Abstract
Lymphatic vessels are well known to participate in the immune response by providing the structural and functional support for the delivery of antigens and antigen presenting cells to draining lymph nodes. Recent advances have improved our understanding of how the lymphatic system works and how it participates to the development of immune responses. New findings suggest that the lymphatic system may control the ultimate immune response through a number of ways which include guiding antigen/dendritic cells (DC) entry into initial lymphatics at the periphery; promoting antigen/DC trafficking through afferent lymphatic vessels by actively facilitating lymph and cell movement; enabling antigen presentation in lymph nodes via a network of lymphatic endothelial cells and lymph node stroma cell and finally by direct lymphocytes exit from lymph nodes. The same mechanisms are likely also important to maintain peripheral tolerance. In this review we will discuss how the morphology and gene expression profile of the lymphatic endothelial cells in lymphatic vessels and lymph nodes provides a highly efficient pathway to initiate immune responses. The fundamental understanding of how lymphatic system participates in immune regulation will guide the research on lymphatic function in various diseases.
1. Overview
Lymphatic vessels have three primary roles in normal human biology. The first is to maintain fluid balance. Fluid that leaks from blood vessels in peripheral tissues is transported through lymphatic vessels and returned to the blood circulation. This is important for regulating the amount and the composition of fluids in circulation and within peripheral tissues. The second role is to absorb dietary fats in the intestine and transport them back into the blood stream. The third function is to facilitate the host's immune defenses. Lymphatic vessels are well recognized as the channels through which antigens and immune cells are transported to their draining lymph nodes for immune protection. When infectious microorganisms invade peripheral tissues, lymphatic vessels transport the pathogens, or the antigen presenting cells that had engulfed the pathogens, to the lymph nodes. This initiates adaptive immunity that lead to production of cells and antibodies that will clear the pathogen and generate memory against it.
Antigens and dendritic cells (DCs) reach the draining lymph node through afferent lymphatic vessels; they must then enter the lymph node and migrate deep into it to activate T cells. Lymph nodes are enclosed in a collagen-rich capsule, which is underlined with lymphatic endothelial cells forming the subcapsular sinus. This structure is directly exposed to the incoming lymph. Lymphatic endothelial cells are also concentrated in the medullary area to form the medullary sinus (Figure 1A). Macrophages are closely integrated between lymphatic endothelial cells in both the subcapsular sinus and the medullary sinus to sample antigens and pathogens present in the lymph [1-3]. Notably, the lymph and cells coming from the afferent lymphatics also maintain peripheral immune tolerance in the lymph node, which depends on the DC activation status and the lymph node stromal cell self-antigen expression [4-6]. Thus, lymphatic vessels participate in immune response either directly, by controlling the antigen/DC transport to the draining lymph node or indirectly, by shaping the lymph node microenvironment. Lymphatic system could support immunity through i) antigen/DC entry into lymphatics ii) antigen/DC trafficking through afferent lymphatic vessels; iii) antigen presentation in lymph nodes and iv) lymphocytes exit from lymph nodes. We will discuss the potential roles of lymphatic endothelial cells in controlling the ultimate immune response. We will also discuss the involvement of these cells in shaping peripheral tolerance.
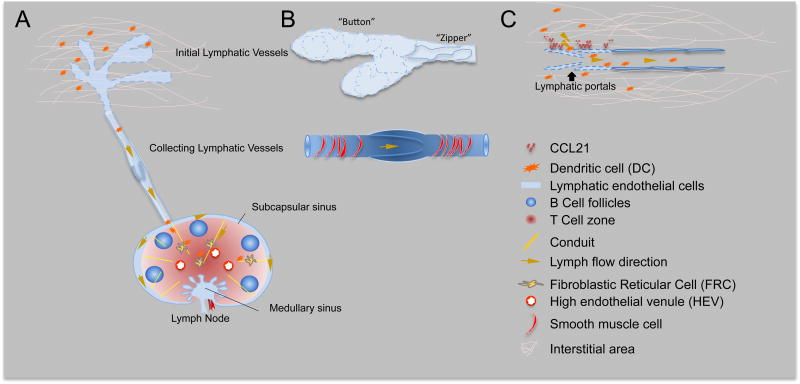
Figure 1. Initial lymphatic vessels, collecting lymphatic vessels and the draining lymph node.
A. Schematic figure of lymphatic system, including initial lymphatic vessels, collecting lymphatic vessel and the draining lymph node. Within the lymph node, fibroblastic reticular cells and high endothelial cells express CCL21 and CCL19 to attract T cells and dendritic cells at the paracortex area of lymph node. The lymph node conduit system, composed of collagen fibers, forms a scaffold to support lymph node architecture and facilitate quick fluid trafficking within the lymph node.
B. Initial lymphatic vessel and collecting lymphatic vessel. The initial lymphatic vessels have oak-leaves shaped cell junctions (Button pattern). Collecting lymphatics have continuous junction molecules and basement membrane and organized smooth muscle cell coverage. Organized smooth muscle coverage in collecting lymphatics allows phasic lymphatic contractions to propel lymph through lymphatic vessels.
C. The button shaped cell junction form lymphatic portals between lymphatic endothelial cells, which allows easy access of fluid and cells. Chemokine CCL21 expressed on lymphatic endothelial cell attract cells to lymphatic vessels and facilitate cell trans-lymphatic endothelial cell migration.
2. Lymphatic transport of antigen and cells to lymph node
2.1 Antigens entry into initial lymphatic vessels
The initial lymphatic vessels are composed of single layer of overlapping, oak leaf-shaped lymphatic endothelial cells expressing the lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1), a typical initial lymphatic endothelial cell marker [7]. Intercellular junction molecules form “button” shaped junctions, with flaps constituting the primary lymphatic valve system (Figure 1B) [8]. Opening of these valves creates a “hole” of approximately 2–3 μm in diameter, which allows fluid and cells to flow through when extracellular fluid pressure is increased. This unique structure provides highly permeable portals that allows quick absorption of extracellular fluid and free access of the molecules and particles less than 1 μm in diameter into the lymphatic vessel lumen (Figure 1C) [4]. Thus, infectious pathogens, such as bacteria and virus particles, could directly enter lymphatic vessels via these portals. The collected lymph is composed of interstitial fluid from the surrounding tissue and contains a pool of self-antigens resulting from homeostatic tissue metabolism and cell turn over [9]. The self-antigens from the lymph may partially activate DCs and these semi-activated DCs play important roles in maintaining peripheral tolerance [10].
2.2 Cell entry into initial lymphatic vessels
DCs are known to be the most potent antigen presenting cells. The peripheral DCs are constantly migrating to the draining lymph node during tissue steady state, carrying self-antigen to maintain peripheral tolerance. They do so by causing self-reactive T cells anergy or clonal depletion [10]. It is estimated that approximately 5% of DCs in lymph nodes are derived from skin during steady state [11]. Upon activation, DCs quickly sample and process foreign antigens, increase expression of co-stimulatory molecules and CCR7 and strikingly accelerate their migration speed towards lymphatic vessels. DC migration through the interstitial area is integrin independent and relies on ameboid movement under chemotaxis of CCL21 [12]. CCL21, the CCR7 ligand, is expressed by lymphatic endothelial cells. CCL21 exhibits cluster pattern on lymphatic endothelial cell and attract DCs migration [13]. Once DCs reach a lymphatic vessel, they seek the endothelial cell portals (Figure 1C), dock on lymphatic by interacting with CCL21 and squeeze through the portal into the lymphatic vessel lumen without any involvement of proteolysis or integrin interaction [12, 14]. The initial lymphatic vessels are critically required for migration of tissue DCs to the draining lymph node, and their absence leads to a deficient induction of immune response or tolerance [15]. However, even a very low density of initial lymphatic vessels is sufficient for DCs to efficiently traffic to the draining lymph node [16].
In addition to DCs, a population of effector-memory T cells also circulates from peripheral tissue to the draining lymph node. While tissue resident memory T cells lack CCR7 expression, the migrating memory T cells express it and enter lymphatic vessels, likely using the same cues as DCs [17, 18]. However, it is not clear how CCR7 expression is induced in the migrating memory T cells. Neutrophils can also enter lymphatic vessels, the mechanism of neutrophil trafficking in lymphatic vessel remains to be clarified [19-21]. Although CCR7 was shown to be involved in neutrophil entry to lymphatic vessels [19], another study claimed that neutrophils rely on macrophage-1 Ag, LFA-1, CXCR4 and sphingosine-1-phosphate receptor 4 for lymphatic trafficking but not on CCR7 [20]. This area of research has gained more attention in the past several years.
2.3 Antigen and cell trafficking through afferent lymphatic vessels
Initial lymphatic vessels merge into pre-collecting and collecting lymphatic vessels while extending to draining lymph node. In collecting lymphatic vessels, junction molecules transit to a continuous “Zipper” pattern, thus dramatically reducing permeability to peripheral fluid (Figure 1B) [8]. Collecting lymphatic vessels gradually loose LYVE-1 expression, gain continuous basement membrane and acquire smooth muscle cell coverage. These morphological changes also contribute to the reduced permeability of collecting lymphatic vessels. It is obvious that this vessel morphology favors transport of lymph and cells rather than material collection from the surroundings However, it is not clear if antigens or cells are able to directly enter collecting lymphatic vessels from peripheral tissue. Collecting lymphatic vessels possess luminal valves, which are strategically distributed to prevent back flow, favoring lymph and cell movement towards lymph nodes (Figure 1B). The vessel sections spanning between two valves is called lymphangion. The lymphangions along the large collecting lymphatic vessels display phasic contractions (lymphatic pumping), which drive lymph transport [22].
Soluble molecules, solid particles and cells traveling through the lymphatic vessels are considered to be passively carried with the lymph to the lymph node sinus. Surprisingly, once they transmigrate into the lymphatic vessel lumen, DCs are found to actively crawl along the initial lymphatic vessel wall. This DC crawling appears to be random and sometimes even opposite to the direction of the lymph [13, 23]. Eventually, DCs detach from the vessel wall, round up and sweep into collecting lymphatic vessels (Figure 1C) [13, 23]. It is not yet clear why DCs linger around the initial lymphatic vessels or how they detach from the lymphatic endothelial cells. One possibility is that while they move deeper into the collecting lymphatic vessels, DCs are flushed away from the vessel wall by the higher lymph flow. It could also be because the CCL21 that attracts DCs docking on the vessel is diluted by the faster lymph flow in the collecting lymphatic vessels. Another speculation could be that collecting lymphatic vessels have different adhesion molecule or chemokine expression profile that favor DCs detachment. DCs may also change their gene expression during their random movement and eventually detach from the lymphatic endothelial wall. However, we observed that even when exposed to higher lymph flow rate driven by the rhythmical lymphatic pumping, some DCs still tether to the lymphatic vessel wall (S. Liao, unpublished observation). Thus, DC intralymphatic migration may be more actively regulated than previously thought and further investigations are needed to have a deeper understanding of this process.
3. Antigen presentation in lymph node
3.1 Migrating DCs
Once soluble molecules, particles and cells reach the lymph node subcapsular sinus from afferent collecting lymphatic vessels, a number of complex steps take place in order to efficiently present antigen and induce adaptive immune responses. First, when entering lymph node subcapsular sinus, the DCs need to transmigrate across the lymphatic endothelial cell layer to reach the lymph node T cell zone. The lymph node lymphatic endothelial cells are known to express CCL1, which binds to CCR8 on DCs [24, 25]. The migration of DCs from the subcapsular sinus to the lymph node paranchyma mainly relies on chemokines CCL21 and CCL19 (another ligand of CCR7) expressed by lymph node fibroblastic reticular cells and high endothelial venule cells (Figure 1A) [26, 27]. The CCL21 and CCL19 gradients are finely regulated in the subcapsular sinus by CCRL1, which has been recently shown to scavenge the two chemokines CCL21/CCL19 in the sinus lumen, thereby shaping the functional chemokine gradient across the subcapsular sinus and guiding DCs entering the lymph nodes [28]. The migrating DCs eventually concentrate in the T-cell zone and survey the circulating naïve T cells. Unlike DCs, when naïve T cells are experimentally injected directly into lymphatic vessels, they preferentially concentrate at the lymph node medullary sinus and further transmigrate through the lymphatic endothelial cell layer into the lymph node parachyma. Similar to DCs, this migration towards the lymph node paracortex depends on CCR7-CCL21/CCL19 chemotaxis [29]. The difference between DC and T cell transmigration in the lymph node sinus may be due to differences in the structure (tightly lined by collagen, lymphatic endothelial cells, macrophages and other innate lymphocytes) of the subcapsular and medullary sinuses. However this area requires further study.
3.2 Small antigens
The free form antigens (soluble factors, molecular weight <70 KDa or diameter <4 nm) from the incoming lymph travel quickly along the lymph node conduit system (Figure 1A). The conduit system extends from the sinus throughout the lymph node, providing a supporting structure and more importantly an efficient route to deliver small antigens deep into lymph node parenchyma (Figure 2) [30]. The conduits consist of collagen bundles, fibrillins and basement membrane components surrounded by fibroblastic reticular cells in the paracortex zone [30, 31]. The population of lymph node resident DCs which are in direct contact with the conduits quickly uptake and process antigens [30, 32]. The conduits also facilitate small molecule delivery into the B cell zone [33] and access of lymph borne factors, such as chemokines, inflammatory cytokines, to the T cell and B cell zone [30, 34].
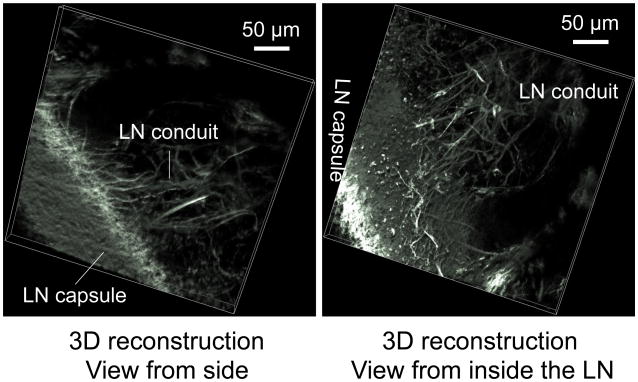
Figure 2. Lymph node conduit system.
Multiphoton microscopy and 3D re-construction images of second harmonic generation, to reveal collagen distribution in lymph node. Collagen forms a dense capsule that surrounds LN and extends into LN to form LN conduit. The 3D side view shows the LN conduits extending from LN capsule. The 3D inside view shows in detail the LN conduits arising from LN capsule.
3.3 Large antigens
The larger antigens or solid particles (molecular weight >70 KDa or diameter >4 nm), including infectious agents such as bacteria and virus particles, are excluded from the conduits. Instead, they flow into the lymph node sinus where they are sampled by the macrophages that bathe in the incoming lymph. Lymph node sinus macrophages (Figure 3) have gained more attention in the past several years because of their unique distribution in lymph nodes and their function of capturing antigens and preventing systemic spread of pathogens [35, 36]. Macrophages are concentrated at the lymphatic endothelial cell area in the lymph node. According to their relative position along the lymph node lymphatic network, they can be divided into two subpopulations, the subcapsular sinus macrophages (SSM) and the medullary sinus macrophages (MSM).
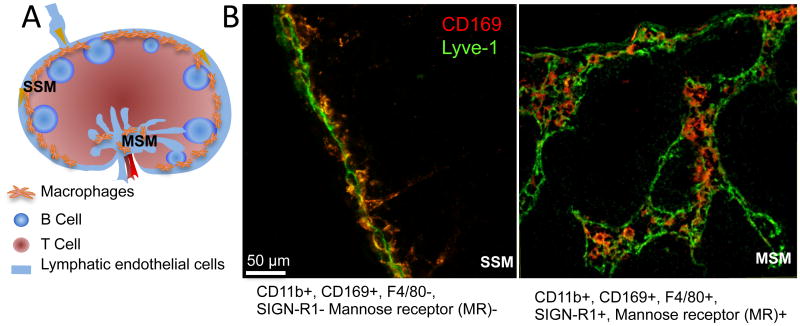
Figure 3. Lymph node macrophages.
A. Schematic illustration of the lymph node macrophages. SSM: subcapsular sinus macrophage; MSM: medullary sinus macrophages.
B. The close interaction between lymphatic endothelial cells and lymph node macrophages illustrated by immune fluorescent staining with Lyve-1 (Green) and CD169 (Red).
While these cells are extremely difficult to distinguish in single cell suspensions, they are relatively easy to be identified in tissue sections after staining with specific markers [2]. Both SSMs and MSMs express CD11b and CD169. While SSMs do not express F4/80, SIGN-R1 or mannose receptor, MSMs are positive for these markers. Given their location in the lymph node sinus, SSMs and MSMs are directly exposed to the incoming lymph and thus are in the front line to capture lymph borne antigens. MSMs are more potent in phagocytosis and express higher endosomal degradation enzymes, while SSMs are more susceptible to virus [36, 37]. SSMs squeeze between subcapsular lymphatic endothelial cells, with their “head” facing the sinus and a “tail” extending into the lymph node cortex. Upon activation, SSMs quickly release prestored IL-18 and activate innate effector lymphoid cells, such as NK cell or γδ T cells to express IFNγ. The quick response of sinus macrophages provides the first layer of immune defense and prevents systemic spread of infectious pathogens [35]. Furthermore, macrophages capture the antigen and shuttle them to B cells in the underlying follicles to initiate adaptive immune response [33, 38, 39].
4. Lymphocyte egress from lymph nodes
After travelling through the lymph node, lymph enters efferent lymphatic vessels, flows through the downstream lymph node(s) and eventually returns to the blood circulation via the subclavian veins. Lymphocytes enter lymph node via high endothelial venule cells and move to T cell or B cell areas. After immune surveillance, the naïve T cells as well as the antigen-activated effector or memory cells also exit the lymph node via efferent lymphatics, returning to the blood circulation and eventually travelling to the inflammatory sites. Immune cell egress from lymph node depends on sphingosine-1-phosphate (S1P) expressed by lymphatic endothelial cells [40]. Lymphocytes recirculate in blood and lymphoid organs. Upon activation, the egress of lymphocytes is transiently blocked to increase naïve lymphocyte retention. The blockade depends on T cell up-regulation of CD69. The egress of cells occurs at the cortex sinus, which is composed of the blind end of lymphatic endothelial cells at the lymph node interfollicular area [41].
5. Lymphatic endothelial cells and peripheral tolerance
In the past several years, accumulating evidences show that lymphatic endothelial cells express peripheral tissue antigens, suggesting that they directly participate in immune regulation [5, 42, 43]. Lymphatic endothelial cells line up the lymph node sinus and are exposed to the incoming lymph. Lymph contains peripheral self-antigens released from tissue homeostatic turn over and metabolism as well as foreign antigens. Lymphatic endothelial cells express MHC class I and II molecules [44-46], which confer them the ability to potentially function as antigen presenting cells. Thanks to this antigen presenting machinery, lymphatic endothelial cells, together with lymph node stroma cells, such as fibroblastic reticular cells, may scavenge antigens and cross present them to self-reactive CD8 T cells, causing clonal depletion [44, 46, 47] rather than T cell activation at steady state [48, 49]. The tolerogenic property of lymphatic endothelial expression and self-antigen presentation is restricted to the lymph node, and depends on LTβR signaling [50]. Similar to semi-activated DCs, lymphatic endothelial cells cause peripheral tolerance by presenting self-antigen with suboptimal co-stimulatory molecules and high level of programmed cell death 1 ligand 1 (PD-L1) [45, 51].
6. Lymphangiogenesis and immune regulation
The growth of lymphatic vessels, termed as lymphangiogenesis, is frequently observed in inflammatory diseases and cancer progression. The expanding lymphatic network presumably provides larger surface area for fluid or cell entry to the lymphatic vessels. However, the role of lymphangiogenesis in regulating the transport of lymph or cells remains unclear.
6.1 Lymphangiogenesis
Lymphangiogenesis occurs in tissue but also in the lymph nodes during inflammation and cancer progression. Factors inducing lymphangiogenesis have been well studied in the past several years. Secreted growth factors, vascular endothelial growth factors (VEGFs) A, C and D can induce lymphatic endothelial cell sprouting and proliferation leading to remodeling of the existing lymphatic vessels [52]. Macrophages contribute largely to tissue and cancer lymphangiogenesis mainly via two pathways: by secreting pro-lymphangiogenic factors and by trans-differentiating into lymphatic endothelial cells [53, 54]. However, the direct trans-differentiation of macrophages into lymphatic endothelial cells requires further study, because it is challenging to determine whether macrophages, which also express the lymphatic endothelial cell marker LYVE-1, are intimately interacting with lymphatic vessels or in fact trans-differentiate into the lymphatic endothelial cells. Using macrophage deficient mouse models, it has been determined that lymphatic progenitor cells are independent of macrophages, further challenging the trans-differentiation hypothesis [55]. Nevertheless, it is well accepted that macrophages contribute to lymphangiogenesis via secretion of growth factors [54, 56-58]. B cells also contribute to lymphangiogenesis by expressing VEGFA, while activated T cells suppress lymphangiogenesis via induction of IFNγ [59, 60].
6.2 Lymphangiogenesis and lymphatic transport
Lymphatic vessels are under active remodeling in diseases. In these conditions, lymphatic drainage of fluid and cells may change if lymphangiogenesis occurs in peripheral tissues or if contractility/drainage is compromised in collecting lymphatics. The functional consequences of lymphangiogenesis occurring in pathological conditions are not clear. During lymphangiogenesis, new lymphatic vessels have larger diameters [61, 62] and display a “Zipper” junction pattern rather than the “button”-like shape characteristic of initial vessels [63]. This structural modification appears to reduce lymphatic vessel permeability to interstitial fluid and materials and is likely to affect fluid and cell access to the lymphatic lumen. However, whether and how this is achieved remains unclear.
Inflammation also alters the contractile function of the lymphatic collecting vessels. Studies in rats, guinea pigs and mice have shown that lymphatic pumping is strongly inhibited during inflammation [62, 64-67]. In these situations, proinflammatory cytokines induce the production and release of vasoactive substances (such as nitric oxide and prostaglandins) from inflammatory cells or lymphatic vessels themselves. These secreted vasoactive substances can inhibit lymphatic muscle contraction and dilate the vessels. Furthermore, cytokines may also increase permeability of lymphatic vessels [68] and allow lymph to leak out hence, compromising lymph drainage.
Presumably, decreasing lymphatic pumping would reduce lymph flow. However, lymph flow is determined by a combination of lymph production and active and passive forces. |The lymph flow velocity and pattern requires further measurement when lymphatic pumping is suppressed during inflammation or cancer. Increased fluid leakage from blood vessels may increase tissue fluid pressure, which forces lymphatic vessels to open up and the fluid to be pushed through without the need of phasic contractions. On the other hand, the lymphatic valves may not efficiently prevent back flow in disease states. Thus, changes of pumping may alter the lymphatic velocity and flow pattern. The overall lymph flow velocity has been shown to be reduced when lymphatic pumping is suppressed in old TNF-Tg mice that serve as a chronic rheumatoid arthritis model [69]. Whether and how these changes affect antigen and cell intralymphatic trafficking during lymphangiogenesis remains to be clarified.
6.3 Lymphatic remodeling and immune regulation
It is getting clearer that the ultimate immune function depends on lymph node microenvironment, which controls the balance of peripheral tolerance and initiation of protective immune defense. Although it is clear that lymphatic vessels undergo dramatic remodeling during inflammation and in cancer metastasis, an understanding of how these changes affect immune function is still lacking. A study showed that complete Freud's adjuvant injection in the mouse footpad increases lymphangiogenesis in draining lymph nodes within 3 days, and increased antigen specific T cell responses [59]. However, CD19 B cells driven overexpression of VEGFA in lymph node reduce the T cell response to ovalbumin or LPS despite artificial increase of lymphatic vessel density in lymph node [70]. On the other hand, during oxazolone-induced lymphangiogenesis, the T cell response is transiently reduced until both tissue and lymph node lymphangiogenesis occurred at day 7 [1, 62]. Lymphatic endothelial cells in tumor draining lymph node may scavenge tumor antigen and cause antigen specific T cell apoptosis [48]. Thus the regulation of lymphangiogenesis and T cell responses may be controlled in a more complex way.
Lymphatic endothelial cells guide each step of the antigen/DCs entry into the lymph node to efficiently initiate adaptive immune responses as discussed above. Thus, interruption of any step of lymphatic trafficking may result in skewed immune responses. Moreover, lymphatics play direct roles in lymph node immune function. Soluble factors and cells draining from the afferent lymph are required to maintain the homeostasis of lymph node architecture. Compromised lymph drainage quickly destroys lymph node's high endothelial venule gene expression profile that is crucial for naïve lymphocytes homing, as well as the lymph node chemokine and cytokine expression. A known consequence of this is the disruption of lymph node cell compartmentalization and macrophage distribution and survival [71-73]. DCs from lymph help to maintain high endothelial venule function and T cell homeostasis via lymphotoxin-β receptor (LTbR) signaling [74, 75]. Thus, lymph borne factors and cells maintain the lymph node microenvironment for initiating adaptive immune responses. Besides attracting DC and T cells via expression of CCL21, lymphatic endothelial cells express chemokine decoy receptor D6, which scavenges inflammatory chemokine CCL2 and CCL5, decreasing their functional level, thus to prevent inappropriate cell adhesion in lymphatic vessels during inflammation [76, 77].
Considering that lymphatic endothelial cells express self-antigen and cause self-reactive T cell tolerance, immune regulation by lymphangiogenesis may be involved in each step of the initiation of T cell response. A fine tuned immune function should generate an effective immune response to foreign antigens, but should not overly damage surrounding tissues. Lymphatic endothelial cells play important roles in suppressing T cell responses in inflammation. Inflamed lymphatic endothelial cells suppress DC maturation, induce TGFβ or indoleamine 2,3-dioxygenase expression and thus further prevent T cell proliferation [78, 79]. The activated T cells release IFNγ and TNFα, inflammatory cytokines, which act upon lymphatic endothelial cells and fibroblastic reticular cells to induce iNOS expression to inhibit further T cell proliferation [80]. With recent technological breakthroughs and our improved understanding of lymphatic functions, the perspective of studying the involvement of lymphatic remodeling during the initiation steps of immune responses and immune regulation seems achievable.
7. Conclusion
Lymphatic vessels serve as the channels sending peripheral antigens and immune cells to the draining lymph nodes to initiate proper immunity. During steady state, lymph nodes maintain peripheral tolerance. Upon activation, lymph nodes quickly initiate protective adaptive immunity to produce antibody, cytotoxic immune cells and memory against the invading foreign antigens. In the past several years, with the improvement of our knowledge on the mechanisms whereby an immune response is initiated during an acute infection or inflammation, we have a better appreciation of the role of the lymphatic system in this process and how it controls each step of the antigen/immune cell migration from the periphery to the draining lymph node, and how it regulates the interaction between antigen presenting cells and T cells. However, important questions such as how lymphangiogenesis and lymphatic pumping affect lymph flow and antigen/antigen presenting cells interaction and migration in lymph node during chronic inflammation and cancer progression still remain to be answered.
Acknowledgments
This work was supported by a University of Calgary start-up funding to SL, provided by the Dianne & Irving Kipnes Foundation and grants from the National Institute of Health (NIH HL096552) and the Canadian Institutes of Health Research to PYvdW.
Footnotes
Conflict of interest: The authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liao S, Ruddle NH. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J Immunol. 2006;177:3369–79. doi: 10.4049/jimmunol.177.5.3369. [DOI] [PubMed] [Google Scholar]
- 2.Gray EE, Cyster JG. Lymph node macrophages. J Innate Immun. 2012;4:424–36. doi: 10.1159/000337007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuka M, Iannacone M. The role of lymph node sinus macrophages in host defense. Ann N Y Acad Sci. 2014 doi: 10.1111/nyas.12387. [DOI] [PubMed] [Google Scholar]
- 4.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nature reviews Immunology. 2005;5:617–28. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 5.Reynoso ED, Lee JW, Turley SJ. Peripheral tolerance induction by lymph node stroma. Advances in experimental medicine and biology. 2009;633:113–27. doi: 10.1007/978-0-387-79311-5_10. [DOI] [PubMed] [Google Scholar]
- 6.Swartz MA, Lund AW. Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat Rev Cancer. 2012;12:210–9. doi: 10.1038/nrc3186. [DOI] [PubMed] [Google Scholar]
- 7.Gale NW, Prevo R, Espinosa J, Ferguson DJ, Dominguez MG, Yancopoulos GD, et al. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Molecular and cellular biology. 2007;27:595–604. doi: 10.1128/MCB.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. The Journal of experimental medicine. 2007;204:2349–62. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clement CC, Rotzschke O, Santambrogio L. The lymph as a pool of self-antigens. Trends Immunol. 2011;32:6–11. doi: 10.1016/j.it.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinman RM, Hawiger D, Liu K, Bonifaz L, Bonnyay D, Mahnke K, et al. Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann N Y Acad Sci. 2003;987:15–25. doi: 10.1111/j.1749-6632.2003.tb06029.x. [DOI] [PubMed] [Google Scholar]
- 11.Tomura M, Yoshida N, Tanaka J, Karasawa S, Miwa Y, Miyawaki A, et al. Monitoring cellular movement in vivo with photoconvertible fluorescence protein “Kaede” transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10871–6. doi: 10.1073/pnas.0802278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–5. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 13.Tal O, Lim HY, Gurevich I, Milo I, Shipony Z, Ng LG, et al. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. The Journal of experimental medicine. 2011;208:2141–53. doi: 10.1084/jem.20102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pflicke H, Sixt M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. The Journal of experimental medicine. 2009;206:2925–35. doi: 10.1084/jem.20091739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas SN, Rutkowski JM, Pasquier M, Kuan EL, Alitalo K, Randolph GJ, et al. Impaired humoral immunity and tolerance in K14-VEGFR-3-Ig mice that lack dermal lymphatic drainage. J Immunol. 2012;189:2181–90. doi: 10.4049/jimmunol.1103545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Platt AM, Rutkowski JM, Martel C, Kuan EL, Ivanov S, Swartz MA, et al. Normal dendritic cell mobilization to lymph nodes under conditions of severe lymphatic hypoplasia. J Immunol. 2013;190:4608–20. doi: 10.4049/jimmunol.1202600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nature immunology. 2005;6:889–94. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nature immunology. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 19.Beauvillain C, Cunin P, Doni A, Scotet M, Jaillon S, Loiry ML, et al. CCR7 is involved in the migration of neutrophils to lymph nodes. Blood. 2011;117:1196–204. doi: 10.1182/blood-2009-11-254490. [DOI] [PubMed] [Google Scholar]
- 20.Gorlino CV, Ranocchia RP, Harman MF, Garcia IA, Crespo MI, Moron G, et al. Neutrophils exhibit differential requirements for homing molecules in their lymphatic and blood trafficking into draining lymph nodes. J Immunol. 2014;193:1966–74. doi: 10.4049/jimmunol.1301791. [DOI] [PubMed] [Google Scholar]
- 21.Abadie V, Badell E, Douillard P, Ensergueix D, Leenen PJ, Tanguy M, et al. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood. 2005;106:1843–50. doi: 10.1182/blood-2005-03-1281. [DOI] [PubMed] [Google Scholar]
- 22.von der Weid PY, Zawieja DC. Lymphatic smooth muscle: the motor unit of lymph drainage. Int J Biochem Cell Biol. 2004;36:1147–53. doi: 10.1016/j.biocel.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Nitschke M, Aebischer D, Abadier M, Haener S, Lucic M, Vigl B, et al. Differential requirement for ROCK in dendritic cell migration within lymphatic capillaries in steady-state and inflammation. Blood. 2012;120:2249–58. doi: 10.1182/blood-2012-03-417923. [DOI] [PubMed] [Google Scholar]
- 24.Qu C, Edwards EW, Tacke F, Angeli V, Llodra J, Sanchez-Schmitz G, et al. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. The Journal of experimental medicine. 2004;200:1231–41. doi: 10.1084/jem.20032152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das S, Sarrou E, Podgrabinska S, Cassella M, Mungamuri SK, Feirt N, et al. Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. The Journal of experimental medicine. 2013;210:1509–28. doi: 10.1084/jem.20111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunn MD, Kyuwa S, Tam C, Kakiuchi T, Matsuzawa A, Williams LT, et al. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. The Journal of experimental medicine. 1999;189:451–60. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annual review of immunology. 2005;23:127–59. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- 28.Ulvmar MH, Werth K, Braun A, Kelay P, Hub E, Eller K, et al. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nature immunology. 2014;15:623–30. doi: 10.1038/ni.2889. [DOI] [PubMed] [Google Scholar]
- 29.Braun A, Worbs T, Moschovakis GL, Halle S, Hoffmann K, Bolter J, et al. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nature immunology. 2011;12:879–87. doi: 10.1038/ni.2085. [DOI] [PubMed] [Google Scholar]
- 30.Sixt M, Kanazawa N, Selg M, Samson T, Roos G, Reinhardt DP, et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Anderson AO, Shaw S. Conduit for privileged communications in the lymph node. Immunity. 2005;22:3–5. doi: 10.1016/j.immuni.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 33.Roozendaal R, Mempel TR, Pitcher LA, Gonzalez SF, Verschoor A, Mebius RE, et al. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity. 2009;30:264–76. doi: 10.1016/j.immuni.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. The Journal of experimental medicine. 2000;192:1425–40. doi: 10.1084/jem.192.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kastenmuller W, Torabi-Parizi P, Subramanian N, Lammermann T, Germain RN. A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell. 2012;150:1235–48. doi: 10.1016/j.cell.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moseman EA, Iannacone M, Bosurgi L, Tonti E, Chevrier N, Tumanov A, et al. B cell maintenance of subcapsular sinus macrophages protects against a fatal viral infection independent of adaptive immunity. Immunity. 2012;36:415–26. doi: 10.1016/j.immuni.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phan TG, Green JA, Gray EE, Xu Y, Cyster JG. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nature immunology. 2009;10:786–93. doi: 10.1038/ni.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez SF, Lukacs-Kornek V, Kuligowski MP, Pitcher LA, Degn SE, Kim YA, et al. Capture of influenza by medullary dendritic cells via SIGN-R1 is essential for humoral immunity in draining lymph nodes. Nature immunology. 2010;11:427–34. doi: 10.1038/ni.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–4. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 40.Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annual review of immunology. 2012;30:69–94. doi: 10.1146/annurev-immunol-020711-075011. [DOI] [PubMed] [Google Scholar]
- 41.Grigorova IL, Panteleev M, Cyster JG. Lymph node cortical sinus organization and relationship to lymphocyte egress dynamics and antigen exposure. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20447–52. doi: 10.1073/pnas.1009968107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Card CM, Yu SS, Swartz MA. Emerging roles of lymphatic endothelium in regulating adaptive immunity. The Journal of clinical investigation. 2014;124:943–52. doi: 10.1172/JCI73316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tewalt EF, Cohen JN, Rouhani SJ, Engelhard VH. Lymphatic endothelial cells - key players in regulation of tolerance and immunity. Frontiers in immunology. 2012;3:305. doi: 10.3389/fimmu.2012.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen JN, Guidi CJ, Tewalt EF, Qiao H, Rouhani SJ, Ruddell A, et al. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. The Journal of experimental medicine. 2010;207:681–8. doi: 10.1084/jem.20092465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tewalt EF, Cohen JN, Rouhani SJ, Guidi CJ, Qiao H, Fahl SP, et al. Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood. 2012;120:4772–82. doi: 10.1182/blood-2012-04-427013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fletcher AL, Lukacs-Kornek V, Reynoso ED, Pinner SE, Bellemare-Pelletier A, Curry MS, et al. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. The Journal of experimental medicine. 2010;207:689–97. doi: 10.1084/jem.20092642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee JW, Epardaud M, Sun J, Becker JE, Cheng AC, Yonekura AR, et al. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nature immunology. 2007;8:181–90. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- 48.Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, Thomas SN, et al. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 2012;1:191–9. doi: 10.1016/j.celrep.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Hirosue S, Vokali E, Raghavan VR, Rincon-Restrepo M, Lund AW, Corthesy-Henrioud P, et al. Steady-State Antigen Scavenging, Cross-Presentation, and CD8+ T Cell Priming: A New Role for Lymphatic Endothelial Cells. J Immunol. 2014;192:5002–11. doi: 10.4049/jimmunol.1302492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen JN, Tewalt EF, Rouhani SJ, Buonomo EL, Bruce AN, Xu X, et al. Tolerogenic properties of lymphatic endothelial cells are controlled by the lymph node microenvironment. PloS one. 2014;9:e87740. doi: 10.1371/journal.pone.0087740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malhotra D, Fletcher AL, Astarita J, Lukacs-Kornek V, Tayalia P, Gonzalez SF, et al. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nature immunology. 2012;13:499–510. doi: 10.1038/ni.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–76. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 53.Kerjaschki D. The crucial role of macrophages in lymphangiogenesis. The Journal of clinical investigation. 2005;115:2316–9. doi: 10.1172/JCI26354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. The Journal of clinical investigation. 2005;115:2363–72. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gordon EJ, Rao S, Pollard JW, Nutt SL, Lang RA, Harvey NL. Macrophages define dermal lymphatic vessel calibre during development by regulating lymphatic endothelial cell proliferation. Development. 2010;137:3899–910. doi: 10.1242/dev.050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. The Journal of clinical investigation. 2005;115:247–57. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jeon BH, Jang C, Han J, Kataru RP, Piao L, Jung K, et al. Profound but dysfunctional lymphangiogenesis via vascular endothelial growth factor ligands from CD11b+ macrophages in advanced ovarian cancer. Cancer Res. 2008;68:1100–9. doi: 10.1158/0008-5472.CAN-07-2572. [DOI] [PubMed] [Google Scholar]
- 58.Kim KE, Koh YJ, Jeon BH, Jang C, Han J, Kataru RP, et al. Role of CD11b+ macrophages in intraperitoneal lipopolysaccharide-induced aberrant lymphangiogenesis and lymphatic function in the diaphragm. Am J Pathol. 2009;175:1733–45. doi: 10.2353/ajpath.2009.090133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M, et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24:203–15. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 60.Kataru RP, Kim H, Jang C, Choi DK, Koh BI, Kim M, et al. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity. 2011;34:96–107. doi: 10.1016/j.immuni.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 61.Hoshida T, Isaka N, Hagendoorn J, di Tomaso E, Chen YL, Pytowski B, et al. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res. 2006;66:8065–75. doi: 10.1158/0008-5472.CAN-06-1392. [DOI] [PubMed] [Google Scholar]
- 62.Liao S, Cheng G, Conner DA, Huang Y, Kucherlapati RS, Munn LL, et al. Impaired lymphatic contraction associated with immunosuppression. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:18784–9. doi: 10.1073/pnas.1116152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yao LC, Baluk P, Srinivasan RS, Oliver G, McDonald DM. Plasticity of button-like junctions in the endothelium of airway lymphatics in development and inflammation. Am J Pathol. 2012;180:2561–75. doi: 10.1016/j.ajpath.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu TF, Carati CJ, Macnaughton WK, von der Weid PY. Contractile activity of lymphatic vessels is altered in the TNBS model of guinea pig ileitis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G566–74. doi: 10.1152/ajpgi.00058.2006. [DOI] [PubMed] [Google Scholar]
- 65.Mathias R, von der Weid PY. Involvement of the NO-cGMP-K(ATP) channel pathway in the mesenteric lymphatic pump dysfunction observed in the guinea pig model of TNBS-induced ileitis. Am J Physiol Gastrointest Liver Physiol. 2013;304:G623–34. doi: 10.1152/ajpgi.00392.2012. [DOI] [PubMed] [Google Scholar]
- 66.Umarova BA, Lelekova TV, Kopylova GN, Goncharova EL, Bakaeva ZV, Samonina GE. The role of protective effects of proline-containing peptides (PGP, PG, and GP) in contractile dysfunction of mesenteric lymphatic vessels in rats with experimental acute peritonitis. Bull Exp Biol Med. 2006;142:279–82. doi: 10.1007/s10517-006-0346-2. [DOI] [PubMed] [Google Scholar]
- 67.Zawieja SD, Wang W, Wu X, Nepiyushchikh ZV, Zawieja DC, Muthuchamy M. Impairments in the intrinsic contractility of mesenteric collecting lymphatics in a rat model of metabolic syndrome. Am J Physiol Heart Circ Physiol. 2012;302:H643–53. doi: 10.1152/ajpheart.00606.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cromer WE, Zawieja SD, Tharakan B, Childs EW, Newell MK, Zawieja DC. The effects of inflammatory cytokines on lymphatic endothelial barrier function. Angiogenesis. 2014;17:395–406. doi: 10.1007/s10456-013-9393-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bouta EM, Wood RW, Brown EB, Rahimi H, Ritchlin CT, Schwarz EM. In vivo quantification of lymph viscosity and pressure in lymphatic vessels and draining lymph nodes of arthritic joints in mice. J Physiol. 2014;592:1213–23. doi: 10.1113/jphysiol.2013.266700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shrestha B, Hashiguchi T, Ito T, Miura N, Takenouchi K, Oyama Y, et al. B cell-derived vascular endothelial growth factor A promotes lymphangiogenesis and high endothelial venule expansion in lymph nodes. J Immunol. 2010;184:4819–26. doi: 10.4049/jimmunol.0903063. [DOI] [PubMed] [Google Scholar]
- 71.Mebius RE, Breve J, Duijvestijn AM, Kraal G. The function of high endothelial venules in mouse lymph nodes stimulated by oxazolone. Immunology. 1990;71:423–7. [PMC free article] [PubMed] [Google Scholar]
- 72.Mebius RE, Streeter PR, Breve J, Duijvestijn AM, Kraal G. The influence of afferent lymphatic vessel interruption on vascular addressin expression. J Cell Biol. 1991;115:85–95. doi: 10.1083/jcb.115.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mebius RE, Dowbenko D, Williams A, Fennie C, Lasky LA, Watson SR. Expression of GlyCAM-1, an endothelial ligand for L-selectin, is affected by afferent lymphatic flow. J Immunol. 1993;151:6769–76. [PubMed] [Google Scholar]
- 74.Moussion C, Girard JP. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature. 2011;479:542–6. doi: 10.1038/nature10540. [DOI] [PubMed] [Google Scholar]
- 75.Wendland M, Willenzon S, Kocks J, Davalos-Misslitz AC, Hammerschmidt SI, Schumann K, et al. Lymph node T cell homeostasis relies on steady state homing of dendritic cells. Immunity. 2011;35:945–57. doi: 10.1016/j.immuni.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 76.McKimmie CS, Singh MD, Hewit K, Lopez-Franco O, Le Brocq M, Rose-John S, et al. An analysis of the function and expression of D6 on lymphatic endothelial cells. Blood. 2013;121:3768–77. doi: 10.1182/blood-2012-04-425314. [DOI] [PubMed] [Google Scholar]
- 77.Lee KM, McKimmie CS, Gilchrist DS, Pallas KJ, Nibbs RJ, Garside P, et al. D6 facilitates cellular migration and fluid flow to lymph nodes by suppressing lymphatic congestion. Blood. 2011;118:6220–9. doi: 10.1182/blood-2011-03-344044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Podgrabinska S, Kamalu O, Mayer L, Shimaoka M, Snoeck H, Randolph GJ, et al. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J Immunol. 2009;183:1767–79. doi: 10.4049/jimmunol.0802167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Norder M, Gutierrez MG, Zicari S, Cervi E, Caruso A, Guzman CA. Lymph node-derived lymphatic endothelial cells express functional costimulatory molecules and impair dendritic cell-induced allogenic T-cell proliferation. FASEB J. 2012;26:2835–46. doi: 10.1096/fj.12-205278. [DOI] [PubMed] [Google Scholar]
- 80.Lukacs-Kornek V, Malhotra D, Fletcher AL, Acton SE, Elpek KG, Tayalia P, et al. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nature immunology. 2011 doi: 10.1038/ni.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]