Abstract
Elucidating the neuronal mechanisms underlying movement disorders is a major challenge due to the intricacy of the relevant neural circuits, which are characterized by diverse cell types and complex connectivity. A major limitation of traditional techniques, such as electrical stimulation or lesions, is that individual elements of a neural circuit cannot be selectively manipulated. Moreover, available treatments are largely based on trial and error rather than a detailed understanding of the circuit mechanisms. Gaps in our knowledge of the circuit mechanisms for movement disorders, as well as mechanisms underlying known treatments such as deep brain stimulation, make it difficult to design new and improved treatment options. In this perspective, we discuss how optogenetics, which allows researchers to use light to manipulate neuronal activity, can contribute to the understanding and treatment of movement disorders. We outline the advantages and limitations of optogenetics and discuss examples of studies that have used this tool to clarify the role of the basal ganglia circuitry in movement.
In large part, our current understanding of the neural circuits underlying movement disorders is based on clinical observations of patients with focal brain pathology, surgical lesions or electrode stimulation, and similar approaches in animal models. These approaches have identified the key brain regions associated with most movement disorders, allowing the formulation of many hypotheses on the circuit mechanisms that are responsible for the observed symptoms. Nevertheless, a key limitation is the lack of selectivity in the conventional tools used to probe neural circuit function. These tools typically affect many circuit elements, and do not permit the formulation of specific mechanistic hypotheses on movement disorders. In recent years, a new technique, optogenetics, has emerged as a tool of choice for examining neural circuit function. Optogenetics enables neuroscientists to probe the functions of discrete circuit elements, such as defined subsets of neurons or axonal projections.
Using optogenetics to study neural circuits
Optogenetics uses light to control genetically encoded light-activated proteins–commonly ion channels–to selectively manipulate the activity of neurons 1, 2. Researchers have attempted to use light to manipulate cells for many years 3, 4, but early optical control of neural activity was slow (seconds to minutes), with poor trafficking of the proteins to the cell membrane, or requiring multiple signaling components. Recent developments have overcome these hurdles. With the discovery of the light-gated cation channel, channelrhodopsin-2 (ChR2), from C. reinhardtii, a light sensing protein was combined with an ion channel that was encoded by a single gene and that could be reliably expressed in mammalian neurons 5. The microbial opsin genes commonly used to manipulate neural activity encode single proteins that form ion channels and bind retinal within the channel (termed ‘rhodopsin’ when retinal is bound). Because retinal is present in sufficient quantities in mammalian tissues, opsins can be introduced into mammalian neurons to manipulate the activity of defined neuronal populations 2. The retinal serves as a light sensor. When photo-activated, it triggers conformational changes within the opsin, leading to opening of the ion channel. In the case of ChR2, light activation permits cation influx through the channel, which depolarizes the cell sufficiently to initiate action potentials and neurotransmitter release. Inhibitory opsins function similarly to hyperpolarize the cell and reduce the probability of action potentials. In recent years 2, 5, numerous opsin variants have been developed to allow reliable membrane trafficking and large channel currents in a variety of neuron types (reviewed in 6).
Optogenetics allows researchers to shine a light on cell bodies and/or axon terminals at a distal site and depolarize or hyperpolarize the neurons or cellular elements that express the opsin. Employing optogenetic techniques in neural systems requires three major steps: 1) developing an opsin to achieve the desired neuronal effect, 2) expressing the opsin in the desired cells, and 3) delivering light to the opsin 7.
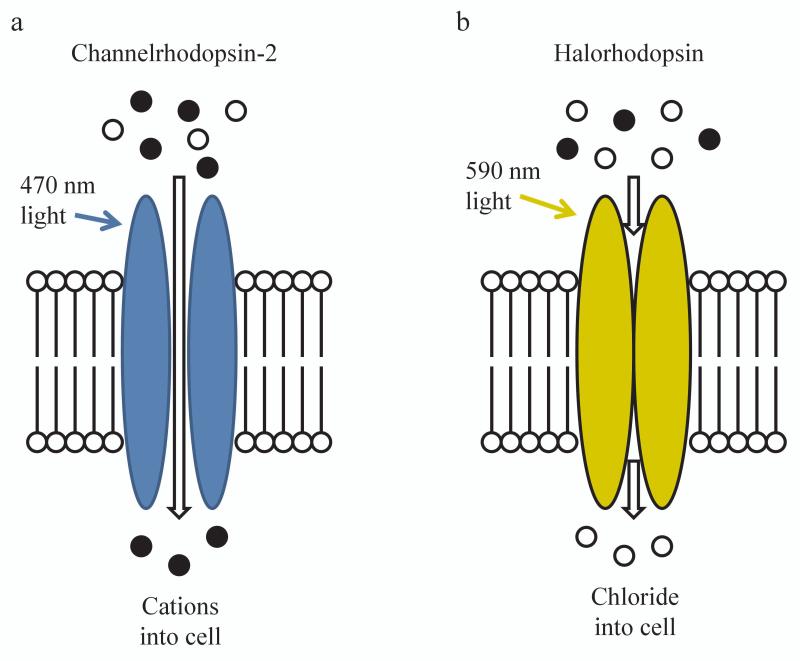
The two major classes of rhodopsins are: 1) excitatory (e.g., ChR2) and 2) inhibitory (e.g., the chloride pump, halorhodopsin). Channelrhodopsin variants are nearly always light-gated cation channels that allow for fast (on the order of milliseconds) depolarization of neurons. Inhibitory opsins take a variety of forms, including proton pumps and chloride pumps (reviewed in 8), which provide relatively inefficient neuronal silencing. Recently, however, ChR2 has been modified into a light-gated anion channel, providing more efficient neuronal inhibition 9. Excitatory opsins, including many channelrhodopsin variants, commonly respond to blue (~470 nm) light (Figure 1). Many inhibitory opsins, including halorhodopsin and the proton pump archaerhodopsin-3 (Arch), respond to yellow-green light (~530-590 nm). Finally, there exist still other opsin variants that operate outside these confines and allow excitation or inhibition with other wavelengths of light 10, 11.
Figure 1. Schematic illustration of common opsins.
(a) When blue light hits channelrhodopsin-2, the channel opens allowing influx of cations. (b) When yellow light hits halorhodopsin, chloride is pumped into the cell. Adapted from 1.
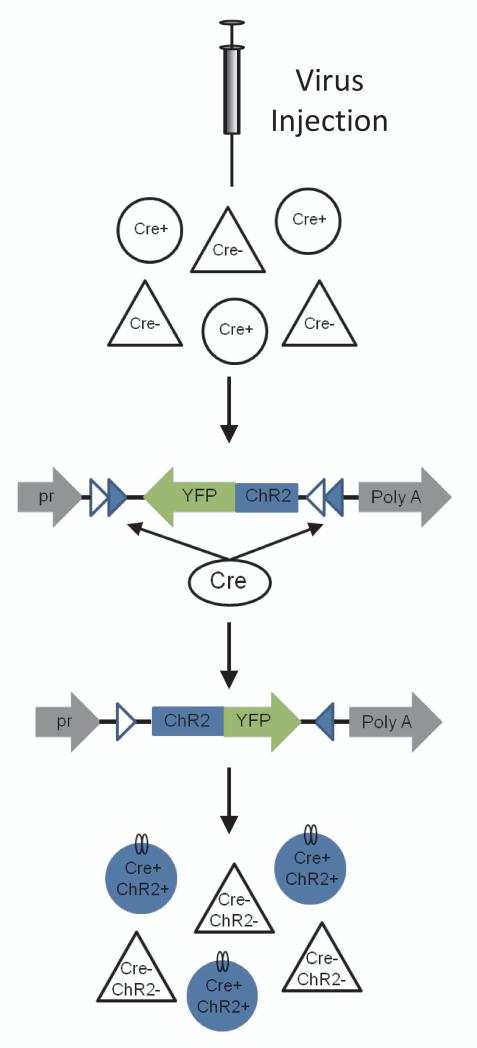
Getting the opsin expressed in the brain and target cell of interest is accomplished with genetic techniques. A common method is viral transduction. The genetic material needed to produce the opsin can be packaged inside a virus for delivery into the neuron, which then utilizes the endogenous cellular machinery to translate the protein and traffic it to the membrane. Cell-type specificity of opsin expression can be achieved with a variety of techniques. One approach is to include a promoter sequence within the virally delivered genetic element that is specific to a molecularly defined neuronal population. The major drawback of this method is that the promoter must be relatively small to fit within the viral packaging constraints, which limits the number and type of cells that can be targeted. Alternatively, the virally delivered genetic element can be conditionally expressed, most commonly only when a Cre recombinase is present to recombine the DNA sequence (Figure 2). Many well-characterized Cre recombinase transgenic mouse lines are available in which the recombinase is under the control of various promoters 12. Lastly, for some opsins, there are transgenic mouse lines that contain the inactive transgene that requires Cre recombinase to activate it. The Cre recombinase may be co-expressed by cross breeding with a Cre transgenic strain or by delivering a Cre-expressing virus 13, 14. In approaches using viral delivery, an additional level of spatial/regional specificity becomes possible because expression will be restricted to neurons near the site of viral delivery. It is also possible to use multiple recombinases (e.g., Cre and Flp) to further limit expression to only a subset of cells that are defined by multiple features 15.
Figure 2. Strategy for Cre-dependent expression of opsins.
The example shown uses the double floxed inverted open reading frame strategy. Opsin-encoding virus can be injected into a transgenic mouse expressing Cre recombinase in a molecularly defined subset of cells. In the presence of Cre recombinase, the opsin-encoding region is inverted, allowing translation into functional protein within these cells. pr, ubiquitous promoter. Adapted from 1.
Since rhodopsins are light-gated, it is necessary to deliver light into the tissue of interest. Light is typically delivered with fiber optics, which pass light from an external source (laser or LED) into the brain 16-18. Light can also be delivered using locally generated light sources (i.e., luminopsin 19 or an implantable microscale light emitting diode 20). For preparations in which dissected brain tissue or cultured cells are studied, the external light source can be shone directly onto the tissue via microscope lenses. When delivering the light, there is again an opportunity to spatially restrict the elements that will be activated. One powerful example of such benefits is when a given neuron sends axon collaterals to multiple spatially distinct brain regions. Opsin expression is sufficiently robust that the experimenter can illuminate axons in a given target region and activate only that subset of the neuron’s projections. Therefore, the combinations of virus, light, and DNA recombinase enable spatially and temporally restricted inhibition or excitation of defined neuronal and glial populations (Figure 3).
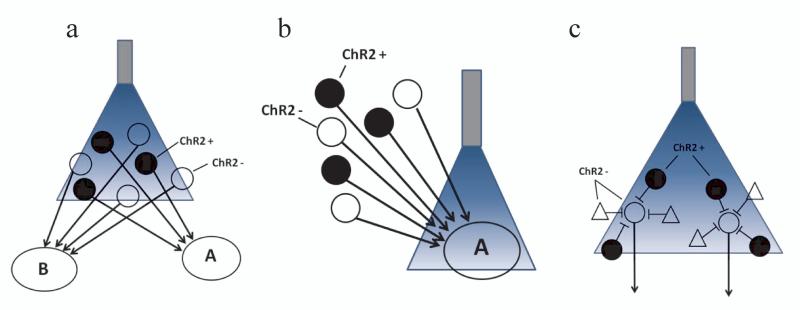
Figure 3. Approaches for dissection of neural circuits using optogenetics.
Schematic illustration of stimulation of molecularly defined neurons using ChR2 as an example. (a) Selective expression of ChR2 in a molecularly defined neuron population allows optical activation of a subset of cell bodies embedded within heterogeneous tissue. (b) When ChR2 is expressed in a molecularly defined subset of projection neurons, distal axon terminals may be targeted with optical stimulation. (c) Stimulation of a molecularly defined interneuron population can be used to inhibit post-synaptic projection neurons.
Optogenetic studies of the neural mechanisms underlying movement disorders
The basal ganglia are implicated in many movement disorders. These subcortical nuclei are interconnected in a complex neural circuit. Many fibers of passage from other brain areas, in particular the cerebral cortex, are found within the basal ganglia. There are also many different types of neurons that are found within a small region without spatial segregation 21.
Much research in the last two decades has been focused on the striatonigral and striatopallidal pathways, the so-called direct and indirect pathways, respectively, which are responsible for regulating motor output. However, whether imbalances in the activity of these pathways leading to behavioral pathology could arise from striatal projection neurons was difficult to directly test because these projection neurons are intermingled and not readily distinguishable by their electrophysiological properties alone. Conventional electrical stimulation affects neurons from both pathways, in addition to fibers of passage. Furthermore, even if electrical stimulation produced reliable behavioral effects, it is still impossible to generate a specific functional model of the neural circuit because it is unclear how the relevant circuit components were affected by the stimulation. In many stimulation paradigms, both activation and inhibition of firing have been proposed 22.
Optogenetic tools have been used to provide evidence that activation of direct and indirect pathways can have different effects on movement. In mice, selective stimulation of striatal dopamine D1 receptor-expressing neurons (striatonigral neurons) resulted in robust inhibition of neurons within the substantia nigra pars reticulata (SNR) and potentiated locomotion 23. Conversely, stimulation of striatal dopamine D2 receptor expressing neurons (striatopallidal neurons) inhibited locomotion 23. Optogenetic stimulation of striatonigral neurons also improved movement in dopamine-depleted mice 23. By targeting discrete neuronal populations, these experiments provide an example of how optogenetics may be employed to test traditional hypotheses about basal ganglia function.
Optogenetic techniques have also been used to elucidate the contributions of the basal ganglia to action initiation and termination, behaviors that are often disrupted in patients with movement disorders. This work shows another useful application of optogenetics, which is in determining cell type during in vivo recording. In this setting, the presence of a light-evoked electrical response was used to identify the type of neurons being recorded. Because the expression of ChR2 was limited to cells expressing Cre recombinase under control of a specific promoter, the presence of a light-evoked response indicates that the cell expresses the gene driven by the promoter. In this way, for example, dopamine neurons in the substantia nigra pars compacta could be identified when the tyrosine hydroxylase promoter is used. Using this approach, Jin et al. found that optogenetically-identified dopamine neurons in the substantia nigra pars compacta show bursts of action potentials prior to the initiation of action sequences 24. Furthermore, using ChR2 to identify striatonigral and striatopallidal pathway neurons in freely behaving mice, researchers have shown that neurons of both pathways are active during initiation and termination of rapid movement sequences 25.
Optogenetics provides many new opportunities to test long-held views of basal ganglia circuitry. For example, it is commonly thought that stimulation of striatonigral neurons will inhibit the basal ganglia output and potentiate movement, whereas stimulation of striatopallidal neurons will facilitate basal ganglia output and inhibit movement. In a test of these ideas, optogenetic activation of either pathway was found to both excite and inhibit basal ganglia output neurons in the SNR 26. However, movement initiation resulting from direct pathway stimulation was more strongly correlated with the inhibited SNR neurons, and movement suppression driven by indirect pathway stimulation was more strongly correlated with the excited SNR neurons. More detailed analysis of movement parameters showed that both the direct and indirect pathways are active during contraversive movements and that activity in both circuits is necessary for movement 27, 28. These results advance our understanding of the direct and indirect pathways to reveal that movement is unlikely to be explained by simple binary activation of one pathway or the other, but rather involves a dynamic interaction between the two. The multitude of downstream circuit effects observed also suggest that our long-held models of basal ganglia function are in need of revision.
As a final example of the application of optogenetics to understand the contributions of basal ganglia circuitry to behavior, we discuss recent work in which optogenetics was applied to help understand the circuits contributing to dystonia. Prior work in a mouse model for Rapid Onset Dystonia Parkinsonism (RDP) due to loss of function of a Na+-K+ATPase had implicated dysfunction between cerebellar and striatal circuits29. In that study, lesioning the centrolateral thalamic nucleus was performed to support a model whereby altered cerebellar outflow via this thalamic relay into the striatum was necessary for dystonia. Recently, the investigators employed optogenetic tools to functionally define this circuitry, demonstrating that cerebellar projections from dentate gyrus modulated short latency striatal responses through connections with the centrolateral thalalmic nucleus30. Thalamic illumination of ChR2-expressing axons that originated in the cerebellar dentate gyrus was shown to modulate striatal responses. However, when thalamic cell bodies were virally transduced to express halorhodopsin and illuminated while ChR2 was activated in the dentate gyrus, striatal responses were blocked. Further, optogenetic silencing of the intralaminar thalamic nuclei alleviated dystonic movements that were triggered by cerebellar infusion of a Na+-K+ATPase inhibitor, ouabain. These results show the application of optogenetic tools to functionally define circuit elements and test their behavioral significance in dystonia.
Mechanisms of DBS
Optogenetics makes it possible for researchers to record from neurons in awake behaving animals while stimulating either the afferent fibers from some other neuronal population or the cell bodies of nearby neurons 31, 32. For this reason, the high spatial and temporal selectivity of optogenetics make it a powerful tool to study the mechanisms of deep brain stimulation (DBS), currently a major treatment option for movement disorders. DBS often shows nearly immediate effects on alleviating tremor in Parkinson’s disease (PD) and essential tremor. By contrast, therapeutic effects for other disorders such as dystonia and Obsessive Compulsive Disorder (OCD) can take much longer to manifest. There is currently no satisfactory explanation for these differences. Moreover, the effective frequency, amplitude, pulse duration, and location of DBS are determined by trial and error for each patient. Because of the numerous unknown factors underlying this treatment modality, animal models may prove useful for performing systematic comparisons aimed at revealing the therapeutic stimulation effects and thereby enhance the ability of practitioners to optimize stimulation parameters.
Notably, the first major application of optogenetics for understanding brain circuits was a study examining which neural elements in the subthalamic nucleus (STN) might underlie the efficacy of DBS in PD 33. PD symptoms can be treated by ablations or high frequency DBS of the STN or the internal segment of the globus pallidus (GPi). Because of the efficacy of both DBS and ablations, it is hypothesized that DBS inactivates the stimulated neurons. However, there is no consensus concerning the mechanism by which DBS affects neural activity and ameliorates PD symptoms. While some studies found inhibition of local neurons during DBS, others showed excitation of efferent projections (discussed in 34).
To elucidate the mechanisms underlying DBS treatment of PD, the light-gated chloride pump, halorhodopsin, was selectively introduced into excitatory neurons within the STN of hemiparkinsonian rats, which show deficits in contralateral limb use and increased frequency of ipsilateral rotations 33. In contrast to electrical DBS, optogenetic inhibition of STN neurons failed to reduce PD symptoms. Thus, inactivating the STN, the goal of traditional DBS, may not be the mechanism responsible for improving motor control in patients. In addition, excitation of STN neurons at high or low frequencies also failed to have significant effects.
Because DBS may activate both local neurons as well as afferent fibers of passage, Gradinaru and colleagues next tested whether stimulation of afferents fibers to the STN can improve motor symptoms. High frequency photo-stimulation of STN afferents reliably reduced PD symptoms, allowing rats to move freely. In anesthetized animals, this manipulation reduced STN neuron spike rates and abolished bursting. Finally, it was shown that ChR2 activation of the cell bodies of a major source of afferents to the STN, layer V pyramidal neurons from the motor cortex, also significantly improved PD symptoms 33. This is in agreement with previous electrical STN DBS studies suggesting that antidromic activation of cortical neurons may contribute to the therapeutic efficacy of DBS 35. However, anatomical differences between the hyperdirect pathway in primates and rodents may still limit the direct translation of these findings 36-38.
According to the traditional model of basal ganglia pathology in PD, excessive activation of STN neurons is responsible for excessive output of the basal ganglia output nuclei (e.g. internal globus pallidus and substrantia nigra pars reticulata). However, alternative proposed mechanisms suggest that DBS may act by disrupting pathological firing patterns 39. The study by Gradinaru et al suggests that reducing the output of STN neurons by direct inhibition does not necessarily alleviate PD symptoms. Rather a more complex interaction between cortical afferents and the STN that inhibited STN bursting and firing rates was found to be the most efficacious. Interestingly, this study failed to replicate the results of pharmacological inactivation studies in which muscimol injection into the STN ameliorated PD symptoms 40. This could be because of species differences, but also might be due to inefficient optogenetic inhibition or ineffective stimulation parameters, emphasizing the importance of electrophysiological validation of putative optogenetic effects in such studies. Interestingly, this study failed to replicate the results of pharmacological inactivation studies in which muscimol injection into the STN ameliorated PD symptoms 40. This could be due to species differences, inefficient optogenetic inhibition, or ineffective stimulation parameters. Despite these caveats, this pioneering study illustrates how the mechanisms of DBS can be dissected using optogenetic manipulations of defined neuronal populations. with significant implications for our understanding of the relevant neural circuitry in humans.
Limitations of optogenetics
While optogenetics provides a powerful tool for studying neural functions, it has a number of limitations: 1) Although opsins are often assumed to have no leak current, it is unlikely that this is true of every channel. Given significant leak current, the opsin may contribute to spontaneous activity; 2) opsins may also build up within cells causing toxicity over time; 3) light stimulation parameters (e.g. stimulation duration, frequency, and intensity) must be chosen carefully and verified in a given cell type to ensure effective neuronal control to avoid problems like depolarization block 41 or rebound excitation 42, 43. Just as stimulation frequency and pulse width of electrode-delivered stimuli can greatly influence neural responses, the light stimulus is another variable that can affect responses. Stimuli used in studies range from continuous illumination over multiple seconds to short millisecond-duration pulses and vary in intensity, introducing a variable that can generate different cellular responses in otherwise similar optogenetic manipulations. Likewise although optogenetic stimulation can achieve high temporal precision (up to ~120 Hz depending on the cell type), whether optogenetic stimulation achieves these rates in any given application must be determined empirically. Even at this relatively high rate, electrical stimulation still offers superior temporal precision (e.g. rates up to ~300 Hz). Altogether, these concerns and the increasing number of opsin variants being made available to researchers emphasize the need to verify the efficacy of expression and stimulation as well as how the opsins may affect the biophysical properties of a given cell type.
In addition to the practical considerations for implementing these techniques, there are caveats that must be taken into account when interpreting the experimental results. Activation of cell bodies can spread to collateral targets, though illuminating the axon terminals in a given target is one way to minimize this possibility. However, even when targeting axon terminals, the possibility of antidromic stimulation is a potential problem. Optogenetic stimulation can also lead to robust synchronized activity that might not reflect natural firing patterns. For example, if high-frequency ChR2-mediated stimulation of a population of neurons increases the frequency of a particular behavior, it is important to keep in mind that this may not hold true when the pattern of stimulation accurately mimics endogenous activity. Similarly, negative results can be difficult to interpret: If stimulation or inhibition of a group of neurons shows no discernible effect on a behavior, it is difficult to conclude that there is truly no effect. The negative result could be due to insensitivity of the behavioral assay, insufficient opsin expression, or difficulty targeting diffuse cell populations with virus injection and optic fibers. Importantly, both opsin expression levels and timing of the expression can vary by virtue of the distinct genetic promoter elements driving its expression or that of the Cre recombinase used to activate the opsin expression cassette. Lastly, the spatial specificity of optogenetic stimulation can be a drawback for investigations of broadly distributed circuits. Thus, as with any single research modality, there are new opportunities as well as limitations.
The future: Optogenetics in humans?
Presently, the primary use of optogenetics is for the dissection of neural circuits that underlie behavioral and physiological phenomena in non-human animals. Optogenetic techniques have been employed in many species, including: C. elegans, Drosophila, zebrafish, birds, mice, rats, and non-human primates. Though optogenetic therapies have not yet been employed in patients, it is conceivable that this may one day be a viable treatment option for movement disorders.
Developing reliable and effective hardware for optogenetic stimulation is one obstacle to overcome before optogenetics can be used in humans. Durable probes that reduce heating of the brain tissue as well as local light and power sources will be necessary. One potential solution to these issues is to use implantable LEDs 20, which produce little heat and consume relatively little power compared to lasers. Although the technical challenge of delivering a reliable light source is quite tractable, the delivery of the opsins presents a major challenge.
Viral delivery of exogenous genes has already been clinically tested in PD patients 44, 45, but the long-term safety and viability of viral delivery of exogenous genes is largely unknown 46, 47. Viral transfection of opsins into the brains of non-human primates has been successful 48-50, and researchers have used optogenetics in in vitro human retina preparations to restore light sensitivity to the previously light-insensitive photoreceptors of blind patients 51. These tools may soon be available for clinical trials. Yet before optogenetics can be used to treat patients, there are still a number of technical obstacles to overcome 52. For example, the potential for long-term burdens on neurons that express high levels of foreign proteins is unknown. Given the potential for opsins to generate leak currents, they may also place additional energy demands on the neurons. It is also unclear whether the small regions that can be targeted with traditional viral delivery will provide opsin expression at levels that are sufficient to affect behavior. While viruses may transduce a sufficient area of tissue to garner clinical efficacy in rodents, the larger size of the human brain and differences in neuroanatomy may present non-trivial obstacles for at least a direct translation of such results. For these reasons, the possibility of treating humans with virally-delivered opsins should be approached with caution. At the least, translation of rodent findings into primate models and extensive characterization of the long-term effects may be needed before clinical trials become feasible.
Although this perspective has focused on optogenetics, another recently developed technique, chemogenetics, is also likely to translate to human therapy. This involves using modified G-protein coupled receptors (GPCRs), which bind synthetic compounds that are otherwise inert within the body. These GPCRs can affect intracellular biochemical signaling cascades and thereby modulate neuronal activity. Getting the receptor into the neurons is accomplished with similar genetic methods as are used for optogenetics. These two methods are potentially complementary. While optogenetics tends to be more useful for spatially and temporally restricted neuronal manipulation, chemogenetics, on the other hand, is more ideal for spatially diffuse applications. Because the synthetic ligand can be administered systemically, chemogenetics provides a means to target diffuse neuronal populations by ingesting a pill. This method also removes the need for hardware delivering light directly into the brain. Of course, the GPCRs of chemogenetics act with different time courses and less direct cellular effects than the ion channels of optogenetics. For example, typically the ligand activates the receptor for minutes to hours after administration 53. Given these differences, a given disease may be more aptly treated by one approach or the other.
While there are still a number of obstacles to overcome before delivering optogenetic stimulation as a human therapy, the information gained from preclinical studies can still greatly accelerate clinical therapy development. Knowledge of functional circuits can guide targeting of treatments currently available such as DBS and transcranial magnetic stimulation. Moreover, when cell populations with expression of relatively unique receptor subtype are found to be key determinants of a behavior, pharmacological compounds can be developed to target them.
Concluding remarks
While no single technique can provide the insights necessary to fully understand the pathogenesis and treatment of movement disorders, optogenetics has become a transformative tool for neuroscience research. Optogenetics provides a way to examine synaptic and circuit properties at a mechanistic level of analysis that was previously unapproachable. This and related techniques have been widely embraced by the neuroscience community. Although there is no shortage of young neuroscientists eager to perform new experiments on the role of specific neural circuits in behavior, neurologists occupy an important position in this process in that they can provide a uniquely informed perspective on those behaviors that are poorly understood, most troubling to patients, and for which treatment options are most limited. Thus, optogenetics also offers a great opportunity for clinical neurologists to reflect back on old questions and refocus them in the light of the research possibilities that optogenetics brings.
Acknowledgements
This work was supported by NIH grants AA021074 to HHY and NS064577 to NC, the Bachmann Strauss Dystonia and Parkinson Foundation, and a National Science Foundation fellowship to MAR.
Footnotes
Conflict of Interest: None.
References
- 1.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8(9):1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 3.Miesenbock G, Kevrekidis IG. Optical imaging and control of genetically designated neurons in functioning circuits. Annu Rev Neurosci. 2005;28:533–563. doi: 10.1146/annurev.neuro.28.051804.101610. [DOI] [PubMed] [Google Scholar]
- 4.Oesterhelt D, Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- 5.Nagel G, Szellas T, Huhn W, et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100(24):13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71(1):9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Zhang F, Gradinaru V, Adamantidis AR, et al. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc. 2010;5(3):439–456. doi: 10.1038/nprot.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang F, Vierock J, Yizhar O, et al. The microbial opsin family of optogenetic tools. Cell. 2011;147(7):1446–1457. doi: 10.1016/j.cell.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berndt A, Lee SY, Ramakrishnan C, Deisseroth K. Structure-guided transformation of channelrhodopsin into a light-activated chloride channel. Science. 2014;344(6182):420–424. doi: 10.1126/science.1252367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow BY, Han X, Dobry AS, et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463(7277):98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang F, Prigge M, Beyriere F, et al. Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri. Nat Neurosci. 2008;11(6):631–633. doi: 10.1038/nn.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. 2008;28(28):7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madisen L, Mao T, Koch H, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15(5):793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng H, Madisen L. Mouse transgenic approaches in optogenetics. Prog Brain Res. 2012;196:193–213. doi: 10.1016/B978-0-444-59426-6.00010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenno LE, Mattis J, Ramakrishnan C, et al. Targeting cells with single vectors using multiple-feature Boolean logic. Nat Methods. 2014;11(7):763–772. doi: 10.1038/nmeth.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sparta DR, Stamatakis AM, Phillips JL, Hovelso N, van Zessen R, Stuber GD. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat Protoc. 2012;7(1):12–23. doi: 10.1038/nprot.2011.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi MA, Sukharnikova T, Hayrapetyan VY, Yang L, Yin HH. Operant self-stimulation of dopamine neurons in the substantia nigra. PLoS One. 2013;8(6):e65799. doi: 10.1371/journal.pone.0065799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossi MA, Hayrapetyan VY, Maimon B, Mak K, Je HS, Yin HH. Prefrontal cortical mechanisms underlying delayed alternation in mice. J Neurophysiol. 2012;108(4):1211–1222. doi: 10.1152/jn.01060.2011. [DOI] [PubMed] [Google Scholar]
- 19.Berglund K, Birkner E, Augustine GJ, Hochgeschwender U. Light-emitting channelrhodopsins for combined optogenetic and chemical-genetic control of neurons. PLoS One. 2013;8(3):e59759. doi: 10.1371/journal.pone.0059759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim TI, McCall JG, Jung YH, et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science. 2013;340(6129):211–216. doi: 10.1126/science.1232437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. J Anat. 2000;196(Pt 4):527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol. 2004;115(6):1239–1248. doi: 10.1016/j.clinph.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 23.Kravitz AV, Freeze BS, Parker PR, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466(7306):622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin X, Costa RM. Start/stop signals emerge in nigrostriatal circuits during sequence learning. Nature. 2010;466(7305):457–462. doi: 10.1038/nature09263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin X, Tecuapetla F, Costa RM. Basal ganglia subcircuits distinctively encode the parsing and concatenation of action sequences. Nat Neurosci. 2014;17(3):423–430. doi: 10.1038/nn.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freeze BS, Kravitz AV, Hammack N, Berke JD, Kreitzer AC. Control of basal ganglia output by direct and indirect pathway projection neurons. J Neurosci. 2013;33(47):18531–18539. doi: 10.1523/JNEUROSCI.1278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tecuapetla F, Matias S, Dugue GP, Mainen ZF, Costa RM. Balanced activity in basal ganglia projection pathways is critical for contraversive movements. Nat Commun. 2014;5:4315. doi: 10.1038/ncomms5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui G, Jun SB, Jin X, et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494(7436):238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calderon DP, Fremont R, Kraenzlin F, Khodakhah K. The neural substrates of rapid-onset Dystonia-Parkinsonism. Nat Neurosci. 2011;14(3):357–365. doi: 10.1038/nn.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen CH, Fremont R, Arteaga-Bracho EE, Khodakhah K. Short latency cerebellar modulation of the basal ganglia. Nat Neurosci. 2014;17(12):1767–1775. doi: 10.1038/nn.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kravitz AV, Owen SF, Kreitzer AC. Optogenetic identification of striatal projection neuron subtypes during in vivo recordings. Brain Res. 2013;1511:21–32. doi: 10.1016/j.brainres.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stuber GD, Britt JP, Bonci A. Optogenetic modulation of neural circuits that underlie reward seeking. Biol Psychiatry. 2012;71(12):1061–1067. doi: 10.1016/j.biopsych.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324(5925):354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agnesi F, Johnson MD, Vitek JL. Deep brain stimulation: how does it work? Handb Clin Neurol. 2013;116:39–54. doi: 10.1016/B978-0-444-53497-2.00004-8. [DOI] [PubMed] [Google Scholar]
- 35.Li S, Arbuthnott GW, Jutras MJ, Goldberg JA, Jaeger D. Resonant antidromic cortical circuit activation as a consequence of high-frequency subthalamic deep-brain stimulation. J Neurophysiol. 2007;98(6):3525–3537. doi: 10.1152/jn.00808.2007. [DOI] [PubMed] [Google Scholar]
- 36.Hamani C, Saint-Cyr JA, Fraser J, Kaplitt M, Lozano AM. The subthalamic nucleus in the context of movement disorders. Brain. 2004;127(Pt 1):4–20. doi: 10.1093/brain/awh029. [DOI] [PubMed] [Google Scholar]
- 37.Joel D, Weiner I. The connections of the primate subthalamic nucleus: indirect pathways and the open-interconnected scheme of basal ganglia-thalamocortical circuitry. Brain Res Brain Res Rev. 1997;23(1-2):62–78. doi: 10.1016/s0165-0173(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 38.Uylings HB, van Eden CG. Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Prog Brain Res. 1990;85:31–62. doi: 10.1016/s0079-6123(08)62675-8. [DOI] [PubMed] [Google Scholar]
- 39.McIntyre CC, Hahn PJ. Network perspectives on the mechanisms of deep brain stimulation. Neurobiol Dis. 2010;38(3):329–337. doi: 10.1016/j.nbd.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wichmann T, Bergman H, DeLong MR. The primate subthalamic nucleus. III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J Neurophysiol. 1994;72(2):521–530. doi: 10.1152/jn.1994.72.2.521. [DOI] [PubMed] [Google Scholar]
- 41.Herman AM, Huang L, Murphey DK, Garcia I, Arenkiel BR. Cell type-specific and time-dependent light exposure contribute to silencing in neurons expressing Channelrhodopsin-2. Elife. 2014;3:e01481. doi: 10.7554/eLife.01481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raimondo JV, Kay L, Ellender TJ, Akerman CJ. Optogenetic silencing strategies differ in their effects on inhibitory synaptic transmission. Nat Neurosci. 2012;15(8):1102–1104. doi: 10.1038/nn.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edgerton JR, Jaeger D. Optogenetic activation of nigral inhibitory inputs to motor thalamus in the mouse reveals classic inhibition with little potential for rebound activation. Front Cell Neurosci. 2014;8:36. doi: 10.3389/fncel.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaplitt MG, Feigin A, Tang C, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369(9579):2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 45.Palfi S, Gurruchaga JM, Ralph GS, et al. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: a dose escalation, open-label, phase 1/2 trial. Lancet. 2014;383(9923):1138–1146. doi: 10.1016/S0140-6736(13)61939-X. [DOI] [PubMed] [Google Scholar]
- 46.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4(5):346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 47.Kotterman MA, Schaffer DV. Engineering adeno-associated viruses for clinical gene therapy. Nat Rev Genet. 2014;15(7):445–451. doi: 10.1038/nrg3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han X, Qian X, Bernstein JG, et al. Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron. 2009;62(2):191–198. doi: 10.1016/j.neuron.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diester I, Kaufman MT, Mogri M, et al. An optogenetic toolbox designed for primates. Nat Neurosci. 2011;14(3):387–397. doi: 10.1038/nn.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galvan A, Hu X, Smith Y, Wichmann T. In vivo optogenetic control of striatal and thalamic neurons in non-human primates. PLoS One. 2012;7(11):e50808. doi: 10.1371/journal.pone.0050808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Busskamp V, Duebel J, Balya D, et al. Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science. 2010;329(5990):413–417. doi: 10.1126/science.1190897. [DOI] [PubMed] [Google Scholar]
- 52.Williams JC, Denison T. From optogenetic technologies to neuromodulation therapies. Sci Transl Med. 2013;5(177):177ps176. doi: 10.1126/scitranslmed.3003100. [DOI] [PubMed] [Google Scholar]
- 53.Sternson SM, Roth BL. Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci. 2014;37:387–407. doi: 10.1146/annurev-neuro-071013-014048. [DOI] [PubMed] [Google Scholar]