1097/PSY.0b013e3181921b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL- 1, and IL-6: A meta-analysis. Psychosom Med. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 32.Kiecolt-Glaser JK, Gouin JP, Hantsoo L. Close relationships, inflammation, and health. Neurosci Biobehav R. 2010;35:33–8. doi: 10.1016/j.neubiorev.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marsland AL, Prather AA, Petersen KL, Cohen S, Manuck SB. Antagonistic characteristics are positively associated with inflammatory markers independently of trait negative emotionality. Brain Behav Immun. 2008;22:753–61. doi: 10.1016/j.bbi.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Donovan A, Slavich GM, Epel ES, Neylan TC. Exaggerated neurobiological sensitivity to threat as a mechanism linking anxiety with increased risk for diseases of aging. Neurosci Biobehav R. 2013;37:96–108. doi: 10.1016/j.neubiorev.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21:901–12. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Appleton AA, McCormick CM, Loucks EB, Buka SL, Koenen KC, Kubzansky LD. The association between childhood emotional functioning and adulthood inflammation is modified by early-life socioeconomic status. Health Psychol. 2012;31:413–22. doi: 10.1037/a0027300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen E, Hanson MD, Paterson LQ, Griffin MJ, Walker HA, Miller GE. Socioeconomic status and inflammatory processes in childhood asthma: the role of psychological stress. J Allergy Clin Immunol. 2006;117:1014–20. doi: 10.1016/j.jaci.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 38.Gruenewald TL, Cohen S, Matthews KA, Tracy RE, Seeman TE. Association of socioeconomic status with inflammation markers in black and white men and women in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Soc Sci Med. 2009;69:451–9. doi: 10.1016/j.socscimed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cacioppo JT, Gardner WL, Berntson GG. The affect system has parallel and integrative processing components form follows function. J Pers Soc Psychol. 1999;76:839–55. [Google Scholar]
- 40.Watson C, Tellegen A. Toward a consensual structure of mood. Psychol Bull. 1985;98:219–35. doi: 10.1037//0033-2909.98.2.219. [DOI] [PubMed] [Google Scholar]
- 41.Larsen JT, McGraw PA. Further evidence for mixed emotions. J Pers Soc Psychol. 2011;100:1095–110. doi: 10.1037/a0021846. [DOI] [PubMed] [Google Scholar]
- 42.Gallo LC, Bogart LM, Vranceanu AM, Matthews KA. Socioeconomic status, resources, psychological experiences, and emotional responses: a test of the reserve capacity model. J Pers Soc Psychol. 2005;88:386–99. doi: 10.1037/0022-3514.88.2.386. [DOI] [PubMed] [Google Scholar]
- 43.Diener E, Tay L, Oishi S. Rising income and the subjective well-being of nations. J Pers Soc Psychol. 2013;104:267–76. doi: 10.1037/a0030487. [DOI] [PubMed] [Google Scholar]
- 44.Diener E. Subjective well-being. In: Diener E, editor. The Science of Well Being. Netherlands: Springer; 2009. pp. 11–58. [Google Scholar]
- 45.Pinquart M, Sorensen S. Influences of socioeconomic status, social network, and competence on subjective well-being in later life: A meta-analysis. Psychol Aging. 2000;15:187–224. doi: 10.1037//0882-7974.15.2.187. [DOI] [PubMed] [Google Scholar]
- 46.Brouwers, Mommersteeg P, Nyklíček I, Pelle AJ, Westerhuis BLWJJM, Szabó BM, Denollet J. Positive affect dimensions and their association with inflammatory biomarkers in patients with chronic heart failure. Biological Psychology. 2013;92:220–6. doi: 10.1016/j.biopsycho.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Steptoe A, O’Donnell K, Badrick E, Kumari M, Marmot M. Neuroendocrine and inflammatory factors associated with positive affect in healthy men and women the Whitehall II Study. Am J Epidemiol. 2008;167:96–102. doi: 10.1093/aje/kwm252. [DOI] [PubMed] [Google Scholar]
- 48.Prather AA, Marsland AL, Muldoon MF, Manuck SB. Positive affective style covaries with stimulated IL-6 and IL-10 prodution in a middle-aged community sample. Brain Behav Immun. 2007;21:1033–7. doi: 10.1016/j.bbi.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steinberg L, Morris AS. Adolescent development. Ann Review Psychol. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- 50.Davey CG, Yücel M, Allen NB. The emergence of depression in adolescence: Development of the prefrontal cortex and the representation of reward. Neurosci Biobehav R. 2008;32:1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 51.Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, Tarr JA, Sciarrillo SR, Dahl RE. Healthy adolescents’ neural response to reward: associations with puberty, positive affect, and depressive symptoms. J Am Acad Child Adolesc Psychiatry. 2010;49:162–72.e5. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Larson RW, Moneta G, Richards MH, Wilson S. Continuity, stability, and change in daily emotional experience across adolescence. Child Dev. 2002;73:1151–65. doi: 10.1111/1467-8624.00464. [DOI] [PubMed] [Google Scholar]
- 53.Patton GC, Viner R. Pubertal transitions in health. Lancet. 2007;369:1130–9. doi: 10.1016/S0140-6736(07)60366-3. [DOI] [PubMed] [Google Scholar]
- 54.Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychol Bull. 2002;128:330–66. [PubMed] [Google Scholar]
- 55.Sawyer SM, Afifi RA, Bearinger LH, Blakemore SJ, Dick B, Ezeh AC, Patton GC. Adolescence: A foundation for future health. Lancet. 2012;379:1630–40. doi: 10.1016/S0140-6736(12)60072-5. [DOI] [PubMed] [Google Scholar]
- 56.Viner RM, Ozer EM, Denny S, Marmot M, Resnick M, Fatusi A, Currie C. Adolescence and the social determinants of health. Lancet. 2012;379:1641–52. doi: 10.1016/S0140-6736(12)60149-4. [DOI] [PubMed] [Google Scholar]
- 57.Almeida DM, Neupert SD, Banks SR, Serido J. Do daily stress processes account for socioeconomic health disparities? J Gerontol B-Psychol. 2005;60:S34–S9. doi: 10.1093/geronb/60.special_issue_2.s34. [DOI] [PubMed] [Google Scholar]
- 58.Grzywacz JG, Almeida DM, Neupert SD, Ettner SL. Socioeconomic status and health: A micro-level analysis o exposure and vulnerability to daily stressors. J Health Soc Behavior. 2004;45:1–16. doi: 10.1177/002214650404500101. [DOI] [PubMed] [Google Scholar]
- 59.Mroczek DK, Stawski RS, Turiano NA, Chan W, Almeida DM, Neupert SD, Spiro A. Emotional reactivity and mortality: Longitudinal findings from the VA Normative Aging Study. J Gerontol B-Psychol. doi: 10.1093/geronb/gbt107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piazza JR, Charles ST, Sliwinski MJ, Mogle J, Almeida DM. Affective reactivity to daily stressors and long-term risk of reporting a chronic physical health condition. Ann Behav Med. 2013;45:110–20. doi: 10.1007/s12160-012-9423-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steptoe A, Wardle J. positive affect measured using ecological momentary assessment and survival in older men and women. Proc Natl Acad Sci. 2011;108:18244–8. doi: 10.1073/pnas.1110892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bolger N, Davis A, Rafaeli E. Diary methods: capturing life as it is lived. Ann Rev Psychol. 2003;54:579–616. doi: 10.1146/annurev.psych.54.101601.145030. [DOI] [PubMed] [Google Scholar]
- 63.Chung GH, Flook L, Fuligni AJ. Daily family conflict and emotional distress among adolescents from Latin American, Asian, and European backgrounds. Dev Psychol. 2009;45:1406–15. doi: 10.1037/a0014163. [DOI] [PubMed] [Google Scholar]
- 64.Nishina A, Juvonen J. Daily reports of witnessing and experiencing peer harassment in middle school. Child Dev. 2005;76:435–50. doi: 10.1111/j.1467-8624.2005.00855.x. [DOI] [PubMed] [Google Scholar]
- 65.McNair D, Lorr M, Droppleman L. Profile of mood states (POMS) 1971 [Google Scholar]
- 66.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 67.Watson D, Clark LA. The PANAS-X: Manual for the Positive and Negative Affect Schedule-Expanded form. Ames: University of Iowa; 1994. [Google Scholar]
- 68.McDade TW, Burhop J, Dohnal J. High-sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clin Chem. 2004;50:652–4. doi: 10.1373/clinchem.2003.029488. [DOI] [PubMed] [Google Scholar]
- 69.Copeland WE, Shanahan L, Worthman C, Angold A, Costello EJ. Cumulative depression episodes predict later C-reactive protein levels: a prospective analyses. Biol Psychiatry. 2010;71:15–21. doi: 10.1016/j.biopsych.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crimmins E, Kim JK, McCreath H, Faul J, Weir D, Seeman T. Validation of blood-based assays using dried blood spots for use in large population studies. Biodemography Soc Biol. 2014;60:38–48. doi: 10.1080/19485565.2014.901885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.CDC. Youth risk behavior surveillance-United States, 2009. Atlanta, Georgia: US Department of Health and Human Services; 2010. [Google Scholar]
- 72.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SCJ, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 73.MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behav Res. 2004;39:99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23:887–97. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–9. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 76.Janicki-Deverts D, Cohen S, Doyle WJ, Turner RB, Treanor JJ. Infection-induced proinflammatory cytokines are associated with decreases in positive affect, but not increases in negative affect. Brain Behav Immun. 2007;21:301–7. doi: 10.1016/j.bbi.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wright CE, Strike PC, Brydon L, Steptoe A. Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav Immun. 2005;19:345–50. doi: 10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 78.Conner TS, Feldman-Barrett L. Trends in ambulatory self-report: The role of momentary experience in psychosomatic medicine. Psychosom Med. 2012;74:327–37. doi: 10.1097/PSY.0b013e3182546f18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sareen J, Afifi TT, McMillan KA, Asmundson GJG. Relationship between household income and mental disorders: Findings from a population-based longitudinal study. Arch Gen Psychiatry. 2011;68:419–27. doi: 10.1001/archgenpsychiatry.2011.15. [DOI] [PubMed] [Google Scholar]
- 80.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav R. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 81.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–97. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matthews KA, Gallo LC, Taylor SE. Are psychosocial factors mediators of socioeconomic status and health connections? A progress report and blueprint for the future. Ann NY Acad Sci. 2010;1186:146–73. doi: 10.1111/j.1749-6632.2009.05332.x. [DOI] [PubMed] [Google Scholar]
- 83.Gallo LC, Shivpuri S, Gonzalez P, Fortmann AL, Esppinosa de los Monteros K, Roesch SC, Talavera GA, Matthews KA. Socioeconomic status and stress in Mexican-American women: a multi-method perspective. J Behav Med. 2013;36:379–88. doi: 10.1007/s10865-012-9432-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fuligni AJ, Telzer EH, Bower J, Irwin MR, Kiang L, Cole SW. Daily family assistance and inflammation among adolescents from Latin American and European backgrounds. Brain Behav Immun. 2009;23:803–9. doi: 10.1016/j.bbi.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller GE, Rohleder N, Cole SW. Chronic interpersonal stress predicts activation of pro- and anti-inflammatory signaling pathways 6 months later. Psychosom Med. 2009;71:57–62. doi: 10.1097/PSY.0b013e318190d7de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psych Sci. 2010;21:848–56. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Low CA, Matthews KA, Hall M. Elevated c-reactive protein in adolescents: roles of stress and coping. Psychosom Med. 2013;75:449–52. doi: 10.1097/PSY.0b013e31828d3f1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cole SW, Arevalo JMG, Manu K, Telzer EH, Kiang L, Bower JE, Irwin MR, Fuligni AJ. Antagonistic pleiotropy at the human IL6 promoter confers genetic resilience to the pro- inflammatory effects of adverse social conditions in adolescence. Dev Psychol. 2011;47:1173–80. doi: 10.1037/a0023871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stalsberg R, Pedersen AV. Effects of socioeconomic status on the physical activity in adolescents: a systematic review of the evidence. Scandinavian Journal of Medicine & Science in Sports. 2010;20:368–83. doi: 10.1111/j.1600-0838.2009.01047.x. [DOI] [PubMed] [Google Scholar]
- 90.Penedo FJ, Dahn JR. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr Opin Psychiatry. 2005;18:189–93. doi: 10.1097/00001504-200503000-00013. [DOI] [PubMed] [Google Scholar]
- 91.Kasapis C, Thompson PM. The effects of physical activity on serum C-reactive protein and inflammatory markers. J Am Coll Cardiol. 2005;45:1563–9. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 92.Kwaijtaal M, van Diest R, Bar FW, van der Ven AJ, Bruggeman CA, de Baets MH, Appels A. Inflammatory markers predict late cardiac events in patients who are exhausted after percutaneous coronary intervention. Atherosclerosis. 2005;182:314–48. doi: 10.1016/j.atherosclerosis.2005.02.022. [DOI] [PubMed] [Google Scholar]


