Abstract
Pulmonary hypertension (PH) is currently defined based on invasive measurements: a resting pulmonary artery pressure ≥ 25 mm Hg. For pulmonary arterial hypertension, a pulmonary arterial wedge pressure ≤ 15 mm Hg and pulmonary vascular resistance > 3 Wood units are also required. Thus, right heart catheterization is inevitable at present. However, the diagnosis, follow-up, and management of PH by noninvasive techniques is progressing. Significant advances have been achieved in the imaging of pulmonary vascular disease and the right ventricle. We review the current sensitivities and specificities of noninvasive imaging of PH and discuss its role and future potential to replace hemodynamics as the primary approach to screening, diagnosing, and following/managing PH.
Résumé
L’hypertension pulmonaire (HP) est actuellement définie selon des méthodes effractives de mesure : une pression artérielle pulmonaire ≥ 25 mm Hg au repos. Pour définir l’hypertension artérielle pulmonaire, une pression artérielle pulmonaire d’occlusion ≤ 15 mm Hg et une résistance vasculaire pulmonaire > 3 unités Wood sont également requises. Par conséquent, le cathétérisme cardiaque droit est inévitable pour le moment. Malgré cela, le diagnostic, le suivi et la prise en charge de l’HP par des techniques non effractives évoluent. L’imagerie des maladies vasculaires pulmonaires et du ventricule droit a connu d’importants progrès. Nous passons en revue la sensibilité et la spécificité actuelles de l’imagerie non effractive de l’HP, et discutons de ses rôles et de son potentiel futur de remplacement de l’hémodynamique comme principale approche en matière de dépistage, de diagnostic, de prise en charge/suivi de l’HP.
Pulmonary arterial hypertension (PAH) is an orphan condition with high morbidity and mortality. Despite increased awareness of pulmonary hypertension (PH), data indicate that the majority of patients are still diagnosed in late stages of the disease. A higher World Health Organization functional class is associated with poorer median survival, illustrating the importance of early diagnosis. In this article, we were asked to defend the value of noninvasive imaging in the diagnosis and follow-up of PH. Although we agree that at this point, invasive assessment remains essential, in the long term it is hoped that noninvasive methods will eliminate the need for invasive assessment. Our original mandate was to discuss PAH; however, because this is a rare condition with relatively little information available, we have broadened our approach to include PH in general.
Limitations of Invasive Assessment
Invasive hemodynamic assessment by right heart catheterization is relatively safe but has technical limitations
At the Nice 5th World Symposium on PH, right heart catheterization (RHC) was confirmed as essential for the diagnostic workup of PH to assess the severity of the disease and to perform a vasoreactivity test.1-3 However, RHC is associated with rare, albeit serious, procedure-related complications, including death. In an analysis of 7218 RHC procedures performed in experienced PH centres, 76 serious adverse events, including 4 fatalities, were observed. The most common serious adverse events were supraventricular and ventricular tachycardia, vagal reactions, and systemic hypotension.4 Although RHC is relatively safe, reports of complications do appear, even in expert centres.5
Data acquisition during RHC requires resting supine patients. There is no standard operating procedure for capturing hemodynamic changes that occur with an upright posture or with physical activity using RHC. In addition, hemodynamic measurements acquired by RHC are subject to intraindividual spontaneous variability and represent only a hemodynamic snapshot.1,6
Routine RHC relies on the use of fluid-filled catheters, which have an insufficient frequency response.7 Standard Swan-Ganz catheter manometry systems used in clinical practice have a frequency response of 12 Hz, whereas a minimum of 50 Hz would be required for the assessment of instantaneous pressure signals.7 Fluid-filled catheters require fast flushes to remove air bubbles in the monitoring system, which account for most of the variability compared with the true gold-standard high-fidelity micromanometer-tipped catheters.7 In contrast to high-fidelity micromanometer-tipped catheter systems, fluid-filled catheter transducers have to be positioned at a “zero reference level,” which is most accurately obtained at midthoracic level or at one third of the thoracic diameter below the anterior thorax surface.8 A deviation of 1 cm of the transducer from zero level affects pressures by 0.78 mm Hg, thus leading to significantly different results if 2 different zero reference levels are used in a single patient.8
Currently used invasive cardiac output measurements estimate but do not measure true cardiac output
The gold standard for the assessment of cardiac output (CO) is the direct Fick method in which CO equals O2 consumption divided by the difference between arterial and venous O2 content. Although O2 consumption can be measured accurately, that measurement is cumbersome, and many laboratories use standard tables for an assumed value instead of direct measurements. Such estimation may cause an error of as much as 40% in the assessment of CO.9 Most laboratories now use thermodilution based on an indicator dilution methodology to measure CO.10 When compared with the direct Fick method, thermodilution measurements show little bias, with a mean difference of 0.1 L/min and a confidence interval of 0.2 L/min, corresponding to excellent accuracy even in the presence of tricuspid regurgitation, but limits of agreement are ± 1 L/min, corresponding to moderate precision.11
Need for an integrated diagnostic approach
Clinically significant information is gained from RHC that helps guide decisions. A restrictive use of RHC may delay a timely diagnosis and treatment.6 Still, the simple distinction between pre- and postcapillary PH is a task that often cannot even be achieved by invasive RHC. In particular, heart failure with preserved ejection fraction is commonly misdiagnosed as precapillary PH.12-14 Unresolved issues are the assessment of precatheterization fluid status, standardization of fluid loading,3,15,16 and mean pulmonary arterial wedge pressure measurements—end-expiratory or as pressure-time integral.16,17 The interpretation of invasive hemodynamics is meaningless outside the context of the clinical picture, in particular echocardiography.1,3 To manage the growing number of PH cases resulting from left heart disease (group 2 PH) and caused by lung disease/hypoxia (group 3 PH) in the general population, successful noninvasive diagnostic algorithms combining multiple parameters have been developed to avoid unnecessary RHC.1,18
Present Value of Noninvasive Techniques
Advanced imaging tools are useful for screening
Transthoracic Doppler echocardiography is the predominant screening modality in early stages of diagnosis to assess right ventricular (RV) structure and function, including the degree of ventricular remodelling as well as the derivation of RV systolic and diastolic pressures and analysis of contraction timing,19-23 thus providing a reliable method for the early detection of PH, with a particularly high sensitivity and specificity in systemic sclerosis (Table 1). Recently, software programs for 2-dimensional (2D) strain analysis by speckle tracking have been applied to evaluate the right ventricle.31 Furthermore, significant progress has been made in the use of knowledge-based reconstruction of 3D RV structure and function from 2D images.32 Studies have suggested that 3D echocardiographic imaging of the right ventricle is feasible, and its results compare well with magnetic resonance imaging (MRI).33,34
Table 1.
Noninvasive imaging to screen for PH
| First author | Technique | Number of patients | Study population/cause | Functional parameter/variable | Screening for PH |
|
|---|---|---|---|---|---|---|
| Sensitivity (95% CI), % |
Specificity (95% CI), % |
|||||
| Denton et al.24 | TTE | 33 | CTD (SSc) | sPAP | 90 | 75 |
| Parent et al.25 | TTE | 385 | Sickle cell disease | Tricuspid regurgitation jet velocity | 100 | 80 |
| Rajaram et al.26 | TTE | 81 | CTD | Tricuspid gradient | 86 | 82 |
| Wang et al.27 | TTE | 123 | CHD | sPAP | 89 | 84 |
| Kuriyama et al.28 | CT | 23 | Suspected PH | MPAD | 69 | 100 |
| Perez-Enguix et al.29 | CT | 71 | Candidates for LTX | MPAD | 66 | 86 |
| Rajaram et al.26 | CT | 81 | CTD | Ventricular mass index | 85 | 82 |
| Stevens et al.30 | MRI | 100 | Suspected PH | PVR | 92.5 | 85.2 |
| Rajaram et al.26 | MRI | 81 | CTD | RV mass index | 85 | 82 |
CHD, congenital heart disease; CT, computed tomography; CTD, connective tissue disease; LTX, lung transplantation; MPAD, main pulmonary artery diameter; MRI, magnetic resonance imaging; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RV, right ventricular; sPAP, systolic pulmonary artery pressure; SSc, systemic sclerosis/scleroderma; TTE, transthoracic echocardiography.
Theoretically, imaging of the pulmonary vasculature should be more sensitive to screening because this is where disease starts; yet, the available methods do not appear to have reached adequate sensitivity and specificity for that purpose.35
Advanced imaging tools are useful for diagnosis
Any patient with unexplained PH should be evaluated for chronic thromboembolic PH (CTEPH). Diagnostic algorithms for PH include ventilation/perfusion (V/Q) scintigraphy,1,36-40 multidetector computed tomography (CT), and cardiac MRI (cMRI).41 Although a mosaic pattern is common in CTEPH, it occurs in up to 12% of patients with PAH.42 MRI of the pulmonary vasculature is still considered inferior to CT but may be preferred according to local practice.43 Recent advances—such as dual-energy CT,44 cone-beam CT, electrocardiographic gated 320-row area detector CT, and lung perfusion MRI—are about to change paradigms in pulmonary vascular imaging. In a pilot study, dynamic contrast-enhanced CT was used to distinguish between patients with and those without PAH by contrast material bolus propagation time and speed in the pulmonary arteries.45 Time differences between bolus peaks correlated with mean pulmonary artery pressures, and discrimination could be achieved with a sensitivity of 100% and specificity of 100% in patients without PH and a sensitivity of 93% and specificity of 80% in patients with PAH, respectively (Table 2).45
Table 2.
Noninvasive imaging to diagnose PH
| First author | Technique | No. of patients | Study population/cause | Functional parameter/variable | Diagnosing PAH |
|
|---|---|---|---|---|---|---|
| Sensitivity (95% CI), % |
Specificity (95% CI), % |
|||||
| Isobe et al.46 | TTE | 77 | Controls vs suspected PH | RV acceleration time | 93 | 97 |
| Tei et al.47 | TTE | 63 | Controls vs iPAH | Tei index | – | – |
| Saba et al.48 | TTE | 26 | Suspected PH | sPAP | 89 | 57 |
| Hsu et al.49 | TTE | 49 | CTD (SSc) | sPAP | 58 | 96 |
| Dahiya et al.50 | TTE | 26 | Suspected PH | Corrected PVR Echocardiographic PVR |
91 93 |
90 91 |
| D'Alto et al.51 | TTE | 161 | Suspected PH | Left atrial pressure Cardiac output mPAP PVR |
85 – – – |
– – – – |
| Gladue et al.52 | TTE | 248 | CTD (SSc) | sPAP | 94∗ | 73∗ |
| Tan et al.53 | CT | 45 | Suspected PH | MPAD | 87 | 89 |
| Chan et al.54 | CT | 101 | Suspected PH | MPAD MPAD/AA ratio MPAD/DA ratio Right descending PA diameter RV/LV lumen ratio RV/LV wall ratio RV free wall True left descending PA diameter True right descending PA diameter |
77 74 77 83 86 79 81 79 83 |
90 92 90 85 86 84 92 92 88 |
| Corson et al.55 | CT | 305 | Suspected PH | Right PA diameter MPAD |
89 (85-94) 89 (84-93) |
82 (74-89) 83 (76-90) |
| Helmberger et al.56 | CT | 24 | Controls vs PH | Pulmonary vessel tortuosity | 83 | 83 |
| Pienn et al.45 | CT | 21 | Controls vs PAH | Propagation contrast medium speed | 100 (77-100) | 100 (48-100) |
| Bouchard et al.57 | MRI | 27 | Controls vs PAH | Left descending PA/DA MPAD MPAD/AA RV wall thickness Septal wall thickness |
– | – |
| Saba et al.48 | MRI | 26 | Suspected PH | Ventricular mass index | 84 | 71 |
| Sanz et al.58 | MRI | 59 | Controls vs PAH | Average blood velocity Minimum PA area |
93 (81-98) 93 (81-98) |
82 (57-96) 88 (64-98) |
| Sanz et al.58 | MRI | 72 | PH | Delayed contrast enhancement | – | – |
| Hsu et al.49 | MRI | 49 | CTD (SSc) | MPAD | 68 | 71 |
| Nogami et al.59 | MRI | 20 | Suspected PH | sPAP Stroke volume |
– – |
– – |
| Shehata et al.60 | MRI | 48 | Controls vs PAH | RV longitudinal strain RV circumferential strain RV tangential strain |
– – – |
– – – |
| Swift et al.61 | MRI | 64 | Suspected PH | sPAP PVR |
87 | 90 |
AA, ascending aorta; CT, computed tomography; DA, descending aorta; CTD, connective tissue disease; iPAH, idiopathic pulmonary arterial hypertension; LV, left ventricular; MPAD, main PA diameter; mPAP, mean PA pressure; MRI, magnetic resonance imaging; PA, pulmonary artery; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RV, right ventricular; sPAP, systolic PA pressure; SSc, systemic sclerosis/scleroderma; TTE, transthoracic echocardiography.
In combination with pulmonary function tests.
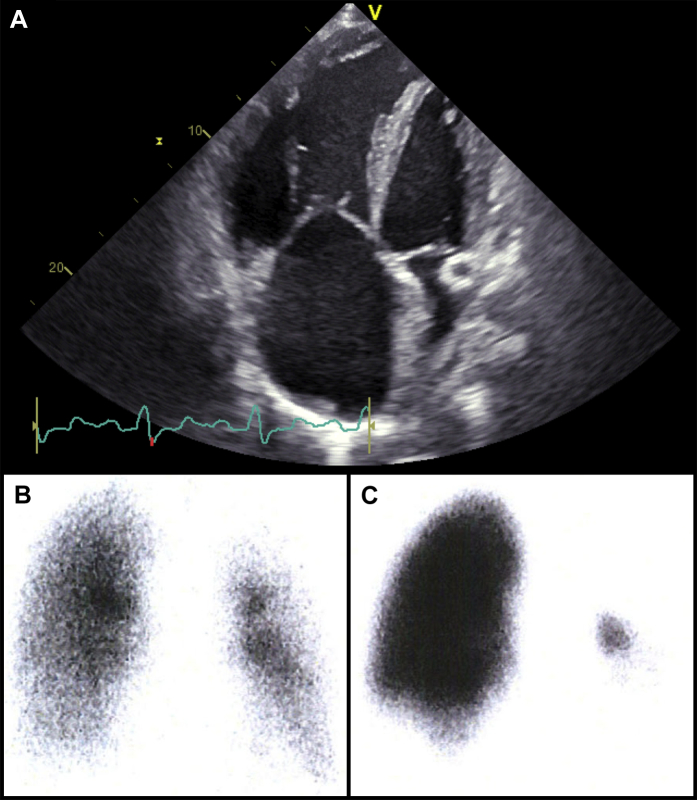
Suspicion should be high when the patient presents with a history of previous venous thromboembolism (VTE). Although formal screening cannot be recommended, CTEPH should be ruled out in any survivor of a pulmonary embolism with persistent dyspnea and > 15% perfusion defects 6 months after the acute VTE after at least 3 months of effective oral anticoagulation.40 V/Q planar images in at least 6 views combined with single-photon emission CT remains the preferred initial diagnostic test for CTEPH. CT pulmonary angiography (CTPA) has a sensitivity of detecting CTEPH of 51%, compared with a > 96% sensitivity of V/Q scanning.62 A normal V/Q, but not a normal CTPA, can exclude CTEPH, although scans tend to normalize as disease progresses.63 CTEPH may be the single PH subset in which advanced imaging and not RHC may be the primary approach to diagnosis, follow-up, and management. In the example shown in Figure 1, the correct diagnosis of CTEPH was made after an echocardiogram and a V/Q scan had been obtained. A CTEPH diagnosis was later confirmed by RHC and pulmonary angiography.
Figure 1.
Imaging in a 24-year-old woman with a history of progressive shortness of breath on exertion, deep vein thrombosis, and recent hemoptysis. (A) Transthoracic echocardiographic 4-chamber view with severe right ventricular dilatation. (B) Technetium-99m–labeled aerosol ventilation and (C) perfusion images show nonmatched perfusion defects of right lower lobe and almost the entire left lung.
Advanced imaging tools are useful for follow-up and management
An important more recent finding is that although PH is a pulmonary vascular disorder, structural and functional assessments of the right ventricle play a central role in both diagnosis and serial follow-up of patients with PAH.23 Therefore, it is reasonable that current guidelines suggest an integrated diagnostic algorithm in which noninvasive modalities are targeted to RV function and can be serially assessed to detect changes (Table 3); such an algorithm will play an ever more important role in the near future.1 For example, the use of 3D speckle tracking to assess area strain, radial strain, longitudinal strain, and circumferential strain correlates with clinical outcomes, with area strain and circumferential strain correlating best with RV ejection fraction.70 Stroke volume and RV ejection fraction measured by cMRI are the most commonly used parameters to evaluate global systolic RV function and to assess response to therapy.74,75,79 However, these parameters are highly dependent on preload and afterload and do not reflect RV contractility.80 RV end-systolic elastance (Ees) is accepted as a load-independent measure of intrinsic myocardial contractility. Ees is usually derived from pressure-volume loops by invasive conductance catheterization. Using this method, arterial elastance (Ea) as a measure of RV afterload can also be determined. RV-to–pulmonary vascular (RV-PV) coupling, the adaptation of the right ventricle to its afterload, can be calculated by Ees divided by Ea (Ees/Ea ratio). However, this method requires the assessment of pressure-volume loops during preload reduction by temporary balloon occlusion of the inferior vena cava, thus making it very invasive and potentially dangerous. As an alternative, Ees/Ea can also be determined by combining measurements from standard RHC and MRI. Studies in healthy individuals and patients with PH have shown good agreement of MRI conductance catheterization data.81,82 The ratio of stroke volume–to–end-systolic volume (SV/ESV) that can be derived completely noninvasively from cMRI was found to correlate well with RV-PV coupling and to be a strong predictor of prognosis.78
Table 3.
Noninvasive imaging to follow-up/detect change in PH
| First author | Technique | No. of patients | Study population/cause | Functional parameter/variable | Detecting change in PAH/CTEPH/PH |
|
|---|---|---|---|---|---|---|
| Sensitivity (95% CI), % |
Specificity (95% CI), % |
|||||
| Chow et al.64 | TTE | 28 | Operable CTEPH before vs after PEA | Acceleration time Tricuspid regurgitation jet velocity |
– – |
– – |
| Eysmann et al.65 | TTE | 26 | iPAH | Pericardial effusion Tricuspid early flow deceleration Pulmonary acceleration time |
– – – |
– – – |
| Tei et al.47 | TTE | 63 | Controls vs iPAH | Tei index | – | – |
| Yeo et al.66 | TTE | 53 | iPAH | Tei index | – | – |
| Raymond et al.19 | TTE | 81 | iPAH | Right atrial area index Diastolic eccentricity index Pericardial effusion |
– – – |
– – – |
| Forfia et al.67 | TTE | 63 | PAH | TAPSE | – | – |
| Dahiya et al.50 | TTE | 10 | PAH | Corrected PVR Echocardiographic PVR |
– – |
– – |
| Fine et al.68 | TTE | 575 | PH | RV longitudinal strain TAPSE |
79 61 |
– – |
| Grünig et al.69 | TTE | 124 | PAH/CTEPH | sPAP; response to exercise | 77 | 53 |
| Smith et al.70 | TTE | 97 | PH | RV ejection fraction TAPSE RV area strain RV circumferential strain RV longitudinal strain RV radial strain |
65 70 80 65 90 75 |
59 59 54 73 52 51 |
| Courand et al.71 | TTE | 100 | PAH | RV ejection fraction | – | – |
| Moledina et al.35 | CT | 31 | Pediatric PAH | Fractal dimension | – | – |
| Zylkowska et al.72 | CT | 264 | PAH/CTEPH | MPAD | 95 | 39 |
| van Wolferen et al.73 | MRI | 64 | PAH | RV ejection fraction RV end-diastolic volume index LV end-diastolic volume index Stroke volume index |
– – – – |
– – – – |
| van de Veerdonk et al.74 | MRI | 76 | PAH | RV ejection fraction | 82 | 75 |
| Freed et al.75 | MRI | 58 | PH | RV ejection fraction RVIP-LGE |
100 | – |
| Ley et al.76 | MRI | 20 | PAH/CTEPH | Cardiac output | – | – |
| Pandya et al.77 | MRI | 50 | Pediatric PAH (CHD) | Septal curvature | 83 (36-99) | 91 (77-97) |
CT, computed tomography; CHD, congenital heart disease; CTEPH, chronic thromboembolic pulmonary hypertension; iPAH, idiopathic pulmonary arterial hypertension; LGE, late gadolinium enhancement; LV, left ventricular; MPAD, mean PA diameter; MRI, magnetic resonance imaging; PA, pulmonary artery; PAH, pulmonary arterial hypertension; PEA, pulmonary thromboendarterectomy; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RV, right ventricular; RVIP, RV insertion point; sPAP, systolic PA pressure; SV/ESV, stroke volume/end-systolic volume ratio; TAPSE, tricuspid annular plane excursion; TTE, transthoracic Doppler echocardiography.
Conclusions
Because of the intrinsic properties of invasive diagnostics, the desire of patients, patient advocates, and physicians is that advanced imaging tools rather than hemodynamics will eventually become the primary approach to diagnosing, following, and managing PH. The values of sensitivities and specificities of available methods shown in Tables 1-3 allow for the selection of the best noninvasive tests for screening, diagnosis, and follow-up in PH, according to testing priorities. Although noninvasive assessment cannot currently replace RHC, it has become an essential part of the management paradigm for PH, and hopefully with further development will 1 day make RHC a historical curiosity.
Funding Sources
This study was supported by FWF KLI209 and FWF F54 and by educational grants from Bayer (Grant No. 15662 [to C.G.]) and United Therapeutics Corporation (Grant No. REG-NC-002 [to M.G.]).
Disclosures
I.M.L. has relationships with AOP Orphan Pharmaceuticals, Actelion, Bayer-Schering, Astra-Zeneca, Servier, Cordis, Medtronic, GSK, Novartis, Pfizer, and United Therapeutics. In addition to being investigator in trials involving these companies, relationships include consultancy service, research grants, and membership on scientific advisory boards. The other authors have no conflicts of interest to disclose.
Footnotes
See article by Maron,pages 515-520of this issue.
See page 525 for disclosure information.
References
- 1.Galie N., Hoeper M.M., Humbert M. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219–1263. doi: 10.1183/09031936.00139009. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin V.V., Archer S.L., Badesch D.B. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 3.Hoeper M.M., Bogaard H.J., Condliffe R. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D42–D50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 4.Hoeper M.M., Lee S.H., Voswinckel R. Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol. 2006;48:2546–2552. doi: 10.1016/j.jacc.2006.07.061. [DOI] [PubMed] [Google Scholar]
- 5.Bonderman D., Pretsch I., Steringer-Mascherbauer R. Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure (DILATE-1): a randomized, double-blind, placebo-controlled, single-dose study. Chest. 2014;146:1274–1285. doi: 10.1378/chest.14-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGoon M., Gutterman D., Steen V. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:14S–34S. doi: 10.1378/chest.126.1_suppl.14S. [DOI] [PubMed] [Google Scholar]
- 7.Nichols W.W., O'Rourke M.F. CRC Press; Philadelphia: 2011. McDonald's Blood Flow in Arteries, 6th Edition: Theoretical, Experimental and Clinical Principles; pp. 129–164. [Google Scholar]
- 8.Kovacs G., Avian A., Olschewski A., Olschewski H. Zero reference level for right heart catheterisation. Eur Respir J. 2013;42:1586–1594. doi: 10.1183/09031936.00050713. [DOI] [PubMed] [Google Scholar]
- 9.Hillis L.D., Firth B.G., Winniford M.D. Analysis of factors affecting the variability of fick versus indicator dilution measurements of cardiac output. Am J Cardiol. 1985;56:764–768. doi: 10.1016/0002-9149(85)91132-4. [DOI] [PubMed] [Google Scholar]
- 10.Ganz W., Donoso R., Marcus H.S., Forrester J.S., Swan H.J. A new technique for measurement of cardiac output by thermodilution in man. Am J Cardiol. 1971;27:392–396. doi: 10.1016/0002-9149(71)90436-x. [DOI] [PubMed] [Google Scholar]
- 11.Hoeper M.M., Maier R., Tongers J. Determination of cardiac output by the Fick method, thermodilution, and acetylene rebreathing in pulmonary hypertension. Am J Respir Crit Care Med. 1999;160:535–541. doi: 10.1164/ajrccm.160.2.9811062. [DOI] [PubMed] [Google Scholar]
- 12.Halpern S.D., Taichman D.B. Misclassification of pulmonary hypertension due to reliance on pulmonary capillary wedge pressure rather than left ventricular end-diastolic pressure. Chest. 2009;136:37–43. doi: 10.1378/chest.08-2784. [DOI] [PubMed] [Google Scholar]
- 13.Robbins I.M., Hemnes A.R., Pugh M.E. High prevalence of occult pulmonary venous hypertension revealed by fluid challenge in pulmonary hypertension. Circ Heart Fail. 2014;7:116–122. doi: 10.1161/CIRCHEARTFAILURE.113.000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bitar A., Selej M., Bolad I., Lahm T. Poor agreement between pulmonary capillary wedge pressure and left ventricular end-diastolic pressure in a veteran population. PLoS One. 2014;9:e87304. doi: 10.1371/journal.pone.0087304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujimoto N., Borlaug B.A., Lewis G.D. Hemodynamic responses to rapid saline loading: the impact of age, sex, and heart failure. Circulation. 2013;127:55–62. doi: 10.1161/CIRCULATIONAHA.112.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LeVarge B.L., Pomerantsev E., Channick R.N. Reliance on end-expiratory wedge pressure leads to misclassification of pulmonary hypertension. Eur Respir J. 2014;44:425–434. doi: 10.1183/09031936.00209313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan J.J., Rich J.D., Thiruvoipati T. Current practice for determining pulmonary capillary wedge pressure predisposes to serious errors in the classification of patients with pulmonary hypertension. Am Heart J. 2012;163:589–594. doi: 10.1016/j.ahj.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 18.Bonderman D., Wexberg P., Heinzl H., Lang I.M. Non-invasive algorithms for the diagnosis of pulmonary hypertension. Thromb Haemost. 2012;108:1037–1041. doi: 10.1160/TH12-04-0239. [DOI] [PubMed] [Google Scholar]
- 19.Raymond R.J., Hinderliter A.L., Willis P.W. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol. 2002;39:1214–1219. doi: 10.1016/s0735-1097(02)01744-8. [DOI] [PubMed] [Google Scholar]
- 20.Ghio S., Klersy C., Magrini G. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int J Cardiol. 2010;140:272–278. doi: 10.1016/j.ijcard.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 21.Rudski L.G., Lai W.W., Afilalo J. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 686-8. [DOI] [PubMed] [Google Scholar]
- 22.Ghio S., Pazzano A.S., Klersy C. Clinical and prognostic relevance of echocardiographic evaluation of right ventricular geometry in patients with idiopathic pulmonary arterial hypertension. Am J Cardiol. 2011;107:628–632. doi: 10.1016/j.amjcard.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 23.Vonk-Noordegraaf A., Haddad F., Chin K.M. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol. 2013;62:D22–D33. doi: 10.1016/j.jacc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 24.Denton C.P., Cailes J.B., Phillips G.D. Comparison of Doppler echocardiography and right heart catheterization to assess pulmonary hypertension in systemic sclerosis. Br J Rheumatol. 1997;36:239–243. doi: 10.1093/rheumatology/36.2.239. [DOI] [PubMed] [Google Scholar]
- 25.Parent F., Bachir D., Inamo J. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. 2011;365:44–53. doi: 10.1056/NEJMoa1005565. [DOI] [PubMed] [Google Scholar]
- 26.Rajaram S., Swift A.J., Capener D. Comparison of the diagnostic utility of cardiac magnetic resonance imaging, computed tomography, and echocardiography in assessment of suspected pulmonary arterial hypertension in patients with connective tissue disease. J Rheumatol. 2012;39:1265–1274. doi: 10.3899/jrheum.110987. [DOI] [PubMed] [Google Scholar]
- 27.Wang B., Feng Y., Jia L.Q. Accuracy of Doppler echocardiography in the assessment of pulmonary arterial hypertension in patients with congenital heart disease. Eur Rev Med Pharmacol Sci. 2013;17:923–928. [PubMed] [Google Scholar]
- 28.Kuriyama K., Gamsu G., Stern R.G. CT-determined pulmonary artery diameters in predicting pulmonary hypertension. Invest Radiol. 1984;19:16–22. doi: 10.1097/00004424-198401000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Perez-Enguix D., Morales P., Tomas J.M., Vera F., Lloret R.M. Computed tomographic screening of pulmonary arterial hypertension in candidates for lung transplantation. Transplant Proc. 2007;39:2405–2408. doi: 10.1016/j.transproceed.2007.07.055. [DOI] [PubMed] [Google Scholar]
- 30.Stevens G.R., Garcia-Alvarez A., Sahni S., Garcia M.J., Fuster V., Sanz J. RV dysfunction in pulmonary hypertension is independently related to pulmonary artery stiffness. JACC Cardiovasc Imaging. 2012;5:378–387. doi: 10.1016/j.jcmg.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 31.Meris A., Faletra F., Conca C. Timing and magnitude of regional right ventricular function: a speckle tracking-derived strain study of normal subjects and patients with right ventricular dysfunction. J Am Soc Echocardiogr. 2010;23:823–831. doi: 10.1016/j.echo.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Bhave N.M., Patel A.R., Weinert L. Three-dimensional modeling of the right ventricle from two-dimensional transthoracic echocardiographic images: utility of knowledge-based reconstruction in pulmonary arterial hypertension. J Am Soc Echocardiogr. 2013;26:860–867. doi: 10.1016/j.echo.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Gopal A.S., Chukwu E.O., Iwuchukwu C.J. Normal values of right ventricular size and function by real-time 3-dimensional echocardiography: comparison with cardiac magnetic resonance imaging. J Am Soc Echocardiogr. 2007;20:445–455. doi: 10.1016/j.echo.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 34.Grapsa J., Gibbs J.S., Cabrita I.Z. The association of clinical outcome with right atrial and ventricular remodelling in patients with pulmonary arterial hypertension: study with real-time three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging. 2012;13:666–672. doi: 10.1093/ehjci/jes003. [DOI] [PubMed] [Google Scholar]
- 35.Moledina S., de Bruyn A., Schievano S. Fractal branching quantifies vascular changes and predicts survival in pulmonary hypertension: a proof of principle study. Heart. 2011;97:1245–1249. doi: 10.1136/hrt.2010.214130. [DOI] [PubMed] [Google Scholar]
- 36.Jaff M.R., McMurtry M.S., Archer S.L. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788–1830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- 37.Mehta S., Helmersen D., Provencher S. Diagnostic evaluation and management of chronic thromboembolic pulmonary hypertension: a clinical practice guideline. Can Respir J. 2010;17:301–334. doi: 10.1155/2010/704258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkens H., Lang I., Behr J. Chronic thromboembolic pulmonary hypertension (CTEPH): updated Recommendations of the Cologne Consensus Conference 2011. Int J Cardiol. 2011;154(suppl 1):S54–S60. doi: 10.1016/S0167-5273(11)70493-4. [DOI] [PubMed] [Google Scholar]
- 39.Kim N.H., Delcroix M., Jenkins D.P. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2013;62:D92–D99. doi: 10.1016/j.jacc.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 40.Lang I.M., Madani M. Update on chronic thromboembolic pulmonary hypertension. Circulation. 2014;130:508–518. doi: 10.1161/CIRCULATIONAHA.114.009309. [DOI] [PubMed] [Google Scholar]
- 41.Lang I.M., Plank C., Sadushi-Kolici R. Imaging in pulmonary hypertension. JACC Cardiovasc Imaging. 2010;3:1287–1295. doi: 10.1016/j.jcmg.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Sherrick A.D., Swensen S.J., Hartman T.E. Mosaic pattern of lung attenuation on CT scans: frequency among patients with pulmonary artery hypertension of different causes. AJR Am J Roentgenol. 1997;169:79–82. doi: 10.2214/ajr.169.1.9207504. [DOI] [PubMed] [Google Scholar]
- 43.Ley S., Ley-Zaporozhan J., Pitton M.B. Diagnostic performance of state-of-the-art imaging techniques for morphological assessment of vascular abnormalities in patients with chronic thromboembolic pulmonary hypertension (CTEPH) Eur Radiol. 2012;22:607–616. doi: 10.1007/s00330-011-2290-4. [DOI] [PubMed] [Google Scholar]
- 44.Hoey E.T., Mirsadraee S., Pepke-Zaba J. Dual-energy CT angiography for assessment of regional pulmonary perfusion in patients with chronic thromboembolic pulmonary hypertension: initial experience. AJR Am J Roentgenol. 2011;196:524–532. doi: 10.2214/AJR.10.4842. [DOI] [PubMed] [Google Scholar]
- 45.Pienn M., Kovacs G., Tscherner M. Non-invasive determination of pulmonary hypertension with dynamic contrast-enhanced computed tomography: a pilot study. Eur Radiol. 2014;24:668–676. doi: 10.1007/s00330-013-3067-8. [DOI] [PubMed] [Google Scholar]
- 46.Isobe M., Yazaki Y., Takaku F. Prediction of pulmonary arterial pressure in adults by pulsed Doppler echocardiography. Am J Cardiol. 1986;57:316–321. doi: 10.1016/0002-9149(86)90911-2. [DOI] [PubMed] [Google Scholar]
- 47.Tei C., Dujardin K.S., Hodge D.O. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr. 1996;9:838–847. doi: 10.1016/s0894-7317(96)90476-9. [DOI] [PubMed] [Google Scholar]
- 48.Saba T.S., Foster J., Cockburn M., Cowan M., Peacock A.J. Ventricular mass index using magnetic resonance imaging accurately estimates pulmonary artery pressure. Eur Respir J. 2002;20:1519–1524. doi: 10.1183/09031936.02.00014602. [DOI] [PubMed] [Google Scholar]
- 49.Hsu V.M., Moreyra A.E., Wilson A.C. Assessment of pulmonary arterial hypertension in patients with systemic sclerosis: comparison of noninvasive tests with results of right-heart catheterization. J Rheumatol. 2008;35:458–465. [PubMed] [Google Scholar]
- 50.Dahiya A., Vollbon W., Jellis C. Echocardiographic assessment of raised pulmonary vascular resistance: application to diagnosis and follow-up of pulmonary hypertension. Heart. 2010;96:2005–2009. doi: 10.1136/hrt.2010.204834. [DOI] [PubMed] [Google Scholar]
- 51.D'Alto M., Romeo E., Argiento P. Accuracy and precision of echocardiography versus right heart catheterization for the assessment of pulmonary hypertension. Int J Cardiol. 2013;168:4058–4062. doi: 10.1016/j.ijcard.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 52.Gladue H., Steen V., Allanore Y. Combination of echocardiographic and pulmonary function test measures improves sensitivity for diagnosis of systemic sclerosis-associated pulmonary arterial hypertension: analysis of 2 cohorts. J Rheumatol. 2013;40:1706–1711. doi: 10.3899/jrheum.130400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan R.T., Kuzo R., Goodman L.R. Utility of CT scan evaluation for predicting pulmonary hypertension in patients with parenchymal lung disease. Medical College of Wisconsin Lung Transplant Group. Chest. 1998;113:1250–1256. doi: 10.1378/chest.113.5.1250. [DOI] [PubMed] [Google Scholar]
- 54.Chan A.L., Juarez M.M., Shelton D.K. Novel computed tomographic chest metrics to detect pulmonary hypertension. BMC Med Imaging. 2011;11:7. doi: 10.1186/1471-2342-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corson N., Armato S.G., 3rd, Labby Z.E. CT-based pulmonary artery measurements for the assessment of pulmonary hypertension. Acad Radiol. 2014;21:523–530. doi: 10.1016/j.acra.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Helmberger M., Pienn M., Urschler M. Quantification of tortuosity and fractal dimension of the lung vessels in pulmonary hypertension patients. PLoS One. 2014;9:e87515. doi: 10.1371/journal.pone.0087515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bouchard A., Higgins C.B., Byrd B.F., 3rd Magnetic resonance imaging in pulmonary arterial hypertension. Am J Cardiol. 1985;56:938–942. doi: 10.1016/0002-9149(85)90408-4. [DOI] [PubMed] [Google Scholar]
- 58.Sanz J., Kuschnir P., Rius T. Pulmonary arterial hypertension: noninvasive detection with phase-contrast MR imaging. Radiology. 2007;243:70–79. doi: 10.1148/radiol.2431060477. [DOI] [PubMed] [Google Scholar]
- 59.Nogami M., Ohno Y., Koyama H. Utility of phase contrast MR imaging for assessment of pulmonary flow and pressure estimation in patients with pulmonary hypertension: comparison with right heart catheterization and echocardiography. J Magn Reson Imaging. 2009;30:973–980. doi: 10.1002/jmri.21935. [DOI] [PubMed] [Google Scholar]
- 60.Shehata M.L., Harouni A.A., Skrok J. Regional and global biventricular function in pulmonary arterial hypertension: a cardiac MR imaging study. Radiology. 2013;266:114–122. doi: 10.1148/radiol.12111599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swift A.J., Rajaram S., Hurdman J. Noninvasive estimation of PA pressure, flow, and resistance with CMR imaging: derivation and prospective validation study from the ASPIRE registry. JACC Cardiovasc Imaging. 2013;6:1036–1047. doi: 10.1016/j.jcmg.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 62.Tunariu N., Gibbs S.J., Win Z. Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J Nucl Med. 2007;48:680–684. doi: 10.2967/jnumed.106.039438. [DOI] [PubMed] [Google Scholar]
- 63.Skoro-Sajer N., Becherer A., Klepetko W. Longitudinal analysis of perfusion lung scintigrams of patients with unoperated chronic thromboembolic pulmonary hypertension. Thromb Haemost. 2004;92:201–207. doi: 10.1160/TH03-11-0727. [DOI] [PubMed] [Google Scholar]
- 64.Chow L.C., Dittrich H.C., Hoit B.D., Moser K.M., Nicod P.H. Doppler assessment of changes in right-sided cardiac hemodynamics after pulmonary thromboendarterectomy. Am J Cardiol. 1988;61:1092–1097. doi: 10.1016/0002-9149(88)90132-4. [DOI] [PubMed] [Google Scholar]
- 65.Eysmann S.B., Palevsky H.I., Reichek N., Hackney K., Douglas P.S. Two-dimensional and Doppler-echocardiographic and cardiac catheterization correlates of survival in primary pulmonary hypertension. Circulation. 1989;80:353–360. doi: 10.1161/01.cir.80.2.353. [DOI] [PubMed] [Google Scholar]
- 66.Yeo T.C., Dujardin K.S., Tei C. Value of a Doppler-derived index combining systolic and diastolic time intervals in predicting outcome in primary pulmonary hypertension. Am J Cardiol. 1998;81:1157–1161. doi: 10.1016/s0002-9149(98)00140-4. [DOI] [PubMed] [Google Scholar]
- 67.Forfia P.R., Fisher M.R., Mathai S.C. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1034–1041. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
- 68.Fine N.M., Chen L., Bastiansen P.M. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circ Cardiovasc Imaging. 2013;6:711–721. doi: 10.1161/CIRCIMAGING.113.000640. [DOI] [PubMed] [Google Scholar]
- 69.Grünig E., Tiede H., Enyimayew E.O. Assessment and prognostic relevance of right ventricular contractile reserve in patients with severe pulmonary hypertension. Circulation. 2013;128:2005–2015. doi: 10.1161/CIRCULATIONAHA.113.001573. [DOI] [PubMed] [Google Scholar]
- 70.Smith B.C., Dobson G., Dawson D. Three-dimensional speckle tracking of the right ventricle: toward optimal quantification of right ventricular dysfunction in pulmonary hypertension. J Am Coll Cardiol. 2014;64:41–51. doi: 10.1016/j.jacc.2014.01.084. [DOI] [PubMed] [Google Scholar]
- 71.Courand P.Y., Pina Jomir G., Khouatra C. Prognostic value of right ventricular ejection fraction in pulmonary arterial hypertension. Eur Respir J. 2015;45:139–149. doi: 10.1183/09031936.00158014. [DOI] [PubMed] [Google Scholar]
- 72.Zylkowska J., Kurzyna M., Florczyk M. Pulmonary artery dilatation correlates with the risk of unexpected death in chronic arterial or thromboembolic pulmonary hypertension. Chest. 2012;142:1406–1416. doi: 10.1378/chest.11-2794. [DOI] [PubMed] [Google Scholar]
- 73.van Wolferen S.A., Marcus J.T., Boonstra A. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J. 2007;28:1250–1257. doi: 10.1093/eurheartj/ehl477. [DOI] [PubMed] [Google Scholar]
- 74.van de Veerdonk M.C., Kind T., Marcus J.T. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011;58:2511–2519. doi: 10.1016/j.jacc.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 75.Freed B.H., Gomberg-Maitland M., Chandra S. Late gadolinium enhancement cardiovascular magnetic resonance predicts clinical worsening in patients with pulmonary hypertension. J Cardiovasc Magn Reson. 2012;14:11. doi: 10.1186/1532-429X-14-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ley S., Fink C., Risse F. Magnetic resonance imaging to assess the effect of exercise training on pulmonary perfusion and blood flow in patients with pulmonary hypertension. Eur Radiol. 2013;23:324–331. doi: 10.1007/s00330-012-2606-z. [DOI] [PubMed] [Google Scholar]
- 77.Pandya B., Quail M.A., Steeden J.A. Real-time magnetic resonance assessment of septal curvature accurately tracks acute hemodynamic changes in pediatric pulmonary hypertension. Circ Cardiovasc Imaging. 2014;7:706–713. doi: 10.1161/CIRCIMAGING.113.001156. [DOI] [PubMed] [Google Scholar]
- 78.Vanderpool R.R., Pinsky M.R., Naeije R. RV-pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart. 2015;101:37–43. doi: 10.1136/heartjnl-2014-306142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Wolferen S.A., van de Veerdonk M.C., Mauritz G.J. Clinically significant change in stroke volume in pulmonary hypertension. Chest. 2011;139:1003–1009. doi: 10.1378/chest.10-1066. [DOI] [PubMed] [Google Scholar]
- 80.Leeuwenburgh B.P., Steendijk P., Helbing W.A., Baan J. Indexes of diastolic RV function: load dependence and changes after chronic RV pressure overload in lambs. Am J Physiol Heart Circ Physiol. 2002;282:H1350–H1358. doi: 10.1152/ajpheart.00782.2001. [DOI] [PubMed] [Google Scholar]
- 81.Kuehne T., Yilmaz S., Steendijk P. Magnetic resonance imaging analysis of right ventricular pressure-volume loops: in vivo validation and clinical application in patients with pulmonary hypertension. Circulation. 2004;110:2010–2016. doi: 10.1161/01.CIR.0000143138.02493.DD. [DOI] [PubMed] [Google Scholar]
- 82.Sanz J., Conroy J., Narula J. Imaging of the right ventricle. Cardiol Clin. 2012;30:189–203. doi: 10.1016/j.ccl.2012.03.001. [DOI] [PubMed] [Google Scholar]