Abstract
IMPORTANCE
Previous studies have indicated a heritable component of the etiology of neurodegenerative diseases such as Alzheimer disease (AD), frontotemporal dementia (FTD), and progressive supranuclear palsy (PSP). However, few have examined the contribution of low-frequency coding variants on a genome-wide level.
OBJECTIVE
To identify low-frequency coding variants that affect susceptibility to AD, FTD, and PSP.
DESIGN, SETTING, AND PARTICIPANTS
We used the Illumina HumanExome BeadChip array to genotype a large number of variants (most of which are low-frequency coding variants) in a cohort of patients with neurodegenerative disease (224 with AD, 168 with FTD, and 48 with PSP) and in 224 control individuals without dementia enrolled between 2005–2012 from multiple centers participating in the Genetic Investigation in Frontotemporal Dementia and Alzheimer’s Disease (GIFT) Study. An additional multiancestral replication cohort of 240 patients with AD and 240 controls without dementia was used to validate suggestive findings. Variant-level association testing and gene-based testing were performed.
MAIN OUTCOMES AND MEASURES
Statistical association of genetic variants with clinical diagnosis of AD, FTD, and PSP.
RESULTS
Genetic variants typed by the exome array explained 44%, 53%, and 57% of the total phenotypic variance of AD, FTD, and PSP, respectively. An association with the known AD gene ABCA7 was replicated in several ancestries (discovery P = .0049, European P = .041, African American P = .043, and Asian P = .027), suggesting that exonic variants within this gene modify AD susceptibility. In addition, 2 suggestive candidate genes, DYSF (P = 5.53 × 10−5) and PAXIP1 (P = 2.26 × 10−4), were highlighted in patients with AD and differentially expressed in AD brain. Corroborating evidence from other exome array studies and gene expression data points toward potential involvement of these genes in the pathogenesis of AD.
CONCLUSIONS AND RELEVANCE
Low-frequency coding variants with intermediate effect size may account for a significant fraction of the genetic susceptibility to AD and FTD. Furthermore, we found evidence that coding variants in the known susceptibility gene ABCA7, as well as candidate genes DYSF and PAXIP1, confer risk for AD.
Genetics studies have revealed a genetic contribution to susceptibility for common or sporadic forms of neurodegenerative disease such as Alzheimer disease (AD), frontotemporal dementia (FTD), and progressive supra-nuclear palsy (PSP, a syndrome characterized by oculomotor and gait abnormalities). In AD, early genetic mapping approaches have identified rare variants in genes such as APP, PSEN1, and PSEN2that cause familial, early-onset forms.1 APOE was also pinpointed as a late-onset AD susceptibility gene.2 Genome-wide association studies3–5 (GWAS) targeted toward common variants in primarily European populations have identified many variants associated with AD, most clearly near APOE but also consistently near ABCA7, BIN1, CLU, CR1, PICALM, SORL1, and other genes. Next-generation sequencing approaches have also found rare variants with strong effect in the MAPT and TREM2 genes.6,7
In FTD, the most frequently observed mutations in familial cases occur in C9ORF72, GRN, MAPT, TARDBP, and other genes.8 In sporadic cases, a haplotype variant on the long arm of chromosome 17 has been repeatedly associated with PSP.9–11 In addition, GWAS have been performed for sporadic cases of FTD, identifying associated single-nucleotide polymorphisms (SNPs) near TMEM106B12 and BTNL2/HLA-DRA/HLA-DRB5 and RAB38/CTSC,13 as well as for PSP, identifying associated SNPs near MAPT, EIF2AK3, STX6, and MOBP.11
Despite progress in understanding the genetics of neurodegenerative diseases, known genetic risk factors cannot explain a large portion of the heritability of these diseases. For example, in AD, all common variants (including known and unknown risk variants) have been predicted to account for less than 25% of disease variance,14 and known high-penetrance rare variants account for few cases, collectively totaling only a fraction of the estimated 58% to 79% heritability of AD.15 Some of this missing heritability may be due to a blind spot in conventional genetic studies to date. A moderately rare variant with moderate effect size would be too uncommon to be tagged by a standard genotyping array and have too small of an effect to be detected by linkage or genome sequencing in practical sample sizes. The exome array bridges this gap by genotyping at low cost more than 200 000 coding variants identified through sequencing studies (Figure 1). This approach has been applied to phenotypes such as insulin homeostasis,16 bronchopulmonary dysplasia,17 and heart disease.18,19 For AD, Chung et al20 recently reported an exome array study in Korean participants that found an association with APOE, APOC1, and TOMM40 variants (near the APOE locus) but did not identify novel genetic variants. Herein, we report findings from the application of the exome array to the multiancestral Genetic Investigation in Frontotemporal Dementia and Alzheimer’s Disease (GIFT) Study cohort to determine the contribution of low-frequency coding variants to susceptibility to sporadic AD, PSP, and FTD.
Figure 1. Comparison of the Exome Array and Related Genotyping and Sequencing Technologies.
The exome array serves as a bridge between conventional single-nucleotide polymorphism (SNP) genotyping array and exome sequencing. The exome array assays primarily variants within exonic regions of the DNA, similar to exome sequencing; however, the location of the variants must be known a priori. The cost of the exome array is typically similar to that of other genotyping arrays and is much less expensive than that of exome sequencing.
Methods
Study Cohort
Patients and healthy control individuals were enrolled between 2005–2012 at the Memory and Aging Center, University of California, San Francisco, as part of the GIFT Study, an investigation of the genetics of neurodegenerative disease.21,22 Written consent was obtained at the participating institutions. The study was approved by the Institutional Review Board of the University of California, Los Angeles. An additional 32 DNA samples from patients with PSP were extracted from postmortem brain tissue from the New York Brain Bank at Columbia University (New York, New York). A subset of these individuals were initially selected for genotyping using the Illumina HumanExome BeadChip array (Table 1). Patients diagnosed as having FTD with motor neuron disease (FTD/ MND) were excluded from further analysis owing to the small sample size and potential genetic heterogeneity.
Table 1.
Demographic Information for the Discovery Cohort
| Characteristic | AD (n = 224) | Control (n = 224) | FTD (n = 168) | FTD/MND (n = 8) | PSP (n = 48) |
|---|---|---|---|---|---|
| Age, median (range), y | 71 (42 to ≥89) | 71 (35 to ≥89) | 67 (35 to ≥89) | 63 (35 to 80) | 76 (55 to ≥89) |
| Sex, No. (%) | |||||
| Male | 12 (56.7) | 94 (42.0) | 95 (56.5) | 8 (100) | 19 (39.6) |
| Female | 97 (43.3) | 130 (58.0) | 73 (43.5) | 0 | 29 (60.4) |
| Ancestry, No. (%) | |||||
| European | 195 (87.1) | 183 (81.7) | 144 (85.7) | 8 (100) | 12 (25.0) |
| African American | 2 (0.9) | 0 | 0 | 0 | 0 |
| Latino | 0 | 4 (1.8) | 1 (0.6) | 0 | 0 |
| Asian | 20 (8.9) | 27 (12.1) | 9 (5.4) | 0 | 1 (2.1) |
| Other | 3 (1.3) | 4 (1.8) | 7 (4.2) | 0 | 2 (4.2) |
| Unknown | 4 (1.8) | 6 (2.7) | 7 (4.2) | 0 | 33 (68.8) |
| APOE genotype, No. (%) | |||||
| E2/E2 | 1 (0.4) | 1 (0.4) | 0 | 0 | 1 (2.1) |
| E2/E3 | 7 (3.1) | 19 (8.5) | 16 (9.5) | 1 (12.5) | 3 (6.3) |
| E2/E4 | 4 (1.8) | 1 (0.4) | 1 (0.6) | 0 | 2 (4.2) |
| E3/E3 | 99 (44.2) | 157 (70.1) | 107 (63.7) | 5 (62.5) | 36 (75.0) |
| E3/E4 | 92 (41.1) | 40 (17.9) | 40 (23.8) | 1 (12.5) | 6 (12.5) |
| E4/E4 | 21 (9.4) | 6 (2.7) | 4 (2.4) | 1 (12.5) | 0 |
| Chromosome 17q21.31 haplotype, No. (%) | |||||
| H1/H1 | 91 (40.6) | 132 (58.9) | 107 (63.7) | 4 (50.0) | 43 (89.6) |
| H1/H2 | 48 (21.4) | 52 (23.2) | 33 (19.6) | 3 (37.5) | 5 (10.4) |
| H2/H2 | 4 (1.8) | 10 (4.5) | 7 (4.2) | 0 | 0 |
| Untyped | 81 (36.2) | 30 (13.4) | 21 (12.5) | 1 (12.5) | 0 |
Abbreviations: AD, Alzheimer disease; FTD, frontotemporal dementia; FTD/MND, FTD with motor neuron disease; PSP, progressive supranuclear palsy.
Replication Cohort
As part of the GIFT Study, individuals were also enrolled from other sites, including Emory University, University of Southern California, and University of California at Berkeley, Davis, Irvine, and Los Angeles. Following initial data analysis, 480 individuals from this additional group of patients, including 240 patients with AD and 240 controls without dementia, were genotyped (Table 2). These individuals were analyzed as above but owing to genetic heterogeneity were divided into 4 general groups (European, African American, Latino, and Asian) based on self-reported ancestry. To ensure proper classification and minimize the inclusion of misplated samples, genetic ancestry was also estimated by multidimensional scaling using the PLINK whole-genome association analysis tool set (http://pngu.mgh.harvard.edu/purcell/plink/) using the entire set of genotyped variants by the exome array. Following this procedure, 44 samples were suspected of misclassification and were removed from further analysis.
Table 2.
Demographic Information for the Replication Cohort
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| European (n = 135) | African American (n = 271) | Latino (n = 50) | Asian (n = 24) | |
| Diagnosis | ||||
| AD | 68 (50.4) | 138 (50.9) | 21 (42.0) | 13 (54.2) |
| Control | 67 (49.6) | 133 (49.1) | 29 (58.0) | 11 (45.8) |
| Sex | ||||
| Male | 68 (50.4) | 73 (26.9) | 19 (38.0) | 8 (33.3) |
| Female | 57 (42.2) | 198 (73.1) | 31 (62.0) | 16 (66.7) |
| Unknown | 10 (7.4) | 0 | 0 | 0 |
| Contributing center | ||||
| Emory University | 21 (15.6) | 223 (82.3) | 0 | 0 |
| University of California, Berkeley | 33 (24.4) | 14 (5.2) | 8 (16.0) | 8 (33.3) |
| University of California, Davis | 3 (2.2) | 32 (11.8) | 23 (46.0) | 5 (20.8) |
| University of California, Irvine | 55 (40.7) | 2 (0.7) | 5 (10.0) | 1 (4.2) |
| University of California, Los Angeles | 2 (1.5) | 0 | 0 | 0 |
| University of California, San Francisco | 20 (14.8) | 0 | 0 | 6 (25.0) |
| University of Southern California | 1 (0.7) | 0 | 14 (28.0) | 4 (16.7) |
| APOE genotype | ||||
| E2/E2 | 1 (0.7) | 2 (0.7) | 0 | 0 |
| E2/E3 | 4 (3.0) | 16 (5.9) | 2 (4.0) | 1 (4.2) |
| E2/E4 | 5 (3.7) | 9 (3.3) | 3 (6.0) | 0 |
| E3/E3 | 41 (30.4) | 87 (32.1) | 34 (68.0) | 8 (33.3) |
| E3/E4 | 21 (15.6) | 86 (31.7) | 9 (18.0) | 3 (12.5) |
| E4/E4 | 9 (6.7) | 12 (4.4) | 1 (2.0) | 2 (8.3) |
| Untyped | 54 (40.0) | 59 (21.8) | 1 (2.0) | 10 (41.7) |
Abbreviation: AD, Alzheimer disease.
Exome Array Genotyping
Exonic and nonexonic variants were genotyped using the Illumina Infinium HumanExome BeadChip kit. While mostly consisting of coding variants from prior sequencing studies, the exome arrays also included markers for previously described GWAS hits, ancestry-informative markers, randomly selected synonymous variants, HLA tag SNPs, and others,16 in total comprising 250 272 genotyped markers per sample. Quality control procedures were enacted to remove suspect variants and minimize the effect of population structure on the data analysis. The eMethods, eFigure 1, and eFigure 2 in the Supplement provide further details on genotyping and data preprocessing procedures.
Statistical Analysis
The total phenotypic (disease) variance explained by the genotyped variants was determined using a restricted maximum likelihood model implemented in Genome-Wide Complex Trait Analysis (GCTA; http://www.complextraitgenomics.com/software/gcta/). Variant-level association with AD, FTD, and PSP was tested using a logistic regression model that corrected for population structure. The association on the gene level was tested using the sequence kernel association test (SKAT),23 a nonburden test that is sensitive in the presence of neutral genetic variants. Genes that showed suggestive associations with AD were also tested in previously described brain messenger RNA (mRNA) expression data sets.24,25 The eMethods in the Supplement provides a more detailed description of the statistical methods used.
Summary statistics and individual-level data are available from the NIA Genetics of Alzheimer’s Disease Data Storage Site (NIAGADS; https://www.niagads.org/, accession number NG00040).
Results
Patient Characteristics
The initial discovery sample included 224 patients with AD, 168 patients with FTD, 8 patients with FTD/MND, 48 patients with PSP, and 224 healthy controls. Demographic characteristics are summarized in Table 1. The ancestral makeup of this sample was predominantly European (80.7% overall). Consistent with their known roles in the respective diseases, individuals classified as having AD showed high prevalence of the APOE ε4 allele (41.1% ε3/ε4 and 9.4% ε4/ε4), and individuals classified as having PSP showed high prevalence of the H1 haplotype (89.6% H1/H1 and 10.4% H1/H2). The replication cohort consisted of a more ancestrally heterogeneous set of patients and controls (Table 2).
Low-Frequency Exonic Variants Explain a Fraction of the Phenotypic Variation in AD and FTD
For each of the 3 diseases (AD, FTD, and PSP), the GCTA software was applied to the data set to estimate the variance explained by the following 3 different classes of variants: all variants, including nonexonic variants; exonic variants only; and low-frequency exonic variants, with minor allele frequency <5%. In each case, a substantial portion of the observed phenotypic variance could be explained by all the typed variants (Table 3). However, owing to the small sample sizes on which each of these estimates is based, the standard error of each measurement is high.
Table 3.
GCTA Explained Variance Analysis
| Variable | Variance Explained (SE) | ||
|---|---|---|---|
| AD | FTD | PSP | |
| All exome array variantsa | 0.44 (0.39) | 0.53 (0.36) | 0.57 (0.44) |
| Exonic fraction | 0.50 (0.36) | 0.45 (0.35) | 0.26 (0.56) |
| Low-frequency exonic fractionb | 0.41 (0.39) | 0.42 (0.37) | 0.03 (0.58) |
Abbreviations: AD, Alzheimer disease; FTD, frontotemporal dementia; GCTA, Genome-Wide Complex Trait Analysis (http://www.complextraitgenomics.com/software/gcta/); PSP, progressive supranuclear palsy.
Includes genome-wide association studies hits, HLA tag single-nucleotide polymorphisms, custom content, ancestry-informative single-nucleotide polymorphisms, and others.
Less than 5% minor allele frequency between all disease cohorts and control subjects.
Variant-Level Association Testing Identifies Significant Associations With Known and Novel Loci
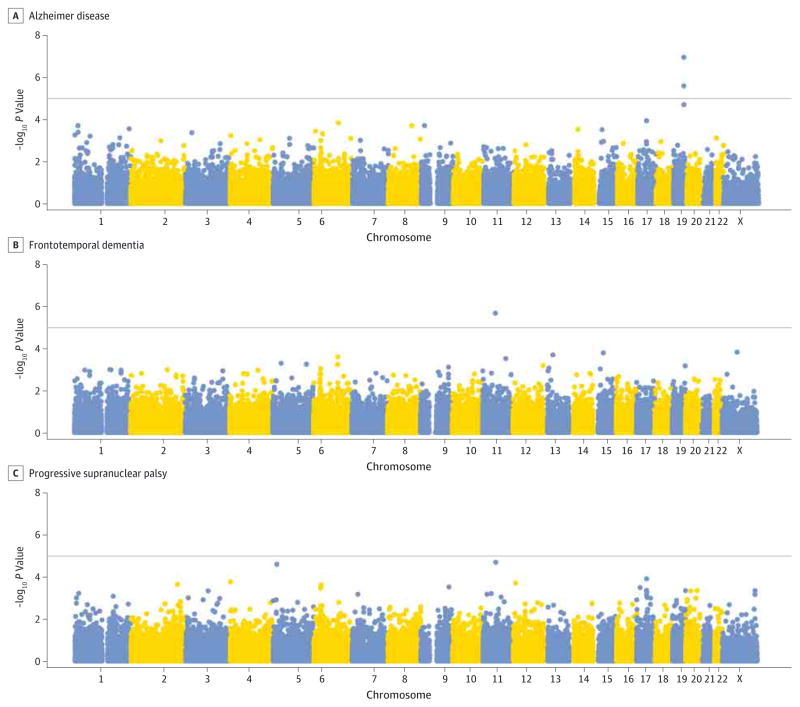
A logistic regression procedure was performed on our discovery cohort to test for an association with AD, FTD, or PSP. Our method largely controlled for genomic inflation due to population stratification in each of the 3 disease categories (eFigure 3 in the Supplement). Two variants were suggestively associated with AD, rs769449 (P = 1.14 × 10−7; minor allele odds ratio [OR], 3.0) and rs4420638 (P = 2.58 × 10−6; minor allele OR, 2.3). Both variants are within the APOE/TOMM40/APOC1 region on chromosome 19 identified in previous genetic studies.2–5 One variant was associated with FTD, exm2250002 (P = 2.08 × 10−6; minor allele OR, 0.8), corresponding to a synonymous exonic variant in the olfactory receptor genes OR9G1 and OR9G9. No variants reached the suggestive P value threshold (1 × 10−5) in the PSP cohort. Manhattan plots depicting associations in AD, FTD, and PSP are shown in Figure 2.
Figure 2. Manhattan Plot of Associations in Alzheimer Disease, Frontotemporal Dementia, and Progressive Supranuclear Palsy.
The association −log10 P values calculated by logistic regression are presentd for for Alzheimer disease, frontotemporal dementia, and progressive supranuclear palsy. The horizontal line indicates the suggestive P value threshold of P = 1 × 10−5. X refers to chromosome X.
Exome Array Genotyping Replicates Some Previous Associations Found in AD and PSP
Thirty-nine polymorphisms previously associated with AD and 9 polymorphisms associated with PSP (National Human Genome Research Institute Genome-Wide Association Studies Catalog; http://www.genome.gov/gwastudies/) were typed by the exome array. Reported susceptibility loci for FTD were not typed on this platform. We tested the association between each of these variants and their respective disease in our cohort, as calculated by the logistic procedure described previously. For AD, the Bonferroni correction for 39 tests at a family wise error rate of .05 yielded a P value threshold at .0013. Two associations near APOE, rs2075650 (P = 2.05 × 10−5) and rs4420638 (P = 2.58 × 10−6), surpassed this predefined P value threshold (eTable 1 in the Supplement). While the other tested GWAS variants were not significantly associated with AD, the overall direction of the association was highly consistent with previously reported results,3–5 and 23 of 32 SNPs for which the risk allele was unambiguous showed the same direction of effect as previously reported (P = .010, binomial test).
For PSP, the Bonferroni correction for 9 tests at a family-wise error rate of .05 yielded a P value threshold at .0056. A single variant exceeded this threshold, rs8070723 (P = .00043) on chromosome 17 near MAPT (eTable 2 in the Supplement). Similar to the AD cohort, the direction of the association was highly consistent with previously reported results,11 with 8 of 9 SNPs showing the same direction of effect (P = .019, binomial test).
Gene-Level Testing Suggests Several AD Candidate Genes
Gene-level hypothesis testing was performed using SKAT-derived P values for 17 141 genes (that contained at least 1 typed variant after quality control). Using a permutation procedure, a false discovery rate of 50% was expected to be controlled at a SKAT-derived P value of 4.54 × 10−4 for AD, 5.06 × 10−4 for FTD, and 9.65 × 10−5 for PSP. For AD, the following 6 genes exceeded this threshold: DYSF, PAXIP1, TOP1MT, C3ORF1, SETDB1, and CRISPLD1 (P = 5.53 × 10−5, P = 2.26 × 10−4, P = 2.29 × 10−4, P = 3.93 × 10−4, P = 4.13 × 10−4, and P = 4.54 × 10−4, respectively). For FTD, the following 8 genes exceeded the threshold: RAB21, AKR1B10, C9ORF6, CD5L, WDR38, OPHN1, ADORA3, and IKBKAP (P = 4.65 × 10−5, P = 4.83 × 10−5, P = 2.55 × 10−4, P = 3.65 × 10−4, P = 3.85 × 10−4, P = 4.78 × 10−4, P = 4.79 × 10−4, and P = 5.06 × 10−4, respectively). For PSP, 2 genes exceeded the threshold, OR1Q1 and VWA3A (P = 3.00 × 10−5 and P = 9.65 × 10−5, respectively).
We attempted to replicate the findings for AD in an additional multiancestral cohort of 240 cases and 240 controls. No further samples from patients with FTD or PSP were available, so those results could not be tested. Using the Bonferroni correction, a P value threshold of .0021 (considering 6 genes times 4 ancestry categories, for a total of 24 tests) was determined to control for a family wise error rate of .05. None of the suggestive genes identified for AD were significant under this threshold in any ancestral category in the replication cohort (eTable 3 in the Supplement). However, several genes trended toward significance in some cases, including DYSF in Europeans (P = .076), PAXIP1 in Latinos and Asians (P = .016 and P = .037, respectively), and TOP1MT in African Americans (P = .0059). Because of previous reports of the involvement of DYSF and PAXIP1 in the AD literature (see the Discussion section below),26,27 these genes were considered interesting candidate genes for AD susceptibility. Overall, we analyzed 38 variants in DYSF (including 3 synonymous and 35 missense) and 5 variants in PAXIP1 (including 1 synonymous and 4 missense) typed by the exome array, demonstrating variation in our cohort, and passing quality control criteria.
We further identified 71 genes previously implicated in genetic studies of AD as categorized in the Human Gene Mutation Database28 (version 2014.1) and extracted the association statistics in the initial discovery set and the 4 replication cohorts. Only ABCA7 (OMIM 605414) (SKAT discovery P = .0049) reached nominal significance. Notably, the SKAT P value was also nominally significant in the European (P = .041), African American (P = .043), and Asian (P = .027) replication cohorts but not the Latino (P = .61) cohort.
DYSF and PAXIP1 Transcripts Are Differentially Expressed in AD Brain
To further solidify whether DYSF (OMIM 603009) and PAXIP1 (OMIM 608254) are involved in the pathogenesis of AD, we examined their relative expression levels in patients with AD and controls without dementia in a published microarray data set.24 The expression of DYSF and PAXIP1 was significantly different between cases and controls in each of the examined brain regions (Figure 3). In the prefrontal cortex, visual cortex, and cerebellum, the expression of DYSF was increased in patients with AD (P < 2.2 × 10−16, P = 2.33 × 10−15, and P = .00080, respectively). These findings were corroborated by independent data,25 which also showed increased expression of DYSF in the cerebral cortex of patients with AD (P = .00023). Similarly, the expression of PAXIP1 in the prefrontal cortex, visual cortex, and cerebellum was increased in patients with AD (P = 3.6 × 10−14, P = .0034, and P = .00095, respectively).
Figure 3. Differential Expression of DYSF and PAXIP1 in Alzheimer Disease (AD) Brain.
Shown is the expression of DYSF (A) and PAXIP1 (B) in a public microarray data set of brain messenger RNA, grouped by brain region, in patients with AD (dark gray) vs healthy control subjects without dementia (light gray). The vertical axis represents the normalized expression residual, corrected for technical covariates. CB indicates cerebellum; PFC, prefrontal cortex; and VC, visual cortex.
Discussion
We evaluated the contribution of exonic variants to neurodegenerative disease susceptibility in a multiancestral cohort totaling 464 patients with AD, 168 patients with FTD, 48 patients with PSP, and 464 controls without dementia. We found that low-frequency (<5%) coding variants explain a sizable proportion of the phenotypic variance in AD and FTD, although the confidence limits for this estimate are large owing to our sample size. Well-known associations with the APOE locus for AD and 17q21.31 haplotype for PSP were replicated, and a novel susceptibility locus was identified at exm2250002 for FTD. Whether this variant is a true genetic signal is questionable given that it was also the most significant signal in the PSP cohort (P = 2.03 × 10−5) and corresponds to a synonymous variant within OR9G1/OR9G9, members of the polymorphic olfactory receptor family. Gene-level testing identified suggestive signals from DYSF and PAXIP1 in AD, and a trend toward significance was observed in a replication cohort in several of the tested ancestral categories. A possible contribution to disease risk from exonic variants in the AD susceptibility gene ABCA7 was also detected in multiple ancestral categories. However, we caution that these results are merely suggestive and await validation in well-powered cohorts and model systems.
The focus of the exome array on coding variation, much of which has low frequency in the general population, means that large sample sizes are needed to observe statistically significant effects, unless the effect sizes are large, as is the case with the association of the APOE ε4 allele with AD. We estimated that a variant at 5% minor allele frequency must have a greater than 4-fold OR to achieve 80% power to identify in our AD discovery cohort. Therefore, our initial cohort of 672 patients and controls and our follow-up cohort of 480 patients and controls are underpowered to detect associations with rare variants of modest or intermediate effect sizes. Taken together with heritability estimates, our analyses indicated that rare variants of low or modest effect have a role in AD, FTD, and PSP, late-onset diseases for which deleterious alleles are presumably under weak selective pressure.
Furthermore, while the GIFT Study cohort enabled testing of an association in multiple ancestral groups simultaneously, our results were limited by the small sample sizes. Therefore, our findings do not exclude the possibility that exonic variants with lower frequency or effect size are present in the general population. In fact, the strong association with ABCA7 (a GWAS-implicated AD susceptibility gene) by SKAT in several ancestral populations strongly suggests that coding variants of modest effect size within this gene are associated with AD risk. Previous GWAS have reported associations with intronic polymorphisms such as rs4147929,5 rs115550680,29 and rs3764650,4 as well as the missense polymorphism rs3752246.3 It is possible that these variants may tag haplotypes containing causal, exonic variants. Therefore, it is reasonable to attempt to identify novel candidate genes containing multiple, low-frequency coding variants that may contribute to AD.
While not strictly genome-wide significant, genewise testing results reinforce prior findings that have implicated both DYSF and PAXIP1 in the pathogenesis of AD. DYSF encodes the protein dysferlin, and mutations in this gene are known to cause autosomal recessive muscle diseases such as Miyoshi myopathy30 and limb-girdle muscular dystrophy type 2B,31 known as dysferlinopathies. In skeletal muscle, dysferlin is thought to have a role in calcium-dependent sarcolemma repair.32,33 Although its function in the central nervous system has not been extensively elaborated, dysferlin has been shown to accumulate in endothelial cells near multiple sclerosis lesions34 and within Aβ plaques of patients with AD.26 The colocalization of dysferlin and Aβ42 aggregates was also demonstrated in sporadic inclusion body myositis, suggesting that Aβ may sequester dysferlin and interfere with its normal repair functions in skeletal muscle.35
The second highlighted gene, PAXIP1, encodes for a nuclear protein with 6 BRCT domains, hinting at its function in DNA repair pathways.36 PAXIP1 may participate in p53 activation mediated by the ataxia-telangiectasia mutated (ATM) serine/ threonine kinase.36–38 Although variants in PAXIP1 have not been definitively associated with disease, Rademakers et al27 identified a significant linkage peak at 7q36 in a large pedigree with multiplex AD. The risk allele of the D7S798 marker also appeared to increase AD risk by 2.7 times in a Dutch population-based cohort.27 Sequencing of the coding exons of 29 candidate genes revealed only a single rare variant, a synonymous Ala626 change in PAXIP1.
To our knowledge, the neuropathological findings by Galvin et al26 and the linkage study by Rademakers et al27 are the only publications to date that implicate DYSF and PAXIP1 in the pathogenesis of AD. Our analysis of published microarray studies indicated increases in DYSF and PAXIP1 mRNA expression in brain regions of patients with AD. However, these results do not provide direct evidence of the roles of these genes in AD. In contrast, the exome array results add additional support for the causal pathogenicity of DYSF and PAXIP1. Although we could not ascertain whether any of the assayed variants directly affected the expression of DYSF and PAXIP1, the fact that these genes were both identified by exome array analysis and by differential expression analysis provides convergent evidence for their involvement in AD. Besides partial, nominal replication within our cohort, our findings are further corroborated by a recently published exome array study20 in AD reporting a strong (but not genome-wide significant) association for DYSF (P = 1.6 × 10−5) with AD in a Korean cohort; the association with PAXIP1 was not reported. The overlap with our suggestive results indicates a high prior probability for the pathogenicity of variants in DYSF (and possibly PAXIP1), and follow-up studies are warranted.
Conclusions
The overall genetic architecture of neurodegenerative diseases is complex and is just beginning to be defined. Our work has strengthened the case for 2 AD candidate genes and provides one of the first glimpses at this genetic variation that heretofore had not been widely studied. We anticipate that the results described herein will provide insight into the genetics of AD, FTD, and PSP and that the data will provide a valuable multiancestral cohort with exome array genotyping data for future studies in each of the 3 diseases. We further expect in the long term that increased understanding of the genetic underpinnings will lead to improvements in diagnosis and management for patients with neurodegenerative diseases.
Supplementary Material
Acknowledgments
Funding/Support: This work was funded by grants from the National Institues of Health: F31 NS084556 (Mr Chen), P50 AG023501 (Dr Miller), RC1 AG035610 (Dr Coppola), R01 MH097268 (Dr Coppola), R01 AG26938 (Dr Geschwind), P01 AG019724 (Drs Levenson, B. L. Miller, and Geschwind), and R01 AG041762 (Dr Levenson); from the John Douglas French Alzheimer’s Foundation (Dr Coppola); the Tau Consortium (Drs Geschwind and Coppola); and the Jim Easton Consortium for Alzheimer’s Drug Discovery and Biomarker Development (Dr Ringman). We acknowledge the support of grant P30 NS062691 from the National Institute of Neurological Disorders and Stroke Informatics Center for Neurogenetics and Neurogenomics; grant P50 AG16570 from the University of California, Los Angeles, Alzheimer’s Disease Research Center; grant P50 AG016573 and POI AG000538 from the University of California, Irvine, Alzheimer’s Disease Research Center; grant P30 AG010129 from the University of California, Davis, Alzheimer’s Disease Center (Dr DeCarli); and grant P50 AG05142 from the University of Southern California Alzheimer’s Disease Research Center. Samples from the National Cell Repository for Alzheimer’s Disease, which receives government support under cooperative agreement grant U24 AG21886 from the National Institute on Aging, were used in this study. The National Alzheimer’s Coordinating Center database is funded by grant U01 AG016979 from the National Institute on Aging.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: None reported.
Author Contributions: Dr Coppola had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chen, Li, Miller, Geschwind, Coppola.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Chen, Geschwind, Coppola.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Chen, Li, Coppola.
Obtained funding: Levenson, Ringman, DeCarli, Miller, Geschwind, Coppola.
Administrative, technical, or material support: Geschwind, Coppola.
Study supervision: Geschwind, Coppola.
Additional Contributions: Iris Vuong, BS, and Joseph DeYoung, BA (University of California, Los Angeles Neurosciences Genomics Core) assisted with array genotyping. Jean Paul Vonsattel, MD (New York Brain Bank at Columbia University) contributed brain specimens from patients with PSP that were used in the study. Bin Zhang, PhD, and Amanda Myers, PhD, assisted with the gene expression data. Margaret Chu, BS, and Maribel Estrada provided administrative support. We thank the contributors who collected samples used in this study. We especially thank the patients and their families, whose help and participation made this work possible and to whom our research is dedicated.
References
- 1.Lendon CL, Ashall F, Goate AM. Exploring the etiology of Alzheimer disease using molecular genetics. JAMA. 1997;277(10):825–831. [PubMed] [Google Scholar]
- 2.Pericak-Vance MA, Bebout JL, Gaskell PC, Jr, et al. Linkage studies in familial Alzheimer disease: evidence for chromosome 19 linkage. Am J Hum Genet. 1991;48(6):1034–1050. [PMC free article] [PubMed] [Google Scholar]
- 3.Naj AC, Jun G, Beecham GW, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;43(5):436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollingworth P, Harold D, Sims R, et al. Alzheimer’s Disease Neuroimaging Initiative; CHARGE Consortium; EADI1 Consortium. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43(5):429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambert JC, Ibrahim-Verbaas CA, Harold D, et al. European Alzheimer’s Disease Initiative (EADI); Genetic and Environmental Risk in Alzheimer’s Disease; Alzheimer’s Disease Genetic Consortium; Cohorts for Heart and Aging Research in Genomic Epidemiology. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45(12):1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coppola G, Chinnathambi S, Lee JJ, et al. Alzheimer’s Disease Genetics Consortium. Evidence for a role of the rare p.A152T variant in MAPT in increasing the risk for FTD-spectrum and Alzheimer’s diseases. Hum Mol Genet. 2012;21(15):3500–3512. doi: 10.1093/hmg/dds161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guerreiro R, Wojtas A, Bras J, et al. Alzheimer Genetic Analysis Group. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368(2):117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rademakers R, Neumann M, Mackenzie IR. Advances in understanding the molecular basis of frontotemporal dementia. Nat Rev Neurol. 2012;8 (8):423–434. doi: 10.1038/nrneurol.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker M, Litvan I, Houlden H, et al. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet. 1999;8(4):711–715. doi: 10.1093/hmg/8.4.711. [DOI] [PubMed] [Google Scholar]
- 10.Pittman AM, Myers AJ, Abou-Sleiman P, et al. Linkage disequilibrium fine mapping and haplotype association analysis of the tau gene in progressive supranuclear palsy and corticobasal degeneration. J Med Genet. 2005;42(11):837–846. doi: 10.1136/jmg.2005.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Höglinger GU, Melhem NM, Dickson DW, et al. PSP Genetics Study Group. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat Genet. 2011;43(7):699–705. doi: 10.1038/ng.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Deerlin VM, Sleiman PMA, Martinez-Lage M, et al. Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet. 2010;42(3):234–239. doi: 10.1038/ng.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrari R, Hernandez DG, Nalls MA, et al. Frontotemporal dementia and its subtypes: a genome-wide association study. Lancet Neurol. 2014;13(7):686–699. doi: 10.1016/S1474-4422(14)70065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SH, Harold D, Nyholt DR, et al. ANZGene Consortium, International Endogene Consortium, the Genetic and Environmental Risk for Alzheimer’s Disease (GERAD1) Consortium. Estimation and partitioning of polygenic variation captured by common SNPs for Alzheimer’s disease, multiple sclerosis and endometriosis. Hum Mol Genet. 2013;22(4):832–841. doi: 10.1093/hmg/dds491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gatz M, Reynolds CA, Fratiglioni L, et al. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry. 2006;63(2):168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- 16.Huyghe JR, Jackson AU, Fogarty MP, et al. Exome array analysis identifies new loci and low-frequency variants influencing insulin processing and secretion. Nat Genet. 2013;45(2):197–201. doi: 10.1038/ng.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, St Julien KR, Stevenson DK, et al. A genome-wide association study (GWAS) for bronchopulmonary dysplasia. Pediatrics. 2013;132 (2):290–297. doi: 10.1542/peds.2013-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peloso GM, Auer PL, Bis JC, et al. NHLBI GO Exome Sequencing Project. Association of low-frequency and rare coding-sequence variants with blood lipids and coronary heart disease in 56,000 whites and blacks. Am J Hum Genet. 2014;94(2):223–232. doi: 10.1016/j.ajhg.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmen OL, Zhang H, Zhou W, et al. No large-effect low-frequency coding variation found for myocardial infarction. Hum Mol Genet. 2014;23 (17):4721–4728. doi: 10.1093/hmg/ddu175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung SJ, Kim MJ, Kim J, et al. Exome array study did not identify novel variants in Alzheimer’s disease. Neurobiol Aging. 2014;35(8):1958.e13–1958.e14. doi: 10.1016/j.neurobiolaging.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Coppola G, Miller BL, Chui H, et al. Genetic investigation in frontotemporal dementia and Alzheimer’s disease: the GIFT Study. [Accessed December 19, 2014];Ann Neurol. 2007 62(suppl 11):S52, Abstract T-10. http://onlinelibrary.wiley.com/doi/10.1002/ana.v62:11%2B/issuetoc. [Google Scholar]
- 22.Li Y, Chen JA, Sears RL, et al. An epigenetic signature in peripheral blood associated with the haplotype on 17q21.31, a risk factor for neurodegenerative tauopathy. PLoS Genet. 2014;10 (3):e1004211. doi: 10.1371/journal.pgen.1004211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu MC, Lee S, Cai T, Li Y, Boehnke M, Lin X. Rare-variant association testing for sequencing data with the sequence kernel association test. Am J Hum Genet. 2011;89(1):82–93. doi: 10.1016/j.ajhg.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang B, Gaiteri C, Bodea LG, et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell. 2013;153(3):707–720. doi: 10.1016/j.cell.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webster JA, Gibbs JR, Clarke J, et al. NACC-Neuropathology Group. Genetic control of human brain transcript expression in Alzheimer disease. Am J Hum Genet. 2009;84(4):445–458. doi: 10.1016/j.ajhg.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galvin JE, Palamand D, Strider J, Milone M, Pestronk A. The muscle protein dysferlin accumulates in the Alzheimer brain. Acta Neuropathol. 2006;112(6):665–671. doi: 10.1007/s00401-006-0147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rademakers R, Cruts M, Sleegers K, et al. Linkage and association studies identify a novel locus for Alzheimer disease at 7q36 in a Dutch population-based sample. Am J Hum Genet. 2005;77(4):643–652. doi: 10.1086/491749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stenson PD, Mort M, Ball EV, Shaw K, Phillips A, Cooper DN. The Human Gene Mutation Database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet. 2014;133(1):1–9. doi: 10.1007/s00439-013-1358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reitz C, Jun G, Naj A, et al. Alzheimer Disease Genetics Consortium. Variants in the ATP-binding cassette transporter (ABCA7), apolipoprotein E ε4, and the risk of late-onset Alzheimer disease in African Americans. JAMA. 2013;309(14):1483–1492. doi: 10.1001/jama.2013.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Aoki M, Illa I, et al. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet. 1998;20(1):31–36. doi: 10.1038/1682. [DOI] [PubMed] [Google Scholar]
- 31.Bashir R, Britton S, Strachan T, et al. A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat Genet. 1998;20(1):37–42. doi: 10.1038/1689. [DOI] [PubMed] [Google Scholar]
- 32.Bansal D, Miyake K, Vogel SS, et al. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423(6936):168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- 33.Han R, Campbell KP. Dysferlin and muscle membrane repair. Curr Opin Cell Biol. 2007;19(4):409–416. doi: 10.1016/j.ceb.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hochmeister S, Grundtner R, Bauer J, et al. Dysferlin is a new marker for leaky brain blood vessels in multiple sclerosis. J Neuropathol Exp Neurol. 2006;65(9):855–865. doi: 10.1097/01.jnen.0000235119.52311.16. [DOI] [PubMed] [Google Scholar]
- 35.Cacciottolo M, Nogalska A, D’Agostino C, Engel WK, Askanas V. Dysferlin is a newly identified binding partner of AβPP and it co-aggregates with amyloid-β42 within sporadic inclusion-body myositis (s-IBM) muscle fibers. Acta Neuropathol. 2013;126(5):781–783. doi: 10.1007/s00401-013-1186-6. [DOI] [PubMed] [Google Scholar]
- 36.Jowsey PA, Doherty AJ, Rouse J. Human PTIP facilitates ATM-mediated activation of p53 and promotes cellular resistance to ionizing radiation. J Biol Chem. 2004;279(53):55562–55569. doi: 10.1074/jbc.M411021200. [DOI] [PubMed] [Google Scholar]
- 37.Munoz IM, Jowsey PA, Toth R, Rouse J. Phospho-epitope binding by the BRCT domains of hPTIP controls multiple aspects of the cellular response to DNA damage. Nucleic Acids Res. 2007;35(16):5312–5322. doi: 10.1093/nar/gkm493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J, Prindle MJ, Dressler GR, Yu X. PTIP regulates 53BP1 and SMC1 at the DNA damage sites. J Biol Chem. 2009;284(27):18078–18084. doi: 10.1074/jbc.M109.002527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.