Abstract
T cell activation and differentiation is a complex process that has evolved beyond the two-signal model to a number of varied and opposing inputs that must be interpreted to make a cell fate decision. While stimulation through the TCR, costimulatory, and cytokine receptors is required, metabolic signaling has emerged not only an activation signal, but one that can influence and shape differentiation. Recent findings have revealed unappreciated roles for glucose, fatty acids, and salt in the function of many T cell subsets. In this review, we will highlight the latest advances in the burgeoning field of immunometabolism, focusing on how the menu of T cell fuels has expanded.
Introduction
During his studies of tumor cells, Otto von Warburg hypothesized that a key transformation event in the development of cancer was the ability to shift their metabolism to support their enhanced proliferation[1]. He observed that, even though tumor cells are in a relatively oxygen-rich environment, they preferentially ferment glucose, producing lactate, rather than consume oxygen and undergo respiration. This type of metabolism, aerobic glycolysis or the “Warburg effect”, is a key characteristic of many cancers[2].
During an immune response, T cells can expand 10–100000 fold during their initial expansion, and need fuel and metabolic intermediates to support their proliferation. Thus, upon activation, conventional T cells, participate in aerobic glycolysis, just like cancer cells[3]. While complete glycolysis, fermenting glucose into lactic acid, is a relatively inefficient means of producing ATP, it is considered to be favorable to highly proliferative cells, as it frees up intermediates for building new cellular components (membranes, proteins, and nucleotides), favoring cell division[4]. In stark contrast, naïve and memory T cells must be able to survive for years, in order to support primary and secondary responses, without undergoing any substantial proliferation. During these periods of quiescence, T cells have been shown to primarily use mitochondrial metabolism to support their survival, utilizing fatty acids, amino acids, and glucose to generate ATP through the TCA cycle and oxidative phosphorylation[5].
Thus, T cells must be able to modulate their metabolism in order to switch between these two distinct proliferative modalities. However, the metabolic requirements of T cells are extraordinarily complex and can vary heavily between individual populations and subsets. Recent studies have attempted to dissect the interplay between fuel and function. In this review, we will focus on these recent insights into what kind of fuel T cells use and how nutrients and nutrient sensors can shape the immune response.
Sugar: memory cells may lack a sweet tooth
Glucose is the predominant (and most studied) fuel source used by somatic cells to generate ATP. Initial studies in cancer cell and T cell metabolism focused on the metabolic “switch” of Warburg metabolism: what factors, signaling pathways, or transcriptional programs could induce this shift away from oxidative metabolism. However, it is becoming increasingly clear that the bioenergetic fate of glucose is not the only factor in cellular metabolism, and that Warburg metabolism may not be the ideal mechanism for generating strong, durable immunity.[6] While T cells dynamically regulate these pathways, and upregulate both oxidative phosphorylation and glycolysis during activation, it is the ability for the cells to engage glycolysis that is critical for the translation and secretion of some cytokines, especially interferon gamma (Ifng).[7]
While the incredible proliferation of an expanding T cell is required to generate an army of effector cells, another important goal of that initial activation is the development of potent memory. Like most highly proliferative cells, Warburg metabolism spares cellular biomass from catabolic metabolism to promote T cell expansion. However, it has not been entirely clear how glycolytic programs might help or hinder T cells’ transition into memory phase. A recent study suggests that the shift to glycolytic metabolism is associated with terminal differentiation and less memory progenitor cells during initial antigen encounter, and that enforced glycolysis can inhibit the memory response [8]. Further, inhibition of glycolysis using 2-deoxyglucose during activation can augment memory cell generation, suggesting that aerobic glycolysis is not necessarily a preferred metabolic modality for T cells. Indeed, inhibition of glycolysis can inhibit the activation of mTOR, a critical nutrient sensor important in dictating effector versus memory fates[9]. Supporting the idea that glycolytic machinery might promote effector T cell differentiation, recent data suggest that glyceraldehyde-3-phosphate dehydrogenase (GADPH), an enzyme that has critical activity in glycolysis, also has functions as an RNA-binding protein for Ifng[7]. This study showed that in T cells, GAPDH could bind to the AU-rich elements of the 3′UTR of Ifng mRNA, repressing transcription, revealing a novel way that metabolism can directly modulate transcription in T cells[7]. At some point during expansion, a cell needs to shift back into an oxidative metabolism in order to preserve and carry out cellular functions outside of proliferation. Reliance on a single source of fuel may lead to skewed or abnormal immune response; invariably, catabolic metabolism will be required to generate intermediates, second messengers, and high ATP levels in order to carry out diverse cellular functions.
Thus, the control and timing of glucose uptake could have major impact on the generation of memory during T cell expansion. The glucose transporter Glut1 is dynamically regulated and critical for glucose influx into T cells, at the levels of transcription, post-translational modification, and cellular localization[10, 11]. Indeed, recent studies have shown that, not only is it the dominant glucose transporter in CD4+ conventional T cells, but that it seems to be dispensable for Treg cell function, consistent with previous data suggesting Treg cells do not rely on glycolytic programs[12]. This could potentially be due to the fact that Treg cells, especially those that are highly suppressive, seek to restrain Akt activation in order to maintain their stability and suppressive function[13]. As Akt is important for Glut1 activity and trafficking, this may explain why Treg cells have limited glycolysis[11, 14]. However, while such metabolic programs may promote the generation and maintenance of T regs, data suggests that “effector-like” T regs may indeed employ increased glycolytic programs similar to their T conventional effector counterparts[15–17].
In addition, Akt may not be the only way T cells control glucose uptake. Other studies have expanded on T cell regulation of glucose transport, including GCN2, a target of the indoleamine 2,3-dioxygenase (IDO) pathway, and leptin, an adipokine that plays a central role in systemic metabolism[18, 19]. These two signaling pathways can modulate Glut1 and Glut3 expression in an Akt-independent manner, suggesting that many distinct pathways may be able to modulate the glycolytic machinery. Future studies should seek to determine how and when, during the effector phase of the immune response, T cells limit glucose uptake to prevent unrestrained glycolysis. Some of these effects may be mediated through the adenosine monophosphate activated protein kinase (AMPK). AMPK acts a critical sensor of energy charge; when AMP is high in the cell, AMPK can be activated through LKB1. AMPK suppresses glycolytic and lypolytic programs, and inhibits mTOR activation directly or indirectly through tuberous sclerosis complex (TSC)[20, 21]. Thus, it stands to reason that AMPK would be important for suppressing unrestrained glycolysis and thus promoting T cell memory. Indeed, using a floxed allele of Ampka1, AMPK has been shown to be a critical regulator of T cell memory [22]. Cells deficient in AMPK had a robust effector response, but failed to transition to memory. While the mechanism is still unclear, these studies suggest that, at some crucial timepoint, glucose uptake and glycolysis must be attenuated in order to effectively transition to a long-lived memory phenotype.
Fat: fuel for the slow burn
Lipids represent a bioenergentically rich fuel source for cells; the oxidation of long fatty acid chains generates acetyl-CoA for the TCA cycle, resulting in the generation of large amounts of ATP[23]. However, in heavily proliferative cells, the utilization of this fuel would come at a cost: fats are vital for the generation of cell membranes and second messengers. So, the utilization of lipids could serve as an important fuel source during periods of long-term T cell quiescence, as needed for naïve and memory cells, but would need to be suppressed during T cell expansion[6]. Fatty acids enter cells a number of ways to undergo oxidation. This pathway consists of a transporter, Octn2 (Slc22a5), to take up free fatty acids (FFA), coupled to an activation step that couples FFAs to coenzyme A to form an acyl-CoA, a carnitine shuttle which translocates the fatty acids into the mitochondria through the rate-limiting enzyme carnitine palmitotransferase I (Cpt1a), and the breakdown into acetyl-CoA molecules to feed into the TCA cycle inside the mitochondria[23].
Previous studies have suggested that memory CD8+ T cells utilize enhanced fatty acid oxidation to function[24]. It was assumed that memory cells took up FFA from their environment, generating a large amount of ATP to fuel their basal functions during quiescent states. However, this had not been proved formally and the source of cellular fatty acids remains unclear[25]. Intriguingly, recent work suggests that memory T cells utilize cell-intrinsic fatty acids they themselves synthesized from carbohydrate sources[26]. In other words, cells utilize glucose to generate fatty acids then feed those fatty acids into the beta oxidation cycle to generate ATP. This “futile cycle” seems paradoxical; cells must utilize ATP and reducing equivalents in order to generate fatty acids that they will then break down for ATP. It has been hypothesized that the futile cycle keeps metabolic machinery “primed” during long periods of quiescence, preventing loss of mitochondria. It will be important to determine how exactly this futile cycle is initiated, what purpose it might serve, and how it might be broken? For instance, other studies have shown that some types of CD8+ T cells, especially alloreactive T cells in GVHD, readily take up and oxidize exogenous free fatty acids[27]. Thus, a question remains as to whether this futile cycle metabolism is important for memory cells specifically or is a more common phenomenon in T cell biology. Along these lines, the cellular control and flux of fatty acids has also been shown to be important for CD4+ helper T cells, specifically the balance between suppressive Treg and IL-17-producing TH17 cells[28]. Acetyl-CoA carboxylase 1 (ACC1), a key regulator of fatty acid synthesis, has been shown to both promote TH17 cell generation and inhibit Treg cell generation[29]. TH17 cells seem to generate their own fatty acids (reminiscent of a futile cycle), while Treg cells take them up from their surrounding environment. However, it is unclear from this study whether fatty acids are being oxidized for ATP or simply being utilized as biomass. Thus, the importance of these futile cycles for fatty acid flux in helper T cells remains to be full understood. Importantly, these findings also suggest that other futile cycles may exist for other metabolites.
Choosing from the menu: metabolites dictate T cell fate through nutrient sensing
As T cells begin to undergo activation and differentiation, they require metabolically rich conditions in order to support their proliferation. Thus, it stands to reason that the ability to sense these conditions could provide a crucial survival or differentiation signal. The macrolide rapamycin, while a poor antibiotic, was shown in the 1990s to be a potent immunosuppressant, whose target protein, mTOR, was revealed to be an evolutionarily conserved protein kinase[30, 31]. mTOR was subsequently shown to play a major role in the ability of somatic cells to sense nutrients, including glucose, amino acids, energy charge, and growth factors, and to use these inputs to make cell fate decisions[32]. In T cells, blockade of mTOR with rapamycin, metabolic deprivation, or genetic deletion, results in a tolerogenic state, characterized by T cell anergy[33–35] and regulatory T cell differentiation[36–39]. In addition, it is now appreciated that, for CD8+ T cells, mTOR activation delivers a signal that promotes effector cell generation and function[9]. Alternatively, when mTOR is inhibited with low doses of rapamycin during T cell activation in vivo, T cell memory is dramatically improved, both in quality and quantity[9, 40]
Research in recent years has dissected further the role of mTOR signaling in regulating T cell differentiation and function[41, 42]. Signaling via mTOR can proceed via two protein complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), which are characterized by distinct scaffolding proteins and downstream substrates[43]. Our group and others have demonstrated a selective role for Rheb-mediated mTORC1 activation in promoting Th1 and Th17 differentiation and a role for mTORC2 in promoting Th2 differentiation[44]. Alternatively, the absence of mTOR promotes Treg cell differentiation even under conditions that would normally lead to T cell activation[36]. Interestingly, recent studies using raptor (a critical scaffolding protein of mTORC1)-deficient T cells suggest a role for raptor-mediated mTORC1 activity in controlling all T helper cell differentiation and function[17, 45]. Indeed, raptor-deficient T cells demonstrate defects in Th1, Th2, Th17 and Treg cell function. The floxed allele of Rptor used in each of these studies results in a more profound loss of mTORC1 activity, unlike previous studies utilizing rapamycin or deletion of Rheb. This may indicate that distinct functions of mTORC1 exist. Some mTORC1 activity is necessary for generic cellular functions (translation initiation, escape from quiescence, and some metabolic programming), but that heightened mTORC1 is required for activating selective differentiation programs. Deconvoluting these two roles for mTORC1 will be critical to fully understanding how this critical nutrient sensor controls T cell fate.
To this end, a next critical step toward dissecting the role of mTOR in T cell differentiation and function is to determine the specific mTORC1 and mTORC2 substrates that promote this regulation. One downstream substrate of mTORC2, the serum and glucocorticoid regulated kinase (SGK1)[46, 47] has been shown to play a major role in promoting Th2 differentiation[48]. T cells deficient in SGK1 demonstrate defective Th2 differentiation. Furthermore, such cells tend to secrete Th1 cytokines even when skewed under Th2 conditions. Interestingly, SGK1 has also been implicated in promoting the generation of pathogenic Th17 T cell differentiation through its regulation of the IL-23 receptor[49].. These studies and another further implicated SGK1 in promoting the ability of T cell to respond to salt concentrations.
Other nutrient sensors cross paths with mTOR
Additional work has focused on how biochemical pathways downstream of mTOR sensing intersect with programs that regulate metabolism and T cell function[41]. The transcription factor Myc is activated in response to TCR stimulation and has a wide variety of cellular functions. Myc has been revealed as a critical regulator upstream of glycolysis and glutaminolysis[50]. In its absence, T cells fail to engage glycolysis or glutamine metabolism, proliferate, or acquire an effector phenotype. Glutaminolysis in T cells is required for the synthesis of polyamines, which are important for a wide variety of cellular functions. Furthermore, the orphan receptor estrogen-related receptor alpha (ERRα) also appears to be a sensor and transcriptional regulator required for effector T cell transitions[51]. In its absence, effector T cells cannot proliferate or modulate their metabolic programming, but Treg cells do not require ERRα to function.
Another sensor that has found a new appreciation in T cells is hypoxia-inducible factor 1, or HIF1α. As oxygen is essential for oxidative phosphorylation and other cellular functions, it is not surprising that a sensor for hypoxia would be important in cellular function. Recently, research from several groups have revealed a complex role for HIF1α in T cell differentiation and function, both along the Treg/TH17 cell axis as well as in the differentiation and migration of CD8+ T cells[52–56]. Interestingly, in spite of its name, HIF1a appears to play a critical role in regulating T cell activation and differentiation independent of its ability to sense oxygen. Nonetheless, these studies suggest that a T cell’s ability to sense an oxygen rich environment may also be critical in making cell fate decisions, and that hypoxic environments (such as tumors) may promote the tolerogenic differentiation of Treg cells.
Finally, new energy sources have been revealed in T cell biology. While autophagy has been previously thought to be a process associated with quality control, cellular aging, or even a prelude to cell death, it has become clear recently that autophagy is a vital cellular process that is not only required for some cell functions, but also can be a source of fuel[57]. By undertaking a genetic approach using T cell-specific deletions of Atg7 or Atg5, key genetic regulators of autophagy, two different groups found that, while autophagy is dispensable for effector T cell proliferation and function, T cells lacking autophagy could not transition into the memory pool and subsequently died by apoptosis[58, 59]. Notably, many forms of autophagy are induced upon mTOR inhibition, which has been shown to promote the effector-to-memory T cell transition. Future studies will need to address what exactly occurs during T cell autophagy, and whether or not autophagy is, indeed, a source of fuel for T cells or has some sort of other function in T cell biology.
In summary, metabolism represents critical cellular processes that can have wide-reaching effects on T cell biology, not simply a switch thrown to decide the fate of glucose. As our appreciation of the role of fuel sources for T cell differentiation and function grows, so too must our appreciation of how distinct microenvironments and tissues might modulate nutrient availability. Nutrient sensing is comprised of ancient signaling pathways heavily conserved from yeast to man. These conserved pathways are emerging as the key components of cellular programs which coordinate T cell differentiation and function and metabolic needs. Understanding and dissecting the evolution of these pathways will not only reveal fundamental insight about T cell biology, but likely identify metabolic targets that could be used to modulate the immune response in autoimmunity or cancer.
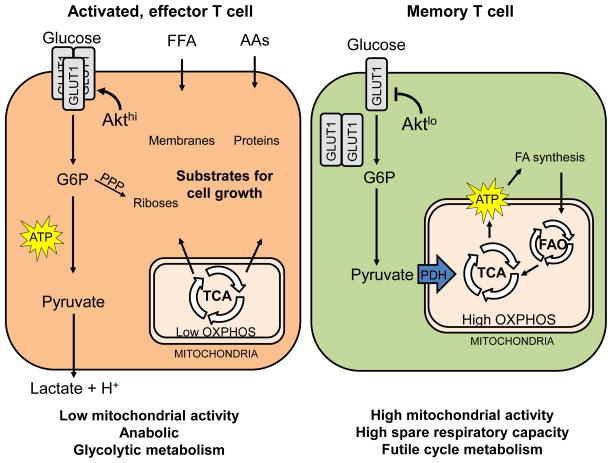
Figure 1. T cells dramatically shift metabolism when in their effector phase.
During T cell expansion, glucose is preferentially fermented into lactic acid, while other metabolites are used to generate intermediates required for cellular growth and proliferation (left). In periods of quiescence, T cells utilize glucose, amino acids, and fatty acids (intrinsic or extrinsic) in order to generate ATP via oxidative phosphorylation (right). While effector cells activate oxidative phosphorylation during T cell activation, aerobic glycolysis is required for optimal effector cell function and cytokine secretion.
HIGHLIGHTS.
T cell can utilize many different sources of fuel
Enforced metabolism or reliance on one pathway can have deleterious effects on T cell function
Nutrient sensing via mTOR represents a key activation and differentiation signal for T cells
Other nutrient sensors also play critical roles in T cell fate, in collaboration with or independent of mTOR
Acknowledgments
This work was supported in part by grants R01AI077610 and R01AI091481 (JDP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Greg M. Delgoffe, Email: delgoffeg@upmc.edu.
Jonathan D. Powell, Email: poweljo@jhmi.edu.
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Kim JW, Dang CV. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66(18):8927–30. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 3.Roos D, Loos JA. Changes in the carbohydrate metabolism of mitogenically stimulated human peripheral lymphocytes. II. Relative importance of glycolysis and oxidative phosphorylation on phytohaemagglutinin stimulation. Exp Cell Res. 1973;77(1):127–35. doi: 10.1016/0014-4827(73)90561-2. [DOI] [PubMed] [Google Scholar]
- 4.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5(11):844–52. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 5.Frauwirth KA, Thompson CB. Regulation of T lymphocyte metabolism. J Immunol. 2004;172(8):4661–5. doi: 10.4049/jimmunol.172.8.4661. [DOI] [PubMed] [Google Scholar]
- 6.Pearce EL, et al. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342(6155):1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7**.Chang CH, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153(6):1239–51. doi: 10.1016/j.cell.2013.05.016. This article demonstrates a secondary role for GAPDH, a key enzyme in glycolysis, as an RNA-binding protein for interferon-gamma, suggesting that metabolism and post-transcriptional control may intersect directly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sukumar M, et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest. 2013;123(10):4479–88. doi: 10.1172/JCI69589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460(7251):108–12. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs SR, et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180(7):4476–86. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell. 2007;18(4):1437–46. doi: 10.1091/mbc.E06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macintyre AN, et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 2014;20(1):61–72. doi: 10.1016/j.cmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delgoffe GM, et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature. 2013;501(7466):252–6. doi: 10.1038/nature12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basu S, Hubbard B, Shevach EM. Foxp3-mediated inhibition of Akt inhibits Glut1 (glucose transporter 1) expression in human T regulatory cells. J Leukoc Biol. 2014 doi: 10.1189/jlb.2AB0514-273RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powell JD, et al. A modified model of T-cell differentiation based on mTOR activity and metabolism. Cold Spring Harb Symp Quant Biol. 2013;78:125–30. doi: 10.1101/sqb.2013.78.020214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smigiel KS, et al. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J Exp Med. 2014;211(1):121–36. doi: 10.1084/jem.20131142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng H, et al. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature. 2013;499(7459):485–90. doi: 10.1038/nature12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eleftheriadis T, et al. Inhibition of indoleamine 2,3-dioxygenase in mixed lymphocyte reaction affects glucose influx and enzymes involved in aerobic glycolysis and glutaminolysis in alloreactive T-cells. Hum Immunol. 2013;74(12):1501–9. doi: 10.1016/j.humimm.2013.08.268. [DOI] [PubMed] [Google Scholar]
- 19.Saucillo DC, et al. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J Immunol. 2014;192(1):136–44. doi: 10.4049/jimmunol.1301158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoki K, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126(5):955–68. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 21.Sofer A, et al. Regulation of mTOR and cell growth in response to energy stress by REDD1. Mol Cell Biol. 2005;25(14):5834–45. doi: 10.1128/MCB.25.14.5834-5845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rolf J, et al. AMPKalpha1: a glucose sensor that controls CD8 T-cell memory. Eur J Immunol. 2013;43(4):889–96. doi: 10.1002/eji.201243008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aon MA, Bhatt N, Cortassa SC. Mitochondrial and cellular mechanisms for managing lipid excess. Front Physiol. 2014;5:282. doi: 10.3389/fphys.2014.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Windt GJ, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36(1):68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearce EL, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460(7251):103–7. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.O’Sullivan D, et al. Memory CD8(+) T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity. 2014;41(1):75–88. doi: 10.1016/j.immuni.2014.06.005. This article reveals a “futile cycle” in metabolic flux used by memory T cells, in which CD8+ cells break down fats that they themselves have synthesized. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byersdorfer CA, et al. Effector T cells require fatty acid metabolism during murine graft-versus-host disease. Blood. 2013;122(18):3230–7. doi: 10.1182/blood-2013-04-495515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagemann S, et al. Nat Med [Google Scholar]
- 29*.Berod L, et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. 2014;20(11):1327–33. doi: 10.1038/nm.3704. This study revealed that the choice and source of metabolic fuel, in this case endogenous versus exogenous fatty acids, can impact cell fate decisions between inflammatory and regulatory fates. [DOI] [PubMed] [Google Scholar]
- 30.Kuo CJ, et al. Rapamycin selectively inhibits interleukin-2 activation of p70 S6 kinase. Nature. 1992;358(6381):70–3. doi: 10.1038/358070a0. [DOI] [PubMed] [Google Scholar]
- 31.Sabatini DM, et al. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78(1):35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 32.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40(2):310–22. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999;162(5):2775–84. [PubMed] [Google Scholar]
- 34.Zheng Y, et al. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J Immunol. 2007;178(4):2163–70. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- 35.Zheng Y, et al. Anergic T cells are metabolically anergic. J Immunol. 2009;183(10):6095–101. doi: 10.4049/jimmunol.0803510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.Delgoffe GM, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30(6):832–44. doi: 10.1016/j.immuni.2009.04.014. This study was the first to identify genetically the role of mTOR in T helper cell differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205(3):565–74. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang J, et al. De novo induction of antigen-specific CD4+CD25+Foxp3+ regulatory T cells in vivo following systemic antigen administration accompanied by blockade of mTOR. J Leukoc Biol. 2008;83(5):1230–9. doi: 10.1189/jlb.1207851. [DOI] [PubMed] [Google Scholar]
- 39.Sauer S, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008;105(22):7797–802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rao RR, et al. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32(1):67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pollizzi KN, Powell JD. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nat Rev Immunol. 2014;14(7):435–46. doi: 10.1038/nri3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Powell JD, et al. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33(3):301–11. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delgoffe GM, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12(4):295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang K, et al. T cell exit from quiescence and differentiation into Th2 cells depend on Raptor-mTORC1-mediated metabolic reprogramming. Immunity. 2013;39(6):1043–56. doi: 10.1016/j.immuni.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem J. 2008;416(3):375–85. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 47.Yan L, Mieulet V, Lamb RF. mTORC2 is the hydrophobic motif kinase for SGK1. Biochem J. 2008;416(3):e19–21. doi: 10.1042/BJ20082202. [DOI] [PubMed] [Google Scholar]
- 48.Heikamp EB, et al. The AGC kinase SGK1 regulates TH1 and TH2 differentiation downstream of the mTORC2 complex. Nat Immunol. 2014;15(5):457–64. doi: 10.1038/ni.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu C, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496(7446):513–7. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang R, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35(6):871–82. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michalek RD, et al. Estrogen-related receptor-alpha is a metabolic regulator of effector T-cell activation and differentiation. Proc Natl Acad Sci U S A. 2011;108(45):18348–53. doi: 10.1073/pnas.1108856108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clambey ET, et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A. 2012;109(41):E2784–93. doi: 10.1073/pnas.1202366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dang EV, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146(5):772–84. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi LZ, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208(7):1367–76. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang H, et al. Negative regulation of Hif1a expression and TH17 differentiation by the hypoxia-regulated microRNA miR-210. Nat Immunol. 2014;15(4):393–401. doi: 10.1038/ni.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu J, et al. Effect of HIF1alpha on Foxp3 expression in CD4+ CD25- T lymphocytes. Microbiol Immunol. 2014;58(7):409–15. doi: 10.1111/1348-0421.12168. [DOI] [PubMed] [Google Scholar]
- 57.Kenific CM, Debnath J. Cellular and metabolic functions for autophagy in cancer cells. Trends Cell Biol. 2014 doi: 10.1016/j.tcb.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58*.Puleston DJ, et al. Autophagy is a critical regulator of memory CD8(+) T cell formation. Elife. 2014:3. doi: 10.7554/eLife.03706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59*.Xu X, et al. Autophagy is essential for effector CD8(+) T cell survival and memory formation. Nat Immunol. 2014;15(12):1152–61. doi: 10.1038/ni.3025. These two articles elegantly demonstrate that autophagy represents a critical process in T cell function, specifically that CD8+ memory cell generation requires an autophagic process. [DOI] [PMC free article] [PubMed] [Google Scholar]