Abstract
We report a patient with Cushing’s disease (CD) and two pituitary adenomas that demonstrated different imaging characteristics and therefore suggest an alternative imaging strategy for these patients. A 42-year-old woman presented with signs and symptoms of CD. Biochemical evaluation confirmed hypercortisolemia and suggested CD. On pituitary MRI with spoiled gradient recalled acquisition in the steady-state and T1-weighted spin echo protocols, a 5 mm hypoenhancing region typical for a pituitary adenoma was identified on the left. However, after surgical resection the patient remained hypercortisolemic and pathology revealed a nonfunctional adenoma. At early repeat surgical exploration a 10 mm adenoma was found in the right side of the gland. Postoperatively the patient became hypocortisolemic and pathology demonstrated an adrenocorticotropic hormone (ACTH)-staining adenoma. On review of the initial MRI this tumor corresponded to a region of contrast retention best visualized on delayed fluid attenuated inversion recovery (FLAIR) imaging. While the incidentaloma in this case demonstrated classical imaging characteristics of a pituitary adenoma the larger ACTH-secreting tumor was best appreciated by contrast retention. This suggests a role for delayed postcontrast FLAIR imaging in the preoperative evaluation of CD. ACTH-secreting tumors causing CD cause significant morbidity. Due to their small size, a pituitary adenoma is frequently not identified on imaging despite endocrinologic testing suggesting CD. Regardless of improvements in MRI, many tumors are only identified at surgical exploration.
Keywords: Cushing’s disease, Delayed MRI, Fluid attenuated inversion recovery imaging, Pituitary adenoma, Transsphenoidal surgery
1. Introduction
MRI is the gold standard for localizing pituitary adenomas in Cushing’s disease (CD) although imaging fails to identify a tumor in as many as 40% of patients.[1,2] Endocrine diagnostic testing and inferior petrosal sinus sampling (IPSS) can help to confirm a pituitary source, however, these tests often do not improve tumor localization within the pituitary gland for surgical planning.[3] To improve preoperative tumor localization and thereby enhance the success of pituitary surgery, several MRI techniques have been applied to patients with CD, including 3D gradient echo and dynamic MRI.[4,5] These techniques have improved imaging sensitivity for pituitary adenomas in CD, although many tumors remain occult before surgery.
We report a patient with CD and two pituitary adenomas. Although the tumors were imaged simultaneously they demonstrated markedly different imaging. Specifically, delayed postcontrast fluid attenuated inversion recovery (FLAIR) was positive for contrast retention in the otherwise MRI-negative endocrine-active tumor suggesting a role for this imaging sequence in patients with CD.
2. Case Report
A 42-year-old woman was evaluated for signs and symptoms of hypercortisolemia over the previous eighteen months including a 50 pound weight gain, hirsutism, abdominal striae, fatigue, amenorrhea and hypertension. Biochemical evaluation revealed elevated urine free cortisol measurements from 91–122 mcg per 24 hours (reference: 3.5–45.0 mcg/24 hours). Inferior petrosal sinus sampling after corticotropin-releasing hormone administration revealed maximal adrenocorticotropic hormone (ACTH) levels of 1397 pg/mL in the right petrosal vein and 1798 pg/mL in the left petrosal vein compared with 126 pg/mL in the peripheral blood.
2.1. Imaging
Pituitary MRI using spoiled gradient recalled acquisition in the steady-state (SPGR) and T1-weighted spin echo (SE) techniques revealed a 5 mm region of hypoenhancement on the left (Fig. 1a–c). There was also an area of subtle hypoenhancement in the right medial gland on early dynamic postcontrast imaging (Fig. 1a). This region was later visualized on delayed SE and FLAIR sequences with subtle and robust contrast enhancement, respectively (Fig. 1c, d).
Fig. 1.
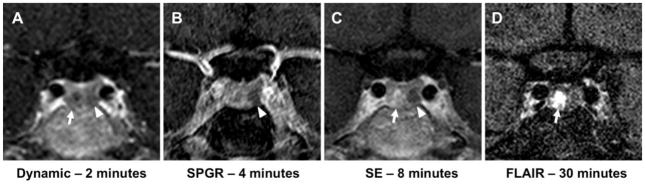
A–D are displayed in the coronal view. A) Dynamic T1-weighted pituitary MRI reveals patchy hypointensity in the left lateral (arrowhead) and right medial pituitary gland (arrow) at 2 minutes after contrast administration. B) On spoiled gradient-recalled acquisition in the steady-state (SPGR), a 3D gradient echo technique, a hypointense area is noted 4 minutes after contrast administration on the left lateral aspect of the gland (arrowhead). C) T1-weighted spin echo (SE) 8 minutes after contrast demonstrates this area of hypointensity corresponding to the non-secreting adenoma more clearly (arrowhead) as well as subtle contrast enhancement in the right medial gland (arrow). D) Delayed fluid attenuated inversion recovery (FLAIR) 30 minutes after contrast demonstrates marked contrast enhancement in the adrenocorticotropic hormone-positive adenoma in the right medial gland (arrow).
2.2. Operation and postoperative course
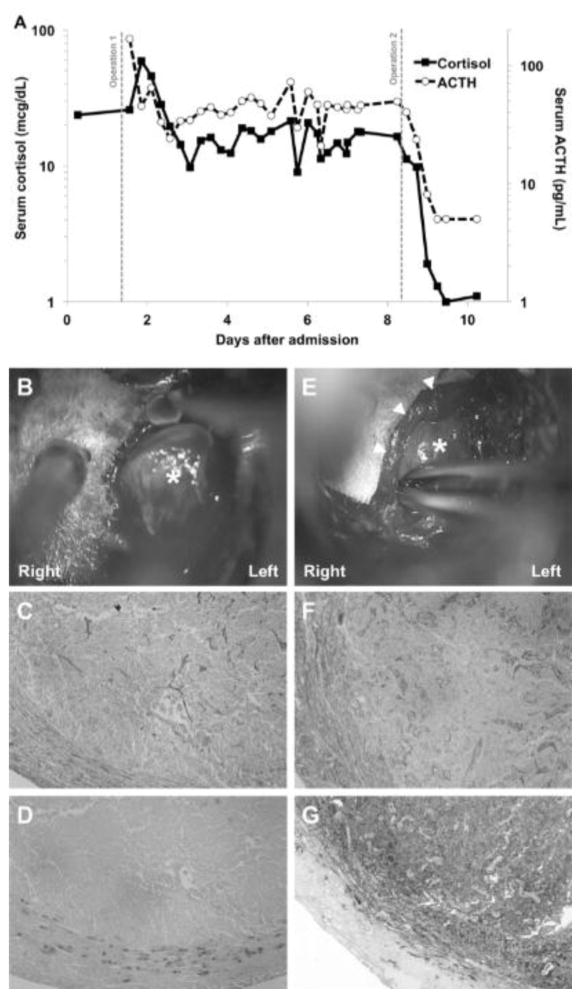
The patient was taken to the operating room for resection of a pituitary adenoma by a microscopic sublabial submucosal transsphenoidal approach. A 6 mm encapsulated adenoma, corresponding to the region of hypointensity on MRI was identified and resected en bloc using pseudocapsular dissection (Fig. 2b). Postoperatively both serum cortisol and ACTH remained elevated (Fig. 2a). Histologic analysis of the resected lesion revealed a tumor by reticulin stain but did not demonstrate ACTH or other pituitary hormone by immunohistochemistry (Fig. 2c, d).
Fig. 2.
A) Measurement of serum cortisol (solid line, left axis) and adrenocorticotropic hormone (ACTH; dashed line, right axis) levels during the admission. Gray vertical lines indicate the relative time points of the two operations. B) At initial surgery an encapsulated 6 mm tumor (*) is resected from the left lateral gland. C–D) This tumor demonstrates loss of typical pituitary acinar cytoarchitecture and a compressed surrounding histologic pseudocapsule on reticulin stain (C) but is negative for ACTH by immunohistochemistry (D). E) At the second operation, a 10 mm tumor (*) is resected from the right medial gland (arrowheads). F–G) This lesion demonstrates similar characteristics of tumor on reticulin stain (F) but is intensely positive for ACTH on immunohistochemistry (G).
Due to the lack of biochemical remission, the patient returned to the operating room for early repeat exploration on postoperative day 7. At surgery, further dissection revealed another tumor in the region of contrast enhancement on delayed FLAIR imaging which was resected en bloc (Fig. 2e). Postoperatively both serum cortisol and ACTH dropped to undetectable levels (Fig. 2a). Histologic analysis once again revealed a tumor by reticulin stain but this tumor was intensely positive for ACTH by immunohistochemistry (Fig. 2f, g).
3. Discussion
3.1. Multiple pituitary adenomas in CD
Multiple sporadic tumors in patients with CD are uncommon and have been identified in only 2% of patients in the largest reported series to date.[6] Most commonly the second tumor is a prolactinoma or a non-secreting tumor.[6–8] Autopsy and imaging studies have identified pituitary adenomas in 8.5 to 27 percent of patients without endocrinopathy.[9–12] Given this frequency of occult adenomas in the general population the incidence of multiple tumors in patients with CD may be underrecognized.
3.2. MRI techniques in CD
Due to their frequently small size at presentation many ACTH-secreting tumors in CD are not identifiable on imaging at the time of diagnosis. Several MRI techniques have been used to improve radiologic identification of pituitary adenomas in these patients. Gadolinium-based contrast agents allow adenomas to be typically visualized as a focal hypointense area on early postcontrast imaging.[4] SPGR imaging (Fig. 1b), a 3D gradient echo technique, allows for greater spatial resolution and greater sensitivity in comparison to traditional postcontrast T1-weighted SE imaging at the expense of slightly decreased specificity (Fig. 1c).[5] The maximum contrast between pituitary and adenoma typically occurs 60–200 seconds after injection of the contrast agent.[4] Dynamic imaging at these early time points can capture this maximal contrast gradient (Fig. 1a). When used for patients with CD, this method has increased sensitivity but reduced specificity in comparison to traditional T1-weighted SE postcontrast imaging.[1]
As pituitary tumors typically take up contrast after normal gland, delayed postcontrast imaging may also identify contrast retention. Given the small size of tumors in CD and the small quantities of contrast that may be retained at late time points, sensitive sequences must be employed to identify such contrast retention. Postcontrast FLAIR imaging has been reported to be particularly sensitive for extra-axial contrast enhancement as seen in leptomeningeal metastases, meningitis and subdural hematomas, and it clearly demonstrated the second adenoma in our patient (Fig. 1d).[13,14] Utility of this sequence in pituitary imaging has not been reported.
In the present case, because the two tumors presented are in a single patient, both tumors received the same contrast dose with the same delay from contrast administration until imaging and the same imaging sequences performed. This case is unique in that it demonstrates two tumors with distinct imaging characteristics despite these same imaging conditions and techniques. Specifically, it demonstrates the potential of delayed postcontrast FLAIR sequences for visualization of contrast retention in a pituitary adenoma. In the current case, the nonfunctioning pituitary tumor demonstrated classic imaging characteristics of a pituitary adenoma (Fig. 1a–c). The larger, pathogenic ACTH-secreting adenoma displayed only subtle hypoenhancement on the early dynamic imaging but was most clearly identified on the delayed postcontrast FLAIR imaging (Fig. 1d).
The biologic basis for the observed difference in contrast enhancement and retention between the tumors is uncertain. The greater contrast retention within the ACTH-secreting adenoma may be related to intrinsic differences between the two tumors including differences in their interstitial spaces or in the pseudocapsule that may result in trapping of contrast within the secreting tumor. Alternatively, the observed differences in imaging may be related to extrinsic factors such as anatomic differences in venous drainage.
4. Conclusions
Based on the different imaging characteristics of the concurrent pituitary adenomas in the present case, delayed postcontrast FLAIR imaging may have utility in identifying delayed contrast retention in pituitary adenomas. Patients with negative MRI but biochemical evidence of CD may benefit from this imaging sequence and further investigation is warranted to examine its utility.
Highlights.
Patients with Cushing’s disease may harbor multiple pituitary tumors.
Concurrent pituitary tumors may display different characteristics on imaging.
Delayed post-contrast FLAIR imaging may identify otherwise imaging-negative tumors.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke at the National Institutes of Health (NIH) and through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc., The Doris Duke Charitable Foundation, The Alexandria Real Estate Equities, Inc. and Mr. and Mrs. Joel S. Marcus, and the Howard Hughes Medical Institute, as well as other private donors.
Footnotes
Conflicts of Interest/Disclosures
The authors declare that they have no financial or other conflicts of interest in relation to this research and its publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tabarin A, Laurent F, Catargi B, Olivier-Puel F, Lescene R, Berge J, et al. Comparative evaluation of conventional and dynamic magnetic resonance imaging of the pituitary gland for the diagnosis of Cushing’s disease. Clin Endocrinol (Oxf) 1998;49:293–300. doi: 10.1046/j.1365-2265.1998.00541.x. [DOI] [PubMed] [Google Scholar]
- 2.Escourolle H, Abecassis JP, Bertagna X, Guilhaume B, Pariente D, Derome P, et al. Comparison of computerized tomography and magnetic resonance imaging for the examination of the pituitary gland in patients with Cushing’s disease. Clin Endocrinol (Oxf) 1993;39:307–13. doi: 10.1111/j.1365-2265.1993.tb02370.x. [DOI] [PubMed] [Google Scholar]
- 3.Wind JJ, Lonser RR, Nieman LK, DeVroom HL, Chang R, Oldfield EH. The lateralization accuracy of inferior petrosal sinus sampling in 501 patients with Cushing’s disease. J Clin Endocrinol Metab. 2013;98:2285–93. doi: 10.1210/jc.2012-3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dwyer AJ, Frank JA, Doppman JL, Oldfield EH, Hickey AM, Cutler GB, et al. Pituitary adenomas in patients with Cushing disease: initial experience with Gd-DTPA-enhanced MR imaging. Radiology. 1987;163:421–6. doi: 10.1148/radiology.163.2.3562821. [DOI] [PubMed] [Google Scholar]
- 5.Patronas N, Bulakbasi N, Stratakis Ca, Lafferty A, Oldfield EH, Doppman J, et al. Spoiled gradient recalled acquisition in the steady state technique is superior to conventional postcontrast spin echo technique for magnetic resonance imaging detection of adrenocorticotropin-secreting pituitary tumors. J Clin Endocrinol Metab. 2003;88:1565–9. doi: 10.1210/jc.2002-021438. [DOI] [PubMed] [Google Scholar]
- 6.Ratliff JK, Oldfield EH. Multiple pituitary adenomas in Cushing’s disease. J Neurosurg. 2000;93:753–61. doi: 10.3171/jns.2000.93.5.0753. [DOI] [PubMed] [Google Scholar]
- 7.McKelvie PA, McNeill P. Double pituitary adenomas: a series of three patients. Pathology. 2002;34:57–60. doi: 10.1080/00313020120105651. [DOI] [PubMed] [Google Scholar]
- 8.Meij BP, Lopes MB, Vance ML, Thorner MO, Laws ER. Double pituitary lesions in three patients with Cushing’s disease. Pituitary. 2000;3:159–68. doi: 10.1023/a:1011499609096. [DOI] [PubMed] [Google Scholar]
- 9.Costello RT. Subclinical Adenoma of the Pituitary Gland. Am J Pathol. 1936;12:205–16.1. [PMC free article] [PubMed] [Google Scholar]
- 10.Burrow GN, Wortzman G, Rewcastle NB, Holgate RC, Kovacs K. Microadenomas of the pituitary and abnormal sellar tomograms in an unselected autopsy series. N Engl J Med. 1981;304:156–8. doi: 10.1056/NEJM198101153040306. [DOI] [PubMed] [Google Scholar]
- 11.Parent AD, Bebin J, Smith RR. Incidental pituitary adenomas. J Neurosurg. 1981;54:228–31. doi: 10.3171/jns.1981.54.2.0228. [DOI] [PubMed] [Google Scholar]
- 12.Hall WA, Luciano MG, Doppman JL, Patronas NJ, Oldfield EH. Pituitary magnetic resonance imaging in normal human volunteers: occult adenomas in the general population. Ann Intern Med. 1994;120:817–20. doi: 10.7326/0003-4819-120-10-199405150-00001. [DOI] [PubMed] [Google Scholar]
- 13.Melhem ER, Bert RJ, Walker RE. Usefulness of optimized gadolinium-enhanced fast fluid-attenuated inversion recovery MR imaging in revealing lesions of the brain. AJR Am J Roentgenol. 1998;171:803–7. doi: 10.2214/ajr.171.3.9725320. [DOI] [PubMed] [Google Scholar]
- 14.Goo HW, Choi C-G. Post-contrast FLAIR MR imaging of the brain in children: normal and abnormal intracranial enhancement. Pediatr Radiol. 2003;33:843–9. doi: 10.1007/s00247-003-1057-8. [DOI] [PubMed] [Google Scholar]