Abstract
Learning through visual exploration often requires orienting of attention to meaningful information in a cluttered world. Previous work has shown that attention modulates visual cortex activity, with enhanced activity for attended targets and suppressed activity for competing inputs, thus enhancing the visual experience. Here we examined the idea that learning may be engaged differentially with variations in attention orienting mechanisms that drive driving eye movements during visual search and exploration. We hypothesized that attention orienting mechanisms that engaged suppression of a previously attended location will boost memory encoding of the currently attended target objects to a greater extent than those that involve target enhancement alone To test this hypothesis we capitalized on the classic spatial cueing task and the inhibition of return (IOR) mechanism (Posner, Rafal, & Choate, 1985; Posner, 1980) to demonstrate that object images encoded in the context of concurrent suppression at a previously attended location were encoded more effectively and remembered better than those encoded without concurrent suppression. Furthermore, fMRI analyses revealed that this memory benefit was driven by attention modulation of visual cortex activity, as increased suppression of the previously attended location in visual cortex during target object encoding predicted better subsequent recognition memory performance. These results suggest that not all attention orienting impacts learning and memory equally.
Keywords: selective attention, recognition memory, distractor suppression, spatial cueing
1. Introduction
Visual exploration involves active scanning of the environment for information gathering. Visual attention during exploration has been traditionally studied as a mechanism that supports resource allocation in a cluttered visual world. We argue that the well-established spatial and temporal dynamics of attention orienting additionally play a critical role in learning and memory during natural visual search and exploration. We present converging eye tracking and neuroimaging data showing that the attention mechanism underlying orienting to a spatial location, and particularly whether suppression of competing information at the previously attended location is engaged, is a determining factor in how well information at the attended location is encoded for subsequent recognition memory.
Evidence of attention/memory interactions at encoding comes from laboratory studies showing enhanced recognition memory for attended versus ignored information (Ballesteros, Reales, Garcia, & Carrasco, 2006), as well as improved encoding when attention is directed to the location of objects prior to their appearance (Broadway, Hilimire, & Corballis, 2011; Hauer & MacLeod, 2006). Neuroimaging studies have identified a distributed network including medial temporal, parietal, and prefrontal regions that are engaged during memory encoding and subsequent retrieval (Brewer, Zhao, Desmond, Glover, & Gabrieli, 1998; Kahn, Davachi, & Wagner, 2004; Kim, 2013; Konishi, Wheeler, Donaldson, & Buckner, 2000; McDermott, Jones, Petersen, Lageman, & Roediger, 2000; Qin, van Marle, Hermans, & Fernandez, 2011; Wagner, Shannon, Kahn, & Buckner, 2005). Parallel research has similarly identified a dorsal attention network that supports attentional selection of relevant stimuli and suppression of distracting or competing information (Corbetta & Shulman, 2002; Squire, Noudoost, Schafer, & Moore, 2013). A recent meta-analysis found that positive subsequent memory effects (i.e., activity elicited during memory encoding that is associated with correct responses at subsequent test) were predominantly associated with involvement of this dorsal attention network, suggesting that top-down attention selection promotes effective encoding (Uncapher & Wagner, 2009).
Growing evidence suggests that modulation of visual cortex activity via the dorsal attention network may mediate the link between attention and enhanced memory encoding (Kim, 2013; Qin et al., 2011; Uncapher & Wagner, 2009). For example, selective attention to specific stimulus features (i.e., color, location) enhanced activity in visual cortical regions dedicated to processing those features, which in turn elicited more effective encoding by medial temporal lobe systems (Uncapher & Rugg, 2009). The authors proposed that selective attention enhanced cortical processing in favor of goal-relevant stimuli, resulting in propagation of higher-fidelity representations to the hippocampus and increased efficacy of memory encoding (Uncapher & Rugg, 2009).
Selective attention involves both this stimulus enhancement and suppression of competing information (Dosher & Lu, 2000; Smith, Singh, & Greenlee, 2000). Thus, activation of dorsal frontoparietal selective attention networks results in enhanced visual cortex activity associated with the attended stimulus (i.e., excitation/enhancement) (Gandhi, Heeger, & Boynton, 1999; Kastner, Pinsk, De Weerd, Desimone, & Ungerleider, 1999) as well as concurrent suppression of the signal associated with information appearing in the surrounding unattended locations (Slotnick, Schwarzbach, & Yantis, 2003; Smith et al., 2000). This distractor suppression can influence the quality of object representations in visual regions such as inferior temporal cortex (IT). Neurophysiological recordings have shown that the neural signal in IT conveys reduced object information in the presence of distractors, relative to when the object was presented in isolation (Zhang et al., 2011). However, when selective attention modulated neural activity, with signal enhancement for the attended object and suppression of the distractors, the object representation was restored to a level as if the object had been presented in isolation (Zhang et al., 2011). The suppression of neural activity elicited by selective attention effectively eliminated the noise introduced by the distractors.
We hypothesize that this suppression of competing interference has benefits that extend beyond object representation in IT: specifically, the presence of distractor suppression will reduce noise in the neural signal of the attended object (Zhang et al., 2011), which will improve memory encoding for the target object. Thus, encoding for subsequent recognition will be improved when target enhancement is paired with distractor suppression. This potential role for suppression in reducing noise and promoting memory encoding may be especially relevant as we execute series of eye movements during visual exploration. With each new eye movement, an attentional trace remains at the previously attended location (Golomb, Pulido, Albrecht, Chun, & Mazer, 2010; Talsma, White, Mathôt, Munoz, & Theeuwes, 2013). Our hypothesis is that suppression of this interference at the previously attended location should enhance the signal at the attended location, in turn benefiting encoding and subsequent recognition memory.
We support this hypothesis with eye tracking and fMRI experiments, all using the spatial cueing task (Posner, 1980). By varying a single timing parameter, the spatial cueing task can be used to compare encoding in the context of attention orienting involving basic target location enhancement versus orienting paired with suppression at the previously attended location. In this task, attention shifts covertly to a peripheral cue, followed by a brief delay and then presentation of a target in either the previously covertly attended ‘cued’ (cued-target trials) or in the ‘noncued’ opposing location (noncued-target trials, Figure 1). The stimulus timing can elicit an orienting bias and enhancement at the cued location (short delay < 250 msec), an effect known as facilitation. Extending the cue-target delay (>250 ms) elicits suppression at the previously attended, cued location, resulting in the well-characterized inhibition of return (IOR) response in which individuals are biased to orient to the opposite, noncued location (Posner & Cohen, 1984; Posner et al., 1985). IOR has long been considered a mechanism relevant for generating non-repetitive sequential eye movements in visual search and exploration, as suppression at previously attended locations promotes orienting to novel locations (Klein & MacInnes, 1999; Klein, 1988, 2000). Previous fMRI studies examining the neural correlates of IOR have found activity in posterior parietal regions, oculomotor regions (e.g., frontal eye fields, supplementary eye fields), middle temporal gyrus, and several frontal regions, including anterior cingulate, medial frontal gyrus, and middle frontal gyrus (Lepsien & Pollmann, 2002; Mayer, Seidenberg, Dorflinger, & Rao, 2004). These regions are consistent with the dorsal attention network that is critical for top-down modulation of visual cortex activity (Corbetta & Shulman, 2002).
Figure 1.
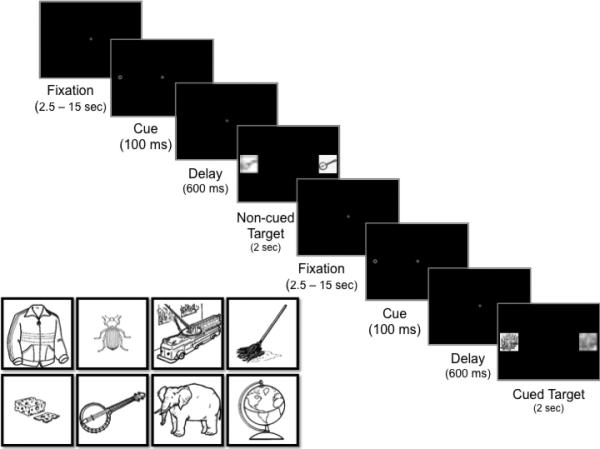
Schematic depiction of the spatial cueing/encoding task and examples of object images used as target stimuli during the task.
In the present study we modified the classic spatial cueing task by placing images of common objects (Figure 1) for encoding in either the cued or opposing locations, without informing participants that they would be performing a subsequent memory test. As such, encoding in this task was entirely incidental. Participants simply oriented to targets images as they appeared either to the right or left of a fixation. The timing of the condition of primary interest was designed to elicit IOR. Thus, critical trials were those in which a distractor appeared in the cued location while the attended target appeared in the noncued location (i.e., noncued target trials), as in this case the target objects were encoded in the context of target enhancement and concurrent suppression at the previously attended, cued location. We additionally conducted a control condition in which the cue-to-target delay was altered to elicit facilitation, rather than suppression, at the cued location. Thus in this case target objects were encoded in the context of target enhancement but without concurrent distractor suppression. Critically, all parameters for the IOR and Facilitation conditions were identical except for the cue-to-target delay length.
This design allowed us to statistically address two questions. First, we examined whether engaging concurrent suppression benefits memory for the attended objects beyond simply biasing attention to a target location alone. To examine this, we compared the noncued target trials (attention bias) from the IOR condition, in which the competing distractor location was suppressed, against Facilitation cued target trials (also attention bias), in which there was target enhancement as a function of the cue but no suppression at the distractor location.
Second, we compared noncued target trials across the IOR and Facilitation conditions to examine whether suppression at a previously attended location benefits memory encoding. This suppression at previously attended locations, characteristic of IOR, contributes to the generation of non-repetitive sequential eye movements during visual search (Klein & MacInnes, 1999; Klein, 1988, 2000). Moreover, previous work has indicated that attention and memory are linked during execution of sequential eye movements. This work demonstrated that visual short-term memory performance was best for the final target in an eye movement sequence, suggesting that there may be differential memory encoding for the final target versus those that were previously attended within the sequence (Gersch, Schnitzer, & Dosher, 2008). By comparing the noncued target trials across the IOR and Facilitation conditions in the present study, we examined whether suppression at a previously attended location might contribute to this enhancement in memory encoding. In the IOR condition participants’ orienting to the noncued target location was coupled with suppression at the previously attended (cued) location. In contrast, participants in the Facilitation condition oriented to the noncued target object location but without sufficient time for the suppression mechanism to engage.
We predicted for both comparisons that concurrent excitation and suppression elicited during the critical noncued target trials of the IOR condition would support more effective encoding and recognition memory compared to encoding of targets appearing in the cued location during the IOR condition, targets encoded in the Facilitation condition, or targets encoded during a no-cue baseline condition. We predicted that there would be no advantage in memory performance for either the cued or noncued targets when encoding occurred in the Facilitation. Furthermore, despite similar overt orienting behaviors, we predicted that encoding of noncued targets in the IOR condition would be associated with better recognition memory for attended objects in the noncued location as well as differential activation in visual cortex reflecting suppression in the cued location, increased activity in visual temporal regions involved in generating and maintaining object representations, and increased functional connectivity between these visual regions and frontal regions typically involved in memory encoding.
2. Material and Methods
2.1 IOR and Facilitation Spatial Cueing Tasks
2.1.1 Participants
Thirty-eight right-handed adults (21 M, 18 F; MAge = 22, SD = 3.1 years, range = 18 – 30 years) participated in a single test session. Participants were recruited from the university community and were compensated for their participation. Prior to enrolling in the study, participants were screened to ensure that they had no previous or current diagnoses of psychiatric or neurological conditions or learning disorders. Based on self-report, 59% of participants were Caucasian, 10.3% were African-American, 5.1% were Hispanic, 23.1% were Asian, and 2.6% were mixed- or unknown race. Four participants’ (2 in each condition) eye tracking data were unavailable due to a recording error. Five additional participants were tested but excluded due to excessive motion artifact (2 in IOR, 3 in Facilitation). Participants’ IQ (MIOR = 123.80, SD = 9.77; MFacilitation = 117.21, SD = 7.22) was assessed using the 2-subtest version of the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999). IQ was not reliably related to any of the reported measures (all ps > .17) and will not be discussed further.
2.1.2 Eye-tracking apparatus
Eye movements were recorded using a remote eye tracker (the SensoMotoric Instruments (SMI) MRI-LR system was used for two-thirds of participants; the SR-Research Eyelink 1000 Plus system was used for the remaining participants). Point-of-gaze was calibrated using a 5-point protocol provided by SMI. Average deviation was 1.78° (SD = 2.0°), suitable for assessing eye movements to the left and right periphery.
2.1.3 Stimuli
Stimuli for the spatial cueing task included a central fixation, peripheral cue, and multiple target stimuli. Targets were line drawings of common objects (Figure 1). All images were drawn from the International Picture Naming Project (Szekely et al., 2004). Each image was presented once on the left and once on the right. A spatial frequency-matched distractor image was created for each target by pixelating the image (cell size = 31) and using a Gaussian blur (radius = 6 pixels) to distort the edges. The distractors always appeared concurrently and opposite the target images. Spatial frequency matching ensured that any observed differences in visual cortex activity could not be attributed to low-level perceptual characteristics of the image. Three sets of 15 object images were used. Two image sets served as targets during the spatial cueing task; one set appeared in the cued location and the second set appeared in the noncued location. The third set of images served as novel objects during the recognition memory task. Set order was counterbalanced across participants.
2.1.4 Procedure
2.1.4.1 Functional visual localizer
Prior to the spatial cueing task, all participants completed a functional visual localizer to identify activations spatiotopically consistent with the left/right target locations in the spatial cueing task. The localizer consisted of a central fixation alternating with a black and white checkerboard presented in the left or right target location every 2 s. Left/right presentation was randomized. Participants were told to look at the central fixation and orient to the checkerboard when it appeared.
2.1.4.2 Spatial Cueing/Encoding Task: Behavioral and Neuroimaging
All task parameters were identical across the IOR condition (N = 20) and Facilitation control condition (N = 19) except for the duration of the delay between cue offset and target onset. Each trial began with presentation of the central fixation (Figure 1). After a variable delay (2.5-15 s), the cue appeared on the left or right for 100 ms, followed by a 67 ms or a 600 ms delay in which only the fixation stimulus was visible. A 67 ms delay between cue offset and target onset elicits a basic orienting response (i.e., facilitation). Although suppression at the cued location (i.e., IOR) may begin at shorter delays (Klein, 2000), a 600 ms delay length reliably elicits IOR and the associated bias in orienting to targets in the noncued location. After this delay the fixation disappeared and a target object appeared in the cued or noncued location (with the distractor concurrently appearing in the opposite location) for 2 s. During cued target trials the target object appeared in the cued location and the distractor appeared in the opposite, noncued location. During noncued target trials the target object appeared in the noncued location and the distractor appeared in the opposite, cued location. In addition to the cued target and noncued target trials, there was a third ‘catch’ trial type in which the fixation, cue, and delay were identical but the target and distractor never appeared. Instead, a blank screen remained for the same duration (2 s) as the targets during cued and noncued target trials. Cued target, noncued target, and catch trials were interleaved and presented in a fixed order; target objects and left/right presentation were randomized across participants. Participants were told to fixate the central stimulus and avoid looking at the cue. They were also asked to orient to the target (the “clear, undistorted image”), to maintain a high level of accuracy (i.e., “make sure you look at the correct image”), and to slow down if they repeatedly looked at the distractor instead of the target. The experimenter monitored participants’ eye movements online during each run and reminded them of the task instructions between runs as necessary. Although the task indexed incidental learning as a function of visual attention orienting mechanisms, we wanted to give participants something to do to stay on task. As such, participants were asked to additionally press a button to indicate if a target was on the left or right. We did not stress speed/accuracy for key presses. Participants were not asked to study the target objects and were never told about the subsequent recognition task during the spatial cueing/encoding phase.
All 45 images were presented during the recognition memory task, including the 15 images that appeared in the noncued location during encoding, the 15 images that appeared in the cued location during encoding, and 15 novel images. Cued, noncued, and new test objects were interleaved and presented in a fixed order; individual test objects were randomized across participants. Participants used a 1 (“definitely old”) to 4 (“definitely new”) scale to indicate if they recognized the target object and rate their confidence. Test objects were visible for 5 s, followed by a variable inter-trial interval (2.5-15 s).
2.1.4.3 Behavioral Data processing
In order to generate the most accurate spatial cueing score per participant, eye movement orienting latencies from individual spatial cueing trials were discarded if the participant looked at the cue prior to target onset, incorrectly looked at the distractor instead of the target, or if orienting data to the target was unavailable. These trials were excluded for analyses of orienting latencies and duration of looking; for fMRI analyses all trials were included except for those in which participants incorrectly looked at the distractor instead of the target. There was no difference across the Facilitation and IOR conditions in the proportion of trials that were excluded (t(33) = 1.10, p = .564). Trials were further filtered to exclude those with saccade latencies that were less than 200 ms or greater than 2 SD above the individual mean. For the recognition memory phase, trials were excluded if participants failed to make a response within 5 s. Recognition memory scores (d’) were generated for each participant based on his/her accuracy for old versus new test objects.
2.2. Behavioral Only Baseline No-Cue Control Task
We ran an additional control task to examine baseline memory performance in the absence of any attention manipulation. Specifically, we conducted a no-cue Baseline task to determine how baseline memory for target objects is affected by our IOR and Facilitation attention orienting manipulations.
2.2.1 Participants
Nineteen adults (9 M, 10 F; MAge = 21.3, SD = 3.2 years, range = 18 – 30 years) participated in a single behavioral test session. Recruitment, screening, and compensation were the same as described previously. Based on self-report, 63.2% were Caucasian, 26.3% were African-American, 5.3% were Asian, and 5.3% were mixed- or unknown race. One additional participant was tested but excluded because she reported explicitly memorizing the target images during encoding.
2.2.2. Procedure
The Baseline experiment was intended to assess participants’ encoding of target images in the absence of spatial cueing. Trials were identical to those in the spatial cueing task except that the cue never appeared. The same images were presented in the Baseline experiment (referred to as Set 1 and Set 2), ensuring that the target images were matched to those presented in the cued and noncued locations in Experiment 1. The recognition memory test for the Baseline experiment included the 30 old objects from Sets 1 and 2 and 15 novel images. As in the spatial cueing task, participants were asked to indicate if the target picture was old or new and to rate their confidence in making their decision.
2.2.2.1 Behavioral data processing
Behavioral data processing was conducted in the same manner described above. Trials were excluded based on missing data, or failure to orient to the target. Trials were further filtered to exclude those with saccade latencies that were less than 200 ms or greater than 2 SD above the individual mean. Recognition memory trials were excluded if participants failed to respond within the 5 s time window.
2.3 fMRI data acquisition
Images were acquired using a 3T Siemens Trio MRI. An initial 3D localizer was collected to position slices, followed by a high-resolution MultiEcho MPRAGE anatomical image (1.0 mm isotropic voxel size, TR = 1900 ms, TI = 900 ms, flip angle = 9°, 160 slices, bandwith = 230 Hz/Px). Echoplanar imaging (EPI) was used to measure BOLD signal during seven functional runs (one for the functional localizer, three for the spatial cueing/encoding phase, and three for the recognition memory phase). EPI images were aligned to the whole brain MPRAGE anatomical image (TR = 2500 ms; TE = 28 ms, flip angle = 90°). Forty-two slices measuring 3 mm thickness and 0 mm gap (64 × 64 in-plane resolution) were collected during each functional run (i.e., 60 repetitions for the functional localizer run, 145 repetitions per spatial cueing/encoding run, 90 repetitions per recognition memory run). One participant in the IOR condition did not finish the last recognition memory run and had fewer than 90 repetitions.
2.3.1 Image processing and analysis
Functional images were processed and analyzed using Analysis of Functional Images (AFNI) software (Cox, 1996). Initial processing included registration to the first image volume (after discarding the first three acquisitions of each run), alignment to the anatomical dataset (MPRAGE), smoothing with an isotropic 6.0 (FWHM) Gaussian kernel, transformation into the standard coordinate Talairach space, and normalization of the time series to percent signal change. General linear model (GLM) analyses fit percent signal change to regressors from each phase of the task and modeled linear and quadratic trends to account for correlated drift. For each subject, separate models were fit for the localizer, encoding, and recognition memory phases of the task. All models included six motion regressors computed during preprocessing (i.e., x, y, z, roll, pitch, and yaw motion dimensions). For the localizer model, regressors of interest included stimulus (i.e., checkerboard) and fixation trial types. For the encoding phase, regressors of interest included cued target, noncued target, and catch trial types, and for the recognition memory phase regressors of interest included cued, noncued, and new test conditions. Beta coefficients derived from the individual-level localizer models were then submitted to group level analyses using ANOVA in AFNI (see below).
2.3.2 Localizer for Visual cortex activity
We used a pre-task localizer to identify the cortical regions topographically corresponding to our target and distractor locations (see 2.1.4.1). For this functional localizer the ANOVA tested for a main effect of Trial type (stimulus vs. fixation). Correction for multiple comparisons was applied at the cluster level based on Monte Carlo simulations using AlphaSim in AFNI. These simulations compute the probability of determining a false positive using individual voxel probability threshold in combination with a cluster size threshold. AlphaSim was conducted at the whole-brain level at 6 mm FWHM, 10,000 simulations, and individual voxel threshold of 0.0001. This simulation revealed that a cluster with a minimum of 8 contiguous voxels was necessary to correct for false positives to p < .05.
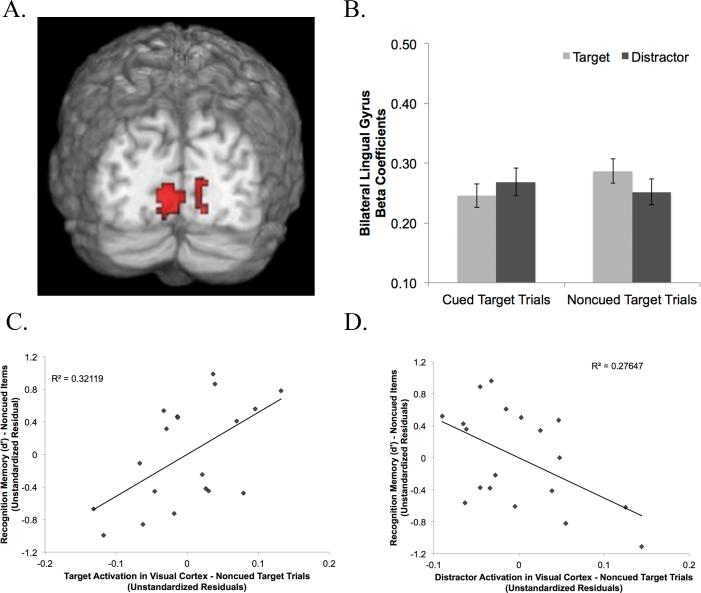
We next examined spatial cueing task-related activity in the localizer-identified visual cortex ROIs corresponding to target and distractor locations (left and right lingual gyrus, Figure 2A). Beta coefficients from the encoding phase of the task were extracted from these ROIs per subject per trial type. This resulted in beta values during spatial cueing for cued targets and the corresponding distractor locations as well as noncued targets and the corresponding distractor locations. We were further interested in isolating activity at target/distractor presentation from previous visual activations resulting from the recently presented cues. During spatial cueing catch trials the cue appeared followed by a blank screen, without any target or distractor stimuli. The beta coefficients for catch trials reflect activity associated with appearance of the cue, independent of activity associated with appearance of the target and distractor stimuli. As such, we removed cue-related activity from cued and noncued target trials by subtracting the beta coefficients for catch trials from the beta coefficients for these trial types. These computations were conducted separately for the left and right visual cortex ROIs to isolate beta coefficients for stimuli that were localized to the left and right visual cortex (i.e., targets & distractors) across cued target and noncued target trials.
Figure 2.
Differential target versus distractor activation in visual cortex during encoding. (A) Bilateral lingual gyrus (visual cortex) ROI identified by the localizer to be topographically consistent with target and distractor locations during the spatial cueing task; (B) interaction between target and distractor activation during cued and noncued target trials in the IOR condition; (C) & (D) partial regression plots reflecting the relationship between recognition memory performance and (C) target-based activity in visual cortex and (D) distractor-based activity in visual cortex during noncued-target trials in the IOR condition.
2.3.3 Spatial Cueing/Encoding: Whole-brain analyses
Beta coefficients were entered into a group-level ANOVA with Condition (Facilitation, IOR) as a between-subjects factor, Trial type (cued-target, noncued-target, catch) as a within-subjects factor, and subject as a random factor. The first contrast examined activity related to encoding of targets appearing in the location of the expected attention bias. This analysis focused on activations associated with the cued target trials in the Facilitation condition compared to noncued target trials in the IOR condition. The final contrast examined activity related to encoding of targets when the previously attended (cued) location was suppressed versus when it was not. This analysis focused on activations associated with noncued target trials in the IOR condition (cued location suppressed) compared to noncued target trials in the Facilitation condition (no suppression at the cued location). As before, correction for multiple comparisons was applied at the cluster level based on Monte Carlo simulations conducted using AlphaSim (using 6 mm FWHM, 10,000 simulations, and individual voxel threshold of 0.025. This simulation revealed that a minimum of 77 contiguous voxels was necessary to correct false positives to p < .05.
2.3.4 Spatial Cueing/Encoding: Functional connectivity
We conducted a psychophysiological interaction (PPI) analysis (Friston, et al., 1997) to identify regions in which activity during encoding correlated with visual cortex activity in a task-dependent manner. We conducted this analysis twice, using two different seed regions, 1) the localized visual cortex ROIs, collapsed across left and right hemispheres, and 2) the inferior temporal (IT) cortex ROI identified by the whole-brain analysis of activations during encoding (see 3.2.2). The same procedure was used for both seeds. GLM analyses were conducted at the individual level with 12 regressors: one regressor reflecting the seed region timeseries, three trial type regressors (cued target, noncued target, catch), two regressors reflecting the interaction of the seed timeseries and relevant trial types (cued target trials × timeseries, noncued target trials × timeseries), and the six motion regressors. The interaction regressors identified brain regions in which the timeseries correlated with the seed timeseries in a task-dependent manner. Beta coefficients derived from the individual-level regressions were entered into an ANOVA with Condition (Facilitation, IOR) as a between-subjects factor, Trial type (cued target, noncued target) as a within-subjects factor, and subject as a random factor. We conducted the same contrast described above (2.3.3) examining activation related to orienting to targets appearing in the location of the expected attention bias (noncued target trials in the IOR condition vs. cued target trials in the Facilitation condition). AlphaSim simulations using 6 mm FWHM, 10,000 simulations, and an individual voxel threshold of 0.01 indicated that a minimum of 42 contiguous voxels was necessary to correct false positives to p < .05.
2.3.5 Recognition memory: Whole-brain analyses
Beta coefficients were entered into a group-level ANOVA with Condition (Facilitation, IOR) as a between-subjects factor, Trial type (cued, noncued, new) as a within-subjects factor, and subject as a random factor. The contrast of primary interest focused on activity related to recognition memory for old objects that had appeared in the location of the attention bias during encoding. As such, we compared recognition memory activation for items that had appeared in the cued location during the Facilitation condition versus those that had appeared in the noncued location during the IOR condition. AlphaSim simulations using 6 mm FWHM, 10,000 simulations, and individual voxel threshold of 0.01 revealed that a minimum of 42 contiguous voxels was necessary to correct false positives to p < .05.
3. Results
3.1 Behavioral Results
3.1.1 IOR and Facilitation Spatial Cueing Tasks
3.1.1.1 Spatial Cueing/Encoding phase
Analysis of eye movement latencies indicated that participants showed the expected suppression at the cued location during the IOR condition, indicated by slower responses to targets presented in that location compared to the noncued target location (MCued = 797.77, SD = 117.15 ms; MNoncued = 754.80, SD = 114.51 ms; F(1,17) = 7.34, p = .015). Results of an ANCOVA with Age included as covariate also revealed a significant difference in orienting latencies in the Facilitation condition, with participants showing faster responses to targets in the cued location (M = 608.09, SD = 243.54 ms) relative to the noncued location (M = 610.13, SD = 252.78 ms; F(1,15) = 6.19, p = .025). Although orienting latencies were our primary focus, we also examined participants’ manual response times. Results showed a reliably faster responses to the noncued location in the IOR condition (MCued = 713.66, SD = 142.39 ms; MNoncued = 685.62, SD = 136.62 ms; F(1,18) = 23.21, p < .001). There were no reliable difference in manual response times in the Facilitation condition (MCued = 629.67, SD = 176.25 ms; MNoncued = 610.48, SD = 172.51 ms; F(1,16) = 2.24, p = .154), which may be due to our lack of emphasis on speeded manual responses during the task.
We conducted control analyses to ensure that any differences in recognition memory were not due to differences in exposure to the targets during encoding. The overall proportion of trials excluded (due to missing data/failure to look at the target), looks to the cue, or incorrect looks) was related to average look duration during encoding (R(35) = −.33, p = .052). As such, we compared average looking times to the noncued and cued targets during encoding using a repeated-measures ANCOVA with proportion of excluded trials treated as a covariate. Results indicated that there were no differences in time spent looking to the target images in the IOR condition (Mcued = 714.25, SD = 311.73 ms, Mnoncued = 747.71 ms, SD = 340.47 ms; F(1,16) = 1.27, p = .277). There was also no reliable difference in looking times in the Facilitation condition, with longer look durations to the noncued targets (Mnoncued = 1006.04, SD = 321.78 ms) relative to the cued targets (Mcued = 978.72, SD = 312.90 ms,; F(1,15) = 3.30, p = .089).
3.1.1.2 Recognition Memory Behavioral Results
Mean recognition accuracy is reported in Table 1. A Trial type (cued, noncued test objects) × Condition (Facilitation, IOR) ANOVA examined d’ as a measure of recognition memory sensitivity. Results indicated a significant Trial type × Condition interaction (F(1,37) = 4.0, p = .053). As predicted, recognition memory scores in the IOR condition were higher for the noncued target test objects (M = 1.26, SD = 0.83) relative to the cued target test objects (M = 0.98, SD = 0.85; t(19) = 2.45, p = .024). Participants were also marginally more confident in identifying noncued target test objects as old (Mcued = 1.67, SD = 0.43, Mnoncued = 1.55, SD = 0.43; t(19) = −1.80, p = .088). In contrast, in the Facilitation condition there were no differences in either recognition memory scores (d’) or confidence ratings across the cued (Md’ = 1.57, SD = 0.96; MCR = 2.04, SD = 0.44) and noncued target test objects (Md’ = 1.53, SD = 0.73, t(18) = −0.31, p = .758; MCR = 2.05, SD = 0.30, t(18) = 0.09, p = .926).
Table 1.
Accuracy and sensitivity in identifying old and new objects at test.
| Accuracy | d' | |||||
|---|---|---|---|---|---|---|
| New Mean (SD) | Old Mean (SD) | Old Mean (SD) | ||||
| New | Overall | Cued | Noncued | Cued | Noncued | |
| Expt. 1 | ||||||
| IOR | 0.61 (0.28) | 0.75 (0.14) | 0.68 (0.28) | 0.83 (0.14) | 1.26 (0.83) | 0.98 (0.83) |
| Facilitation | 0.82 (0.13) | 0.67 (0.14) | 0.67 (0.19) | 0.67 (0.13) | 1.57 (0.96) | 1.53 (0.73) |
3.1.2 Control Task Recognition Memory Behavioral Results
Results of the no-cue Baseline experiment showed that there were no differences in recognition memory sensitivity (MSet1 = 1.63, SD = 1.01, MSet2 = 1.62, SD = 1.10; t(18) = 0.05, p = .959) or confidence ratings (MSet1 = 1.97, SD = 0.52, MSet2 = 1.98, SD = 0.52; t(18) = −0.05, p = 0.964) across the two sets of images presented as the cued and noncued targets in the spatial cueing task, indicating that the two image sets were equally memorable when attention was not manipulated via spatial cueing.
3.2 Neuroimaging Results
3.2.1 Spatial cueing visual cortex modulation
Our prediction was that the benefits of attention on memory encoding during the IOR condition stem from the role of the dorsal attention network in driving target enhancement and distractor suppression in visual cortex (Kastner et al., 1999; Smith et al., 2000). As such, we first verified the purported modulatory effects of the attention cueing manipulation on visual cortex activity. Figure 2A illustrates the right and left visual cortex (lingual gyrus) regions of interest (ROIs) identified by the localizer (see 2.3.2) to be active at the left and right target locations (t = 4.36, p < .01 corrected). A Trial type (cued target, noncued target) × Stimulus Location (Target, Distractor) × Condition (Facilitation, IOR) ANOVA indicated a main effect of Condition (F(1,37) = 4.20, p = .048), with higher overall activation in the Facilitation condition (M = 0.44, SD = 0.17) relative to the IOR condition (M = 0.26, SD = 17). Results also indicated a significant Trial type (Cued/Noncued) × Condition (IOR/Facilitation) interaction (F(1,37) = 6.77, p = .013); however, this interaction was further moderated by a trend-level Trial type (Cued/Noncued) × Stimulus Location (Target/Distractor) × Condition (IOR/Facilitation) interaction (F(1,37) = 2.69, p = .11).
Follow-up planned comparisons indicated that in the Facilitation condition there was no difference in activation across the targets and distractors during either cued target trials (MTarget = 0.51, SD = 0.18; MDistractor = 0.47, SD = 0.20; t(18) = 1.36, p = .19) or noncued target trials (MTarget = 0.39, SD = 0.20, MDistractor = 0.38, SD = 0.18; t(18) = 0.40, p = .691). In contrast, during IOR noncued target trials the target location was more active than the relatively suppressed distractor location (MTarget = 0.29, SD = 0.22, MDistractor = 0.25, SD = 0.21; t(19) = 2.30, p = .033, Figure 2B) consistent with behavioral reaction time attention bias to the noncued location. There was no difference in activity for the target and distractor during the cued target trials (MTarget = 0.25, SD = 0.17, MDistractor = 0.27, SD = 0.17; t(19) = −1.29, p = .211).
3.2.2 Network Activations at Encoding
The reported contrasts are derived from the whole brain ANOVA with Condition (Facilitation, IOR) as a between-subjects factor and Trial type (cued, noncued-target) as a within-subjects factor (see 2.3.3). Table 2 reports the results of contrasts examining the main effect of Condition and a subset of follow-up contrasts examining the Condition × Trial type interaction. Results indicated overall greater activation for the IOR condition relative to the Facilitation condition in left medial frontal gyrus (t = 2.45, p < .05, corrected).
Table 2.
Regions showing differential activity for IOR versus Facilitation trials during encoding.
| Spatial Cueing/Encoding Phase | ||||
|---|---|---|---|---|
| Contrast Region | Brodmann Area | Peak Voxel | Cluster Size (# voxels) | T (p < .05) |
| IOR > Facilitation | ||||
| Left medial frontal gyrus | 11 | 10.5, −28.5, −21.5 | 77 | 3.05 |
| IOR Noncued > Facilitation Cued | ||||
| Bilateral thalamus/caudate body | 1.5, 13.5, 14.5 | 117 | 2.88 | |
| Left middle frontal gyrus/anterior cingulate | 9/10 | 13.5, −55.5, 26.5 | 85 | 2.56 |
| Facilitation Cued > IOR Noncued | ||||
| Bilateral lingual gyrus | 18/19 | −13.5, 58.5, 2.5 | 703 | 4.98 |
| Left cuneus | 19 | 7.5, 85.5, 32.5 | 158 | 2.64 |
| IOR Noncued > Facilitation Noncued | ||||
| Right caudate/hippocampus | −19.5, 40.5, 14.5 | 305 | 4.28 | |
| Right inferior temporal cortex | 20 | −55.5, 16.5, −24.5 | 121 | 3.07 |
| Left inferior frontal gyrus | 47 | 43.5, −34.5, −9.5 | 81 | 2.74 |
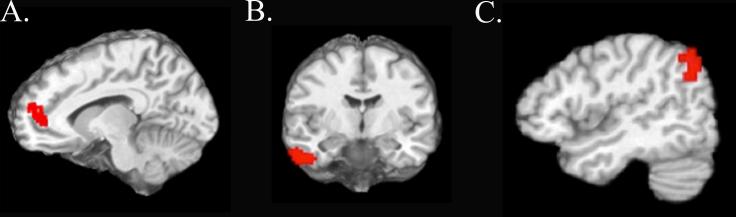
We next compared activation patterns associated with encoding of targets appearing in the location of the expected attention bias in each condition (i.e., cued target trials in the Facilitation condition versus noncued target trials in the IOR condition, Figure 1). One cortical region, the left middle frontal gyrus extending to the anterior cingulate, showed greater activation during IOR noncued target trials, relative to Facilitation cued target trials (Figure 3A). Two regions in occipital cortex, bilateral lingual gyrus and left cuneus, showed greater activation for Facilitation cued target trials than the IOR noncued target trials (t = 2.45, p < .05, corrected).
Figure 3.
Regions showing greater activity for (A) IOR noncued trials relative to Facilitation cued trials during encoding, (B) IOR noncued trials relative to Facilitation noncued trials during encoding, and (C) IOR noncued trials relative to Facilitation cued trials during recognition memory.
Our next contrast compared activity associated with encoding of noncued targets in the Facilitation and IOR conditions. This analysis revealed greater activity during IOR noncued target trials in right inferior temporal (IT) cortex (Figure 3B) as well as the right caudate tail extending into the right hippocampus (t = 2.34, p < .05, corrected). IT cortex is involved in tracking the strength of object representations (Emadi & Esteky, 2013), suggesting that increased activity in this region during IOR noncued target trials reflects a more robust representation of the target objects.
3.2.3 Network Connectivity at Encoding
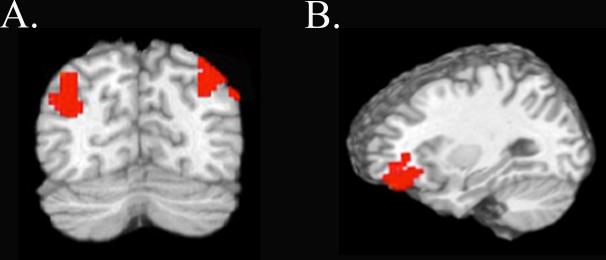
Theoretically, if differential target/distractor activations in visual cortex (Figure 2B) impact encoding efficacy by eliminating distractor interference, we should observe stronger functional connectivity between visual cortex and relevant attention/memory networks for noncued target trials in the IOR condition relative to cued target trials in the Facilitation condition. That is, even when targets in both conditions are in the location of the attention bias, the presence of concurrent suppression in the IOR condition should drive stronger connectivity between attention, visual, and memory networks. To test this hypothesis we performed a psychophysiological interaction analysis (PPI) (see 2.3.4) for each participant using the lingual gyrus ROI as a single visual cortex seed region. We again examined activation related to encoding of targets appearing in the location of the attention bias in each condition (IOR noncued targets vs. Facilitation cued targets). This analysis verified our prediction, revealing that visual cortex during encoding of IOR noncued targets had greater functional connectivity with left inferior parietal lobule (IPL) and right superior parietal lobule (t = 2.45, p < .05, corrected; Figure 4A/Table 3), Importantly, connectivity between these regions and the visual cortex seed was stronger during IOR noncued trials than Facilitation cued trials, despite the fact that participants were biased to attend to the target in both of these cases. These results thus suggest that the presence of both target enhancement and concurrent suppression in the IOR condition contributed to stronger network connectivity.
Figure 4.
Regions showing greater connectivity with the visual cortex seed (A) and right IT cortex seed (B) during IOR noncued target trials relative to Facilitation cued target trials.
Table 3.
Regions showing differential connectivity with visual and IT cortex regions during IOR versus Facilitation encoding trials.
| PPI: Visual Cortex Seed | ||||
|---|---|---|---|---|
| Contrast Region | Brodmann Area | Peak Voxel | Cluster Size (# voxels) | T (p < .05) |
| Facilitation > IOR | ||||
| Left inferior parietal lobule/postcentral gyrus | 40 | 43.5, 46.5, 56.5 | 397 | 2.95 |
| Left precuneus | 7 | 16.5, 73.5, 41.5 | 135 | 3.07 |
| Left fusiform gyrus | 37 | 37.5, 55.5, −18.5 | 114 | 2.54 |
| IOR Noncued > Facilitation Cued | ||||
| Left inferior parietal lobule | 40 | 46.5, 58.5, 50.5 | 129 | 3.45 |
| Right superior parietal lobule | 7 | −40.5 70.5, 44.5 | 93 | 2.69 |
| PPI: IT Cortex Seed | ||||
|---|---|---|---|---|
| Contrast Region | Brodmann Area | Peak Voxel | Cluster Size (# voxels) | T (p < .05) |
| IOR > Facilitation | ||||
| Left inferior temporal gyrus | 37 | 52.5, 67.5, −9.5 | 269 | 2.66 |
| Left cuneus | 19 | 28.5, 91.5, 23.5 | 113 | 2.60 |
| IOR Noncued > Facilitation Cued | ||||
| Left middle frontal gyrus | 11 | 40.5, −49.5, −9.5 | 94 | 3.43 |
Given the important role of inferior temporal (IT) cortex in representing object information, we repeated this PPI analysis using right IT cortex as a seed region. The IT seed ROI was identified using the region of activation identified in the analysis of activation patterns during encoding, as described above. Results of the contrast revealed that activity in right IT cortex was more functionally coupled with activity in left middle frontal gyrus (t = 3.34, p < .05, corrected) during encoding of IOR noncued target trials relative to Facilitation cued target trials (Figure 4B/Table 3).
3.2.4 Recognition Memory Phase
We examined neural activation patterns during an unannounced subsequent recognition memory test for objects that had previously been encoded in the IOR and Facilitation conditions. This analysis again utilized a whole brain ANOVA with Condition (Facilitation, IOR) as a between-subjects factor and Trial type (cued, noncued-target, new) as a within-subjects factor. Table 4 reports the results of contrasts examining the main effect of Condition and a subset of follow-up contrasts examining the Condition × Trial type interaction. There was overall greater activation for the IOR condition relative to the Facilitation condition in a number of frontal and parietal regions, including left inferior parietal lobule, right medial frontal gyrus, right middle frontal gyrus, and left prefrontal gyrus. (t = 2.45, p < .05, corrected).
Table 4.
Regions showing differential activity for IOR versus Facilitation trials during the recognition memory test.
| Recognition Memory Phase | ||||
|---|---|---|---|---|
| Contrast Region | Brodmann Area | Peak Voxel | Cluster Size (# voxels) | T (p < .05) |
| IOR > Facilitation | ||||
| Right medial frontal gyrus | 6 | −1.5, −4.5, 62.5 | 490 | 3.67 |
| Right lingual gyrus | 18 | −4.5, 85.5, −6.5 | 243 | 3.37 |
| Left precentral gyrus/inferior frontal gyrus | 6/9 | 55.5, −4.5, 32.5 | 182 | 4.66 |
| Right caudate/putamen | −13.5, −7.5, −9.5 | 149 | 4.00 | |
| Right insula | 13 | −34.5, −16.5, 5.5 | 133 | 4.29 |
| Left middle occipital gyrus | 18 | 25.5, 94.5, 8.5 | 81 | 4.56 |
| Left inferior parietal lobule | 7 | 37.5, 64.5, 47.5 | 75 | 3.65 |
| Right middle frontal gyrus | 10 | −37.5, −52.5, 8.5 | 72 | 3.15 |
| Left precentral gyrus | 4 | 34.5, 16.5, 53.5 | 66 | 2.98 |
| IOR Noncued > Facilitation Cued | ||||
| Bilateral caudate | −1.5, −13.5, 11.5 | 161 | 4.90 | |
| Right IPL/angular gyrus | 39 | −49.5, 67.5, 41.5 | 140 | 2.47 |
| Right fusiform gyrus | 19 | −25.5, 64.5, −12.5 | 103 | 3.91 |
| Bilateral cingulate gyrus | 32 | −1.5, −16.5, 38.5 | 95 | 2.70 |
| Bilateral medial frontal gyrus | 6 | −1.5, 1.5, 62.5 | 85 | 2.83 |
We next compared activation for objects that had previously been encoded in the cued location during the Facilitation condition relative to those encoded in the noncued location during the IOR condition. Results of this contrast revealed greater activation for the IOR noncued test objects in bilateral medial frontal gyrus, right angular gyrus/inferior parietal lobule, right fusiform gyrus, and bilateral cingulate cortex (t = 2.45, p < .05, corrected; Figure 3B), regions commonly active during recognition memory (Kuhl, Dudukovic, Kahn, & Wagner, 2007; Sestieri, Capotosto, Tosoni, Romani, & Corbetta, 2013; Vann, Aggleton, & Maguire, 2009).
3.3 Predictors of Memory Performance
Finally, we were interested in isolating the neural systems involved in encoding and recognition memory that best contributed to effective recognition of noncued targets encoded in the context of distractor suppression (i.e., IOR) and cued targets encoded in the Facilitation condition. We used multiple regression to model predictors of recognition sensitivity (d’) for noncued target test objects encoded in the IOR condition. Predictors were based on neural activations during encoding and recognition memory that were greater for noncued trials. These predictors included 1) beta coefficients reflecting target/distractor activity in visual cortex ROIs during encoding (Figure 2C) and 2) beta coefficients from ROIs identified by the contrasts during the encoding and retrieval analyses. Any predictors contributing to high multicollinearity were removed from the model. All predictors are listed in Table 5. Preliminary examination of standardized residuals revealed one outlier; these data were excluded to preserve model assumptions. The target- and distractor-based visual cortex ROIs were entered first and all remaining ROIs were entered second in a stepwise manner. The model with the visual cortex ROIs alone accounted for a significant proportion of variance in recognition memory performance (R2 = .33, F(2,16) = 3.91, p = .042). In addition, both target- and distractor-based visual cortex activity during noncued target encoding trials predicted recognition memory accuracy for noncued test objects (tTarget(16) = 2.75, p = .014; tDistractor(16) = −2.47, p = .025). Specifically, increased enhancement at the target location (i.e., increased activation) and increased suppression at the distractor location (i.e. reduced activation) were related to enhanced recognition sensitivity for noncued objects (Figure 2C and 2D). This suggests that distractor suppression accounted for variance in recognition memory that was independent of target enhancement. Target- and distractor-based visual cortex activity during cued target trials did not similarly predict recognition memory performance for cued test objects in the IOR condition (R2 = .10, F(2,16) = 0.86, p = .440; tTarget(16) = −0.35, p = .972; tDistractor(16) = 0.63, p = .540). The full model including ROIs from encoding and test accounted for a marginally significant proportion of variance in recognition memory performance (R2 = .51, F(5,13) = 2.69, p = .07). In addition, recognition memory-based activity in the right angular gyrus (IPL) was a trend-level significant predictor of recognition memory performance for noncued test objects (t(12) = 1.75, p = .104).
Table 5.
Encoding- and recognition memory-based predictors of recognition memory (d') for noncued test objects presented during the IOR condition.
| Task Phase | Variable | B (SE) | |
|---|---|---|---|
| Model 1 | Constant | 0.94** (0.21) | |
| Encoding: Visual cortex activity | |||
| Target-based activity: noncued target trials | 5.15* (1.87) | ||
| Distractor-based activity: noncued target trials | −5.03* (2.04) | ||
| R2 | .33* | ||
| N | 20 | ||
| Model 2 | Constant | 0.71* (0.23) | |
| Encoding: Visual cortex activity | |||
| Target-based activity: noncued target trials | 4.45* (1.81) | ||
| Distractor-based activity: noncued target trials | −3.74t (2.03) | ||
| Encoding-based activity | |||
| Left middle frontal gyrus: noncued target trials | −1.86 (1.17) | ||
| Right inferior temporal cortex: noncued target trials | −0.72 (0.68) | ||
| Retrieval-based activity | |||
| Right angular gyrus: noncued trials | 1.78t (1.02) | ||
| R2 | .51* | ||
| Δ R2 | .18 | ||
| N | 20 | ||
p < .01
p < .05
p < .01
A second analysis modeled predictors derived from ROIs identified by the encoding PPI analyses (see 3.2.3). ROIs identified from the visual cortex seed were entered first followed by those identified by the IT seed. None of the ROIs were reliable predictors and neither model accounted for a significant proportion of variance in recognition memory performance for noncued test objects in the IOR condition.
We repeated these regression analyses to model predictors of recognition memory performance for cued target test objects encoded in the Facilitation condition. These targets appeared in the location of the attention bias, but were not encoded in the context of concurrent suppression. Predictors again included target/distractor activity in visual cortex ROIs during cued target encoding trials and beta coefficients from cortical ROIs with neural activations that were greater for cued relative to noncued target trials during encoding. As before, the target- and distractor-based visual cortex ROIs were entered first and all other ROIs were included in a second model. Neither model explained a significant proportion of variance in recognition memory (R21 = .19, F(2,16) = 1.85, p = .189; R22 = .28, F(4,14) = 1.36, p = .297) and none of the ROIs were reliable predictors of recognition memory for cued test objects. A final regression modeled the ROI predictors derived from PPI analysis. As before, the ROIs identified from the visual cortex seed were entered followed by the ROIs identified by the IT seed (Table 6). Here, the first model was significant (R2 = .50, F(2,16) = 8.09, p = .004) and the degree of connectivity between visual cortex and left inferior parietal lobule (IPL) was a reliable predictor of recognition memory for cued objects (t(16) = 3.96, p = .001). The addition of the ROI derived from the IT cortex seed did not significantly improve the model (ΔR2 < .001, F(1,15) = 0.01, p = .923) and left IPL remained the only reliable predictor of recognition memory (t(15) = 3.77, p = .002). .
Table 6.
PPI-based predictors of recognition memory (d') for cued test objects presented during the Facilitation condition.
| Task Phase | Variable | B (SE) | |
|---|---|---|---|
| Model 1 | Constant | 1.96** (0.24) | |
| Encoding PPI: Visual cortex seed | |||
| Left inferior parietal lobule: cued target trials | 0.04* (0.07) | ||
| Right superior parietal lobule: cued target trials | −0.02t (0.07) | ||
| R2 | .50* | ||
| N | 19 | ||
| Model 2 | Constant | 1.95** (0.29) | |
| Encoding PPI: Visual cortex seed | |||
| Left inferior parietal lobule: cued target trials | 0.04* (0.07) | ||
| Right superior parietal lobule: cued target trials | −0.02t (0.07) | ||
| Encoding PPI: Inferior temporal cortex seed | |||
| Left middle frontal gyrus: cued targer trials | −0.001 (0.01) | ||
| R2 | .50** | ||
| Δ R2 | .001 | ||
| N | 19 | ||
p < .01
p < .05
p < .01
4. Discussion
Not all attention orienting is equal with respect to learning and memory. The dynamics of the mechanisms driving attention orienting, and specifically whether suppression of previously attended, competing information is engaged, are tightly coupled with the efficacy of memory formation at the attended location. We capitalized on a classic spatial cueing task and IOR mechanisms to behaviorally show that objects encoded in the presence of suppression of distractors appearing in the previously attended location were encoded and remembered better than objects encoded as a function of cueing enhancement alone or in a baseline memory condition. We further showed that this memory benefit was linked to modulation of visual cortex activity: recognition accuracy for objects encoded in the context of distractor suppression was predicted by both target location enhancement and by the extent to which competing distractor locations were suppressed during encoding (Figure 2C & 2D). Specifically, increased target location enhancement and reduced distractor interference from the previously attended location during encoding contributed to better memory for the attended target objects at test. In contrast, the extent of activity in visual cortex was unrelated to memory performance when the objects were encoded in the context of target location enhancement alone (i.e., Facilitation).
Additionally, differential activation in IT cortex during the IOR condition suggests that encoding in the context of suppression at the previously attended location may have contributed to less noisy, more robust representation of the target objects. Neural signal in IT cortex tracks the strength of object representations (Emadi & Esteky, 2013). As noted earlier, selective attention can modulate neural activity in IT cortex; when the signal associated with distractors was suppressed effectively, the target object representation in IT cortex was restored to the same level observed when the object was presented in the absence of distractors (Zhang et al., 2011). Additional work has identified increased connectivity between inferior temporal regions and frontal cortex when object features were attended versus unattended (Baldauf & Desimone, 2014). The present results are consistent with these findings. The suppression present in the IOR condition was associated with greater activation in IT cortex during encoding, suggesting that there may have been a stronger signal/representation for the target objects in this condition relative to the Facilitation condition. Furthermore, the increased connectivity between IT cortex and frontal cortex during the IOR condition may similarly reflect increased attention-based modulation of the object representation/signal in the IOR condition.
We additionally found greater activity in frontal and parietal regions during a subsequent recognition memory test when objects were encoded in the context of concurrent enhancement and suppression (i.e., IOR), relative to when they were encoded in the context of enhancement alone (i.e., Facilitation), including the left medial frontal gyrus, bilateral cingulate cortex, and right inferior parietal lobule/angular gyrus. Left middle frontal cortex also showed greater functional connectivity with IT cortex when encoding involved both target enhancement and distractor suppression. Middle frontal gyrus has previously been identified as a potential source of top-down modulation that supports distractor suppression in visual cortex (Clapp, Rubens, & Gazzaley, 2010; Gazzaley, Rissman, & D’Esposito, 2004), perhaps making it a critical component during encoding in the IOR condition. Notably, the differential profile of activation and functional connectivity during the IOR condition was observed despite the fact that the target objects appeared in the location of the expected attention bias in both conditions.
The present results are consistent with previous work demonstrating a role of distractor suppression in enhanced working memory performance. Top-down attention modulation, and suppression of irrelevant distractors in particular, and connectivity between frontal and visual cortical regions has been linked to efficacy of working memory (Clapp et al., 2010; Gazzaley et al., 2004; Kuo, Stokes, Murray, & Nobre, 2014). For example, directing attention to a previously relevant location during the delay period of a visual short-term memory task leads to modulation of visual cortex activity, with greater modulation predicting more efficient performance on the subsequent memory probe (Kuo et al., 2014). Conversely, poor working memory performance is associated with lack of ERP components associated with suppression of irrelevant stimuli (Zanto & Gazzaley, 2009).
Our functional connectivity analyses, with visual cortex seed regions, shed light on the networks involved in the attention/memory interaction during encoding trials (Figure 4). This analysis indicated that, relative to the Facilitation condition, target encoding in the IOR condition engaged increased visual cortex functional connectivity with inferior and superior parietal regions, as well as increased IT cortex connectivity with middle frontal gyrus. This network maps on to regions previously identified as a “control” or “enhancement” network in the context of attending to task-relevant objects (Chadick & Gazzaley, 2011; Gazzaley & Nobre, 2012).
Within this distributed network, particular regions may have distinct contributions to memory encoding. The involvement of frontoparietal regions is likely attributable in part to the demands of attention selection invoked by the spatial cueing manipulation (Lepsien & Pollmann, 2002). Recent work has also identified a link between frontoparietal activity and cortical pattern similarity, suggesting that frontoparietal regions support effective memory encoding by reducing noise in neural representations and allowing for greater reliability of cortical patterns, and thus more effective memory encoding (Xue, Dong, Lu, Mumford, & Poldrack, 2013). This is similar to our argument that modulation of visual cortex activity, and distractor suppression in particular, via frontoparietal attention networks generates a more robust, less noisy signal for memory encoding.
Our analyses revealed overall greater connectivity between visual and parietal cortical regions during target encoding in the IOR condition relative to the Facilitation condition. Within this context, the extent of connectivity between visual cortex and left inferior parietal lobule predicted individual differences in recognition memory performance in the Facilitation condition, but not in the IOR condition. Instead, variability in recognition memory performance in the IOR condition was best predicted by the extent of modulation of visual cortex activity. Dorsal parietal regions, including IPL, have consistently been implicated in spatial orienting of attention (Corbetta, Kincade, Ollinger, Mcavoy, & Gordon, 2000; Greenberg, Esterman, Wilson, Serences, & Yantis, 2010; Yantis & Serences, 2003), suggesting that greater engagement of the orienting network may have supported more effective learning in general. However, the extent to which IOR engaged suppression at the previously attended location predicted the most optimal learning and memory, consistent with the idea that suppression of the visual cortex signal associated with competing distractors supported a more robust representation of the target objects.
We designed this work to ask whether distractor suppression in the context of attention orienting is a mechanism that is critical to learning during visual exploration. Previous work has highlighted links between attention and memory as sequential eye movements are executed, demonstrating that the final target of an eye movement sequence is remembered better than any target that was previously attended along the path of the eye movement sequence (Gersch et al., 2008). The present study revealed greater activity in IT cortex when the previously attended location was suppressed in the IOR condition, suggesting a potential mechanism that allows data from the previously attended location to be suppressed so that data at the current location can be best encoded. This may also be a more general-purpose mechanism, where suppression of any distracting object in the surround supports enhanced memory encoding. In this work, the distractor is both currently present and in a previously attended location. As such, this is a question for future research. In either case, active vision and exploration requires a sequence of saccades and fixations in a temporal order. The present work suggests that as we move through the world the ability to suppress competing information will afford greater efficacy to encode and remember the information that we encounter.
5. Conclusions
The present results underscore the idea that memory benefits afforded by selective attention are not simply due to enhanced processing of attended objects alone. More generally, these results provide insight into the mechanisms that link attention and memory as functionally integrated systems and highlight broader implications for developmental and patient populations. We showed here that eye movements that otherwise look the same result in very different learning and memory profiles. The specific mechanisms driving IOR emerge over the first year of life (Johnson & Tucker, 1996; Richards, 2000) and the more general ability to effectively suppress interfering information continues to develop into childhood (Rueda, Fan, McCandliss, & Halparin, 2004). The present results suggest that these developments will not only affect how infants and children select information for learning but will also affect how well that information is learned. Attention and memory problems are also common to many psychiatric and neurologic diagnoses. IOR and/or the ability to suppress distracting information are disrupted in several patient populations, including those diagnosed with schizophrenia, autism, ADHD, and Parkinson’s disease (Gouzoulis-Mayfrank et al., 2004; Murphy, Foxe, Peters, & Molholm, 2014; Possin, Filoteo, Song, & Salmon, 2009). Although more work is needed, these findings linking distractor suppression with efficacy of memory encoding have potentially broad relevance for understanding the emergence of these disruptions, suggesting that by examining the mechanisms underlying ineffective distractor suppression we may also gain insight into mechanisms contributing to poorer learning and memory outcomes within these populations. As such, these findings also suggest that this approach also holds potential for developing interventions that harness attention systems to improve learning and memory in these populations.
Highlights.
Suppression at a previously attended location enhanced encoding and recognition memory.
Distractor suppression increased activity in ventral temporal object recognition areas.
Distractor suppression increased coupling of visual & frontal cortex activity.
Extent of distractor suppression in visual cortex predicted memory performance.
Acknowledgements
The authors gratefully acknowledge the National Institutes of Health (NIGMS-NIH (P20 GM103645) to DA) and the James S. McDonnell Foundation (Scholar Award in Understanding Human Cognition to DA) for their generous support of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldauf D, Desimone R. Neural mechanisms of object-based attention. Science. 2014;344(6182):424–7. doi: 10.1126/science.1247003. doi:10.1126/science.1247003. [DOI] [PubMed] [Google Scholar]
- Ballesteros S, Reales JM, Garcia E, Carrasco M. Selective attention affects implicit and explicit memory for familiar pictures at different delay conditions. Psicothema. 2006;18(1):88–99. [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JDE. Making memories: Brain activity the predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. doi:10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Broadway JM, Hilimire MR, Corballis PM. Orienting to external versus internal regions of space: Consequences of attending in advance versus after the fact. Psychophysiology. 2011 doi: 10.1111/j.1469-8986.2011.01307.x. doi:10.1111/j.1469-8986.2011.01307.x. [DOI] [PubMed] [Google Scholar]
- Chadick JZ, Gazzaley A. Differential coupling of visual cortex with default or frontal-parietal network based on goals. Nature Neuroscience. 2011;14(7):830–2. doi: 10.1038/nn.2823. doi:10.1038/nn.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp WC, Rubens MT, Gazzaley A. Mechanisms of working memory disruption by external interference. Cerebral Cortex. 2010;20(4):859–872. doi: 10.1093/cercor/bhp150. doi:10.1093/cercor/bhp150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, Mcavoy MP, Gordon L. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience. 2000;3(3):292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. doi:10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(0014):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dosher BA, Lu Z-L. Noise exclusion in spatial attention. Psychological Science. 2000;11:139. doi: 10.1111/1467-9280.00229. doi:10.1111/1467-9280.00229. [DOI] [PubMed] [Google Scholar]
- Emadi N, Esteky H. Neural representation of ambiguous visual objects in the inferior temporal cortex. PloS One. 2013;8(10):e76856. doi: 10.1371/journal.pone.0076856. doi:10.1371/journal.pone.0076856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi SP, Heeger DJ, Boynton GM. Spatial attention affects brain activity in human primary visual cortex. Proceedings of the National Academy of Sciences. 1999;96(6):3314–3319. doi: 10.1073/pnas.96.6.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Nobre AC. Top-down modulation: bridging selective attention and working memory. Trends in Cognitive Sciences. 2012;16(2):129–135. doi: 10.1016/j.tics.2011.11.014. doi:10.1016/j.tics.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, D'Esposito M. Functional connectivity during working memory maintenance. Cognitive, Affective, & Behavioral Neuroscience. 2004;4(4):580–599. doi: 10.3758/cabn.4.4.580. [DOI] [PubMed] [Google Scholar]
- Gersch TM, Schnitzer BS, Dosher BA. Visual memory during pauses between successive saccades. Journal of Vision. 2008;8(16):1–18. doi: 10.1167/8.16.15. doi:10.1167/8.16.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb JD, Pulido VZ, Albrecht AR, Chun MM, Mazer JA. Robustness of the retinotopic attentional trace after eye movements. Journal of Vision. 2010;10(3):1–12. doi: 10.1167/10.3.19. doi:10.1167/10.3.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Heekeren K, Voss T, Moerth D, Thelen B, Meincke U. Blunted inhibition of return in schizophrenia - evidence from a longitudinal study. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2004;28:389–396. doi: 10.1016/j.pnpbp.2003.11.010. doi:10.1016/j.pnpbp.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Esterman M, Wilson D, Serences JT, Yantis S. Control of spatial and feature-based attention in frontoparietal cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30(43):14330–9. doi: 10.1523/JNEUROSCI.4248-09.2010. doi:10.1523/JNEUROSCI.4248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauer BJA, MacLeod CM. Endogenous versus exogenous attentional cuing effects on memory. Acta Psychologica. 2006;122:305–320. doi: 10.1016/j.actpsy.2005.12.008. doi:10.1016/j.actpsy.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Tucker LA. The development and temporal dynamics of spatial orienting in infants. Journal of Experimental Child Psychology. 1996;63:171–188. doi: 10.1006/jecp.1996.0046. doi:10.1006/jecp.1996.0046. [DOI] [PubMed] [Google Scholar]
- Kahn I, Davachi L, Wagner A. Functional-neuroanatomic correlates of recollection: Implications for models of recognition memory. The Journal of Neuroscience. 2004;24(17):4172–4180. doi: 10.1523/JNEUROSCI.0624-04.2004. doi:10.1523/JNEUROSCI.0624-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kim H. Differential neural activity in the recognition of old versus new events: An activation likelihood estimation meta-analysis. Human Brain Mapping. 2013;34:814–836. doi: 10.1002/hbm.21474. doi:10.1002/hbm.21474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RM. Inhibitory tagging system facilitates visual search. Nature. 1988;334:430–431. doi: 10.1038/334430a0. [DOI] [PubMed] [Google Scholar]
- Klein RM. Inhibition of return. Trends in Cognitive Sciences. 2000;4(4):138–147. doi: 10.1016/s1364-6613(00)01452-2. doi:10.1016/S1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- Klein RM, MacInnes WJ. Inhibition of return is a foraging facilitator in visual search. Psychological Science. 1999;10(4):346–352. doi:10.1111/1467-9280.00166. [Google Scholar]
- Konishi S, Wheeler ME, Donaldson DI, Buckner RL. Neural correlates of episodic retrieval success. NeuroImage. 2000;12:276–286. doi: 10.1006/nimg.2000.0614. doi:10.1006/nimg.2000.0614. [DOI] [PubMed] [Google Scholar]
- Kuhl BA, Dudukovic NM, Kahn I, Wagner A. Decreased demands on cognitive control reveal the neural processing benefits of forgetting. Nature Neuroscience. 2007;10(7):908–914. doi: 10.1038/nn1918. doi:10.1038/nn1918. [DOI] [PubMed] [Google Scholar]
- Kuo B-C, Stokes MG, Murray A, Nobre AC. Attention biases visual activity in visual STM. Journal of Cognitive Neuroscience. 2014:1–13. doi: 10.1162/jocn_a_00577. doi:10.1162/jocn_a_00577. [DOI] [PubMed] [Google Scholar]
- Lepsien J, Pollmann S. Covert reorienting and inhbition of return: An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14(2):127–144. doi: 10.1162/089892902317236795. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Seidenberg M, Dorflinger JM, Rao S. An event-related fMRI study of exogenous orienting: Supporting evidence for the cortical basis of inhibition of return? Journal of Cognitive Neuroscience. 2004;16(7):1262–1271. doi: 10.1162/0898929041920531. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Jones TC, Petersen SE, Lageman SK, Roediger HLIII. Retrieval success is accompanied by enhanced activation in anterior prefrontal cortex during recognition memory: An event-related fMRI study. Journal of Cognitive Neuroscience. 2000;12(6):965–976. doi: 10.1162/08989290051137503. [DOI] [PubMed] [Google Scholar]
- Murphy JW, Foxe JJ, Peters JB, Molholm S. Susceptibility to distraction in autism spectrum disorder: Probing the integrity of oscillatory alpha-band suppression mechanisms. Autism Research. 2014:1–17. doi: 10.1002/aur.1374. doi:10.1002/aur.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. doi:10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Cohen Y. Components of visual orienting. In: Bouma H, Bouwhuis D, editors. Attention and Performance. X. Erlbaum Lawrence Associates; Hillsdale, NJ: 1984. pp. 531–556. [Google Scholar]
- Posner MI, Rafal RD, Choate LS. Inhibition of return: Neural basis and function. Cognitive Neuropsychology. 1985;2(3):211–228. doi:10.1080/02643298508252866. [Google Scholar]
- Possin KL, Filoteo JV, Song DD, Salmon DP. Space-based but not object- based inhibition of return is impaired in parkinson's disease. Neuropsychologia. 2009;47(7):1694–1700. doi: 10.1016/j.neuropsychologia.2009.02.006. doi:10.1016/j.neuropsychologia.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, van Marle HJF, Hermans EJ, Fernandez G. Subjective sense of memory strength and the objective amount of information accurately remembered are related to distinct neural correlates at encoding. The Journal of Neuroscience. 2011;31(24):8920–8927. doi: 10.1523/JNEUROSCI.2587-10.2011. doi:10.1523/JNEUROSCI.2587-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE. Localizing the development of covert attention in infants with scalp event-related potentials. Developmental Psychology. 2000;36(1):91–108. doi:10.1037/0012-1649.36.1.91. [PubMed] [Google Scholar]
- Rueda MR, Fan J, McCandliss BD, Halparin JD. Development of attentional networks in childhood. Neuropsychologia. 2004;42:1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. doi:10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Sestieri C, Capotosto P, Tosoni A, Romani GL, Corbetta M. Interference with episodic memory retrieval following transcranial stimulation of the inferior but not the superior parietal lobule. Neuropsychologia. 2013;51:900–906. doi: 10.1016/j.neuropsychologia.2013.01.023. doi:10.1016/j.neuropsychologia.2013.01.023. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Schwarzbach J, Yantis S. Attentional inhibition of visual processing in human striate and extrastriate cortex. NeuroImage. 2003;19(4):1602–1611. doi: 10.1016/s1053-8119(03)00187-3. [DOI] [PubMed] [Google Scholar]
- Smith AT, Singh KD, Greenlee MW. Attentional suppression of activity in the human visual cortex. Neuroreport. 2000;11(2):271–277. doi: 10.1097/00001756-200002070-00010. [DOI] [PubMed] [Google Scholar]
- Squire RF, Noudoost B, Schafer RJ, Moore T. Prefrontal contributions to visual selective attention. Annual Review of Neuroscience. 2013;36:451–466. doi: 10.1146/annurev-neuro-062111-150439. doi:10.1146/annurev-neuro-062111-150439. [DOI] [PubMed] [Google Scholar]
- Szekely A, Jacobsen T, D'Amico S, Devescovi A, Andonova E, Herron D, Bates E. A new on-line resource for psycholinguistic studies. Journal of Memory and Language. 2004;51(2):247–250. doi: 10.1016/j.jml.2004.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talsma D, White BJ, Mathôt S, Munoz DP, Theeuwes J. A retinotopic attentional trace after saccadic eye movements: evidence from event-related potentials. Journal of Cognitive Neuroscience. 2013;25(9):1563–77. doi: 10.1162/jocn_a_00390. doi:10.1162/jocn_a_00390. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Rugg MD. Selecting for memory? The influence of selective attention on the mnemonic binding of contextual information. The Journal of Neuroscience. 2009;29(25):8270–8279. doi: 10.1523/JNEUROSCI.1043-09.2009. doi:10.1523/JNEUROSCI.1043-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, Wagner A. Posterior parietal cortex and episodic encoding: Insights from fMRI subsequent memory effects and dual-attention theory. Neurobiology of Learning and Memory. 2009;91:139–154. doi: 10.1016/j.nlm.2008.10.011. doi:10.1016/j.nlm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nature Reviews Neuroscience. 2009;10:792–802. doi: 10.1038/nrn2733. doi:10.1038/nm2733. [DOI] [PubMed] [Google Scholar]
- Wagner A, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences. 2005;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. doi:10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) Harcourt Assessment; San Antonio, TX: 1999. [Google Scholar]
- Xue G, Dong Q, Lu Z-L, Mumford JA, Poldrack RA. Complementary role of frontoparietal activity and cortical pattern similarity in successful episodic memory encoding. Cerebral Cortex. 2013;23:1562–1571. doi: 10.1093/cercor/bhs143. doi:10.1093/cercor/bhs143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S, Serences JT. Cortical mechanisms of space-based and object-based attentional control. Current Opinion in Neurobiology. 2003;13(2):187–193. doi: 10.1016/s0959-4388(03)00033-3. doi:10.1016/S0959-4388(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Zanto TP, Gazzaley A. Neural suppression of irrelevant information underlies optimal working memory performance. The Journal of Neuroscience. 2009;29(10):3059–3066. doi: 10.1523/JNEUROSCI.4621-08.2009. doi:10.1523/JNEUROSCI.4621-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Meyers EM, Bichot N, Serre T, Poggio TA, Desimone R. Object decoding with attention in inferior temporal cortex. Proceedings of the National Academy of Sciences. 2011;108(21):8850–8855. doi: 10.1073/pnas.1100999108. doi:10.1073/pnas.1100999108. [DOI] [PMC free article] [PubMed] [Google Scholar]