Abstract
The swim bladder is a gas-filled organ that is used for regulating buoyancy and is essential for survival in most teleost species. In zebrafish, swim bladder development begins during embryogenesis and inflation occurs within 5 days post fertilization (dpf). Embryos exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) before 96 hours post fertilization (hpf) developed swim bladders normally until the growth/elongation phase, at which point growth was arrested. It is known that TCDD exposure causes heart malformations that lead to heart failure in zebrafish larvae, and that blood circulation is a key factor in normal development of the swim bladder. The adverse effects of TCDD exposure on the heart occur during the same period of time that swim bladder development and growth occurs. Based on this coincident timing, and the dependence of swim bladder development on proper circulatory development, we hypothesized that the adverse effects of TCDD on swim bladder development were secondary to heart failure. We compared swim bladder development in TCDD-exposed embryos to: (1) silent heart morphants, which lack cardiac contractility, and (2) transiently transgenic cmlc2:caAHR-2AtRFP embryos, which mimic TCDD-induced heart failure via heart-specific, constitutive activation of AHR signaling. Both of these treatment groups, which were not exposed to TCDD, developed hypoplastic swim bladders of comparable size and morphology to those found in TCDD-exposed embryos. Furthermore, in all treatment groups swim bladder development was arrested during the growth/elongation phase. Together, these findings support a potential role for heart failure in the inhibition of swim bladder development caused by TCDD.
Keywords: TCDD, zebrafish, swim bladder, heart failure, development, aryl hydrocarbon receptor
1. Introduction
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD or dioxin) is a lipophilic, halogenated aromatic hydrocarbon that is persistent, bioaccumulative, and ubiquitously found in the environment. It is the most potent of the halogenated aromatic hydrocarbons (HAHs) (Hankinson, 1995). These compounds are agonists of the aryl hydrocarbon receptor (AHR). When activated by TCDD, AHR translocates into the nucleus, forms a heterodimer with aryl hydrocarbon nuclear translocator (ARNT), and binds to recognition sites on the DNA sequence, leading to regulation of gene expression (Nguyen and Bradfield, 2008; Schmidt and Bradfield, 1996; Tanguay et al., 2005).
TCDD has been observed to adversely affect the development of many vertebrate species, and fish are especially sensitive to TCDD developmental toxicity (Elonen et al., 1998; Peterson et al., 1993; Walker and Peterson, 1994). Adverse developmental effects of TCDD in fish larvae include heart malformations and heart failure, pericardial edema, yolk sac edema, meningeal edema, hemorrhage, craniofacial malformations, growth arrest, and mortality (reviewed in King-Heiden et al., 2012). One of the hallmark effects of TCDD developmental toxicity in fish larvae is failure of the swim bladder to inflate properly (Henry et al., 1997; Oritz-Delagado et al., 2004).
The swim bladder is present in approximately half of all modern teleost fish species (Denton, 1961). It is a gas-filled sac located dorsal to the gut, used to regulate buoyancy and occasionally for acoustic sensation (Alexander, 1993; Evans, 1925; Zeddies and Fay, 2005). It is crucial for survival in most fish species because it minimizes energy required to maintain vertical position in the water column (Alexander, 1972). Aside from TCDD, exposure to other AHR agonists such as PCB126 also inhibits swim bladder inflation in zebrafish (Jönsson et al., 2012).
In zebrafish, development of the swim bladder occurs in three phases: budding, growth/elongation, and inflation (Winata et al., 2009). Budding phase lasts from 36-65 hours post fertilization (hpf) and involves initiation of the swim bladder bud, which forms as an evagination of the foregut and consists entirely of epithelial cells. The growth/elongation phase (65-96 hpf) involves elongation of the swim bladder bud to form the pneumatic duct, and growth of the swim bladder. At this stage three distinct tissue layers form in the swim bladder: an epithelial layer (from the bud), surrounded by a mesenchymal layer, followed by an outer mesothelium (Finney et al., 2006; Winata et al., 2009). Each layer has unique expression of gene markers and coordinated growth and organization occurs via crosstalk between the layers using signals, such as hedgehog and Wnt (Winata et al. 2009; Winata et al., 2010). The final inflation phase involves inflation of the single-chambered swim bladder (presumptive posterior chamber) at 96-120 hpf via air-gulping (Goolish and Okutake, 1999; Winata et al., 2009). Later, at 20-21 days post fertilization (dpf), inflation of the anterior chamber occurs, and development of the two-chambered swim bladder as seen in adults is complete (Robertson et al., 2007; Winata et al., 2009).
While it is well known that TCDD exposure inhibits inflation of the swim bladder, the potential mechanisms underlying this observed phenotype have not been elucidated. It was previously assumed that TCDD acts directly on the swim bladder, either by interference with development of presumptive swim bladder cells or by causing cellular necrosis in the swim bladder. However, Winata et al. (2010) showed that normal blood circulation plays an important role in swim bladder development. This is significant because TCDD causes heart malformations that culminate in heart failure and a complete loss of circulation (Antkiewicz et al., 2005; Belair et al., 2001; Henry et al., 1997). Therefore, we hypothesized that TCDD-induced heart failure impairs development of the swim bladder secondary to circulatory failure.
Here we show that TCDD impairs development of the swim bladder in zebrafish larvae by arresting swim bladder development during the growth/elongation phase. We propose that this effect may be secondary to TCDD-induced heart failure because the two effects temporally coincide. In support of this hypothesis, we show that silent heart morphant larvae and transiently transgenic cmlc2:caAHR-2AtRFP larvae, neither one exposed to TCDD, also develop heart failure and disrupted swim bladder development. In addition, the impaired swim bladder development of these larvae temporally coincides with effects observed in TCDD-exposed embryos. Furthermore, these larvae phenocopied the gross morphology and histology of disrupted swim bladder development in TCDD-exposed embryos.
2. Materials and Methods
2.1. Zebrafish and TCDD exposure
Embryos were obtained from adult zebrafish (Danio rerio) housed and maintained according to methods described by Westerfield (2000). AB wild-type strain zebrafish were used in all experiments unless otherwise indicated. Eggs were collected within 4 h of spawning and fertilized eggs were placed into a large petri dish with egg water (60 μg/ml Instant Ocean Sea Salts with 0.2 ppm methylene blue) until appropriate age for use in experiments was reached. Clean water changes were made daily.
Zebrafish embryos or larvae were statically exposed in water to either TCDD (1 ng/ml) or vehicle (0.1% dimethyl sulfoxide, DMSO) for 1 h in 4 ml glass scintillation vials, with gentle rocking. Ten embryos or larvae were present per ml of dosing solution, with a total of 20 embryos or larvae in each vial. After 1 h exposure to TCDD or vehicle, embryos or larvae were rinsed with TCDD-free water at least three times and placed in 100 mm petri dishes with clean water. Embryos and larvae were raised, with daily water changes, until the age when measurements were made (48, 72, 96, or 120 hpf).
All procedures involving zebrafish were approved by the Animal Care and Use Committee of the University of Wisconsin-Madison and adhered to the National Institute of Health’s “Guide for the Care and Use of Laboratory Animals.”
2.2. Silent heart morphants
Silent heart (sih; cardiac troponin T2, tnnt2) morpholino was obtained from Gene Tools (Philomath, OR). A 2 nM morpholino solution was prepared and microinjected into fertilized AB strain eggs at the 1-2 cell stage, as previously described (Carney et al., 2004; Antkiewicz et al. 2006). The Gene Tools standard control morpholino (control MO) was used as a control. Embryos were screened for incorporation of morpholino at 48 hpf.
2.3. Heart-specific constitutively activated AHR transient transgenic fish, cmlc2:caAHR-2AtRFP
Newly fertilized AB strain eggs were microinjected with either cmlc2:caAHR-2AtRFP or cmlc2:caAHR−dbd-2AtRFP DNA plasmid construct (negative control), designed and generously donated by Dr. Kevin Lanham (Lanham et al., 2014). Briefly, the cmlc2:caAHR-2AtRFP construct constitutively activates AHR signaling in cells expressing the cardiomyocyte-specific cmlc2 (myosin light chain 7, myl7) promoter, and production of red fluorescent protein (RFP) is used as an indicator of successful construct incorporation and therefore activation of AHR signaling. The cmlc2:caAHR−dbd-2AtRFP construct served as a negative control and is similar to the cmlc2:caAHR-2AtRFP construct except for an insertion mutation in the Rgs domain that disrupts DNA binding and hence there is no constitutive activation of AHR signaling. Construct injections were done as previously described by Lanham et al. (2012). Embryos were screened at 48 hpf for use in experiments.
2.4. Histology
Embryos or larvae were euthanized using tricaine (MS 222, Sigma) and fixed in 4% parafomaldehyde in phosphate buffered solution (PBS) overnight at 4 °C. Embryos were then dehydrated in a graded ethanol series and stored at −20 °C until time for processing. To align samples for sagittal sectioning, stored embryos were re-hydrated into PBS in a graded series, oriented laterally in 0.5% agarose under a dissecting microscope, and completely dehydrated into ethanol before embedding into paraffin. Sections were made in 8 μm thickness, stained with hematoxylin and eosin (H&E), and mounted on glass microscope slides with permount as previously described (King Heiden et al., 2009).
2.5. Imaging and analysis of swim bladder areas
At age 120 hpf, DMSO- and TCDD-treated larvae were placed in 3% methylcellulose to characterize TCDD-induced effects on the swim bladder. Live imaging and imaging of H&E sections were done using an Olympus SZX16 camera mounted on an Olympus DP72 epifluorescent microscope with cellSens Digital Imaging software.
Swim bladder area was measured using Image J software from images of H&E sections taken at 12.4× magnification. Only images of sections where the pneumatic duct was clearly visible were used for swim bladder area analysis, and such a section image from an individual fish that met this requirement was considered n = 1 for that cohort for statistics.
2.6. Statistics
Student’s t-test was used to compare mean swim bladder area in TCDD-treated embryos dosed at 4, 24, 48, 72, or 96 hpf to DMSO-treated embryos (control) dosed at the same time. Data were square-root transformed and checked with the F-test before analysis in order to meet the condition of homoscedasticity required for Student’s t-test. Significance was set at p ≤ 0.01. One-way analysis of variance (ANOVA) followed by Tukey’s HSD test was conducted to compare swim bladder area in TCDD-treated embryos, silent heart morphants, cmlc2:caAHR-2AtRFP transient transgenic embryos, and their respective controls. These data were not transformed for analysis, as all required conditions for ANOVA were met. Significance was set at p ≤ 0.01. All cohorts had a sample size of n = 10 to 13. All statistical analyses were done using GraphPad Prism statistics software.
3. Results
3.1. TCDD exposure impairs swim bladder development
Exposure to TCDD causes developmental toxicity in zebrafish larvae, which is clearly manifested by 120 hpf (Fig. 1). Many of these effects were reviewed by King-Heiden et al. (2012), and include heart malformations culminating in heart failure and vasculature malformations (not shown), craniofacial and jaw malformations, pericardial edema, yolk sac edema, and meningeal edema (Fig. 1B, black arrow heads). Of particular interest for the present study is the inflated swim bladder, which is clearly visible in control embryos exposed to DMSO (Fig. 1A, white arrow head). Conversely, the swim bladder is either absent or uninflated in the representative TCDD-exposed larvae (Fig. 1B, white arrow head).
Figure 1. Zebrafish embryos exposed to TCDD do not develop an inflated swim bladder by 120 hpf.
Embryos exposed to (A) 0.1% DMSO for 1 h at 4 hpf develop a functional, inflated, single-chamber swim bladder by age 120 hpf (white arrow head). In contrast, embryos exposed to (B) TCDD (1 ng/ml) for 1 h at 4 hpf have an undeveloped, uninflated swim bladder (white arrow head). In addition, there is craniofacial malformation and meningeal, pericardial, and yolk sac edema (B, black arrow heads). Scale bars represent 500 μm.
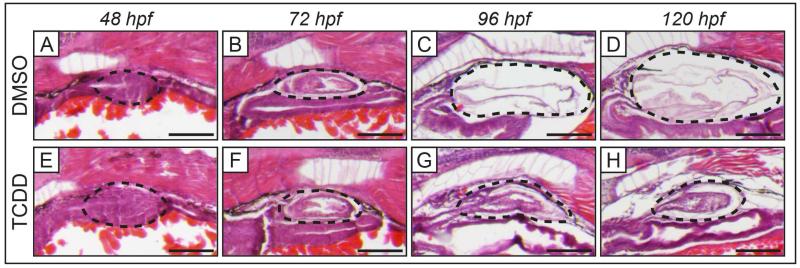
To investigate the effect of TCDD on development of the swim bladder, zebrafish embryos were exposed to DMSO (vehicle control) or TCDD for 1 h beginning at 4 hpf and collected at different stages of swim bladder development (Fig. 2). In control fish the swim bladder bud is observed forming from a dorsal evagination of the foregut at 48 hpf (Fig. 2A). The bud elongates to form the pneumatic duct that connects to the primitive swim bladder by 72 hpf, which shows a thick-walled morphology (Fig. 2B). The swim bladder then enters a period of growth where it increases in size and layers of the swim bladder wall become thinner (Fig. 2C). Between 96 and 120 hpf the swim bladder inflates, at which point it is easily identifiable in free-swimming larvae (Fig. 1A, Fig. 2D). Embryos exposed to TCDD seem to develop a swim bladder normally until 72 hpf (Fig. 2E-H). More specifically, initiation of the swim bladder bud at 48 hpf, followed by bud elongation and initial growth at 72 hpf appears to occur normally (Fig. 2E-F). However, further development is severely impaired beyond 72 hpf, such that by 120 hpf the swim bladder is significantly smaller than in similarly-aged controls and is not inflated (Fig. 2G-H). The swim bladder of the TCDD-exposed embryo at 120 hpf has morphology similar to a developing swim bladder at 72 hpf: a small, uninflated sac with thick walls (compare Fig. 2B and 2H). Overall, this suggests that TCDD does not affect initiation, elongation, or early growth of the swim bladder bud; rather arrests development of the swim bladder at 72 hpf, just before it enters the phase of extensive growth.
Figure 2. Exposure to TCDD arrests development of the swim bladder during the growth/elongation phase in embryonic zebrafish.
Histology of swim bladder development in embryos at 48, 72, 96, and 120 hpf when exposed for 1 h beginning at 4 hpf to DMSO (control, A-D) or TCDD (1 ng/ml, E-H). Normal swim bladder development is observed in embryos exposed to DMSO: initiation of swim bladder bud from foregut (A), bud elongation and growth (B), and inflation (C-D). TCDD-exposed embryos have developing swim bladder morphology indistinguishable from control at 48 and 72 hpf (E-F), suggesting that TCDD does not interrupt budding and initial growth of the swim bladder bud. Swim bladder development in TCDD-exposed larvae is impaired beyond 72 hpf (G-H). By 120 hpf the TCDD-exposed swim bladder is smaller, uninflated, and has morphology more similar to the 72 hpf swim bladder than control at 120 hpf. Black dotted outlines indicate developing swim bladder, scale bars represent 100 μm. In all panels, the representative embryos are positioned laterally, with anterior to the left.
3.2. Swim bladder development is sensitive to the time of TCDD exposure
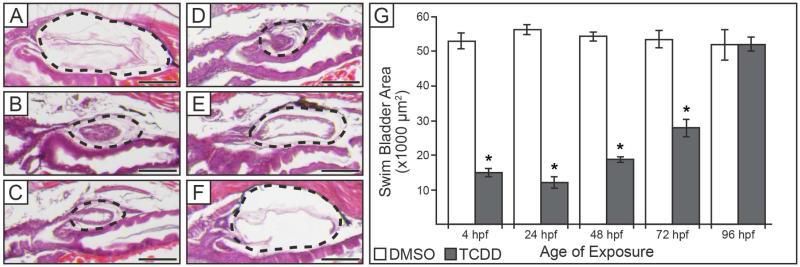
To determine whether there is a specific window of time in which swim bladder development is particularly sensitive to TCDD, zebrafish embryos were exposed to either DMSO or TCDD at different ages, and swim bladder size and histology were assessed at 120 hpf. All embryos exposed to DMSO developed fully inflated swim bladders of comparable size by 120 hpf (Fig. 3A, G). In contrast, embryos exposed to TCDD at 4, 24, or 48 hpf had hypoplastic swim bladders at 120 hpf (Fig. 3B-D). These swim bladders had morphology comparable to developing swim bladders at 72 hpf, and were significantly smaller than their respective DMSO-exposed controls at 120 hpf (Fig. 3G, p ≤ 0.01). Embryos exposed to TCDD at 96 hpf were able to develop an inflated swim bladder that was comparable in size and morphology to control at both 120 hpf (Fig. 3F, G), and up until mortality at approximately 168 hpf when assessment of swim bladder development ended (data not shown). Embryos exposed to TCDD at 72 hpf, when extensive growth of the swim bladder begins, were able to develop a small, partially-inflated swim bladder by 120 hpf (Fig. 3E). These swim bladders were still significantly smaller than control (Fig. 3G, p ≤ 0.01) and displayed morphology intermediate between TCDD-treated embryos exposed at earlier ages (4, 24, or 48 hpf) and control embryos (compare Fig. 3E to 3A-D).
Figure 3. TCDD inhibits swim bladder development when exposure occurs before 96 hpf.
(A-F) Histology of swim bladders at 120 hpf when exposed to either (A) DMSO, or (B-F) TCDD. Exposure to TCDD was started at either: (B) 4, (C) 24, (D) 48, (E) 72, or (F) 96 hpf. Black dotted outlines indicate swim bladder, scale bars represent 100 μm. (G) Mean area of swim bladders (± SEM, n = 10 to 13) at 120 hpf when exposed to DMSO or TCDD at 4, 24, 48, 72, or 96 hpf. Swim bladder areas were measured from H&E sections where the pneumatic duct was clearly visible. Embryos exposed to TCDD before 96 hpf had swim bladders significantly reduced in size, while embryos exposed to TCDD beginning at 96 hpf had swim bladder areas similar to control. Presence of an asterisk indicates swim bladder area in the TCDD-treated group is significantly less than control (p ≤ 0.01).
Overall, these results suggest that the developing swim bladder is most sensitive to TCDD-induced disruption of development when TCDD exposure occurs prior to 72 hpf. TCDD exposure at 96 hpf is ineffective in disrupting swim bladder development, while exposure at 72 hpf is only partially effective.
3.3. Swim bladder development is impaired in the silent heart morphant
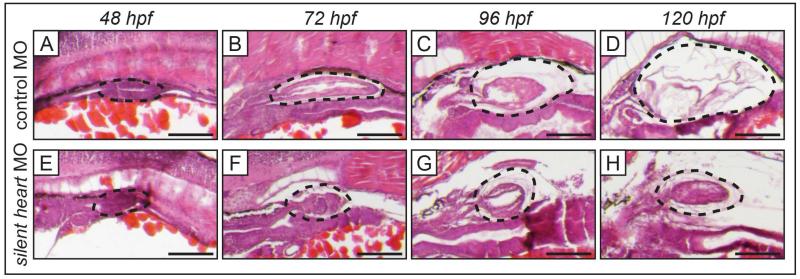
The silent heart morphant lacks cardiac contractility and develops many gross morphological characteristics by 120 hpf that are similar to embryos exposed to TCDD at 4 hpf, including an un-looped heart, heart failure, pericardial edema, and an uninflated swim bladder. In the present study, embryos injected with control MO developed swim bladders normally, such that by 120 hpf an inflated swim bladder was clearly visible (Fig. 4A-D). In contrast, silent heart morphants had a hypoplastic swim bladder at 120 hpf (Fig. 4E-H). In the silent heart morphant, initiation of swim bladder bud, elongation, and initial growth of the primitive swim bladder progressed normally through 48 and 72 hpf (Fig. 4E-F). At these ages the silent heart morphant had morphology indistinguishable from control (compare Fig. 4A-B and 4E-F). Beginning at 96 hpf, the silent heart morphant swim bladder did not increase in size, nor did it transition to the thinner-walled morphology typically seen in controls at 120 hpf (compare Fig. 4C-D and 4G-H).
Figure 4. Swim bladder development is impaired in silent heart morphants.
Histology of swim bladder development of silent heart morphants with heart failure (E-H), and embryos injected with control MO (A-D), at 48 to 120 hpf. Control embryos developed swim bladders normally. Silent heart morphants underwent normal swim bladder budding (E), elongation, and initial growth (F), however, subsequent growth beginning at 96 hpf was disrupted (G-H). By 120 hpf the silent heart morphant swim bladder is significantly smaller and has thick-walled morphology (H) compared to control (D). Black dotted outlines indicate developing swim bladder, scale bars represent 100 μm.
3.4. Swim bladder development is impaired in transient transgenic fish where AHR signaling is constitutively activated in the heart
Since impaired development of the swim bladder in silent heart morphants resembled impaired swim bladder development in TCDD-exposed embryos, we investigated whether TCDD-induced heart failure was sufficient to impair swim bladder development. However, it is not possible to limit TCDD exposure to only one organ, such as the heart, without simultaneously exposing other organs, such as the developing swim bladder, to TCDD as well. TCDD causes embryo toxicity in zebrafish by activating AHR signaling (Prasch et al., 2003; Prasch et al. 2006). The cmlc2:caAHR-2AtRFP transient transgenic zebrafish embryo phenocopies TCDD-induced heart malformations and heart failure, in the absence of TCDD, by constitutively activating AHR signaling in only the heart myocytes (Lanham et al., 2014). It is important to note that AHR signaling is not activated in any other cell types, including the developing swim bladder, in embryos expressing this heart-specific, constitutively active, AHR construct (Lanham et al., 2014).
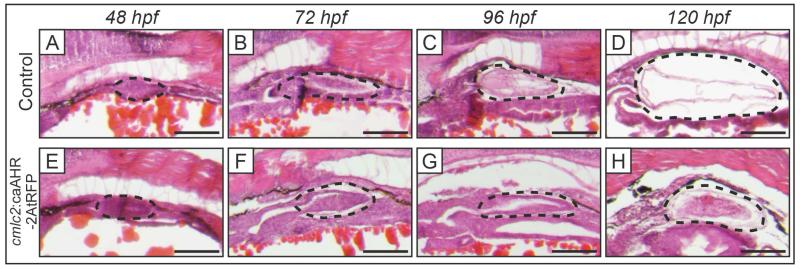
Control embryos injected with a nonfunctional construct, cmlc2:caAHR−dbd-2AtRFP, which contains a mutated DNA binding domain that prevents activation of constitutively activated AHR signaling in the heart (Lanham et al., 2014), develop normal swim bladders that inflate by 120 hpf (Fig. 5A-D). In contrast, at 120 hpf, cmlc2:caAHR-2AtRFP embryos with constitutively active AHR signaling in the heart produced the previously reported heart failure and malformation (not shown) and developed hypoplastic, uninflated swim bladders (Fig. 5H). The latter, transient transgenic embryos, displayed normal initiation of swim bladder bud formation, elongation, and initial growth of the swim bladder until 72 hpf, after which further development was significantly impaired (Fig. 5E-H). The cmlc2:caAHR-2AtRFP larvae swim bladder at 96 and 120 hpf had similar size and thick-walled morphology to the 72 hpf developing swim bladder, suggesting development had been arrested at this age (Fig. 5G-H).
Figure 5. Swim bladder development is impaired in cmlc2:caAHR-2AtRFP transient transgenic fish.
Histology of swim bladder development in transient transgenic fish where AHR signaling was constitutively activated only in heart myocytes expressing the cardiomyocyte-specific gene cmlc2 (cmlc2:caAHR-2AtRFP, E-H), and embryos injected with a nonfunctional cmlc2:caAHR−dbd-2AtRFP control plasmid (Control, A-D), at 48 to 120 hpf. Control embryos developed swim bladders normally (A-D). Conversely, cmlc2:caAHR-2AtRFP embryos had normal swim bladder budding, elongation, and initial growth (E-F), but development was disrupted beginning at 96 hpf (G-H). By 120 hpf the cmlc2:caAHR-2AtRFP larvae had distinctly smaller swim bladders whose morphology was different from control. Black dotted outlines indicate developing swim bladder, scale bars represent 100 μm.
3.5. Swim bladder development is inhibited in zebrafish larvae when heart failure is induced by: TCDD exposure, silent heart morpholino, or heart-specific constitutively activated AHR signaling
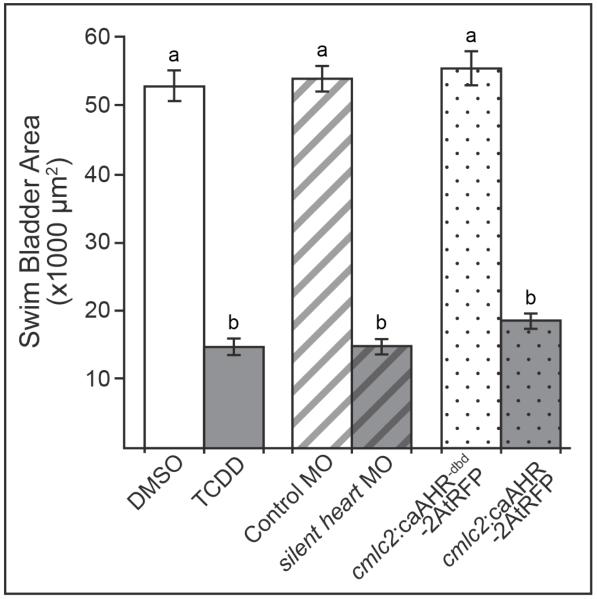
It is well known that circulatory failure due to TCDD-induced heart malformation and heart failure plays an important role in TCDD developmental toxicity in zebrafish larvae (Carney et al., 2006; King-Heiden et al., 2012; Lanham et al., 2014). To determine if impaired cardiac function is capable of inhibiting swim bladder development, we compared the area of the swim bladder at 120 hpf, relative to control, in larvae with TCDD-, silent heart morphant-, and cmlc2:caAHR-2AtRFP-induced heart failure. In all three treatment groups, swim bladder area was significantly reduced at 120 hpf compared to the respective control group (Fig. 6). In addition, swim bladder areas in all control groups were not significantly different from each other, nor were swim bladder areas in the three treatment groups significantly different from one another (Fig. 6).
Figure 6. Swim bladder area in TCDD-exposed embryos is similar to embryos with impaired cardiac function.
Swim bladder area (mean ± SEM, n = 10 to 13) was measured at 120 hpf in embryos exposed to: TCDD at 4 hpf (TCDD, solid gray), silent heart morphants (silent heart MO, gray with black stripes), and cmlc2:caAHR-2AtRFP transient transgenic larvae (cmlc2:caAHR-2AtRFP, gray with black dots). Embryos exposed to DMSO at 4 hpf (solid white), injected with a control morpholino (Control MO, white with gray stripes), or control plasmid (cmlc2:caAHR−dbd-2AtRFP, white with gray dots) served as a control for each treatment group, respectively. Different lower case letters indicate significant difference between groups (p ≤ 0.01).
4. Discussion
4.1. TCDD impairs swim bladder development during the growth/elongation phase
When TCDD exposure began as early as 4 hpf, the initial steps in swim bladder development did not seem to be affected; swim bladder bud initiation, elongation, and early growth of the primitive swim bladder occurred on time. The gross morphology and histology of the TCDD-exposed developing swim bladder was indistinguishable from control up to 72 hpf. However, beyond this stage of development swim bladder growth was significantly impaired; TCDD-exposed larvae at 96 and 120 hpf had abnormally small, underdeveloped swim bladders that were similar in gross morphology and histology to the 72 hpf swim bladder of control larvae.
The effects of TCDD on swim bladder development were found to depend on the timing of exposure. Exposure to TCDD beginning at 96 hpf, when the growth phase has been completed and the swim bladder is beginning inflation, failed to impair swim bladder development. Additionally, TCDD exposure beginning at 96 hpf did not cause the inflated swim bladder to become uninflated, which suggests that TCDD does not affect cells and tissues involved in maintaining the inflated swim bladder.
Embryos exposed to TCDD before the beginning of the growth/elongation phase (at 4, 24, or 48 hpf) had swim bladders with severely impaired development at 120 hpf. More specifically, swim bladder area was significantly smaller, and gross morphology and histology of the swim bladder at 120 hpf were strikingly abnormal, resembling the control swim bladder at 72 hpf. Larvae exposed to TCDD beginning at 72 hpf, had swim bladders that displayed an intermediate phenotype when evaluated at 120 hpf; these swim bladders at 120 hpf were only partially inflated, significantly reduced in size, and had walls that were thicker than control.
Taken together, these results highlight the early growth/elongation phase, between 65 and 72 hpf when formation and organization of the three swim bladder tissue layers occurs, as the sensitive window for TCDD-induced inhibition of swim bladder development. TCDD exposure beyond this time, at 96 hpf, is not effective in disrupting swim bladder development.
4.2. Potential role of TCDD-induced decrease in cardiac output on swim bladder development
Exposure to TCDD at 4 hpf did not produce detrimental effects on the swim bladder until after 72 hpf. Consistent with this observation, embryos exposed to TCDD at 4, 24, or 48 hpf exhibit normal blood circulation until 72 hpf, at which time a significant decrease in peripheral blood flow is observed (Henry et al., 1997; Prasch et al., 2003; Teraoka et al., 2002). These reductions may be the result of TCDD interference with heart valve formation, which leads to blood regurgitation, an effect that begins to manifest at 60 hpf, and is significantly observed at 72 hpf (Mehta et al., 2008). In addition, stroke volume and cardiac output in embryos exposed to TCDD at 4 hpf may begin to decline at 60 hpf and progressively worsen with time. TCDD exposure also interferes with formation of the heart epicardium beginning around this time, which plays an important role in normal heart function and development (Plavicki et al. 2013). Normal peripheral circulation is known to be important for swim bladder development (Winata et al., 2010). Considering this, it is possible that TCDD-induced impairment of blood circulation, which manifests specifically when the swim bladder begins to undergo growth and organization of the three tissue layers, plays an important role in inhibiting swim bladder development.
Swim bladder development in embryos exposed to TCDD at 72 hpf was less affected than in embryos exposed to TCDD at earlier ages. Previously, Carney et al. (2006) showed that exposing embryos to TCDD beginning at 72 hpf did not seem to have any deleterious effects on cardiac output 4 hours later. However, beginning 8 hours post exposure, at 80 hpf, there were significant decreases in stroke volume and cardiac output, which continued to worsen with time. Thus, it is likely that embryos exposed to TCDD at 72 hpf had significant decreases in blood circulation, that began after the most sensitive stage of swim bladder development, leading to the formation of less severely affected swim bladders at 120 hpf.
In another study by Jönsson et al. (2012), zebrafish embryos injected with an Ahr2 morpholino were exposed to PCB126, a full AHR agonist. Although the Ahr2 morpholino loses effectiveness at approximately 72 to 96 hpf, this partial knockdown of Ahr2 was still sufficient to block the adverse effects of PCB126 on swim bladder development (Jönsson et al., 2012).
4.3 Sensitive window for TCDD inhibition of swim bladder development coincides with formation of mesenchymal and outer mesothelial swim bladder tissues
The growth/elongation phase in swim bladder development (65-96 hpf) may be particularly sensitive because it is when the mesenchymal and outer mesothelial layers are forming and crosstalk of signals between these layers, and the existing epithelial layer, play an important role in their organization and growth. Disorganization of the mesenchymal layer caused by impaired hedgehog signaling leads to a disorganized and underdeveloped outer mesothelium (Winata et al., 2009). At the same time, Wnt signals from both mesenchyme and outer mesothelium seem to play an important role in coordinating growth of the epithelial layer (Yin et al., 2011). Henry et al. (1997) observed a lack of mesenchymal layer in the uninflated swim bladders of TCDD-exposed zebrafish embryos at 104 hpf, and Winata et al. (2010) observed significantly disorganized mesenchymal and outer mesothelial layers in both the cloche mutant and silent heart morphant.
4.4. Is inhibition of swim bladder development by TCDD secondary to TCDD-induced heart failure?
Due to the similar timing during zebrafish embryo/larval development of TCDD-induced heart and circulatory failure and arrested swim bladder development, we hypothesized that TCDD arrests swim bladder development secondary to heart failure. Previous research has shown that these responses to TCDD are mediated through AHR signaling (Prasch et al., 2003; Prasch et al. 2006). In support of our hypothesis, we show that heart-specific constitutive activation of AHR signaling, which phenocopies heart failure caused by TCDD, but in the absence of TCDD exposure (Lanham et al. 2014), inhibited swim bladder development in a manner that was essentially indistinguishable from that of TCDD when assessed at 48, 72, 96 and 120 hpf. This demonstrates that it is not necessary to activate AHR signaling in the developing swim bladder itself to disrupt swim bladder development. Rather, constitutive activation of AHR signaling in the heart alone, leading to circulatory failure, is sufficient to impair swim bladder development. This, of course, does not eliminate the possibility that TCDD-induced activation of AHR signaling in the swim bladder itself may be capable of disrupting swim bladder development. Rather, it shows that swim bladder development can be inhibited indirectly by causing circulatory failure that coincides temporally with vulnerable stages of swim bladder development.
To investigate this latter possibility further, we assessed how swim bladder development was affected in the silent heart morphant, which lacks cardiac contractility and therefore also lacks circulation (Sehnert et al., 2002). We showed that heart failure produced in silent heart morphant larvae also was accompanied by impaired swim bladder development that follows a similar time course to that of TCDD, and when AHR signaling was constitutively activated in the heart. These results are consistent with observations by Winata et al. (2010), who found that impaired swim bladder development occurred secondary to circulatory failure in cloche mutants, which lack circulation due to inhibition of endothelial cell and hematopoietic progenitor differentiation (Stainier et al., 1995; Winata et al., 2010).
Thus, zebrafish larvae assessed at 120 hpf, where heart failure was induced by TCDD exposure, silent heart MO, or heart-specific constitutive activation of AHR signaling, developed swim bladders smaller than their respective controls. Gross morphology and histology of the 120 hpf swim bladders in each treatment group resembled each other, and a control swim bladder at 72 hpf. These findings suggest that swim bladder development was arrested in all groups at this stage of development.
It should be noted that the work presented here does not prove that inhibition of swim bladder development in TCDD-exposed zebrafish was entirely secondary to heart failure, or that direct effects of TCDD on the swim bladder were not involved. TCDD was not present in experiments involving the silent heart morphant and the cmcl2:caAHR-2AtRFP larvae. The only condition that was common to these two treatment groups and the TCDD group was heart failure. In this context it is striking that the: time course of inhibited swim bladder development, reduced swim bladder area, swim bladder gross morphology and histology, and lack of swim bladder inflation in silent heart morphant and cmcl2:caAHR-2AtRFP larvae were essentially similar to that of larvae with TCDD-induced heart failure. Together, these various results strongly suggest that the observed inhibitory effects of TCDD on swim bladder development could be secondary to heart malformation culminating in heart failure.
5. Conclusions
It has been widely documented that embryonic exposure to TCDD in the developing zebrafish results in failure of the swim bladder to inflate. Here we propose that TCDD produces this effect on the swim bladder by arresting development early in the growth/elongation stage (65-72 hpf), which occurs prior to inflation. Embryos exposed to TCDD before this stage develop hypoplastic swim bladders that are significantly reduced in size. However, swim bladder development rapidly becomes less sensitive to the effects of TCDD exposure after 72 hpf, such that embryos exposed to TCDD at 96 hpf have swim bladders that seem to develop and function normally. Thus, TCDD does not block initiation of swim bladder formation, nor the survival or differentiation of mature swim bladder cells after inflation.
The timing during development, for TCDD-induced disruption of swim bladder development, is similar to that for TCDD-induced circulation failure. However, what cannot be determined from experiments using TCDD alone is whether the effects of TCDD on swim bladder development are direct or indirect. In support of an indirect effect, we investigated the effect of circulatory failure on swim bladder development in silent heart morphants and larvae with heart-specific constitutively activated AHR signaling (cmlc2:caAHR-2AtRFP) that were not exposed to TCDD. Swim bladder development in these larvae was compared to larvae exposed to TCDD. We found that in all treatment groups circulatory failure was sufficient to arrest swim bladder development; the resultant phenotype and time course of effects was similar among all treatment groups. Thus, it is possible for TCDD to impair development of one organ, the swim bladder, secondary to causing dysfunction in a different organ, the heart. These findings provide new insights into the complex mechanisms by which TCDD induces developmental toxicity.
Highlights.
Arrested swim bladder development is a hallmark sign of TCDD developmental toxicity.
The growth/elongation phase of swim bladder development is inhibited.
TCDD-induced heart failure and -arrested swim bladder formation temporally coincide.
Genetically induced heart failure, without TCDD, arrests swim bladder development.
TCDD-arrested swim bladder development may be secondary to heart failure.
Acknowledgements
This research was funded by the National Institute for Environmental Health Sciences training grant (T32 ES007015) and (R01 ES012716) to W.H. and R.E.P. The authors wish to thank Dr. Kevin Lanham for providing DNA plasmid constructs for use in experiments, as well as his helpful advice. We also wish to thank Dr. J. Plavicki, Dr. P. Hofsteen, Dr. F. Burns, K. Behrens, and D. Nesbit for expert advice and general support in conducting this research and preparing this publication.
Abbreviations
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- HAHs
halogenated aromatic hydrocarbons
- AHR
aryl hydrocarbon receptor
- ARNT
aryl hydrocarbon receptor nuclear translocator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander RM. The energetics of vertical migration by fishes. Symp. Soc. Exp. Biol. 1972;26:273–294. [PubMed] [Google Scholar]
- Alexander RM. Buoyancy. In: Evans DH, editor. The physiology of fishes. CRC Press; Boca Raton: 1993. pp. 75–97. [Google Scholar]
- Antkiewicz DS, Burns CG, Carney SA, Peterson RE, Heideman W. Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol. Sci. 2005;84:368–377. doi: 10.1093/toxsci/kfi073. [DOI] [PubMed] [Google Scholar]
- Antkiewicz DS, Peterson RE, Heideman W. Blocking expression of AHR2 and ARNT1 in zebrafish larvae protects against cardiac toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2006;94:175–182. doi: 10.1093/toxsci/kfl093. [DOI] [PubMed] [Google Scholar]
- Belair CD, Peterson RE, Heideman W. Disruption of erythropoiesis by dioxin in the zebrafish. Dev. Dyn. 2001;222:581–594. doi: 10.1002/dvdy.1213. [DOI] [PubMed] [Google Scholar]
- Carney SA, Chen J, Burns CG, Xiong KM, Peterson RE, Heideman W. Aryl hydrocarbon receptor activation produces heart-specific transcriptional and toxic responses in developing zebrafish. Mol. Pharmacol. 2006;70:549–561. doi: 10.1124/mol.106.025304. [DOI] [PubMed] [Google Scholar]
- Carney SA, Peterson RE, Heideman W. 2,3,7,8-Tetrachlorodibenzo-p-dioxin activation of the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator pathway causes developmental toxicity through a CYP1A-independent mechanism in zebrafish. Mol. Pharmacol. 2004;66:512–521. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- Denton EJ. The buoyancy of fish and cephalopods. Prog. Biophys. Biophys. Chem. 1961;11:177–234. [PubMed] [Google Scholar]
- Elonen GE, Sphear RL, Holcombe GW, Johnson RD. Comparative toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin to seven freshwater species during early life-stage development. Environ. Toxicol. Chem. 1998;17:472–483. [Google Scholar]
- Evans HM. A contribution to the anatomy and physiology of the air-bladder and Weberian ossicles in cyprinidae. Proc. R. Soc. London, Ser. B. 1925;97:545–576. [Google Scholar]
- Finney JL, Robertson GN, McGee CA, Smith FM, Croll RP. Structure and autonomic innervation of the swim bladder in the zebrafish (Danio rerio) J. Comp. Neurol. 2006;495:587–606. doi: 10.1002/cne.20948. [DOI] [PubMed] [Google Scholar]
- Goolish EM, Okutake K. Lack of gas bladder inflation by the larvae of zebrafish in the absence of an air-water interface. J. Fish Biol. 1999;55:1054–1063. [Google Scholar]
- Hankinson O. The aryl hydrocarbon receptor complex. Annu. Rev. Pharmacol. Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- Henry TR, Spitsbergen JM, Hornung MW, Abnet CC, Peterson RE. Early life stage toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish (Danio rerio) Toxicol. Appl. Pharmacol. 1997;142:56–68. doi: 10.1006/taap.1996.8024. [DOI] [PubMed] [Google Scholar]
- Jönsson ME, Kubota A, Timme-Laragy AR, Woodin B, Stegeman JJ. Ahr2-dependence of PCB126 effects on the swim bladder in relation to expression of CYP1 and cox-2 genes in developing zebrafish. Toxicol. Appl. Pharmacol. 2012;265:166–174. doi: 10.1016/j.taap.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Heiden TC, Mehta V, Xiong KM, Lanham KA, Antkiewicz DS, Ganser A, Heideman W, Peterson RE. Reproductive and developmental toxicity of dioxin in fish. Mol. Cell. Endocrinol. 2012;354:121–138. doi: 10.1016/j.mce.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King Heiden TC, Spitsbergen J, Heideman W, Peterson RE. Persistent adverse effects on health and reproduction caused by exposure of zebrafish to 2,3,7,8-tetrachlorodibenzo-p-dioxin during early development and gonad differentiation. Toxicol. Sci. 2009;109:75–87. doi: 10.1093/toxsci/kfp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanham KA, Peterson RE, Heideman W. Sensitivity to dioxin decreases as zebrafish mature. Toxicol. Sci. 2012;127:360–70. doi: 10.1093/toxsci/kfs103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanham KA, Plavicki J, Peterson RE, Heideman W. Cardiac Myocyte-Specific AHR Activation Phenocopies TCDD-Induced Toxicity in Zebrafish. Toxicol. Sci. 2014;141:141–154. doi: 10.1093/toxsci/kfu111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta V, Peterson RE, Heideman W. 2,3,7,8-Tetrachlorodibenzop-dioxin exposure prevents cardiac valve formation in developing zebrafish. Toxicol. Sci. 2008;104:303–311. doi: 10.1093/toxsci/kfn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem. Res. Toxicol. 2008;21:102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Delgado JB, Sarasquete C. Toxicity, histopathological alterations and immunohistochemical CYP1A induction in the early life stages of the seabream, Sparus aurata, following waterborne exposure to B(a)P and TCDD. J. Mol. Histol. 2004;35:29–45. doi: 10.1023/b:hijo.0000020952.20386.16. [DOI] [PubMed] [Google Scholar]
- Peterson RE, Theobald HM, Kimmel GL. Developmental and reproductive toxicity of dioxins and related compounds: Cross-species comparisons. Crit. Rev. Toxicol. 1993;23:283–335. doi: 10.3109/10408449309105013. [DOI] [PubMed] [Google Scholar]
- Plavicki J, Hofsteen P, Peterson RE, Heideman W. Dioxin inhibits zebrafish epicardium and proepicardium development. Toxicol. Sci. 2013;131:558–567. doi: 10.1093/toxsci/kfs301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasch AL, Tanguay RL, Mehta V, Heideman W, Peterson RE. Identification of zebrafish ARNT1 homologs: 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity in the developing zebrafish requires ARNT1. Mol. Pharmacol. 2006;69:776–787. doi: 10.1124/mol.105.016873. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Teraoka H, Carney SA, Dong W, Hiraga T, Stegeman JJ, Heideman W, Peterson RE. Aryl hydrocarbon receptor 2 mediates 2,3,7,8-tetrachlorodibenzo-p-dioxin developmental toxicity in zebrafish. Toxicol. Sci. 2003;76:138–150. doi: 10.1093/toxsci/kfg202. [DOI] [PubMed] [Google Scholar]
- Robertson GN, McGee CAS, Dumbarton TC, Croll RP, Smith FM. Development of the swimbladder and its innervation in the zebrafish, Danio rerio. J. Morphol. 2007;268:967–985. doi: 10.1002/jmor.10558. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Annu. Rev. Cell Dev. Biol. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- Sehnert AJ, Huq A, Weinstein BM, Walker C, Fishman M, Stainier DYR. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat. Genet. 2002;31:106–10. doi: 10.1038/ng875. [DOI] [PubMed] [Google Scholar]
- Stainier DY, Weinstein BM, Detrich HW, 3rd, Zon LI, Fishman MC. Cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development. 1995;121:3141–50. doi: 10.1242/dev.121.10.3141. [DOI] [PubMed] [Google Scholar]
- Tanguay RL, Andreasen EA, Walker MK, Peterson RE. Dioxin toxicity and aryl hydrocarbon receptor signaling in fish. In: Schecter A, Gasiewicz TA, editors. Dioxins and Health. John Wiley & Sons, Inc.; New York: 2005. pp. 603–628. [Google Scholar]
- Teraoka H, Dong W, Ogawa S, Tsukiyama S, Okuhara Y, Niiyama M, Ueno N, Peterson RE, Hiraga T. 2,3,7,8-Tetrachlorodibenzo-p-dioxin toxicity in the zebrafish embryo: altered regional blood flow and impaired lower jaw development. Toxicol. Sci. 2002;65:192–199. doi: 10.1093/toxsci/65.2.192. [DOI] [PubMed] [Google Scholar]
- Walker MK, Peterson RE. Aquatic toxicity of dioxins and related chemicals. In: Schecter A, editor. Dioxins and Health. Plenum Press; New York: 1994. pp. 347–387. [Google Scholar]
- Westerfield M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio) fourth ed. Univ. of Oregon Press; Eugene, OR: 2000. [Google Scholar]
- Winata CL, Korzh S, Kondrychyn I, Korzh V, Gong Z. The role of vasculature and blood circulation in zebrafish swimbladder development. BMC Dev. Biol. 2010;10:3. doi: 10.1186/1471-213X-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winata CL, Korzh S, Kondrychyn I, Zheng W, Korzh V, Gong Z. Development of zebrafish swimbladder: The requirements of Hedgehog signaling in specification and organization of the three tissue layers. Dev. Biol. 2009;331:222–36. doi: 10.1016/j.ydbio.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Yin A, Korzh S, Winata CL, Korzh V, Gong Z. Wnt signaling is required for early development of zebrafish swimbladder. PLoS One. 2011;30:e18431. doi: 10.1371/journal.pone.0018431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeddies DG, Fay RR. Development of the acoustically evoked behavioral response in zebrafish to pure tones. J. Exp. Biol. 2005;208:1363–1372. doi: 10.1242/jeb.01534. [DOI] [PubMed] [Google Scholar]