Abstract
Nanomaterials exhibit unique properties that are absent in the bulk material because decreasing material size leads to an exponential increase in surface area, surface area to volume ratio, and effective stiffness, resulting in altered physiochemical properties. Diverse categories of nanomaterials such as nanoparticles, nanoporous scaffolds, nanopatterned surfaces, nanofibers and carbon nanotubes can be generated using advanced fabrication and processing techniques. These materials are being increasingly incorporated in tissue engineering scaffolds to facilitate the development of biomimetic substitutes to replace damaged tissues and organs. Long term success of nanomaterials in tissue engineering is contingent upon the inflammatory responses they elicit in vivo. This review seeks to summarize the recent developments in our understanding of biochemical and biophysical attributes of nanomaterials and the inflammatory responses they elicit, with a focus on strategies for nanomaterial design in tissue engineering applications.
Introduction
Nanomaterials in Tissue Engineering
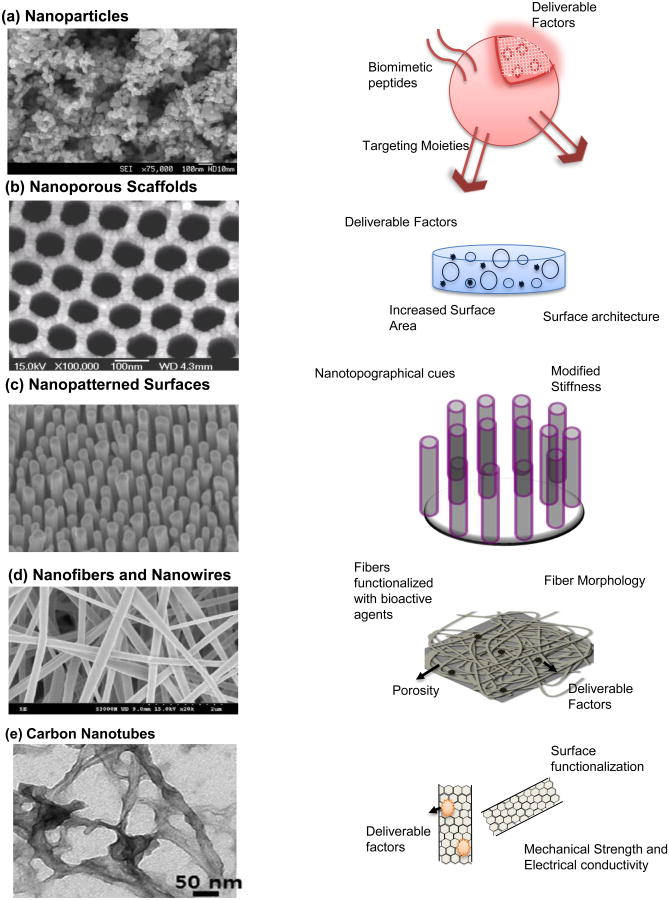
Tissue engineering aims to develop constructs consisting of cells and biomaterials to replace damaged tissues and organs and circumvent the time-consuming, complicated, and rarely available organ transplants. Nanoscale design of tissue engineering constructs facilitates generation of biocompatible scaffolds that precisely resemble the native extracellular matrix, provides physiologically relevant biomechanical cues and enables spatiotemporal release of biological factors necessary for functional tissue replacements.1, 2 Recent advances in nanotechnology and nanofabrication techniques have enabled formation of biocompatible nanomaterials such as nanoparticles, nanoporous scaffolds, nanopatterned surfaces, nanofibers, nanowires and carbon nanotubes.3, 4 (Figure 1)
Figure 1. Nanomaterials in tissue engineering.
(a) (Left) SEM of ZnO nanoparticles. Reprinted with permission from Ref. 44 Copyright 2011 Springer (Archives of Toxicology); (Right) Schematic of a functional nanoparticle, which provides a reliable conduit for delivering drugs, growth factors, cytokines and other factors. These can be functionalized with biomimetic peptides, targeting moieties and functional groups for degradation in response to appropriate physiological stimuli. (b) (Left) SEM image of nanoporous alumina. Reprinted with permission from Ref. 69 Copyright 2007 Elsevier Ltd. (Biomaterials); (Right) Schematic of a nanoporous scaffold. These scaffolds make for a three dimensional construct with increased surface area, surface nanoarchitecture and an ability for controlled delivery of factors. (c) (Left) SEM image of metallic glass nanopillars. Reprinted with permission from Ref. 39 Copyright 2014 ACS Nano ; (Right) Shematic of nanopatterned Surfaces, which present nanotopographical and biomechanical cues to engineer cellular response to tissue engineering scaffolds. (d) (Left) SEM image of PLGA/Collagen nanofibers. Reprinted with permission from Ref. 90 Copyright 2010 Elsevier Ltd. (Journal of Membrane Science); (Right) Schematic of a nanofibrous scaffold. Nanofibers and nanowires contribute to creation of biomimetic scaffolds that recreate the native extracellular matrix structure. These can also be functionalized/loaded with drugs and other factors for controlled localized delivery. (e) (Left) TEM image of a gelatin-methacrylate coated nanotube. Reprinted from Ref. 24 Copyright 2013 ACS Nano.; (Right) Schematic of carbon nanotubes. These are used to reinforce mechanical strength and promote electrical conductivity in polymers and hydrogels used in tissue engineering applications. They can also serve as excellent drug delivery vehicles.
Each of these classes of nanomaterials is used for specialized applications in advanced tissue engineering. Nanoparticles are primarily used as vehicles for targeted and controlled delivery of growth factors, drugs, and oligonucleotides in vivo. In addition, nanoparticles can be used in composite scaffolds to enhance mechanical properties, corrosion rate, biodegradation and other traits.5-8 Nanoporous materials, generated using sol-gel techniques, etching processes, anodization and other electrochemical methods, provide increased surface area, pore-size dependent diffusion properties, improved protein adhesion, cellular integration and have found particular success in bone tissue engineering.9-13 Nanopatterned surfaces incorporate structures such as pillars, ridges and other topographical features with tunable mechanical properties and surface area. These surfaces have been shown to elicit extensive cellular responses ranging from stem cell differentiation to prevention of tissue fibrosis.9, 14-17 Nanofibrous biomaterials, produced predominantly by electrospinning techniques, have been used to recreate the architecture of the extracellular matrix with demonstrated therapeutic potential.18-21 Carbon nanotubes are used as reinforcements in tissue engineering scaffolds for mechanical strength and can aid in both electrical conductivity and controlled drug delivery. These properties make carbon nanotubes favorable for cardiac and neural tissue engineering purposes.22-25 In addition to the categories of nanomaterials described above, compound nanomaterials incorporating two or more categories, such as nanoparticle loaded nanofibers and nanopatterns imprinted on electrospun fibers, are also being investigated.26, 27
The success of nanomaterials in tissue engineering will be determined, in part by the nature of inflammatory responses they evoke. Inflammatory responses towards tissue engineering scaffolds ranging from neutrophil infiltration to macrophage recruitment and ultimately fibrous capsule formation, are influenced by both material biochemistry and the size-features/nanoscale design of scaffold material.28 A variety of materials including polymers, hydrogels, extracellular matrix proteins and even metals and carbon-based elements are used to generate nanomaterials. Biological and inflammatory responses elicited by diverse biomaterials have been reviewed previously28-30, but size-dependent changes in material properties convolute the findings. Decreasing size alters the physiochemical properties of materials and their effects on cellular and subcellular elements at the nanoscale. Therefore, properties such as surface area and topography hold the potential for countless cures as well as contrary consequences.1, 31, 32 The surge of nanomaterials in tissue engineering necessitates a careful reevaluation of inflammatory responses, particularly the molecular mechanisms that orchestrate the integration/rejection of tissue engineering constructs with nanoscale features.
Inflammation and Tissue Engineering
In general terms, biomaterials, are recognized as foreign bodies and evoke an array of inflammatory responses.29 Inflammatory reactions to implanted nanomaterials are initiated by protein interactions at the surface. Adsorption of proteins such as albumin, fibronectin, fibrinogen and vitronectin lead to varying levels of inflammation.28 Chemical composition of materials, surface charge and topography are known to affect adsorption of plasma proteins and extracellular matrix components. Surface-adsorbed proteins as well as the injury caused by implantation of biomaterials result in recruitment of neutrophils and activation of resident mast cells, constituting the acute inflammatory response.1, 28. These cells release reactive oxygen species (ROS) and cytokines such as interleukin-4 (IL-4) and interleukin-13 (IL-13) prompting oxidative stress and recruitment of monocytes from the bloodstream. In addition, implanted biomaterials stimulate production of inflammasomes, which are intracellular multiprotein complexes that mediate activation of pro-inflammatory cytokines such as IL-1β and IL-18.33, 34 Inflammasomes can be produced as a result of phagosome rupture after material uptake by phagocytosis or by particulate interaction with plasma membrane causing changes in cholesterol rafts as described elsewhere.33, 34. Regardless of the mode of activation, inflammasomes contribute to increased levels of proinflammatory cytokines and eventual tissue fibrosis. In parallel, dendritic cells can mediate generation of antibodies against antigens presented by tissue engineered constructs, which subsequently lead to targeting and removal of the specific antigens.35
Biomaterial-induced inflammation progresses to development of the foreign body response (FBR) and is characterized by the extended presence of monocytes, which mature into macrophages and foreign body giant cells, and other lymphocytes.28 Recruited monocytes and resident macrophages produce chemoattractant proteins such as granulocyte macrophage colony stimulating factor (GM-CSF), monocyte chemotactic protein-1 (MCP-1) and platelet derived growth factor (PDGF) leading to further recruitment of monocytes. These cells also increase expression of cell surface receptors including Mac-1 and other integrins that aid in migration and adhesion to surface-adsorbed proteins. Integrin engagement by adsorbed proteins induces changes in the expression and organization of focal adhesion proteins like vinculin, paxillin and α-actinin. Changes in focal adhesion formation are translated into cytoskeletal remodeling and downstream effects including activation of focal adhesion kinase (FAK) and extracellular signal-regulated kinase (ERK). These signaling events determine the phenotype of macrophages and subsequent inflammatory responses. As mentioned above, macrophages can fuse to form FBGCs in the context of the FBR. Fusion of macrophages, induced by IL-4 and mediated by Rac-1, leads to formation of FBGCs that release a host of factors including degradative reactive oxygen intermediates, remodeling proteins like matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs), inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and other ILs, as well as transforming growth factor-β (TGF-β). Prolonged inflammation, characterized by the presence of macrophages and FBGCs and the release of these factors, is followed by infiltration of fibroblasts and production of collagen. Inflammation-mediated formation of collagen-rich fibrotic capsule impedes tissue integration and implant function.29
While inflammation is more often associated negative consequences such as fibrosis and biomaterial rejection, some inflammatory reactions can promote biomaterial-tissue integration.35 This dichotomy originates from the overlap between the processes of inflammation and tissue repair that follow the injury caused by implantation of tissue engineered constructs.36 Tissue repair involves formation of new blood vessels via angiogenesis and matrix remodeling. Activated macrophages have been shown to produce pro-angiogenic factors such as fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF), which are known to promote migration and proliferation of endothelial cells and vessel formation.36, 37 The newly formed vessels are critical for integration of implanted biomaterials with the surrounding tissue.37 In addition, proteases such as MMPs released by macrophages and FBGCs, contribute to matrix remodeling to accommodate the implanted biomaterial. Furthermore, dendritic cell-mediated antigen tolerance leading to improved biomaterial performance and increased regeneration have also been reported.35 Material properties contributing to changes in inflammatory cell behavior are still under investigation, but it is appreciated that modulation, rather than complete evasion of inflammation will lead to successful tissue engineering constructs.
Investigators examine a host of factors to determine inflammatory response to tissue engineered constructs. Interactions of biomaterials with cell types that mediate inflammation such as neutrophils, monocytes, and macrophages are analyzed in vitro to detect cellular responses such as change in morphology, activation of receptors (e.g., Mac-1 receptor in monocytes), release of ROS and pro-inflammatory cytokines such as specific ILs, TNF-α and MCP-1. Inflammatory responses to nanomaterials in vivo are evaluated by measuring the recruitment of neutrophils and monocytes/macrophages, the levels of pro-inflammatory cytokines for short-term studies. For long term studies, accumulation of material degradation products and thickness of fibrous capsule formation are examined.
Inflammatory responses to nanomaterials used in tissue engineering
Nanomaterials used in tissue engineering are susceptible to the same inflammatory apparatus as other biomaterials, i.e. inflammatory cells, cytokines and enzymes, but elicit unique responses from each component in the cascade. Biomaterials with nanoscale features possess surface nanotopography which influences inflammatory cell response. For example, pro-inflammatory cytokine production in macrophages has been shown to be influenced by the surface architecture of nanoporous scaffolds.38 Investigation of cell-nanotopography interactions has been relatively recent and limited, but some early trends have emerged. Firstly, nanotopographical cues can induce both pro-inflammatory and anti-inflammatory responses, and the outcome is sensitive to the size features of the scaffold surface. For example, levels of IL-1-β and TNF-α produced by macrophages grown on nanoporous alumina were found to be influenced by the pore-size of the alumina surface.38 Similarly, length of nanopillars has been linked to collagen production in fibroblasts cultured on those nanopillars.14 Secondly, cellular response to nanotopography is cell type specific. For instance, macrophages, endothelial cells and fibroblasts displayed differential responses to identical nanopatterns.39 It is believed that detection of nanotopography by cells is mediated by differential protein adsorption by biomaterials with nanoscale surface features.14, 39 Nanopillars made of polymers and metallic glass have been shown to influence serum protein deposition in vitro which leads to changes in morphology and function of cells cultured on these materials.
It is important to note that nanoparticles and carbon nanotubes are often used as individual entities, rather than as part of bulk biomaterials. Hence, these two classes of nanomaterials are distinct from biomaterials with nanoscale features (nanoporous scaffolds, nanofibers and nanopatterned surfaces) in that they are taken up by cells by phagocytosis or other means. Consequently, specific responses elicited by uptake determine the activation of inflammatory cells. In contrast, cells in contact with surfaces with nanoscale features are activated via adsorbed protein-receptor interactions. Nevertheless, cellular responses to nanoparticles and nanotubes have exhibited similar trends as described for other classes of nanomaterials. Nanoparticle size and shape influence the inflammatory response they elicit in vivo.40 Additionally, macrophages and neutrophils have been shown to respond to distinct ranges of nanoparticle size.40, 41
It is clear that nanomaterial size and shape regulate the inflammatory response. In addition to characterizing size-feature ranges that attenuate or modulate inflammation, traditional methods of promoting material biocompatibility such as chemical modifications and release of anti-inflammatory drugs also constitute important design parameters to be optimized.
This review seeks to summarize recent developments in our understanding of inflammatory responses towards nanomaterials used in tissue engineering. Analysis of these interactions will provide insight into the immunomodulatory effects of nanomaterials and inform future design.
Nanoparticles
Nanoparticles are synthesized using a variety of natural and artificial polymers, lipids, dendrimers, ceramics and metals. Regardless of the core material, nanoparticles can access intracellular components via phagocytosis or endocytosis and provide for an efficient and reliable conduit to deliver therapeutics. While a decrease in particle size endows materials with the ability to penetrate biological barriers, there are some reports that indicate that nanoparticles can incite increased inflammatory response. In vitro experiments have suggested that these particles can trigger production of ROS in lung epithelial cells and macrophages, which may lead to lung injury.42 Therefore, it is important to review strategies to attenuate inflammatory responses to nanoparticles.
Inflammatory response to nanoparticles is particle-size dependent. For instance, inflammation induced by nanoparticles made of poly-acryl-acid coated gold nanoparticles is contingent upon their hydrodynamic diameters.41 When these particles were less than 20 nm in size, they were shown to increase fibrinogen-mediated activation of Mac-1 receptor in monocytes in vitro. Mac-1 receptor activation in monocytes which can lead to stimulation of the NF-kB pathway and upregulation of other downstream inflammatory cytokines, was absent when nanoparticles of 20 nm or larger in size were used. The effect of nanoparticle size on inflammatory responses has also been established in vivo. For example, recruitment of polymorphonucleocyte (PMN) induced by Poly(d,L-lactide-co-glycolic acid) (PLGA) nanoparticles has been shown to be size-dependent.40 75 nm particles, in contrast to 200 nm particles, elicited limited PMN recruitment in bronchial alveolar lavage fluid. In addition to size features, nanoparticle shape is a determinant of inflammatory responses. For example, cellular uptake of rod-shaped particles larger than 100nm has been found to be greater than spheres, cube-shaped and cylindrical particles43 .In contrast, sub-100nm particles with smaller aspect ratios like spheres were taken up more readily than nanoparticles with higher aspect ratio such as nanorods or nanosheets.43, 44. The effect of increased cellular uptake of nanoparticles on inflammatory responses is not clear. Zinc oxide (ZnO) nanosheets have been shown to induce higher levels of TNF-α production in murine dendritic cells, while nanospheres of similar chemical composition induced higher levels of TNF-α production in murine macrophages.44 In contrast, shape of ZnO nanoparticles did not significantly alter ROS production in epithelial cells at low concentrations.44 It is speculated that the size and shape of nanoparticles contribute to the effective surface area available for ligand-receptor interactions, which in turn dictates cellular uptake and consequential inflammation evoked in vivo.43 It should be noted that nanoparticles could induce inflammatory responses independent of cell interactions. Radical electrons released from the nanoparticle surface endow these particles with ROS-generating capacity when appropriate substrates are present.45. For example, platinum nanoflowers, have been shown to generate increased ROS levels as compared to platinum multipod nanoparticles in cell free systems.45
Chemical composition of nanoparticles has also been implicated in exaggerated inflammatory responses. Non-biodegradable nanoparticles and cationic polymers are known to induce more inflammation than biodegradable and anionic surrogates respectively.46, 47 Titanium- and silicon-based nanoparticles as opposed to zinc containing nanoparticles led to activation of the inflammasome and increased release of cytokines such as IL-1β in macrophages and keratinocytes.47
Nanoparticles can be used as vehicles to deliver anti-inflammatory drugs, thereby attenuating unfavorable biological responses.46, 48, 49 Poly(d,L-lactide-co-glycolide)-block-poly(ethylene glycol) (PLGA-PEG) nanoparticles conjugated with Col IV peptides can effectively deliver drugs mimicking anti-inflammatory mediators such as lipocortin-1 and reduce neutrophil infiltration in peritonitis.50 PLGA nanoparticles embedded in hydrogel coatings on neural electrodes have been used to deliver dexamethasone to mitigate glial inflammation resulting in extended biomaterial lifespan in vivo.51 PLGA, chitosan and thioketal nanoparticles have been employed to deliver drugs and siRNA to alleviate levels of TNF-α produced by macrophages and combat intestinal inflammation and rheumatoid arthritis.52-54 Thioketal nanoparticles, such as poly-(1,4-phenyleneacetone dimethylene thioketal), loaded with siRNA against TNF- α have been designed to degrade and release drugs in response to ROS production, thus enabling targeted silencing of TNF-α expression at the site of inflammation.54 Besides TNF-α, cell cycle regulatory molecule cyclin D, immunomodulatory protein complex NF-kB and cell adhesion protein P-selectin have also been identified as potent targets for suppressing inflammation using nanoparticle-assisted drug delivery.55-57
Although the majority of these strategies have been developed for drug delivery applications, nanoparticles can be easily incorporated into implant design to modulate inflammatory responses. Incorporation of nanoparticles in electrospun fibers, macroporous hydrogels and other polymers for development of multifunctional scaffolds has been described previously.58, 59 It is appreciated that nanoparticle-assisted delivery of drugs provides increased spatiotemporal control and enhanced efficacy resulting in improved biomimetic scaffolds. As the translation of tissue engineering approaches to clinical interventions evolves, anti-inflammatory drugs will form a critical component of the cocktail of factors delivered from constructs.
In summary, 20 nm – 75 nm nanoparticles with minimal aspect ratio, synthesized using inert, anionic and biodegradable polymers are well-suited to attenuate inflammatory responses. Additionally, controlled and targeted silencing of inflammatory cytokines such as TNF-α or NF-kB using drug-loaded nanoparticles hold maximal potential for tissue engineering applications. Future research should be directed towards determining the relative contribution of size features and chemical composition of nanoparticles to inflammation. Development of in vitro evaluation protocols for inflammatory properties of nanoparticles will help expedite the screening process. For example, 3D co-cultures of multiple cell types are more representative of native tissue, and could help investigate unique inflammatory processes.60 An in vitro culture of epithelial cells sandwiched between macrophages and dendritic cells revealed that dendritic cells, rather than macrophages, extend processes between epithelial tight junctions to collect inflammatory nanoparticles.60 Moreover, cell-cell interactions in co-cultures of epithelial cells, macrophages and dendritic cells have been found to affect the inflammatory response to nanoparticles42. The levels of pro-inflammatory cytokine TNF-α produced by the co-culture of cells was found to be higher than the levels expected from the data collected using individual monocultures.42 Such experiments suggest that validated tissue-specific co-culture models can help screening methods and evaluate nanoparticulate formations.
Nanoporous Scaffolds
Porosity of materials constitutes an important design specification in the engineering of scaffolds because it can influence the infiltration of cells and blood vessels leading to acceleration of integration with the surrounding tissue. Pore sizes ranging from hundreds of micrometers to tens of nanometers are relevant for biomimetic models. In fact, hybrid materials containing macro, micro and nanopores such as tailored amorphous multi-porous (TAMP) scaffolds have been developed.13 In this regard, nanoporosity of materials is unique because nanopores do not allow for cellular infiltration and tissue integration. But nanopores and micropores are similar in that they both grant an exponential increase in surface area and confer a sharp increase in capacity for controlled local delivery of drugs, cytokines and other factors.
Additionally, nanoporous scaffolds, by resembling native tissue/matrix architecture, could evade the host's inflammatory system. For example, synthetic bone grafts made of nanoporous silica and hydroxyapatite have been shown to induce minimal leukocyte activation and rolling on the walls of microcapillaries surrounding the implant, comparable to that induced by cancellous bone substitutes.61 These biomimetic substitutes induce fusion of macrophages forming multinucleated giant cells, which resemble fused osteoclasts, and help in bone resorption and remodeling, thereby eliciting ‘favorable’ inflammatory responses. In contrast, nanoporous polymers such as polycaprolactone (PCL) with nanopits can exacerbate inflammation-mediated fibrous capsule formation in vivo.62 Yet other groups have reported that the activation of immune cells induced by nanoporous silicon and nanoporous alumina is similar to that induced by non-porous surfaces with the same chemical composition, thus concluding that nanoporosity does not affect inflammatory responses appreciably in vitro.62 Reconciling these contradicting reports in the literature requires a revisit of cellular responses induced by nanopore architecture.
Interactions between inflammatory cells and nanoporous materials are largely governed by the pore size. In vitro experiments studying the effect of pore-size on inflammatory cells have provided some insight. Alumina surfaces with nanopores 20 nm in diameter have been found to attract more macrophages that produced lower levels of pro-inflammatory cytokines such as IL-1β and TNF-α.38 In contrast, surfaces with 200 nm sized pores exhibited lower cell attachment and increased levels of pro-inflammatory cytokines. In a separate study, 200 nm pores, as opposed to 20 nm pores, were shown to activate a variety of pro-inflammatory cytokines in vivo.63
In addition to modulating cellular response, material pore-size is critical for the controlled release of drugs. Nanoporous drug vehicles have been developed using PEG, titanium surfaces, alumina filters, alginate gels and other materials to engineer the delivery of cytokines, antioxidants, non-steroidal anti-inflammatory drugs (NSAIDs) and a host of other factors.64-67 Controlled, long term release of anti-inflammatory drugs might be key to biomaterial acceptance. Nanopores can be incorporated into tissue engineering scaffolds to release growth factors, angiogenic factors and drugs to stimulate implant-tissue integration. Devising pore-sizes of tissue engineering scaffolds will depend on the dual deliberations of cellular interactions and the demand for drug delivery.
Lastly, material chemistry and surface modifications can help reduce the pro-inflammatory properties of nanoporous scaffolds. Native proteins such as collagen have also been used to decrease nanoporous scaffold induced ROS production in neutrophils in vitro.68 Some groups have successfully used chemical/surface modifications to attenuate inflammatory responses in vivo as well. For example, a layer of PEG coating on the surface of nanoporous alumina membranes has been shown to decrease serum albumin deposition on the material leading to suppressed inflammation.69 In addition, nanoporous poly(1,8-octanediol-co-citrate) (POC) has been shown to recruit fewer inflammatory cells and induce less fibrosis compared to the commonly used PLGA.70 Since the effect of biomaterial composition on associated inflammatory properties is beyond the scope of this review, the reader is directed to a recently published comprehensive review on biocompatibility of commonly used polymers in tissue engineering.71
In conclusion, nanoporous scaffolds fabricated using native matrix proteins or coated with inert polymers such as PEG and loaded with antioxidants and NSAIDs for long term sustained release are best suited to minimize inflammation. There is no consensus on the ideal pore size, but preliminary in vivo studies indicate that 20 nm pores reduce inflammatory responses.60
Nanopatterned Surfaces
Nanotopographical cues and associated cellular signals in the form of integrin receptor motifs are ubiquitous in native extracellular matrix that supports cells and tissues. Similarly, tissue engineered scaffolds with surfaces that incorporate three dimensional nanoscale architecture such as nano-pillars, grooves and ridges alter the nature of cell-substrate adhesions. Nanopatterned surfaces have been shown to evoke responses ranging from changes in cell morphology and motility to modifications in gene expression and phenotypic switches.9, 14, 39, 72 The biomechanical cues inherent to nanotopography are not completely understood, and constitute an active area of research. In addition to biomolecular signals inherent to topography, which are contingent upon size features and spacing between ordered features, nanopatterned surfaces possess additional unique properties. For example, similar to other nanomaterials, they exhibit an exponential increase in surface area. In addition, nanoscale features on the material surface provide a means to manipulate mechanical properties such as effective stiffness. For example, scaffold stiffness can be engineered to match that of the target tissue and could help attenuate compliance mismatch, fibrosis and implant failure.
These “smart” surfaces could be incorporated into scaffold design to attenuate inflammatory responses and biomaterial rejection. Recent in vitro models have aided in elucidating the effect of surface nanotopography on inflammatory cell response. Nanopatterned surfaces such as polystyrene nanopillars, metallic glass nanorods and titanium oxide nano-hemispherical structures have been shown to modify adsorption of a variety of serum proteins leading to changes in cell adhesion, morphology and function.14, 39, 73 Substrates with Mac-1 integrin receptors with variable inter-receptor spacings such as 60 nm, 100 nm and 200 nm were found to differentially influence neutrophils.74 Specifically, neutrophils were found to spread the least on substrates with 100 nm inter-receptor spacing, as opposed to substrates with lower or higher inter-receptor spacings. In a separate study, decreased TNF-α production was observed when macrophages were cultured on nanopatterned poly(dimethyl siloxane) (PDMS) substrates for 48 hours.75 Similarly, neurons and inflammatory glial cells in organotypic brain slices expressed reduced levels of genes for cytokines such as TGF-β and TNF-α when exposed to nanogrooved PDMS.76 Moreover, high aspect ratio nanorods have been shown to reduce cell spreading and matrix production by fibroblasts.14, 39 The anti-inflammatory effects of nanopatterned polymers have also been demonstrated in in vivo experiments. Polyacrylamide hydrogels with nanoridges with an average inter-ridge spacing of 600 nm exhibited reduced adhesion of inflammatory macrophages in vivo.77 Polystyrene nanogrooves with an average spacing of 150 nm were shown to decrease inflammatory cytokine production and macrophage cell fusion to form multinucleated giant cells as compared to similar nanogrooves with varied pitch such as 300 nm and 1000 nm in vivo.78 Taken together, these studies indicate that cell type-specific responses to nanopatterned surfaces could be used to control inflammatory and fibrotic cell behavior and promote biomaterial integration.
While the evidence summarized above indicates that nanopatterned surfaces can be used to attenuate inflammation towards tissue engineering constructs, there are some contrarian reports as well. Low aspect ratio polymer nanopillars, tantalum oxide nanodots as well as certain designs of nanopatterned titanium have been shown to be less effective in controlling fibrotic response in vitro.14, 72, 79 It should be noted that cytokine secretion in macrophages induced by nanopatterned surfaces is time-dependent, convoluting the long term applicability of these biophysical anti-inflammatory cues.67 The labyrinth of cues inherent to topography such as size features, stiffness, and material chemistry contribute to cell behavior, further confounding observed cellular response to nanopatterned surfaces. Some evidence exists to suggest that topography overrides material chemistry in dictating cell behavior, but further studies are necessary to disentangle the effect of multiple topographical signals.14, 75 For example, it has been observed that nanotopography increases the contact angle of both polystyrene and polypropylene, regardless of material chemistry.14 Similarly, adhesion and elongation of macrophage cells on nanopatterned PCL, poly(lactic acid) (PLA) and PDMS has been found to be similar, independent of material chemistry.75
Such studies, combined with long term in vivo evaluation of nanopatterned surfaces, will lay the foundation for rational design of nanopattern geometry and size-features to modulate inflammatory responses. Taken together, these studies suggest that nanopatterned substrates consisting of high aspect ratio features (e.g nanopillars with aspect ratio of > 5), and inter-feature spacing of 100-150nm (e.g nanogrooves/ridges spaced 100nm apart) are ideal to attenuate inflammatory responses. Chemical composition of these surfaces does not appear to be a significant contributor to inflammation.
Nanofibers and Nanowires
Ordered structure of native extracellular components in the form of nanofibers has been shown to direct tissue morphogenesis in vivo.80 Biomimetic approaches to recreate nanofibrous architecture, employing electrospinning, hydrothermal treatment of metals and other techniques, have proved efficient in producing bioactive scaffolds for tissue engineering.81, 82 Nanofibers and nanowires make for attractive scaffolds because they emulate matrix features such as surface nanotopography and interconnected nanopores. In addition, the compatibility of electrospinning with a variety of extracellular matrix proteins and other biopolymers permits efficient control of mechanical strength, material chemistry and functionalization. Moreover, these versatile materials can be designed to acquire significant anti-inflammatory attributes.
Firstly, electrospun polymer nanofibers can be loaded or functionalized with a variety of anti-inflammatory drugs such as NSAIDs, curcumin and other therapeutic moieties for site-specific drug delivery.83-85 Secondly, nanofiber morphology, characterized by fiber diameter, pore size and fiber alignment can affect cellular responses. A decrease in fiber diameter has been correlated to reduction of pro-inflammatory cytokine secretion by macrophages in vitro.86 Aligned PCL nanofibers, in contrast to random PCL nanofibers nanofibers have been used to create scaffolds that can promote fibroblast cell elongation, migration and organization, leading to accelerated tissue regeneration in vitro.87 Moreover, an increasing number of studies corroborate the anti-inflammatory effects of nanofiber length and alignment in tissue engineering applications in vivo. Silver nanowires, which were about 120 nm in diameters, were shown to induce minimal pulmonary inflammatory cell infiltration if the fiber length was below 14 μm.88 Nanowires with fiber lengths of 14 um or higher were observed to induce increased lymphocyte and granulocyte infiltration. A similar threshold size of 5 μm was described for silver nanowire-induced inflammation in the pleural cavity.88 Aligned nanofibers synthesized using PCL have been shown to decrease macrophage infiltration and fibrous capsule formation in vivo.89 PLGA/collagen nanofibers have been shown to be more effective than commercially available hydrocolloid dressings in decreasing inflammatory response and accelerating wound healing of full thickness wounds in mice.90
Lastly, choice of material used for producing nanofibers has also been shown to affect inflammatory responses. PCL nanofibers, as opposed to polyglycolide (PGA) and PLA based fibers, exhibit a slower rate of degradation, which prevented the accumulation of acidic byproducts.87 Gelatin or denatured collagen, in contrast to collagen, was shown to elicit a higher degree of inflammatory response in vivo.91 Finally, subcutaneously implanted nanofiber gels made of heparin-binding peptide amphiphiles and heparin sulfate induced minimal macrophage infiltration and multinucleated giant cell formation. 92
In summary, nanofibers and nanowires show great potential for use in tissue engineering. Specifically, aligned fibers/wires with diameters of 600nm or less and aspect ratios below 40, synthesized using slowly degrading polymers such as PCL and loaded with anti-inflammatory drugs for site-specific delivery, constitute optimal nanofibrous scaffolds. Key challenges in the field include identifying the molecular and biophysical mechanisms underlying the effect of nanofiber morphology on inflammatory response. Further research will help explore the influence of nanofiber length, diameter, alignment and scaffold porosity on inflammatory responses in order to facilitate informed design for tissue replacements. Nanofibers can also be functionalized with nanoparticles in order to deliver anti-inflammatory drugs. In addition, nanofibers have found applications in blood vessel tissue engineering, bone replacements and fibrous extracellular matrix substitutes.82, 93
Carbon Nanotubes
Long, cylindrical carbon tubes with nano-sized diameters, known as carbon nanotubes, constitute a versatile class of nanomaterials.22 These materials can be considered as a special form of nanofibers, but their inimitable mechanical properties, specialized applications and wide usage requires a more detailed discussion. Carbon nanotubes are primarily utilized to enhance mechanical integrity and strength of biopolymers, improve electrical conductivity of scaffolds and as drug delivery vehicles.22, 94, 95 Both single nanotubes and multiple concentric nanotubes are used for tissue engineering applications. While carbon nanotubes assist in development of mechanically robust tissue engineering scaffolds, the consequential inflammatory responses, non-biodegradability and nanotube cytotoxicity pose challenges.
Polymers such as poly(propylene fumarate) (PPF) reinforced with single walled carbon nanotubes were observed to induce inflammatory responses to the same extent as pristine PPF based on histological evaluation of explants.96 However, in hard tissue implants, nanotube reinforced polymer scaffolds induced more collagen production and a thicker fibrous capsule in vivo as compared to pristine polymer scaffolds.96 Moreover, single walled carbon nanotubes have been shown to induce a high degree of pulmonary inflammation in mice.97 These nanotubes, when aspirated by mice, caused increased infiltration of neutrophils and macrophages, higher levels of pro-inflammatory cytokines such as TNF-α, IL-1β, TGF-β and led to accelerated occurrence of granulomas and fibrosis. Similarly, carbon nanotubes instilled in the intratracheal region of mice led to pulmonary and systemic inflammation identified by increased neutrophil accumulation in broncheoalveolar lavage, upregulation of cytokines and increase in inflammatory B-cells in the blood.98 There is no clear consensus on whether carbon nanotubes increase or reduce ROS production in inflammatory cells.99, 100 Long carbon nanotubes in the peritoneal cavity in mice have been shown to induce foreign body giant cell formation and granuloma formation, resembling inflammatory responses evoked by highly toxic asbestos fibers.101
Reminiscent of previously discussed nanomaterial design strategies, carbon nanotube length has been implicated in inflammatory reactions. A decrease in nanotube aspect ratio has been shown to attenuate inflammatory responses in the intraperitoneal cavity and pleural space of mice.101, 102 Similarly, the concentration of nanotubes is also critical in scaffold design since cellular responses to these materials have been shown to be dose-dependent.22, 97 Carbon nanotubes at a concentration of 0.6 μg/ml have been demonstrated to affect fibroblast growth and differentiation in vitro.22 The effect of nanotube length or dose might be convoluted due to aggregation of nanotubes in vivo. A threshold size of 10 μm has been established for the fiber-like aggregates to induce granuloma formation.103 Thus, along with a reduced dose and lower aspect ratio nanotubes, strategies to prevent nanotube aggregation will be critical to avoid exacerbated inflammation.
Additionally, carbon nanotubes have been functionalized and loaded with anti-inflammatory drugs such as dexamethasone and ketoprofen for localized delivery in in vitro and in vivo settings.104-106 Functionalization of nanotubes has been exploited in a variety of ways to enhance their performance in vivo. For example, non-functionalized and IgG-functionalized nanotubes were targeted for neutrophil internalization and biodegradation with 30 and 100% efficiency, respectively.107 Ammonium functionalized nanotubes generated using 1,3-dipolar cycloaddion reaction and oxidation/amidation methods displayed decreased cell cytotoxicity as compared to non-functionalized nanotubes, thereby increasing the efficiency and safety of nanotubes as drug delivery vehicles.108 Single-walled carbon nanotubes functionalized with surfactants such as triton or PEG exhibited minimal non-specific binding of proteins, which could be utilized to attenuate inflammatory responses to nanotubes in vivo.109 Moreover, chemical treatments such as nitrogen doping has also been shown to be beneficial in controlling inflammation.110 Furthermore, host animals can also be treated in order to increase nanotube acceptance in vivo. For example, diet-controlled vitamin E deficiency in the host animal has been shown to attenuate inflammatory responses.111 Nanotube biodegradation mediated by host neutrophil myeloperoxidase has also been suggested as an alternative to avoid the unfavorable biological responses to carbon nanotubes.107
To summarize, low aspect ratio nanotubes with aggregate sizes lower than 10 μm at concentrations below 0.6 μg/ml, functionalized with surfactants or inert polymers such as PEG and loaded with anti-inflammatory drugs such as dexamethasone are best-suited to attenuate inflammatory responses.
Conclusion
Nanomaterials can be designed to attenuate inflammatory responses towards tissue engineered constructs. Major design parameters for optimization include size-features of nanomaterials, apparent material stiffness, rate of material biodegradation, and drug release. We have summarized five major categories of nanomaterials used in tissue engineering. Strategies to modulate inflammatory responses elicited by various nanomaterials are summarized in table 1. For each category of nanomaterials, the effect of individual design parameters on inflammatory responses needs to be further examined. This approach might also help identify signals and cues presented by native extracellular matrix.
Table 1. Strategies to modulate inflammatory responses toward nonomaterials.
| Size & Shape Considerations | Chemical Modifications | Drug Loading & Release | |
|---|---|---|---|
| Nanoparticles |
|
||
| Nanoporous Scaffolds | |||
| Nanopatterned Surfaces |
|
______ | |
| Nanofibers & Nanowires | |||
| Carbon Nanotubes |
To optimize nanomaterial size, the threshold size features of nanomaterials for minimal inflammatory responses should be probed, characterized and validated. As noted above, upper threshold fiber length of 14 μm for minimal inflammatory responses has been suggested for silicon nanowires.88 Similarly, polymer coated goal particles, smaller than a threshold size of 20 nm, were found to exacerbate inflammatory responses.41 Such defined range of “anti-inflammatory” nanomaterial morphology will aid design of tissue engineered scaffolds.
Additionally, surface nanoarchitecture can be used as a tool to modulate apparent stiffness of tissue engineered scaffolds to match that of the microenvironment and attenuate inflammatory cell activation and implant-induced fibrosis. In general, delayed biodegradation with less acidic byproducts is considered beneficial for long term implants.87 Moreover, nanomaterial-assisted loading and local delivery of anti-inflammatory drugs can augment tissue-scaffold integration.
Finally, in addition to the materials discussed above, hybrid nanomaterials such as nanoparticle containing electrospun fibers and nanopatterned fibers are also being developed as multifunctional scaffolds. Moreover, cellular responses to nanomaterials can be exploited for a variety of applications in tissue engineering such as enhanced tissue regeneration and scaffold functionality. Exploring the molecular basis for nanomaterial-cell interactions and optimizing scaffold design parameters could lead to efficient, inexpensive, and safe solutions to current challenges in tissue engineering.
Acknowledgments
This work was supported by CRISP (NSF MRSEC DMR 1119826) & NIH Grant (GM-072194).
Footnotes
The authors declare no competing financial interest.
References
- 1.Dvir T, Timko BP, Kohane DS, Langer R. Nanotechnological strategies for engineering complex tissues. Nat Nanotechnol. 2011;6:13–22. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi J, Votruba AR, Farokhzad OC, Langer R. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett. 2010;10:3223–3230. doi: 10.1021/nl102184c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park H, Cannizzaro C, Vunjak-Novakovic G, Langer R, Vacanti CA, Farokhzad OC. Nanofabrication and microfabrication of functional materials for tissue engineering. Tissue Eng. 2007;13:1867–1877. doi: 10.1089/ten.2006.0198. [DOI] [PubMed] [Google Scholar]
- 4.Biswas A, Bayer IS, Biris AS, Wang T, Dervishi E, Faupel F. Advances in top-down and bottom-up surface nanofabrication: techniques, applications & future prospects. Adv Colloid Interface Sci. 2012;170:2–27. doi: 10.1016/j.cis.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Dang JM, Leong KW. Natural polymers for gene delivery and tissue engineering. Adv Drug Deliv Rev. 2006;58:487–499. doi: 10.1016/j.addr.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Kim K, Fisher JP. Nanoparticle technology in bone tissue engineering. Journal of Drug Targeting. 2007;15:241–252. doi: 10.1080/10611860701289818. [DOI] [PubMed] [Google Scholar]
- 7.Kretlow JD, Klouda L, Mikos AG. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:263–273. doi: 10.1016/j.addr.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Armentano I, Dottori M, Fortunati E, Mattioli S, Kenny JM. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polymer Degradation and Stability. 2010;95:2126–2146. [Google Scholar]
- 9.Stevens MM, George JH. Exploring and engineering the cell surface interface. Science. 2005;310:1135–1138. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 10.Ramiro-Gutierrez ML, Will J, Boccaccini AR, Diaz-Cuenca A. Reticulated bioactive scaffolds with improved textural properties for bone tissue engineering: Nanostructured surfaces and porosity. J Biomed Mater Res A. 2013 doi: 10.1002/jbm.a.34968. [DOI] [PubMed] [Google Scholar]
- 11.Jones JR. New trends in bioactive scaffolds: The importance of nanostructure. Journal of the European Ceramic Society. 2009;29:1275–1281. [Google Scholar]
- 12.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 13.Wang SJ, Kowal TJ, Marei MK, Falk MM, Jain H. Nanoporosity Significantly Enhances the Biological Performance of Engineered Glass Tissue Scaffolds. Tissue Engineering Part A. 2013;19:1632–1640. doi: 10.1089/ten.tea.2012.0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kam KR, Walsh LA, Bock SM, Ollerenshaw JD, Ross RF, Desai TA. The effect of nanotopography on modulating protein adsorption and the fibrotic response. Tissue Eng Part A. 2014;20:130–138. doi: 10.1089/ten.tea.2012.0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barkam S, Saraf S, Seal S. Fabricated micro-nano devices for in vivo and in vitro biomedical applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5:544–568. doi: 10.1002/wnan.1236. [DOI] [PubMed] [Google Scholar]
- 16.Kim DH, Kshitiz, Smith RR, Kim P, Ahn EH, Kim HN, Marban E, Suh KY, Levchenko A. Nanopatterned cardiac cell patches promote stem cell niche formation and myocardial regeneration. Integr Biol (Camb) 2012;4:1019–1033. doi: 10.1039/c2ib20067h. [DOI] [PubMed] [Google Scholar]
- 17.Teo BK, Wong ST, Lim CK, Kung TY, Yap CH, Ramagopal Y, Romer LH, Yim EK. Nanotopography modulates mechanotransduction of stem cells and induces differentiation through focal adhesion kinase. ACS Nano. 2013;7:4785–4798. doi: 10.1021/nn304966z. [DOI] [PubMed] [Google Scholar]
- 18.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 19.Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Ramakrishna S. Electrospun poly(epsilon-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials. 2008;29:4532–4539. doi: 10.1016/j.biomaterials.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Holzwarth JM, Ma PX. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials. 2011;32:9622–9629. doi: 10.1016/j.biomaterials.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H, Li X, Zhou G, Fan H, Fan Y. Electrospun sulfated silk fibroin nanofibrous scaffolds for vascular tissue engineering. Biomaterials. 2011;32:3784–3793. doi: 10.1016/j.biomaterials.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Harrison BS, Atala A. Carbon nanotube applications for tissue engineering. Biomaterials. 2007;28:344–353. doi: 10.1016/j.biomaterials.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 23.Kostarelos K, Bianco A, Prato M. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nat Nanotechnol. 2009;4:627–633. doi: 10.1038/nnano.2009.241. [DOI] [PubMed] [Google Scholar]
- 24.Shin SR, Jung SM, Zalabany M, Kim K, Zorlutuna P, Kim SB, Nikkhah M, Khabiry M, Azize M, Kong J, et al. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano. 2013;7:2369–2380. doi: 10.1021/nn305559j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cha C, Shin SR, Annabi N, Dokmeci MR, Khademhosseini A. Carbon-based nanomaterials: multifunctional materials for biomedical engineering. ACS Nano. 2013;7:2891–2897. doi: 10.1021/nn401196a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nandakumar A, Truckenmuller R, Ahmed M, Damanik F, Santos DR, Auffermann N, de Boer J, Habibovic P, van Blitterswijk C, Moroni L. A fast process for imprinting micro and nano patterns on electrospun fiber meshes at physiological temperatures. Small. 2013;9:3405–3409. doi: 10.1002/smll.201300220. [DOI] [PubMed] [Google Scholar]
- 27.Xie Z, Paras CB, Weng H, Punnakitikashem P, Su LC, Vu K, Tang L, Yang J, Nguyen KT. Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomater. 2013;9:9351–9359. doi: 10.1016/j.actbio.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapekar MS. Tissue engineering: challenges and opportunities. J Biomed Mater Res. 2000;53:617–620. doi: 10.1002/1097-4636(2000)53:6<617::aid-jbm1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 30.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8:457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 31.Liang XJ, Chen C, Zhao Y, Jia L, Wang PC. Biopharmaceutics and therapeutic potential of engineered nanomaterials. Curr Drug Metab. 2008;9:697–709. doi: 10.2174/138920008786049230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang LJ, Webster TJ. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today. 2009;4:66–80. [Google Scholar]
- 33.Demento SL, Eisenbarth SC, Foellmer HG, Platt C, Caplan MJ, Mark Saltzman W, Mellman I, Ledizet M, Fikrig E, Flavell RA, et al. Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine. 2009;27:3013–3021. doi: 10.1016/j.vaccine.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malik AF, Hoque R, Ouyang X, Ghani A, Hong E, Khan K, Moore LB, Ng G, Munro F, Flavell RA, et al. Inflammasome components Asc and caspase-1 mediate biomaterial-induced inflammation and foreign body response. Proc Natl Acad Sci U S A. 2011;108:20095–20100. doi: 10.1073/pnas.1105152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan G, Mooney DJ. New materials for tissue engineering: towards greater control over the biological response. Trends Biotechnol. 2008;26:382–392. doi: 10.1016/j.tibtech.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Kirkpatrick CJ, Krump-Konvalinkova V, Unger RE, Bittinger F, Otto M, Peters K. Tissue response and biomaterial integration: the efficacy of in vitro methods. Biomol Eng. 2002;19:211–217. doi: 10.1016/s1389-0344(02)00019-9. [DOI] [PubMed] [Google Scholar]
- 37.Dagtekin G, Schiffer R, Klein B, Jahnen-Dechent W, Zwadlo-Klarwasser G. Modulation of angiogenic functions in human macrophages by biomaterials. Biomaterials. 2003;24:3395–3401. doi: 10.1016/s0142-9612(03)00201-1. [DOI] [PubMed] [Google Scholar]
- 38.Ferraz N, Hong J, Santin M, Karlsson Ott M. Nanoporosity of alumina surfaces induces different patterns of activation in adhering monocytes/macrophages. Int J Biomater. 2010;2010:402715. doi: 10.1155/2010/402715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Padmanabhan J, Kinser ER, Stalter MA, Duncan-Lewis C, Balestrini JL, Sawyer AJ, Schroers J, Kyriakides TR. Engineering Cellular Response Using Nanopatterned Bulk Metallic Glass. ACS Nano. 2014 doi: 10.1021/nn501874q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dailey LA, Jekel N, Fink L, Gessler T, Schmehl T, Wittmar M, Kissel T, Seeger W. Investigation of the proinflammatory potential of biodegradable nanoparticle drug delivery systems in the lung. Toxicol Appl Pharmacol. 2006;215:100–108. doi: 10.1016/j.taap.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 41.Deng ZJ, Liang M, Monteiro M, Toth I, Minchin RF. Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat Nanotechnol. 2011;6:39–44. doi: 10.1038/nnano.2010.250. [DOI] [PubMed] [Google Scholar]
- 42.Muller L, Riediker M, Wick P, Mohr M, Gehr P, Rothen-Rutishauser B. Oxidative stress and inflammation response after nanoparticle exposure: differences between human lung cell monocultures and an advanced three-dimensional model of the human epithelial airways. J R Soc Interface. 2010;7(Suppl 1):S27–40. doi: 10.1098/rsif.2009.0161.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 44.Heng BC, Zhao X, Tan EC, Khamis N, Assodani A, Xiong S, Ruedl C, Ng KW, Loo JS. Evaluation of the cytotoxic and inflammatory potential of differentially shaped zinc oxide nanoparticles. Arch Toxicol. 2011;85:1517–1528. doi: 10.1007/s00204-011-0722-1. [DOI] [PubMed] [Google Scholar]
- 45.Elder A, Yang H, Gwiazda R, Teng X, Thurston S, He H, Oberdorster G. Testing nanomaterials of unknown toxicity: An example based on platinum nanoparticles of different shapes. Advanced Materials. 2007;19:3124–+. [Google Scholar]
- 46.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 47.Yazdi AS, Guarda G, Riteau N, Drexler SK, Tardivel A, Couillin I, Tschopp J. Nanoparticles activate the NLR pyrin domain containing 3 (Nlrp3) inflammasome and cause pulmonary inflammation through release of IL-1alpha and IL-1beta. Proc Natl Acad Sci U S A. 2010;107:19449–19454. doi: 10.1073/pnas.1008155107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parveen S, Misra R, Sahoo SK. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine. 2012;8:147–166. doi: 10.1016/j.nano.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 49.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 50.Kamaly N, Fredman G, Subramanian M, Gadde S, Pesic A, Cheung L, Fayad ZA, Langer R, Tabas I, Farokhzad OC. Development and in vivo efficacy of targeted polymeric inflammation-resolving nanoparticles. Proc Natl Acad Sci U S A. 2013;110:6506–6511. doi: 10.1073/pnas.1303377110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim DH, Martin DC. Sustained release of dexamethasone from hydrophilic matrices using PLGA nanoparticles for neural drug delivery. Biomaterials. 2006;27:3031–3037. doi: 10.1016/j.biomaterials.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 52.Lamprecht A, Ubrich N, Yamamoto H, Schafer U, Takeuchi H, Maincent P, Kawashima Y, Lehr CM. Biodegradable nanoparticles for targeted drug delivery in treatment of inflammatory bowel disease. J Pharmacol Exp Ther. 2001;299:775–781. [PubMed] [Google Scholar]
- 53.Howard KA, Paludan SR, Behlke MA, Besenbacher F, Deleuran B, Kjems J. Chitosan/siRNA nanoparticle-mediated TNF-alpha knockdown in peritoneal macrophages for anti-inflammatory treatment in a murine arthritis model. Mol Ther. 2009;17:162–168. doi: 10.1038/mt.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson DS, Dalmasso G, Wang L, Sitaraman SV, Merlin D, Murthy N. Orally delivered thioketal nanoparticles loaded with TNF-alpha-siRNA target inflammation and inhibit gene expression in the intestines. Nat Mater. 2010;9:923–928. doi: 10.1038/nmat2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peer D, Park EJ, Morishita Y, Carman CV, Shimaoka M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science. 2008;319:627–630. doi: 10.1126/science.1149859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gadde S, Even-Or O, Kamaly N, Hasija A, Gagnon PG, Adusumilli KH, Erakovic A, Pal AK, Zhang XQ, Kolishetti N, et al. Development of Therapeutic Polymeric Nanoparticles for the Resolution of Inflammation. Adv Healthc Mater. 2014 doi: 10.1002/adhm.201300688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.John AE, Lukacs NW, Berlin AA, Palecanda A, Bargatze RF, Stoolman LM, Nagy JO. Discovery of a potent nanoparticle P-selectin antagonist with anti-inflammatory effects in allergic airway disease. FASEB J. 2003;17:2296–2298. doi: 10.1096/fj.03-0166fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kobsa S, Kristofik NJ, Sawyer AJ, Bothwell AL, Kyriakides TR, Saltzman WM. An electrospun scaffold integrating nucleic acid delivery for treatment of full-thickness wounds. Biomaterials. 2013;34:3891–3901. doi: 10.1016/j.biomaterials.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen FM, Zhang M, Wu ZF. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 2010;31:6279–6308. doi: 10.1016/j.biomaterials.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 60.Blank F, Rothen-Rutishauser B, Gehr P. Dendritic cells and macrophages form a transepithelial network against foreign particulate antigens. American Journal of Respiratory Cell and Molecular Biology. 2007;36:669–677. doi: 10.1165/rcmb.2006-0234OC. [DOI] [PubMed] [Google Scholar]
- 61.Abshagen K, Schrodi I, Gerber T, Vollmar B. In vivo analysis of biocompatibility and vascularization of the synthetic bone grafting substitute NanoBone. J Biomed Mater Res A. 2009;91:557–566. doi: 10.1002/jbm.a.32237. [DOI] [PubMed] [Google Scholar]
- 62.Ainslie KM, T RG, Bernards DA, Desai TA. Inflammatory response to implanted nanostructured materials. In: Puleo DA, B R, editors. Biological Interactions on Material Surfaces. New York: Springer; 2009. pp. 355–371. [Google Scholar]
- 63.Pujari S, Hoess A, Shen J, Thormann A, Heilmann A, Tang L, Karlsson-Ott M. Effects of nanoporous alumina on inflammatory cell response. J Biomed Mater Res A. 2013 doi: 10.1002/jbm.a.35048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He HY, Grignol V, Karpa V, Yen C, LaPerle K, Zhang XL, Jones NB, Liang MI, Lesinski GB, Ho WSW, et al. Use of a nanoporous biodegradable miniature device to regulate cytokine release for cancer treatment. Journal of Controlled Release. 2011;151:239–245. doi: 10.1016/j.jconrel.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orosz K, Gupta S, Hassink M, Abdel-Rahman M, Moldovan L, Davidorf FH, Moldovan NI. Delivery of antiangiogenic and antioxidant drugs of ophthalmic interest through a nanoporous inorganic filter. Molecular Vision. 2004;10:555–565. [PubMed] [Google Scholar]
- 66.Gultepe E, Nagesha D, Sridhar S, Amiji M. Nanoporous inorganic membranes or coatings for sustained drug delivery in implantable devices. Adv Drug Deliv Rev. 2010;62:305–315. doi: 10.1016/j.addr.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 67.Del Gaudio P, Auriemma G, Mencherini T, Della Porta G, Reverchon E, Aquino RP. Design of alginate-based aerogel for nonsteroidal anti-inflammatory drugs controlled delivery systems using prilling and supercritical-assisted drying. Journal of Pharmaceutical Sciences. 2013;102:185–194. doi: 10.1002/jps.23361. [DOI] [PubMed] [Google Scholar]
- 68.Karlsson M, Tang L. Surface morphology and adsorbed proteins affect phagocyte responses to nano-porous alumina. J Mater Sci Mater Med. 2006;17:1101–1111. doi: 10.1007/s10856-006-0537-4. [DOI] [PubMed] [Google Scholar]
- 69.La Flamme KE, Popat KC, Leoni L, Markiewicz E, La Tempa TJ, Roman BB, Grimes CA, Desai TA. Biocompatibility of nanoporous alumina membranes for immunoisolation. Biomaterials. 2007;28:2638–2645. doi: 10.1016/j.biomaterials.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoshi RA, Behl S, Ameer GA. Nanoporous Biodegradable Elastomers. Advanced Materials. 2009;21:188–+. [Google Scholar]
- 71.Chen QZ, Liang SL, Thouas GA. Elastomeric biomaterials for tissue engineering. Progress in Polymer Science. 2013;38:584–671. [Google Scholar]
- 72.Mohiuddin M, Pan HA, Hung YC, Huang GS. Control of growth and inflammatory response of macrophages and foam cells with nanotopography. Nanoscale Res Lett. 2012;7:394. doi: 10.1186/1556-276X-7-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rice JM, Hunt JA, Gallagher JA, Hanarp P, Sutherland DS, Gold J. Quantitative assessment of the response of primary derived human osteoblasts and macrophages to a range of nanotopography surfaces in a single culture model in vitro. Biomaterials. 2003;24:4799–4818. doi: 10.1016/s0142-9612(03)00381-8. [DOI] [PubMed] [Google Scholar]
- 74.Kruss S, Erpenbeck L, Amschler K, Mundinger TA, Boehm H, Helms HJ, Friede T, Andrews RK, Schon MP, Spatz JP. Adhesion maturation of neutrophils on nanoscopically presented platelet glycoprotein Ibalpha. ACS Nano. 2013;7:9984–9996. doi: 10.1021/nn403923h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen S, Jones JA, Xu Y, Low HY, Anderson JM, Leong KW. Characterization of topographical effects on macrophage behavior in a foreign body response model. Biomaterials. 2010;31:3479–3491. doi: 10.1016/j.biomaterials.2010.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ereifej ES, Cheng MM, Mao G, VandeVord PJ. Examining the inflammatory response to nanopatterned polydimethylsiloxane using organotypic brain slice methods. J Neurosci Methods. 2013;217:17–25. doi: 10.1016/j.jneumeth.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 77.Takahashi M, Heo YJ, Shibata H, Satou H, Kawanishi T, Okitsu T, Takeuchi S. Nano-Patterned Hydrogel Reduced Inflammatory Effects in Subcutaneous Tissue. 2012 Ieee 25th International Conference on Micro Electro Mechanical Systems (Mems) 2012 [Google Scholar]
- 78.Lamers E, Walboomers XF, Domanski M, Prodanov L, Melis J, Luttge R, Winnubst L, Anderson JM, Gardeniers HJGE, Jansen JA. In vitro and in vivo evaluation of the inflammatory response to nanoscale grooved substrates. Nanomedicine-Nanotechnology Biology and Medicine. 2012;8:308–317. doi: 10.1016/j.nano.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 79.Schwartz-Filho HO, Morandini AC, Ramos-Junior ES, Jimbo R, Santos CF, Marcantonio E, Jr, Wennerberg A, Marcantonio RA. Titanium surfaces with nanotopography modulate cytokine production in cultured human gingival fibroblasts. J Biomed Mater Res A. 2012;100:2629–2636. doi: 10.1002/jbm.a.34200. [DOI] [PubMed] [Google Scholar]
- 80.Patel S, Kurpinski K, Quigley R, Gao H, Hsiao BS, Poo MM, Li S. Bioactive nanofibers: synergistic effects of nanotopography and chemical signaling on cell guidance. Nano Lett. 2007;7:2122–2128. doi: 10.1021/nl071182z. [DOI] [PubMed] [Google Scholar]
- 81.Park EJ, Shim HW, Lee GH, Kim JH, Kim DW. Comparison of toxicity between the different-type TiO2 nanowires in vivo and in vitro. Archives of Toxicology. 2013;87:1219–1230. doi: 10.1007/s00204-013-1019-3. [DOI] [PubMed] [Google Scholar]
- 82.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12:1197–1211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 83.Kenawy ER, Abdel-Hay FI, El-Newehy MH, Wnek GE. Processing of polymer nanofibers through electrospinning as drug delivery systems. Materials Chemistry and Physics. 2009;113:296–302. [Google Scholar]
- 84.Merrell JG, McLaughlin SW, Tie L, Laurencin CT, Chen AF, Nair LS. Curcumin-loaded poly(epsilon-caprolactone) nanofibres: diabetic wound dressing with anti-oxidant and anti-inflammatory properties. Clin Exp Pharmacol Physiol. 2009;36:1149–1156. doi: 10.1111/j.1440-1681.2009.05216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoo HS, Kim TG, Park TG. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv Drug Deliv Rev. 2009;61:1033–1042. doi: 10.1016/j.addr.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 86.Saino E, Focarete ML, Gualandi C, Emanuele E, Cornaglia AI, Imbriani M, Visai L. Effect of electrospun fiber diameter and alignment on macrophage activation and secretion of proinflammatory cytokines and chemokines. Biomacromolecules. 2011;12:1900–1911. doi: 10.1021/bm200248h. [DOI] [PubMed] [Google Scholar]
- 87.Xie JW, MacEwan MR, Ray WZ, Liu WY, Siewe DY, Xia YN. Radially Aligned, Electrospun Nanofibers as Dural Substitutes for Wound Closure and Tissue Regeneration Applications. Acs Nano. 2010;4:5027–5036. doi: 10.1021/nn101554u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schinwald A, Chernova T, Donaldson K. Use of silver nanowires to determine thresholds for fibre length-dependent pulmonary inflammation and inhibition of macrophage migration in vitro. Particle and Fibre Toxicology. 2012;9 doi: 10.1186/1743-8977-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cao HQ, Mchugh K, Chew SY, Anderson JM. The topographical effect of electrospun nanofibrous scaffolds on the in vivo and in vitro foreign body reaction. Journal of Biomedical Materials Research Part A. 2010;93A:1151–1159. doi: 10.1002/jbm.a.32609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu SJ, Kau YC, Chou CY, Chen JK, Wu RC, Yeh WL. Electrospun PLGA/collagen nanofibrous membrane as early-stage wound dressing. Journal of Membrane Science. 2010;355:53–59. [Google Scholar]
- 91.Telemeco TA, Ayres C, Bowlin GL, Wnek GE, Boland ED, Cohen N, Baumgarten CM, Mathews J, Simpson DG. Regulation of cellular infiltration into tissue engineering scaffolds composed of submicron diameter fibrils produced by electrospinning. Acta Biomater. 2005;1:377–385. doi: 10.1016/j.actbio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 92.Ghanaati S, Webber MJ, Unger RE, Orth C, Hulvat JF, Kiehna SE, Barbeck M, Rasic A, Stupp SI, Kirkpatrick CJ. Dynamic in vivo biocompatibility of angiogenic peptide amphiphile nanofibers. Biomaterials. 2009;30:6202–6212. doi: 10.1016/j.biomaterials.2009.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murugan R, Ramakrishna S. Nano-featured scaffolds for tissue engineering: a review of spinning methodologies. Tissue Eng. 2006;12:435–447. doi: 10.1089/ten.2006.12.435. [DOI] [PubMed] [Google Scholar]
- 94.Ramon-Azcon J, Ahadian S, Estili M, Liang X, Ostrovidov S, Kaji H, Shiku H, Ramalingam M, Nakajima K, Sakka Y, et al. Dielectrophoretically aligned carbon nanotubes to control electrical and mechanical properties of hydrogels to fabricate contractile muscle myofibers. Adv Mater. 2013;25:4028–4034. doi: 10.1002/adma.201301300. [DOI] [PubMed] [Google Scholar]
- 95.Hirata E, Uo M, Takita H, Akasaka T, Watari F, Yokoyama A. Multiwalled carbon nanotube-coating of 3D collagen scaffolds for bone tissue engineering. Carbon. 2011;49:3284–3291. [Google Scholar]
- 96.Sitharaman B, Shi XF, Walboomers XF, Liao HB, Cuijpers V, Wilson LJ, Mikos AG, Jansen JA. In vivo biocompatibility of ultra-short single-walled carbon nanotube/biodegradable polymer nanocomposites for, bone tissue engineering. Bone. 2008;43:362–370. doi: 10.1016/j.bone.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 97.Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, Tyurina YY, Gorelik O, Arepalli S, Schwegler-Berry D, et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L698–708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- 98.Park EJ, Cho WS, Jeong J, Yi J, Choi K, Park K. Pro-inflammatory and potential allergic responses resulting from B cell activation in mice treated with multi-walled carbon nanotubes by intratracheal instillation. Toxicology. 2009;259:113–121. doi: 10.1016/j.tox.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 99.Crouzier D, Follot S, Gentilhomme E, Flahaut E, Arnaud R, Dabouis V, Castellarin C, Debouzy JC. Carbon nanotubes induce inflammation but decrease the production of reactive oxygen species in lung. Toxicology. 2010;272:39–45. doi: 10.1016/j.tox.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 100.Murray AR, Kisin E, Leonard SS, Young SH, Kommineni C, Kagan VE, Castranova V, Shvedova AA. Oxidative stress and inflammatory response in dermal toxicity of single-walled carbon nanotubes. Toxicology. 2009;257:161–171. doi: 10.1016/j.tox.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 101.Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WA, Seaton A, Stone V, Brown S, Macnee W, Donaldson K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3:423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 102.Murphy FA, Poland CA, Duffin R, Al-Jamal KT, Ali-Boucetta H, Nunes A, Byrne F, Prina-Mello A, Volkov Y, Li S, et al. Length-dependent retention of carbon nanotubes in the pleural space of mice initiates sustained inflammation and progressive fibrosis on the parietal pleura. Am J Pathol. 2011;178:2587–2600. doi: 10.1016/j.ajpath.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kolosnjaj-Tabi J, Hartman KB, Boudjemaa S, Ananta JS, Morgant G, Szwarc H, Wilson LJ, Moussa F. In vivo behavior of large doses of ultrashort and full-length single-walled carbon nanotubes after oral and intraperitoneal administration to Swiss mice. ACS Nano. 2010;4:1481–1492. doi: 10.1021/nn901573w. [DOI] [PubMed] [Google Scholar]
- 104.Luo XL, Matranga C, Tan SS, Alba N, Cui XYT. Carbon nanotube nanoreservior for controlled release of anti-inflammatory dexamethasone. Biomaterials. 2011;32:6316–6323. doi: 10.1016/j.biomaterials.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sung JY, Barone PW, Kong HJ, Strano MS. Sequential delivery of dexamethasone and VEGF to control local tissue response for carbon nanotube fluorescence based micro-capillary implantable sensors. Biomaterials. 2009;30:622–631. doi: 10.1016/j.biomaterials.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 106.Vashist SK, Zheng D, Pastorin G, Al-Rubeaan K, Luong JHT, Sheu FS. Delivery of drugs and biomolecules using carbon nanotubes. Carbon. 2011;49:4077–4097. [Google Scholar]
- 107.Kagan VE, Konduru NV, Feng WH, Allen BL, Conroy J, Volkov Y, Vlasova II, Belikova NA, Yanamala N, Kapralov A, et al. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nature Nanotechnology. 2010;5:354–359. doi: 10.1038/nnano.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dumortier H, Lacotte S, Pastorin G, Marega R, Wu W, Bonifazi D, Briand JP, Prato M, Muller S, Bianco A. Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano Lett. 2006;6:1522–1528. doi: 10.1021/nl061160x. [DOI] [PubMed] [Google Scholar]
- 109.Shim M, Kam NWS, Chen RJ, Li YM, Dai HJ. Functionalization of carbon nanotubes for biocompatibility and biomolecular recognition. Nano Letters. 2002;2:285–288. [Google Scholar]
- 110.Carrero-Sanchez JC, Elias AL, Mancilla R, Arrellin G, Terrones H, Laclette JP, Terrones M. Biocompatibility and toxicological studies of carbon nanotubes doped with nitrogen. Nano Letters. 2006;6:1609–1616. doi: 10.1021/nl060548p. [DOI] [PubMed] [Google Scholar]
- 111.Shvedova AA, Kisin ER, Murray AR, Gorelik O, Arepalli S, Castranova V, Young SH, Gao F, Tyurina YY, Oury TD, et al. Vitamin E deficiency enhances pulmonary inflammatory response and oxidative stress induced by single-walled carbon nanotubes in C57BL/6 mice. Toxicology and Applied Pharmacology. 2007;221:339–348. doi: 10.1016/j.taap.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further Reading/Resources
- 1.Ilinskaya AN, Dobrovolskaia MA. Immunosuppressive and anti-inflammatory properties of engineered nanomaterials. Br J Pharmacol. 2014 doi: 10.1111/bph.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krpetic Z, Anguissola S, Garry D, Kelly PM, Dawson KA. Nanomaterials: impact on cells and cell organelles. Adv Exp Med Biol. 2014;811:135–156. doi: 10.1007/978-94-017-8739-0_8. [DOI] [PubMed] [Google Scholar]
- 3.Zhao C, Tan A, Pastorin G, Ho HK. Nanomaterial scaffolds for stem cell proliferation and differentiation in tissue engineering. Biotechnol Adv. 2013;31:654–668. doi: 10.1016/j.biotechadv.2012.08.001. [DOI] [PubMed] [Google Scholar]