Abstract
This technical report addresses the problem of endotoxin contamination of apolipoprotein reagents. Using a bromodeoxyuridine incorporation cell proliferation assay, we observed that human plasma ApoA-I as low as 1 μg/ml resulted in a >90% inhibition in macrophage proliferation. However, not all ApoA-I from different sources showed this effect. We considered the possibility that endotoxin contamination of the apolipoproteins contributed to the differential inhibition of macrophage cell proliferation. Endotoxin alone very potently inhibited macrophage proliferation (0.1 ng/ml inhibited macrophage proliferation >90%). Measurement of endotoxin levels in the apolipoprotein products, including an analysis of free versus total endotoxin, the latter which included endotoxin that was masked due to binding to protein, suggested that free endotoxin mediated inhibition of macrophage proliferation. Despite the use of an advanced endotoxin removal procedure and agents commonly used to inhibit endotoxin action, the potency of endotoxin precluded successful elimination of endotoxin effect. Our findings show that endotoxin contamination can significantly influence apparent apolipoprotein-mediated cell effects (or effects of any other biological products), especially when these products are tested on highly endotoxin-sensitive cells, such as macrophages.
Keywords: endotoxin, macrophages, cell proliferation, apolipoproteins, atherosclerosis
Investigators employ many biological agents such as cytokines, hormones, and in our case, apolipoproteins, during research investigations. We were interested to study the effect of apolipoprotein (Apo)A-I on macrophage biology. ApoA-I is the major protein component of high-density lipoprotein, the plasma lipoprotein that is considered to be atheroprotective, in part, by mediating mobilization of excess cholesterol from tissues. Biological effects are often attributed to the factor under study (in our case, ApoA-I) with the assumption that the factor is of sufficient purity that only the factor could mediate the biological effect under study. However, endotoxin contamination of reagents is a potentially significant problem that can lead to erroneous conclusions in experiments. This is because endotoxin itself has many effects on cell functions such as activating cells and stimulating release of cellular cytokines. Many cell types are susceptible to endotoxin effects. These cell types include macrophages, neutrophils, dendritic cells, lymphocytes, hepatocytes, endothelial cells, platelets and others.1
In this technical report, we describe our experience with endotoxin contamination of apolipoprotein reagents as a potential problem in the study of the effects of apolipoproteins and other agents on macrophage biology.
Materials and Methods
See supplemental data.
Results
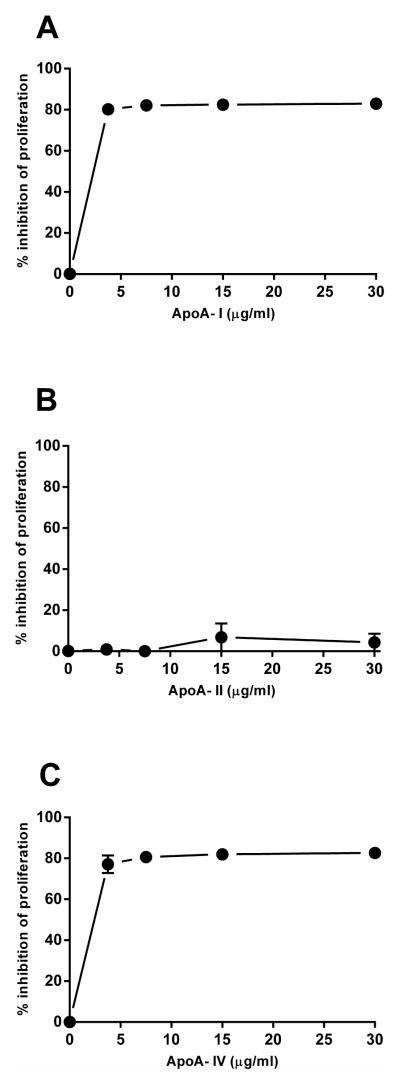
During initial experiments meant to test the effect of ApoA-I (human plasma ApoA-I obtained from Company A) on macrophage cholesterol metabolism, we observed that ApoA-I treated macrophages became round and that macrophage cultures were less dense than control cultures (Supplemental Figure 1). Sometimes some cell detachment occurred. The detached cells were >90% viable by trypan blue dye exclusion assay. However, the consistent decrease in cell density suggested that ApoA-I was possibly inhibiting macrophage proliferation. To examine this possibility, we examined the effect of this ApoA-I on macrophage proliferation by monitoring BrdU incorporation into the total cells, both attached and non-adherent. The results showed that Company A ApoA-I indeed did inhibit macrophage proliferation by 80% (Figure 1A). Company A ApoA-II did not inhibit macrophage proliferation (Figure 1B), while Company A ApoA-IV also inhibited macrophage proliferation by 80% (Figure 1C).
Figure 1. Company A ApoA-I and ApoA-IV inhibited macrophage proliferation, but Company A ApoA-II did not.
Macrophages were incubated 24 hours in RPMI-1640 medium and 50 ng/ml M-CSF with either Company A ApoA-I (A), Company A ApoA-II (B), or Company A ApoA-IV (C) all derived from human plasma. Macrophage proliferation during incubations was determined by monitoring BrdU incorporation.
Next we wanted to confirm that ApoA-I obtained from other sources would also show inhibition of macrophage proliferation. E. coli bacterial recombinant ApoA-I obtained from Company B but not HEC 293 human cell-derived recombinant ApoA-I obtained from Company C inhibited macrophage proliferation (Supplemental Figure 2). Given that the bacterial-derived recombinant could be contaminated with endotoxin from the bacteria, we considered the possibility that the inhibitory effect of the ApoA-I preparations could be due to contaminating endotoxin. We directly tested this possibility by incubating the macrophage cultures with one of two different purified endotoxins, E. coli 0111:B4 and 055:B5 (Supplemental Figure 3). Both endotoxins were potent inhibitors of macrophage proliferation: 75% inhibition at 0.01 ng/ml and nearly 100% inhibition at 0.1 ng/ml.
This prompted us to examine the endotoxin contents of the various apolipoproteins. One complicating factor was that ApoA-I can bind endotoxin under certain conditions and this could mask endotoxin activity.2–5 However, the degree of this interaction would be uncertain in each apolipoprotein preparation. Thus, we measured total endotoxin activity following protease treatment of the ApoA-I in order to eliminate any potential endotoxin inhibition by ApoA-I. Endotoxin levels in the different apolipoprotein preparations showed a large variation (Table 1). Company A ApoA-II had the lowest endotoxin level (0.01 EU/μg) and this preparation as described above did not inhibit macrophage proliferation. Company A ApoA-I endotoxin levels for digested (i.e., total) and undigested (i.e., potentially masked) were 1.63 and 0.46 EU/μg, indicating that endotoxin in this ApoA-I preparation was partially inhibited by its protein content. On the other hand, Company C HEC 293 human cell-derived recombinant ApoA-I showed a digested endotoxin concentration of 1.31 EU/μg, but no endotoxin was detected in the undigested sample suggesting that some protein component (not necessarily just ApoA-I as discussed below) in this preparation completely masked endotoxin activity. Consistent with the lack of endotoxin activity, this ApoA-I preparation did not inhibit macrophage proliferation. The level of endotoxin in Company B ApoA-I was very high, some 15-times that of any of the other apolipoprotein product, likely due to the bacterial origin of this recombinant ApoA-I.
Table 1.
Comparison of endotoxin content of different apolipoprotein reagents
| source of apolipoprotein | digested (EU/μg) | undigested (EU/μg) | endotoxin in culture medium (ng/ml)1 | inhibited proliferation |
|---|---|---|---|---|
| Company A human plasma ApoA-I | 1.63 | 0.46 | 0.163 | yes |
| Company B E. coli recombinant ApoA-I | >20.00 | -- | >2.000 | yes |
| Company C HEC 293 recombinant ApoA-I | 1.31 | 0.00 | 0.131 | no |
| Company A human plasma ApoA-II | 0.01 | -- | 0.001 | no |
| Company A human plasma ApoA-IV | 0.22 | -- | 0.022 | yes |
total endotoxin when apolipoprotein was added at a concentration of 1 μg/ml of culture medium. Assumes 1 EU = 0.1 ng endotoxin.
Samples were assayed for endotoxin using the Lonza PyroGene™ assay either without or following protease digestion. Protease treatment allows for determination of total endotoxin content including endotoxin whose activity may be masked by associated protein. Under certain conditions, ApoA-I can bind endotoxin and neutralize its activity. Without protease treatment, only endotoxin that is freely available to inhibit macrophage proliferation would be measured. The endotoxin concentrations of the apolipoprotein preparations that inhibited macrophage proliferation were sufficiently high to inhibit macrophage proliferation. Interestingly, Company C ApoA-I did not inhibit macrophage proliferation, presumably because its endotoxin content was completely masked (compare protease-digested and -undigested endotoxin levels).
To further implicate endotoxin as the responsible agent mediating inhibition of macrophage proliferation and to eliminate its effect, we employed the following strategies: we reduced the endotoxin level in Company A ApoA-I by repetitive treatments with EndoBind-RTM (an affinity chromatography media utilizing a peptide comprising the sushi 3 domain of horseshoe crab Factor C that binds endotoxin6); we utilized polymyxin B, a cationic cyclic antibiotic peptide that binds and inactivates endotoxin7; and we employed the endotoxin antagonist, lipid IVa (a precursor to and antagonist of the lipid A component of endotoxin8, 9). All these approaches to minimizing endotoxin activity decreased but failed to eliminate the endotoxin effect on macrophage proliferation (Supplemental Figures 4-7).
Discussion
Research investigation often depends on the use of reagents that investigators assume are free of contaminating endotoxin. This bacterial product has many biological effects that can confound interpretations of experimental results.10–13 In the worst case, investigators wrongly attribute a biological effect to an agent under investigation rather than recognizing that the effect is due to contaminating endotoxin. Macrophages are a particularly sensitive cell type with respect to endotoxin stimulation. We have found that endotoxin contamination of apolipoproteins obtained from various commercial sources was responsible for potent inhibition of macrophage proliferation. Endotoxin was previously shown to inhibit proliferation of mouse macrophages, and as we observed here, this effect also occurs with human macrophages.14–17
Endotoxin can contaminate any reagent. Because of the extreme potency of endotoxin (sub ng/ml concentrations can be active), even seemingly low levels may be sufficient to stimulate biological responses. Reagent products labeled as endotoxin-free may only mean that the endotoxin level was below the threshold level of detection for the assay used to quantify endotoxin. Even if endotoxin is substantially removed from a reagent product, it can be reintroduced during downstream processing and packaging of the product. Thus, it would be prudent that investigators always consider that the results they observe could be due to contaminating endotoxin. However, it is likely that endotoxin contamination of reagents continues to compromise many research investigations.
One approach to examine for endotoxin effects is to check whether the reagents from different sources give the same results. Another approach is to try to reduce any endotoxin that may be present in the reagent being tested as we did in our study using EndoBind-RTM. Unfortunately, no endotoxin reduction procedure can eliminate endotoxin, and their success depends on how much endotoxin must be removed. For example, in our study, despite an 80-fold reduction in endotoxin level to a seemingly low level of 0.002 ng endotoxin/ml, some inhibition of macrophage proliferation still occurred because of the potency of endotoxin. Lastly, endotoxin contamination can be neutralized with reagents (e.g., polymyxin B) that bind to the endotoxin. However, as we show here, this approach also failed to eliminate the endotoxin effect.
Since the 1970’s, the vast majority of endotoxin detection has been done using the Limulus amebocyte lysate assay, although recently other assays have been introduced. However, all of the available assays are limited by endotoxin masking. Endotoxin is an amphipathic molecule with a net negative charge known to bind to many common proteins. Under certain conditions, ApoA-I and other amphipathic peptides bind endotoxin and block its biological action.18–22 Thus, the effect on macrophage proliferation could be the net result of the amount of endotoxin contaminating the specific ApoA-I preparation tested and the degree of ApoA-I binding to the endotoxin that neutralizes its activity. Therefore, we digested the ApoA-I reagent with protease and then assayed endotoxin activity to assess the degree of masking. Company A ApoA-I showed only partial masking of endotoxin with sufficient remaining endotoxin activity to inhibit cell proliferation. On the other hand, the Company C ApoA-I preparation completely masked its endotoxin activity, and as a result, this preparation did not inhibit cell proliferation. The reason for the difference in the degree of endotoxin masking for Company A and Company C ApoA-I preparations may be the different source of the ApoA-I. Company A ApoA-I was human plasma-derived, while Company C ApoA-I was human cell-derived recombinant ApoA-I. The recombinant ApoA-I contained a polyhistidine peptide tag, as is commonly the case to facilitate recombinant protein purification. In this regard, histidine and histidine-rich peptides bind endotoxin and neutralize its biological effects, and probably contributed to the complete masking of endotoxin in the Company C ApoA-I preparation. While Company B E. coli-derived recombinant ApoA-I also has a polyhistidine tag, the level of endotoxin in this ApoA-I was >15-fold higher than Company C human cell-derived ApoA-I, possibly superseding any neutralizing effect of the polyhistidine tag in this ApoA-I preparation.
In conclusion, apparent apolipoprotein-mediated cell effects, or effects of any other biological products, can be due to endotoxin contamination, especially when these products are tested on highly endotoxin-sensitive cells such as macrophages. Investigators should routinely consider endotoxin as a possible confounding variable in their studies. However, our findings show that removing or completely neutralizing endotoxin in contaminated reagents is not readily achieved.
Materials
RPMI-1640 medium and L-glutamine were obtained from Mediatech (Herndon, VA); fetal bovine serum (FBS)(catalog number 16000-036) was from GIBCO (Grand Island, NY); CellBIND tissue culture plates were from Corning (Corning, NY); a colorimetric bromodeoxyuridine (BrdU) cell proliferation ELISA assay kit (catalog number 11647229001) was from Roche (Indianapolis, IN); human macrophage-colony stimulating factor (M-CSF) and human interleukin-10 (IL-10) were from PeproTech (Rocky Hill, NJ); human plasma ApoA-I, ApoA-II, ApoA-IV were from Company A; E. coli recombinant ApoA-I was from Company B; HEC 293 human cell-derived recombinant ApoA-I was from Company C; lipid A and lipid IVa were from Peptide Institute (Osaka, Japan); polymyxin B, E. coli O111:B4 (L4391) and from E. coli O55:B5 (L6529) endotoxins (i.e., lipopolysaccharides) were from Sigma (St. Louis, MO); EndoPrep™ and EndoBind-R™ endotoxin preparation reagent kits were from BioDtech (Birmingham, AL); PyroGene™ endotoxin assay was from Lonza (Allendale, NJ).
Macrophage proliferation assay
Mononuclear cells were obtained from human donors by monocytopheresis and subsequently purified using counterflow centrifugal elutriation. Monocytopheresis was carried out under a human subjects research protocol approved by a National Institutes of Health institutional review board. Cells were seeded in 48-well Cellbind plates containing 0.4 ml per well at a density of 2 × 105 monocytes/cm2 in RPMI-1640 medium containing 10% FBS, 2 mM L-glutamine, 50 ng/ml M-CSF and 25 ng/ml IL-10. After 2 days incubation at 37°C with 5% CO2/95% air, cells were rinsed three times with 0.5 ml RPMI-1640 medium to remove nonadherent cells, and then cultured further with 0.4 ml of RPMI-1640 medium containing 10% FBS, 2 mM L-glutamine, 50 ng/ml M-CSF and 25 ng/ml IL-10. After an additional 3 days in culture, cells were rinsed three times with 0.5 ml RPMI-1640 medium, then cultured another day with 0.4 ml of RPMI-1640 medium containing 10% FBS, 2 mM L-glutamine, and 50 ng/ml M-CSF but without IL-10. Then, experiments were performed with IL-10 and FBS-free RPMI-1640 medium containing 50 ng/ml M-CSF and the indicated additions. Apolipoproteins were dialyzed against RPMI-1640 medium before their addition to incubations. Following 24-hour incubations, 40 μl/well BrdU labeling solution was added to the wells and the cells were incubated for 24 hours at 37°C. Cell culture plates were centrifuged at 300 xg for 10 minutes, labeling medium was removed, and culture plates were dried for 1 hour. Then, cells were fixed to avoid loss of any non-adherent cells. Fixation was carried out for 30 minutes at room temperature with 800 μl/well fixative provided with the assay kit. Next, incorporated BrdU was detected with anti-BrdU using kit reagents. The substrate product was quantified by measuring its absorbance using a scanning multiwell spectrophotometer (Vector2, PerkinEImer, Santa Clara, CA).
Cell proliferation data are presented as the mean ± SEM. Means were determined with five replicate wells. An unpaired, two-tailed Student’s t test was used for statistical comparisons. A p value = 0.05 was considered significant.
Apolipoprotein EndoPrep™ Digestion and Endotoxin Testing
In some cases, apolipoprotein samples were treated with protease to eliminate possible protein inhibition of endotoxin activity. Apolipoprotein samples were diluted to 0.1 mg/ml in EndoPrep™ digestion buffer. Then, samples were verified for appropriate pH for digestion. 270 μl of each sample was removed to a new tube. 30 μl of EndoPrep™ protease solution was added to each tube and vortexed. Samples were incubated in a 37° shaking water bath for >120 minutes, then diluted as needed in a neutral buffer.
All samples, both protease-digested and not digested, were tested in triplicate with positive product controls (PPC), two sets of standards, blanks, and appropriate controls using the PyroGeneTM assay according to the manufacturer’s specifications. Standard curves were prepared from the endotoxin standards using both linear and polynomial fitting equations. The estimated endotoxin content of each sample was calculated using both of these equations. The generation of the standard curves and the determination of endotoxin content were performed using Gen5 software associated with the BioTek Synergy2 plate reader.
PPC were prepared by mixing 270 μl of diluted sample post-digestion with 30 μl of the 10 endotoxin units (EU)/ml standard endotoxin stock provided with the PyroGene™ assay kit. Therefore, all PPC values are 1 EU/ml. The percent accuracy of each PPC reaction was determined by comparing the results with multiple PPC controls. PPC values from 50-200% were considered acceptable.
ApoA-I Detoxification with EndoBind-R™
Experiments were carried out in which we sought to reduce the endotoxin content of human plasma ApoA-I in order to learn whether endotoxin was contributing to the cell proliferation inhibition we observed with ApoA-I. Samples were prepared by combining vials of ApoA-I from Company A for starting material. The total starting material contained 2.3 mg ApoA-I in 10 mM NH4HCO3, pH 7.4. The combined solution was diluted to 1 mg/ml in 10 mM NaOAc, pH 6.0. Then, the pH was adjusted to pH 5.0 with 1 M HCl, and the molarity was adjusted to 400 mM with 5 M NaCl.
Next, a volume of 400 μl EndoBind-R™ was transferred to a microcentrifuge tube and spun at 13,000 xg for 2 minutes. The supernatant was removed and the beads washed with 400 μl 10 mM NaOAc and spun at 13,000 xg for 2 minutes. Then, the supernatant was removed and the ApoA-I sample was added to the beads and mixed at room temperature for 60 minutes. The bead/sample mixture was spun at 13,000 xg for 2 minutes and the supernatant was removed to a clean tube. This series of steps was repeated a total of 4 times. After each series, a sample was removed and tested in triplicate for protein content using absorbance at optical density 280 nm and endotoxin content using a combination of EndoPrep™ digestion and PyroGene™ assay.
Supplementary Material
Supplemental Figure 1. ApoA-I changed macrophage morphology and cell density. Macrophages were incubated 24 hours in RPMI-1640 medium and 50 ng/ml M-CSF either without (A) or with (B) human plasma-derived ApoA-I (10 μg/ml) from Company A. Macrophages became round and cultures were less dense than control macrophages suggesting that ApoA-I decreased macrophage proliferation. Bar in A also applies to B.
Supplemental Figure 2. Company B Apo A-I inhibited macrophage proliferation but Company C ApoA-I did not. Macrophages were incubated 24 hours in RPMI-1640 medium and 50 ng/ml M-CSF with 1 μg/ml of either E. coli bacterial-derived recombinant ApoA-I obtained from Company B or HEC 293 human cell-derived recombinant ApoA-I obtained from Company C. Macrophage proliferation during incubations was determined by monitoring BrdU incorporation.
Supplemental Figure 3. Endotoxin inhibited macrophage proliferation. Macrophages were incubated 24 hours in RPMI-1640 medium and 50 ng/ml M-CSF with varying concentrations of endotoxins, E. coli O111:B4 (A) and E. coli O55:B5 (B). Macrophage proliferation during incubations was determined by monitoring BrdU incorporation. Both endotoxins completely inhibited macrophage proliferation at the low dose of 0.1 ng/ml.
Supplemental Figure 4. Reducing endotoxin in ApoA-I reduced its inhibition of macrophage proliferation. Endotoxin was reduced in Company A human plasma-derived ApoA-I by repetitive treatments with EndoBind-R™ (a chromatography medium containing the sushi 3 domain of horseshoe crab Factor C peptide bound to a 4% cross-linked beaded agarose support resin). Purification resulted in a decrease in ApoA-I starting material from 2.3 mg to 0.8 mg. At the same time, the endotoxin content was reduced from 1.63 EU/μg to 0.02 EU/μg. Macrophages were incubated 24 hours in RPMI-1640 medium and 50 ng/ml M-CSF with either ApoA-I or endotoxin-reduced ApoA-I. Macrophage proliferation during incubations was determined by monitoring BrdU incorporation. Reducing the endotoxin content of ApoA-I reduced ApoA-I inhibition of macrophage proliferation, consistent with endotoxin mediating the inhibition.
Supplemental Figure 5. Polymyxin B decreased Company A ApoA-I inhibition of macrophage proliferation. Macrophages were incubated 24 hours in RPMI-1640 medium, 50 ng/ml M-CSF, and Company A human plasma-derived ApoA-I (1μg/ml) either without or with polymyxin B (10 μg/ml), a known inhibitor of endotoxin. During the incubations, macrophage proliferation was determined by monitoring BrdU incorporation. Polymyxin B substantially decreased Company A ApoA-I inhibition of macrophage proliferation, further confirming that endotoxin mediated Company A ApoA-I inhibition of macrophage proliferation.
Supplemental Figure 6. Lipid A component of endotoxin inhibits macrophage proliferation. Macrophages were incubated 24 hours in RPMI-1640 medium with 50 ng/ml M-CSF and either the indicated concentration of lipid A or its precursor, lipid IVa. During the incubations, macrophage proliferation was determined by monitoring BrdU incorporation. Lipid A substantially decreased macrophage proliferation (>85%), while lipid IVa showed little effect on macrophage proliferation (<10% inhibition).
Supplemental Figure 7. Endotoxin competitive inhibitor, lipid IVa, decreased ApoA-I inhibition of macrophage proliferation. Macrophages were incubated 24 hours in RPMI-1640 medium with 50 ng/ml M-CSF and either Company A human plasma-derived ApoA-I (1 μg/ml), lipid IVa (1 μg/ml), or ApoA-I plus lipid IVa. During the incubations, macrophage proliferation was determined by monitoring BrdU incorporation. Lipid IVa substantially decreased ApoA-I inhibition of macrophage proliferation. Lipid IVa added alone showed no effect on macrophage proliferation.
Highlights.
Apolipoproteins may be contaminated with endotoxin
Endotoxin contamination of apolipoproteins confounds interpretation of experimental results
Endotoxin inhibits macrophage proliferation
Acknowledgments
We thank the Department of Transfusion Medicine, Clinical Center, National Institutes of Health, for providing elutriated monocytes. The Intramural Research Program, National Heart, Lung, and Blood Institute, National Institutes of Health supported this work.
This work was supported by the Intramural Research Program, National Heart, Lung, and Blood Institute, National Institutes of Health.
Abbreviations
- Apo
apolipoprotein
- BrdU
bromodeoxyuridine
- EU
endotoxin units
- FBS
fetal bovine serum
- IL-10
interleukin-10
- M-CSF
macrophage-colony stimulating factor
- PPC
positive product controls
Footnotes
Conflict of interest statement
Keith Champion is an employee of BioDtech Inc. that offers products and services related to endotoxin analysis and removal, some of which were utilized in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zanoni I, Granucci F. Differences in lipopolysaccharide-induced signaling between conventional dendritic cells and macrophages. Immunobiology. 2010;215:709–712. doi: 10.1016/j.imbio.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Ma J, Liao XL, Lou B, et al. Role of apolipoprotein A-I in protecting against endotoxin toxicity. Acta biochimica et biophysica Sinica. 2004;36:419–424. doi: 10.1093/abbs/36.6.419. [DOI] [PubMed] [Google Scholar]
- 3.Henning MF, Herlax V, Bakas L. Contribution of the C-terminal end of apolipoprotein AI to neutralization of lipopolysaccharide endotoxic effect. Innate Immun. 2011;17:327–337. doi: 10.1177/1753425910370709. [DOI] [PubMed] [Google Scholar]
- 4.Park CT, Wright SD. Plasma lipopolysaccharide-binding protein is found associated with a particle containing apolipoprotein A-I, phospholipid, and factor H-related proteins. The Journal of biological chemistry. 1996;271:18054–18060. doi: 10.1074/jbc.271.30.18054. [DOI] [PubMed] [Google Scholar]
- 5.Massamiri T, Tobias PS, Curtiss LK. Structural determinants for the interaction of lipopolysaccharide binding protein with purified high density lipoproteins: role of apolipoprotein A-I. J Lipid Res. 1997;38:516–525. [PubMed] [Google Scholar]
- 6.Tan NS, Ng ML, Yau YH, et al. Definition of endotoxin binding sites in horseshoe crab factor C recombinant sushi proteins and neutralization of endotoxin by sushi peptides. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2000;14:1801–1813. doi: 10.1096/fj.99-0866com. [DOI] [PubMed] [Google Scholar]
- 7.Cooperstock MS. Inactivation of endotoxin by polymyxin B, Antimicrob. Agents Chemother. 1974;6:422–425. doi: 10.1128/aac.6.4.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loppnow H, Brade H, Durrbaum I, et al. IL-1 induction–capacity of defined lipopolysaccharide partial structures. J Immunol. 1989;142:3229–3238. [PubMed] [Google Scholar]
- 9.Golenbock DT, Hampton RY, Qureshi N, et al. Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. The Journal of biological chemistry. 1991;266:19490–19498. [PubMed] [Google Scholar]
- 10.Majde JA. Microbial cell-wall contaminants in peptides: a potential source of physiological artifacts. Peptides. 1993;14:629–632. doi: 10.1016/0196-9781(93)90155-a. [DOI] [PubMed] [Google Scholar]
- 11.Wakelin SJ, Sabroe I, Gregory CD, et al. “Dirty little secrets”--endotoxin contamination of recombinant proteins. Immunol Lett. 2006;106:1–7. doi: 10.1016/j.imlet.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Smulders S, Kaiser JP, Zuin S, et al. Contamination of nanoparticles by endotoxin: evaluation of different test methods. Part Fibre Toxicol. 2012;9:41. doi: 10.1186/1743-8977-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wakelin SJ, Forsythe JL, Garden OJ, et al. Commercially available recombinant sonic hedgehog up-regulates Ptc and modulates the cytokine and chemokine expression of human macrophages: an effect mediated by endotoxin contamination? Immunobiology. 2008;213:25–38. doi: 10.1016/j.imbio.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Vairo G, Argyriou S, Knight KR, et al. Inhibition of colony-stimulating factor-stimulated macrophage proliferation by tumor necrosis factor-alpha, IFN-gamma, and lipopolysaccharide is not due to a general loss of responsiveness to growth factor. J Immunol. 1991;146:3469–3477. [PubMed] [Google Scholar]
- 15.Chang CY, Tucci M, Baker RC. Lipopolysaccharide-stimulated nitric oxide production and inhibition of cell proliferation is antagonized by ethanol in a clonal macrophage cell line. Alcohol. 2000;20:37–43. doi: 10.1016/s0741-8329(99)00054-3. [DOI] [PubMed] [Google Scholar]
- 16.Raschke WC, Baird S, Ralph P, et al. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978;15:261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- 17.Xaus J, Cardo M, Valledor AF, et al. Interferon gamma induces the expression of p21waf-1 and arrests macrophage cell cycle, preventing induction of apoptosis. Immunity. 1999;11:103–113. doi: 10.1016/s1074-7613(00)80085-0. [DOI] [PubMed] [Google Scholar]
- 18.Flegel WA, Baumstark MW, Weinstock C, et al. Prevention of endotoxin-induced monokine release by human low- and high-density lipoproteins and by apolipoprotein A-I, Infect. Immun. 1993;61:5140–5146. doi: 10.1128/iai.61.12.5140-5146.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang GH, Baek L, Bertelsen T, et al. Quantification of the endotoxin-neutralizing capacity of serum and plasma. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 1995;103:721–730. doi: 10.1111/j.1699-0463.1995.tb01429.x. [DOI] [PubMed] [Google Scholar]
- 20.Ogata M, Fletcher MF, Kloczewiak M, et al. Effect of anticoagulants on binding and neutralization of lipopolysaccharide by the peptide immunoglobulin conjugate CAP18(106-138)-immunoglobulin G in whole blood, Infect. Immun. 1997;65:2160–2167. doi: 10.1128/iai.65.6.2160-2167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bocharov AV, Baranova IN, Vishnyakova TG, et al. Targeting of scavenger receptor class B type I by synthetic amphipathic alpha-helical-containing peptides blocks lipopolysaccharide (LPS) uptake and LPS-induced pro-inflammatory cytokine responses in THP-1 monocyte cells. The Journal of biological chemistry. 2004;279:36072–36082. doi: 10.1074/jbc.M314264200. [DOI] [PubMed] [Google Scholar]
- 22.Emancipator K, Csako G, Elin RJ. In vitro inactivation of bacterial endotoxin by human lipoproteins and apolipoproteins, Infect. Immun. 1992;60:596–601. doi: 10.1128/iai.60.2.596-601.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. ApoA-I changed macrophage morphology and cell density. Macrophages were incubated 24 hours in RPMI-1640 medium and 50 ng/ml M-CSF either without (A) or with (B) human plasma-derived ApoA-I (10 μg/ml) from Company A. Macrophages became round and cultures were less dense than control macrophages suggesting that ApoA-I decreased macrophage proliferation. Bar in A also applies to B.
Supplemental Figure 2. Company B Apo A-I inhibited macrophage proliferation but Company C ApoA-I did not. Macrophages were incubated 24 hours in RPMI-1640 medium and 50 ng/ml M-CSF with 1 μg/ml of either E. coli bacterial-derived recombinant ApoA-I obtained from Company B or HEC 293 human cell-derived recombinant ApoA-I obtained from Company C. Macrophage proliferation during incubations was determined by monitoring BrdU incorporation.
Supplemental Figure 3. Endotoxin inhibited macrophage proliferation. Macrophages were incubated 24 hours in RPMI-1640 medium and 50 ng/ml M-CSF with varying concentrations of endotoxins, E. coli O111:B4 (A) and E. coli O55:B5 (B). Macrophage proliferation during incubations was determined by monitoring BrdU incorporation. Both endotoxins completely inhibited macrophage proliferation at the low dose of 0.1 ng/ml.
Supplemental Figure 4. Reducing endotoxin in ApoA-I reduced its inhibition of macrophage proliferation. Endotoxin was reduced in Company A human plasma-derived ApoA-I by repetitive treatments with EndoBind-R™ (a chromatography medium containing the sushi 3 domain of horseshoe crab Factor C peptide bound to a 4% cross-linked beaded agarose support resin). Purification resulted in a decrease in ApoA-I starting material from 2.3 mg to 0.8 mg. At the same time, the endotoxin content was reduced from 1.63 EU/μg to 0.02 EU/μg. Macrophages were incubated 24 hours in RPMI-1640 medium and 50 ng/ml M-CSF with either ApoA-I or endotoxin-reduced ApoA-I. Macrophage proliferation during incubations was determined by monitoring BrdU incorporation. Reducing the endotoxin content of ApoA-I reduced ApoA-I inhibition of macrophage proliferation, consistent with endotoxin mediating the inhibition.
Supplemental Figure 5. Polymyxin B decreased Company A ApoA-I inhibition of macrophage proliferation. Macrophages were incubated 24 hours in RPMI-1640 medium, 50 ng/ml M-CSF, and Company A human plasma-derived ApoA-I (1μg/ml) either without or with polymyxin B (10 μg/ml), a known inhibitor of endotoxin. During the incubations, macrophage proliferation was determined by monitoring BrdU incorporation. Polymyxin B substantially decreased Company A ApoA-I inhibition of macrophage proliferation, further confirming that endotoxin mediated Company A ApoA-I inhibition of macrophage proliferation.
Supplemental Figure 6. Lipid A component of endotoxin inhibits macrophage proliferation. Macrophages were incubated 24 hours in RPMI-1640 medium with 50 ng/ml M-CSF and either the indicated concentration of lipid A or its precursor, lipid IVa. During the incubations, macrophage proliferation was determined by monitoring BrdU incorporation. Lipid A substantially decreased macrophage proliferation (>85%), while lipid IVa showed little effect on macrophage proliferation (<10% inhibition).
Supplemental Figure 7. Endotoxin competitive inhibitor, lipid IVa, decreased ApoA-I inhibition of macrophage proliferation. Macrophages were incubated 24 hours in RPMI-1640 medium with 50 ng/ml M-CSF and either Company A human plasma-derived ApoA-I (1 μg/ml), lipid IVa (1 μg/ml), or ApoA-I plus lipid IVa. During the incubations, macrophage proliferation was determined by monitoring BrdU incorporation. Lipid IVa substantially decreased ApoA-I inhibition of macrophage proliferation. Lipid IVa added alone showed no effect on macrophage proliferation.