Abstract
Objective
To assess in participants in the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study (DPP/DPPOS) whether diagnosis of diabetes predicted: elevated depressive symptoms (DS) or antidepressant medicine (ADM) use after diagnosis; diabetes status or duration had significant effect on DS or ADM use; and associations between A1C, fasting plasma glucose (FPG), normalization of FPG and DS or ADM use post diagnosis.
Methods
DPP participants in 3 treatment arms [intensive lifestyle (ILS), metformin (MET), placebo (PLC)] were assessed semiannually or annually for diabetes, glucose control, ADM use, and DS. DS was measured using Beck Depression Inventory (BDI) questionnaire. Among the total 3234 enrolled participants, 1285 developed diabetes whose levels of depression were measured before and after their diabetes diagnosis.
Results
Neither DS nor ADM use increased significantly following diabetes diagnosis. After diabetes diagnosis, higher FPG was associated with greater ADM use in the ILS arm independent of potential confounders; a 10 mg/dl higher in FPG is associated with 8.8% more odds of ADM use. Higher FPG, and higher A1C were associated with higher BDI scores in all three arms. On average, a participant with 10 mg/dl higher rise in FPG had a 0.07 increase in BDI score. Similarly, 1% higher A1c was associated with a 0.21 point increase in BDI score. On contrary, normalization of FPG was associated with lower BDI scores. In participants with FPG that had normalized, there was a decrease of 0.30 points in the BDI score compared to those whose FPG had not normalized.
Conclusions
Contrary to clinical attributions, the diagnosis of diabetes did not show an immediate impact on BDI scores or ADM use. However, higher glucose levels after diagnosis were associated with small but significant higher BDI score and more ADM use.
Index/key words: diagnosis of diabetes, depressive symptoms, antidepressant medication, pre-diabetes, type 2 diabetes mellitus, prevention
Introduction
Elevated depressive symptoms are associated with type 2 diabetes mellitus with evidence suggesting a bi-directional relationship (1,2). Individuals with type 2 diabetes have been shown to have a higher risk of developing depression (3–10). Conversely, individuals with a lifetime history of depression as well as those with antidepressant medication use (ADM) have been shown to be at higher risk for developing type 2 diabetes (3,9–10). This relationship is hypothesized to be bi-directional, but few studies have been capable of assessing this relationship from a lifespan perspective (11). In one meta analysis exploring this issue, the presence of depression was shown to be associated with the onset of type 2 diabetes whereas the diagnosis of the disease was only modestly associated with the onset of depression (11). Previous research investigating these relationships is mitigated by the fact that it is difficult to know when the onset of diabetes occurred. Indeed, most studies have used cross-sectional samples in which it cannot be determined accurately when diabetes began. In many cases, diabetes may have been present for years before a clinical diagnosis was made. Thus, it is impossible to ascertain if there is a dose or duration effect of either diabetes or depressive symptoms.
There is a paucity of literature characterizing the psychosocial impacts, including depressive symptoms, of new onset diabetes among adults (12) with the majority of psychosocial studies utilizing samples with a wide range of diabetes duration. Development theories have posited that the onset of diabetes is characterized by emotional adjustment that may include a period of grief and mourning or denial of the illness and its consequences (13). These theories suggest that the onset of diabetes may set the stage for elevated depressive symptoms that may have preceded or resulted from the onset of diabetes. In a study of adults newly diagnosed with either type 1 (N=41) or type 2 diabetes (N=48), Rane and colleagues found that 41% of participants reported psychosocial problems ranging from relationships, work, personal finances and social support. More than 17% of patients reporting psychosocial problems also reported depressive symptoms representing a significantly higher rate than participants without psychosocial problems (12).
The literature documenting the relationship of depressive symptoms to glycemic control has been mixed with evidence suggesting a small hyperglycemic effect of depressive symptoms (14). More recent studies in which depressive symptoms, depressive disorders and diabetes-distress were assessed and compared directly within the same participant sample have shown no association between depressive symptoms and A1c, but significant relationships between diabetes-related distress and glycemic outcomes (15). In this study, participants had an average duration of type 2 diabetes of 8.1 years. To date, no studies have examined the relationship of fasting plasma glucose (FPG), A1c and depression symptom scores before and after diabetes onset using a precise definition of diabetes diagnosis. Glucose may play a role in the relationship between depression and diabetes. In addition, glucose control may influence depressive symptoms and ADM use. In addition, the onset of diabetes may influence changes to mood that could have an impact on glycemic control downstream.
The Diabetes Prevention Program (DPP) and the Diabetes Prevention Program Outcomes Study (DPPOS) offer an opportunity to investigate the impact a diagnosis of diabetes on development of depressive symptoms or the onset of ADM use, and the association between glucose control and depressive symptoms or ADM use. Unlike previous studies where it is not possible to ascertain when clinical diabetes began, the onset of diabetes in this sample can be determined within six months of its occurrence. In addition, since the DPP is a longitudinal study, it can be used to investigate how depressive symptoms and ADM use change over time. Finally, since glucose levels were tracked over time, the relationship between variations in glucose, depressive symptoms and antidepressant use can be explored.
The current study had two aims: 1) to examine changes in depressive symptoms and ADM use immediately following diagnosis of diabetes in the DPP cohort; 2) to examine relationships between depressive symptoms, ADM use and glycemic control (A1c, FPG) in the DPPOS study cohort at follow-up assessment time points post-diabetes diagnosis. We hypothesized that levels of depressive symptoms and ADM use would increase immediately following diabetes diagnosis and a small but significant relationship would be observed between depressive symptoms, ADM use and glycemic control in subsequent study assessment time points.
Design and Methods
Study Population
The DPP enrolled individuals at high risk for type 2 diabetes and was conducted at 27 U.S. centers. DPP methods and results are described in detail elsewhere (16–18). The protocol is available at http://www.bsc.gwu.edu/dpp. At each DPP center, an institutional review board approved the protocol and all participants gave written informed consent. Participants were 25 years of age or older, had a BMI of ≥24 kg/m2 (≥22 kg/m2 in Asian Americans), and had a plasma glucose concentration of 95–125 mg/dl (5.3–6.9 mmol/l) in the fasting state (≤125 mg/dl in American Indians) and 140–199 mg/dl (7.8–11.0 mmol/l) 2 hours after a 75-g oral glucose load. Exclusion criteria included antidepressant medication use that might contribute to weight loss, conditions that could seriously reduce ability to participate in the DPP (including major psychiatric disorders or serious clinical depressive symptoms), or inability to complete the 3-week run-in period during which participants took placebo medicines and recorded their usual eating and physical activity patterns. Eligible participants were randomly assigned to one of three interventions: standard lifestyle recommendations plus metformin at a dose of 850 mg twice daily (MET arm), standard lifestyle recommendations (i.e., annual brief advice) plus a placebo pill twice daily (PLC arm), or an intensive lifestyle modification program (ILS arm). Goals for ILS participants were to achieve and maintain a reduction of ≥7% of initial body weight through a calorie-controlled, low-fat diet and to engage in physical activity of moderate intensity, such as brisk walking, for ≥150 min per week (18).
This study includes the 1285 participants who developed diabetes during the original DPP study (1996–2002, including the bridge period) or DPPOS phase I (2002–2008) and had Beck Depression Inventory scores before and after diagnosis using a data lock on August 27, 2008. The baseline characteristics of the sample are shown in Table 1. Overall, the 1285 participants were followed for 6.0 years (IQR 3.0–7.9) after their diabetes diagnosis.
Table 1.
Baseline Characteristics of Participants diagnosed with diabetes
| Characteristics | Lifestyle | Metformin | Placebo | |
|---|---|---|---|---|
| N | 360 | 430 | 495 | |
| Age at randomization* | Median(IQR) | 48.6 (41.6- 56.2) | 50.7 (44.5–58.5) | 49.0 (43.0–56.5) |
| Sex | Male | 114 (31.7%) | 141 (32.8%) | 160 (32.3%) |
| Female | 246 (68.3%) | 289 (67.2%) | 335 (67.7%) | |
| Race/ethnicity | Caucasian | 184 (51.1%) | 227 (52.8%) | 243 (49.1%) |
| African American | 78 (21.7%) | 106 (24.7%) | 123 (24.8%) | |
| Hispanic | 59 (16.4%) | 62 (14.4%) | 77 (15.6%) | |
| American Indian | 19 (5.3%) | 21 (4.9%) | 26 (5.3%) | |
| Asian | 20 (5.6%) | 14 (3.3%) | 26 (5.3%) | |
| Household Income# | <50,000 | 180 (53.6%) | 223 (55.8%) | 250 (54.6%) |
| 50,000+ | 156 (46.4%) | 177 (44.3%) | 208 (45.4%) | |
| Marital Status | Living together/Married | 236 (65.6%) | 269 (62.6%) | 344 (69.5%) |
| Others | 124 (34.4%) | 161 (37.4%) | 151 (30.5%) | |
| Education Level | 12 yrs or less | 93 (25.8%) | 127 (29.5%) | 120 (24.2%) |
| 13–16 yrs | 177 (49.2%) | 199 (46.3%) | 247 (49.9%) | |
| 17+ yrs | 90 (25.0%) | 104 (24.2%) | 128 (25.9%) | |
| Leisure activity(met-hours) | Median(IQR) | 9.8 (3.3–22.1) | 9.7 (4.2–20.5) | 10.2 (3.9–21.7) |
| BMI | Median(IQR) | 33.9 (29.8–38.5) | 33.0 (29.3- 37.9) | 33.8 (29.4–38.9) |
| Quality of Life | Median(IQR) | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) |
| BDI score | Median(IQR) | 4.0 (2.0–7.0) | 3.0 (1.0–6.0) | 3.0 (1.0–7.0) |
| BDI>=11# | No | 318 (88.8%) | 385 (90.6%) | 441 (90.2%) |
| Yes | 40 (11.2%) | 40 (9.4%) | 48 (9.8%) | |
| Taking Anti-depressant | No | 339 (94.2%) | 409 (95.1%) | 469 (94.7%) |
| Yes | 21 (5.8%) | 21 (4.9%) | 26 (5.3%) |
Only age at randomization is significantly different by the treatment groups (p<0.05).
There are missing values in these two variables; percentages are the number in cells divided by the non-missing observations.
Measurements
The primary measures were the diagnosis of diabetes, the quality of glucose control, depression symptom total score (BDI), and the use of anti-depression medications (ADM). Diabetes was diagnosed according to the 1997 American Diabetes Association criteria (20) based on an annual oral glucose tolerance test or a semiannual fasting plasma glucose test. Diagnosis required a confirmation glucose test usually within 6 weeks (16). The quality of glucose control was assessed by measuring fasting plasma glucose (FPG) semi-annually and glycosylated hemoglobin A1c measured annually at a centralized laboratory. Depressive symptoms were measured using the Beck Depression Inventory (BDI), which was assessed annually. A score ≥ 11 was used as the definition of clinically meaningful depressive symptoms. Use of ADM’s was assessed by subject interview conducted semi-annually and examination of pills or prescriptions brought to the clinic for that purpose.
Statistical Analysis
BDI scores that were measured right before or at the visit (if assessed at the same time as diabetes diagnosis) of the diabetes diagnosis and at the first visit after the diabetes diagnosis were compared using Wilcoxon’s signed rank test to assess the influence of onset of diabetes (21). The BDI was also dichotomized at 11 (≥11 vs. <11), and compared using McNemar’s test of agreement (22). Antidepressant use at diabetes diagnosis and at the first visit after the diabetes diagnosis was compared using McNemar’s test of agreement. We also analyzed associations between glycemic measurements and depression after a participant was diagnosed with diabetes. FPG, A1C and normalization of FPG were assessed for their association with BDI score and ADM use using repeated measures analyses (23). In a longitudinal analysis including both participants with a diabetes diagnosis and those without, we modeled whether diabetes status or diabetes duration was associated with depressive symptoms or ADM use using repeated measures analysis.
In addition to these measures, data were collected for variables that have been shown to be independently associated with depressive symptoms. These include baseline covariates age, sex, ethnicity, income level, marital status, education, physical activity, BMI, and quality of life (measured by use of the SF 6d subscale of the SF-36) (23). These variables were used as covariates in the models predicting depressive symptoms or ADM use.
Results
Table 1 presents the baseline characteristics of the 1285 participants who were diagnosed as having diabetes during the study, by treatment arm. More people were diagnosed with diabetes in the placebo group, reflecting the preventive effect of intensive lifestyle and metformin medication. The three groups were comparable at baseline, the metformin participants were slightly older (median at 50.7) than the other two groups (48.6 for ILS and 49.0 for PLC).
Table 2 shows the status of subjects in each study intervention with regard to their age at diagnosis, their time in the study, their mean BMI, FPG, and BDI scores and the percentage with depressive symptoms and the proportion taking ADM at or before diabetes diagnosis. This is based on data collected at the annual visit at or preceding diabetes diagnosis with a valid BDI score. Metformin participants were older (median age 54.9) than Lifestyle (53.2) or Placebo (52.5). Due to the intervention effect, by the time of diabetes diagnosis, lifestyle participants who developed diabetes were in the study longer than persons with diabetes in the metformin group and again longer than the placebo group. Overall, the median depressive symptom score was low (median [IQR] = 3[1–6]) and not significantly different (p=0.78) across the treatment groups (median [IQR] = 3[0–7] for ILS, 3[1–6] for MET, 3[1–6] for PLC.)
Table 2.
Characteristics of participants at/before diabetes diagnosis (N=1285).
| Characteristics | Lifestyle | Metformin | Placebo | |
|---|---|---|---|---|
| N | 360 | 430 | 495 | |
| Age at diagnosis* | Median(IQR) | 53.2 (46.2–61.0) | 54.9 (48.0–62.5) | 52.5 (46.4–60.0) |
| Time in study* | Median(IQR) | 4.4 (2.5- 6.1) | 3.0 (2.0–6.0) | 2.6 (1.1–5.0) |
| BMI | Median(IQR) | 34.5 (29.7–39.6) | 33.3 (29.1–38.6) | 34.8 (30.0–40.1) |
| Fasting plasma glucose* | Median(IQR) | 117.0 (109.0–127.0) | 114.0 (104.0–125.0) | 118.0 (110.0–127.0) |
| BDI score | Median(IQR) | 3.0 (0.0–7.0) | 3.0 (1.0–6.0) | 3.0 (1.0–6.0) |
| BDI ≥ 11 | No | 323 (89.7%) | 393 (91.4%) | 441 (89.1%) |
| Yes | 37 (10.3%) | 37 (8.6%) | 54 (10.9%) | |
| Taking Anti-depressant | No | 316 (87.8%) | 393 (91.4%) | 445 (89.9%) |
| Yes | 44 (12.2%) | 37 (8.6%) | 50 (10.1%) |
This is based on the annual visit at or within six months before diabetes diagnosis with a valid BDI score.
Age, Time in study and FPG are significantly different in the three treatment groups (p<0.05).
Relationship between Diabetes Diagnosis and Depressive Symptoms and ADM Use
One year after diabetes diagnosis, participants’ BDI scores remained low (median [IQR] =3[0–6]) and was not significantly different (p=0.59) across the treatment arms (median[IQR] = 3[0–7] for ILS, 3[1–6] for MET and 3[1–6] for PLC). Table 3 shows the cross-tab of BDI scores ≥ 11 indicating the presence of DS and ADM use before (or at) and following the diagnosis of diabetes. The proportion of subjects with BDI scores indicating clinically meaningful depressive symptoms did not change significantly immediately after diagnosis (p=0.28); nor did ADM use (p=0.76). Evaluation of the use of ADM use over time indicated that ADM use increased significantly with study duration (p<0.0001) as shown in Figure 1. However, this change may reflect a secular increase in the prescription and use of antidepressants nationally during this time frame. BDI scores did not change significantly over time (p =0.72) and there were no differences among the three treatment groups (p=0.43). The results remained the same when BDI score was evaluated as a dichomomized variable (BDI≥ 11).
Table 3.
BDI Scores BDI≥11 and antidepressant use before and after diabetes diagnosis
| Before Diagnosis | After Diagnosis | |||
|---|---|---|---|---|
| BDI ≥11 | Anti-depressant Use | |||
| No | Yes | No | Yes | |
| No | 1090 | 67 | 1131 | 22 |
| Yes | 55 | 73 | 20 | 112 |
| Total | 1145 | 140 | 1151 | 134 |
Figure 1.
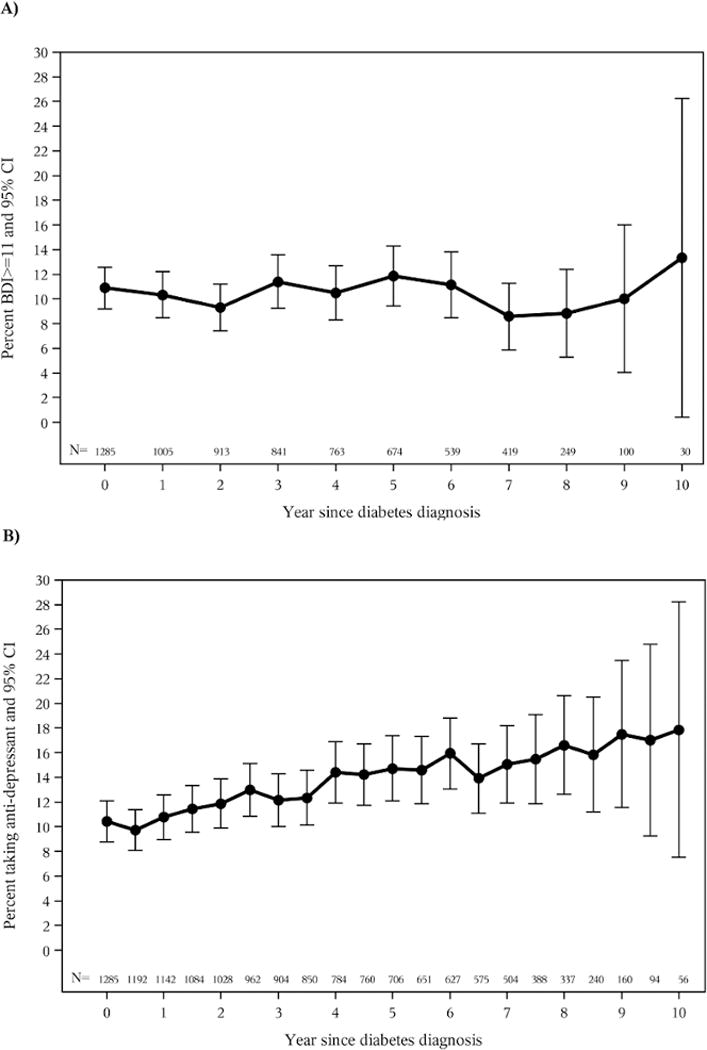
BDI≥11 and Antidepressant Medication Use Over time for Participants Diagnosed with Diabetes. Error bars represent 95% confidence intervals for the % estimated. “0” on the X axis indicates the time of diabetes diagnosis. N represents number of participants at each annual (or semi-annual) visits.
A) %BDI≥11 overtime for participants with diabetes.
B) Anti-depressant use over time for participants with diabetes.
Relationship between Depressive Symptoms, ADM use and Glycemic Indicators
Analyses were conducted to assess the relationship between BDI score, as a continuous and dichotomous measure, and glucose levels as measured by FPG and A1c. As shown in Figure 2A), normalization of FPG was associated with lower BDi scores; a higher FPG and higher A1C (%)were associated with higher BDI scores. On average, for participants with a FPG that had normalized, there was a decrease of 0.3031 point in the BDI score (95% CI 0.0924 to 0.5119; p=0.005). A 10 mg/dl rise in FPG was associated with a 0.073 increase in BDI score (95% CI 0.036–0.111, p=0.001). A 1% rise in A1c was associated with a 0.2185 point increase in BDI score (95% CI 0.075–0.3620; p=0.003). Assessing BDI as a dichotomous variable (≥ 11 yes or no) did not change these outcomes.
Figure 2.
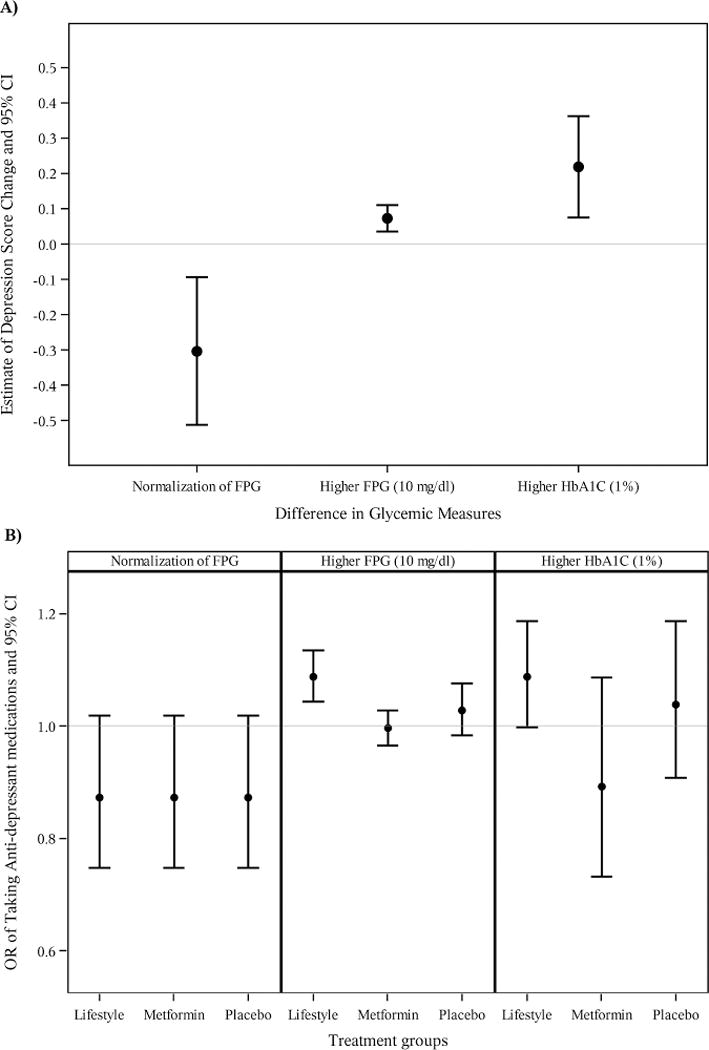
Association between glycemic measures and depression. Error bars represent 95% confidence interval of the parameter estimates.
A) Difference in BDI scores by glycemic measures.
B) Difference in Antidepressant use and glycemic measures
There was no treatment interaction with any of the three predictors. Treatment groups were not significantly associated with BDI scores. Covariates entered into the above models included age, gender, ethnicity, income level, marital status, education level, physical activity, BMI, and quality of life at baseline of the DPP.
Finally, the relationship between different measures of glycemic levels and likelihood of taking antidepressant medications for the three treatment group were analyzed and shown in Figure 2B. Normalization of FPG has no significnat effect on ADM use (p=0.084). Higher fasting plasma glucose (FPG) levels were associated with greater ADM use only in the ILS intervention (Odds ratio [95%CI] = 1.088 [1.044–1.135], p<0.0001). There was significant treatment interaction with two predictors, FPG (p=0.01) and A1C (p=0.01). Age, gender, ethnicity, income level, marital status, education level, baseline activity, BMI, QOL (SF-36) at entrance to DPP were entered into the models as covariates.
We also looked at the depressive symptoms and ADM use over the course of the DPP/DPPOS phase I. Neither diabetes status nor diabetes duration was significantly associated with ADM use or depressive symptoms.
Discussion
In the current study, a well-characterized sample of adults at high risk for diabetes was followed longitudinally in the DPP and DPPOS. Contrary to expectations about the emotional impact of newly diagnosed diabetes, we observed that the diagnosis of diabetes did not predict the onset of clinically meaningful depressive symptoms or new use of ADM. Even though previous research suggests that there is only a modest association with diagnosis and onset of depression, it is still argued that diabetes may increase risk of depression because of the general sense of threat associated with the diagnosis of a chronic condition, the sudden onset of lifestyle demands that are not freely chosen, and the threat of developing complications [11]. In the study sample, over 65% reported experience with a first degree relative (mother, father, sister, brother) who they observed struggle with diabetes. Thus, they were motivated to try and prevent the disease by participating in a rigorous and demanding trial. Thus, it is plausible that the diagnosis of diabetes, in spite of their efforts, may have had a more significant and distressing impact on their psychological state. In this context, this study contributes an important negative finding to the limited literature that documents the psychosocial experience of adults newly diagnosed with diabetes and in particular to those who had a clear awareness of their increased risk and had invested considerable effort to reduce it.
In this study we also found that glucose levels post-diagnosis were positively associated with depressive symptoms. On average, the BDI score was 0.073 (95% CI 0.036–0.111, p=0.001) points higher with 10 mg/dl higher FPG, 0.2185 points (95% CI 0.075–0.3620; p=0.0028) higher with 1% higher A1C, and 0.3031 points (95% CI 0.0924 to 0.5119; p=0.0045) higher if FPG had not normalized. This finding demonstrates a positive relationship between depressive symptoms and fasting plasma glucose and A1c and demonstrates the interrelationship of depressive symptoms on glucose outcomes even if clinical thresholds for depression are not met. Higher fasting plasma glucose was also associated with greater ADM use in the lifestyle arm post-diagnosis. Whether this is an effect of frustration with and poor response to initial treatment efforts, a biological artifact or simply the cause of poor control is not clear. It is possible that self management may have been mitigated by initial reactions having “failed” in their efforts reduce their known diabetes risk resulting in lower motivation for self-management.
We observed that diabetes duration was not significantly associated with ADM use or having depressive symptoms. This reflects the fact that the emotional response to diagnosis from the point of diagnosis for a relatively short period of time is a different psychological phenomenon than depressive symptoms that persist or develop after the adjustment to a chronic illness (i.e., psychiatric syndromes or disorders). Thus, the 1-year time period distinguishes adjustment to diagnosis from psychiatric depressive syndromes. The reason for this is that there are a number of psychological constructs that change in the context of a new diagnosis – incorporation (or not) of the disease as part of one’s identity, behavioral adjustments to self and family routines to accommodate self-care behaviors (or not), accommodation of the meaning of the disease and its complications to expectations of life and mortality, etc. While these can be associated with depressive symptoms, adjustment issues tend to be transitory and resolve as the individual and his/her family makes psychological accommodation. Depression as a psychiatric syndrome, on the other hand, is typically independent of diagnosis of diabetes but may be triggered by the stress associated with the diagnosis. It does not necessarily remit following psychological adjustment.
The strengths of this study include a large sample size of adults at high risk for diabetes whose glycemic status over a 10-year period of time was precisely defined by multiple measures of glucose to establish the onset of diabetes. A limitation of the study is the low levels of depressive symptoms present in the sample at the time of study enrollment. To be eligible for inclusion in the DPP/DPPOS study, participants had to be free of serious clinical depression. In this regard, the subjects reported a lower level of depressive symptoms than might be expected in a similar population of middle age, overweight persons. Clearly, this sample is not reflective of the broader population with increased risk for developing type 2 diabetes. Several estimates suggest that the there is a higher prevalence rate of depression and depressive symptoms in this cohort [25–27]. The fact that we excluded persons with serious depressive symptoms or using ADMs that could aid weight loss (a primary outcome measures for the ILS condition) caution over generalizing the findings of this study. It is possible that the low incidence of depressive symptoms reported here are reflective of a more proactive and motivated group than may be encountered in the general population. This being said, it is equally plausible that one would expect a more pronounced reaction to diagnosis in this group that had actively worked at reducing their risk only to have diabetes diagnosed despite their best efforts.
In summary, the current study demonstrated that the diagnosis of diabetes did not result in an immediate increase of depressive symptoms or antidepressant medication use. However, a linear relationship observed between BDI scores, FPG and A1c underscores the importance of monitoring depressive symptoms and glycemic indicators in order to maximize health and mental health outcomes in patients newly diagnosed with diabetes.
Supplementary Material
Acknowledgments
The Research Group gratefully acknowledges the commitment and dedication of the participants of the DPP and DPPOS. During the DPPOS, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health provided funding to the clinical centers and the Coordinating Center for the design and conduct of the study, and collection, management, analysis, and interpretation of the data (U01 DK048489). The Southwestern American Indian Centers were supported directly by the NIDDK, including its Intramural Research Program, and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources, and the Department of Veterans Affairs supported data collection at many of the clinical centers. Funding was also provided by the National Institute of Child Health and Human Development, the National Institute on Aging, the National Eye Institute, the National Heart Lung and Blood Institute, the Office of Research on Women’s Health, the National Institute on Minority Health and Health Disparities, the Centers for Disease Control and Prevention, and the American Diabetes Association. Bristol-Myers Squibb and Parke-Davis provided additional funding and material support during the DPP, Lipha (Merck-Sante) provided medication and LifeScan Inc. donated materials during the DPP and DPPOS. LifeScan Inc., Health O Meter, Hoechst Marion Roussel, Inc., Merck-Medco Managed Care, Inc., Merck and Co., Nike Sports Marketing, Slim Fast Foods Co., and Quaker Oats Co. donated materials, equipment, or medicines for concomitant conditions. McKesson BioServices Corp., Matthews Media Group, Inc., and the Henry M. Jackson Foundation provided support services under subcontract with the Coordinating Center. The opinions expressed are those of the investigators and do not necessarily reflect the views of the funding agencies. For a complete list of centers, investigators, and staff, please see Supplemental Digital Content 1.
D.G.M. and Y.M. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. D.G.M., Y.M., M.D.G., E.B.C. and W.C.K. wrote the manuscript and researched data. E.H., D.P., and M.C. also researched data and contributed to the discussion and reviewed and edited the manuscript.
The authors gratefully acknowledge the contributions of Richard R. Rubin (Johns Hopkins School of Medicine) to the development of this manuscript. Dr. Rubin passed away in 2013.
Acronyms
- ADM
antidepressant medicine
- BDI
Beck Depression Inventory
- DPP
Diabetes Prevention Program
- DPPOS
Diabetes Prevention Program Outcomes Study
- DS
depressive symptoms
- FPG
fasting plasma glucose
- ILS
intensive lifestyle
- MET
Metformin
- PLC
Placebo
Footnotes
Conflicts of Interest: The authors have no relevant conflicts of interest to disclose.
Clinicaltrials.gov Registry: DPPOS: NCT00038727 DPP: NCT00004992
References
- 1.Golden SH, Lazo M, Carnethon M, Bertoni AG, Schreiner PJ, Diez-Roux AV, Hochang BL, Lyketsos C. Examining a bidirectional association between depressive symptoms and Diabetes. JAMA. 2008;299(23):2751–2759. doi: 10.1001/jama.299.23.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palinkas LA, Barrett-Connor E, Wingard DL. Type 2 diabetes and depressive symptoms in older adults: a population-based study. Diabet Med. 1991 Jul;8(6):532–9. doi: 10.1111/j.1464-5491.1991.tb01646.x. [DOI] [PubMed] [Google Scholar]
- 3.Knol MJ, Twisk JWR, Beekman ATF, Helne RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes: A meta-analysis. Diabetologia. 2006;49(5):837–845. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- 4.Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Archives of Internal Medicine. 2000;160(21):3278–85. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- 5.Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabetic Medicine. 2006;23(11):1165–1173. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- 6.de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman P. Association of depression and diabetes complications: A meta-analysis. Psychosomatic Medicine. 2001;63:619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Lustman P, Anderson R, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: A meta-analytic review of the literature. Diabetes Care. 2000;23:934–942. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 8.Musselman DL, Betan E, Larsen H, Phillips LS. Relationship of depression to diabetes types 1 and 2: epidemiology, biology, and treatment. Biological Psychiatry. 2003;54(3):317–329. doi: 10.1016/s0006-3223(03)00569-9. [DOI] [PubMed] [Google Scholar]
- 9.Rubin RR, Ma Y, Marrero DG, Peyrot M, Barrett-Connor EL, Kahn SE, Haffner SM, Price DW, Knowler WC, for the Diabetes Prevention Program Research Group Elevated depression symptoms, antidepressant medicine use and risk of developing diabetes during the Diabetes Prevention Program. Diabetes Care. 2008;(3):420–6. doi: 10.2337/dc07-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubin RR, Marrero D, Price D, Barrett-Connor E, Carnethon M, Crandall J, et al. Antidepressant Medicine Use and Risk of Developing Diabetes During the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study. Diabetes Care. 2010;12(33):2549–51. doi: 10.2337/dc10-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31(12):2383–2390. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rane K, Wajngot A, Wandell PE, Gafvels C. Psychosocial problems in patients with newly diagnosed diabetes: number and characteristics. Diab Res Clin Pract. 2011;93:371–8. doi: 10.1016/j.diabres.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Weinger K, Welch G, Jacobson AM. Psychological and psychiatric issues in diabetes mellitus. In: Poretsky L, editor. Principles of Diabetes Mellitus. Norwell, Massachusetts: Kluwer Academic Publishers; 2003. [Google Scholar]
- 14.Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic Control: a meta-analytic review of the literature. Diabetes Care. 2000;23(7):934–42. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 15.Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care. 2010;33(5):1034–6. doi: 10.2337/dc09-2175. Epub 2010 Feb 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program: design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22:623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program: recruitment methods and results. Controlled Clinical Trials. 2002;23:157–171. doi: 10.1016/s0197-2456(01)00184-2. [DOI] [PubMed] [Google Scholar]
- 18.The Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:623–634. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–119. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 21.Wilcoxon F. Individual comparisons by ranking methods. Biometrics Bulletin. 1945;1(6):80–83. [Google Scholar]
- 22.McNemar Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika. 1947;12(2):153–157. doi: 10.1007/BF02295996. [DOI] [PubMed] [Google Scholar]
- 23.Kung-Yee L, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 24.Brazier J, Roberts J, Tsuchiya A, Busschbach J. A comparison of the EQ-5D and SF-6D across seven patient groups. Health Economics. 2004;13:873–84. doi: 10.1002/hec.866. [DOI] [PubMed] [Google Scholar]
- 25.de Groot M, Pinkerman B, Wagner J, Hockman E. Depression screening and treatment attitudes in a multicultural sample of type 1 and type 2 diabetes patients. Diabetes Care. 2006;29(3):549–553. doi: 10.2337/diacare.29.03.06.dc05-1396. [DOI] [PubMed] [Google Scholar]
- 26.Peyrot M, Rubin RR. Persistence of depressive symptoms in diabetic adults. Diabetes Care. 1999;22(3):448–452. doi: 10.2337/diacare.22.3.448. [DOI] [PubMed] [Google Scholar]
- 27.Katon W, Russo J, Lin EH, Heckbert SR, Ciechanowski P, Ludman, Von Korff M. Depression and diabetes: factors associated with major depression at five-year follow-up. Psychosomatics. 2009;50:570–9. doi: 10.1176/appi.psy.50.6.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


