Abstract
Objective
Stiffness and viscosity represent passive resistances to joint motion related with the structural properties of the joint tissue and of the musculotendinous complex. Both parameters can be affected in patients with spinal cord injury (SCI). The purpose of this study was to measure passive knee stiffness and viscosity in patients with SCI with paraplegia and healthy subjects using Wartenberg pendulum test.
Design
Non-experimental, cross-sectional, case–control design.
Setting
An outpatient physical therapy clinic, University of social welfare and Rehabilitation Science, Iran.
Patients
A sample of convenience sample of 30 subjects participated in the study. Subjects were categorized into two groups: individuals with paraplegic SCI (n = 15, age: 34.60 ± 9.18 years) and 15 able-bodied individuals as control group (n = 15, age: 30.66 ± 11.13 years).
Interventions
Not applicable.
Main measures
Passive pendulum test of Wartenberg was used to measure passive viscous-elastic parameters of the knee (stiffness, viscosity) in all subjects.
Results
Statistical analysis (independent t-test) revealed significant difference in the joint stiffness between healthy subjects and those with paraplegic SCI (P = 0.01). However, no significant difference was found in the viscosity between two groups (P = 0.17). Except for first peak flexion angle, all other displacement kinematic parameters exhibited no statistically significant difference between normal subjects and subjects with SCI.
Conclusions
Patients with SCI have significantly greater joint stiffness compared to able-bodied subjects.
Keywords: Spinal cord injury, Paraplegia, Stiffness, Viscosity, Passive pendulum test, Viscous-elastic
Introduction
Spinal cord injury (SCI) refers to any injury to the spinal cord that results in loss of function and motor and/or sensory disturbances below the point of the injury. SCI often occurs unexpectedly and most frequently because of trauma (motor vehicle accident, falls, diving into lakes, etc.). In the UK, incidences of SCI are 10–15 per million which includes about 600–900 new cases per year.1 Investigators reported more than 200 000 patients with SCI in the USA and 10 000 new cases in each year.2 The majority of all SCI (80%) occurs in the individuals who are about 30 years of age.
SCI causes loss of sensation, loss of movement (physical inactivity), loss of muscle function (weakness and paralysis), spasticity, and stiffness.2 Spasticity is one of the most common symptoms in persons with SCI.3,4 Previous studies indicated that about 30% of a regional SCI population reported problematic spasticity.3 Altered muscle tone and change in tendon or exteroceptive reflexes are the components commonly included in the spastic syndrome.3 It has been reported that spasticity might reduce the range through which the muscle can contract.5 Considering muscle spasticity and hyper-activity in SCI, contracture represents a muscle adaptation in which the muscle increases passive stiffness such that range of motion (ROM) around a joint is limited without active force production of the muscle. Thus, the persons with SCI are susceptible to the changes in viscous-elastic properties of the tissues, tightness and shortening of ligaments, stiffening of joint capsules, shortening of muscles, joint contractures, and ROM limitation.3–7
Different procedures might affect muscle tone and viscous-elastic properties. For example, lack of movement is thought to cause stiffness whereas passive movements are commonly performed by physical therapists to decrease muscles or joints stiffness.
The quantitative analysis of the viscous-elastic properties (stiffness, viscosity, joint flexibility) in subjects with SCI could be useful for rehabilitation programs and to follow-up the effects of physical therapy interventions and rehabilitation programs.
The passive pendulum test is a biomechanical method commonly used to measure the viscous-elastic parameters (stiffness, viscosity) during passive swing of the lower limb to represent the passive resistances to joint motion associated with structural properties of the joint tissues and the musculotendinous complex. It is simple and easy to use, specific to the quadriceps (an important muscle for functional activities), and has shown to give reliable and consistent results.8–11 Oatis8 evaluated the reliability of this method in 96 healthy adults and concluded that the method was reliable and quick measure of stiffness in the knee joint. Previous studies used this technique to calculate stiffness and viscosity in the patients with different neuromusculoskeletal disorders compared with healthy subjects.8–12 Katz and Rymer 13 suggested the pendulum test as a robust and practical measure of spasticity with minimal variability in repeated measures.
The knee joint is the most measured and standardized joint using this technique by various researchers. The trajectory of the oscillating leg from passive pendulum test presents a set of kinematic parameters such as peak angular values, which is useful to monitor the changes in the knee ROM. Some investigators used this test to understand the underlying neurophysiological disturbances in spasticity.14,15 The kinematic outcome depends on a combination of forces acting at a joint. Stiffness and viscosity represent the passive resistances provided by tissues to the angular motion. While stiffness is considered the resistance of an elastic body to resist deformation, viscosity is related to the friction between adjacent layers of tissues. Both parameters may influence the ROM of a knee joint affecting angular displacement.
Some investigators measured kinematic variables using the pendulum test and evaluated changes in knee angular displacement, passive stiffness, and viscosity in a paraplegic SCI patient to design a dynamic leg model for estimation of passive stiffness and viscosity.16
Considering the importance of evidence-based practice, the purpose of this study was to measure the passive knee stiffness and viscosity in SCI patients with paraplegia and healthy subjects using the Wartenberg pendulum test to determine any changes in viscous-elastic properties of the tissues in these patients relative to healthy controls.
We hypothesize that:
-
•
The passive knee stiffness and viscosity are significantly different between healthy subjects and SCI patients with paraplegia.
-
•
The kinematic data assessed during the pendulum test are significantly different between subjects with and without paraplegic SCI.
Methods
Subjects
Experiments were performed on 15 individuals with paraplegic SCI (age: 34.60 ± 9.18 years; height: 169.80 ± 11.02 cm; weight: 70.86 ± 14.45 kg) and 15 able-bodied individuals (age: 30.66 ± 11.13 years; height: 168.67±11.02 cm; weight: 67.10 ± 16 kg) as control group.The subject population in this study was a sample of convenience made up of the subjects who were between the ages of 21 and 55 years. They were consecutive subjects who agreed to participate and fulfilled the inclusion criteria. The subjects with SCI (n=15) all had clinically stable lesions between the neurological levels of T4–L1, signified by a lack of sensation and voluntary movement below the level of injury. Considering the American Spinal Injury Association (ASIA) Impairment Scale (AIS) for SCI, patients were classified as having incomplete injury (grade C, AIS-C). Patients with higher level injuries were excluded to provide a group of patients with similar physiological characteristics. In this study, SCI occurred following motor vehicle accident, falls, gunshot wounds, lifting heavy objects during a parade and from Myelin degeneration. Detailed information is provided in the Results section. Healthy subjects, matched in age, were selected as control. All the subjects signed an informed consent form approved by the human subjects committee at the University of Social Welfare and Rehabilitation Sciences before participating in the study. Physical characteristics of the subjects in each group are shown in Table 1.
Table 1 .
Demographic data of the subjects (mean±SD)
| Variables | Patients with paraplegia and SCI (n=15) | Healthy subjects (n=15) | |
|---|---|---|---|
| Age (years) | 36.22 ± 11.16 | 30.16 ± 12.08 | |
| Weight (kg) | 69.55 ± 17.61 | 65.83 ± 8.83 | |
| Height (m) | 1.71 ± 0.15 | 1.7 ± 0.03 | |
| Sex | Male | (n = 8) 53.3% | (n = 8) 53.3% |
| Female | (n = 7) 46.7% | (n = 7) 46.7% | |
| Cause of SCI | Motor vehicle accident | (n = 9) 60% | NA |
| Fall | (n = 2) 13.33% | NA | |
| Gunshot | (n = 1) 6.66% | NA | |
| Lifting heavy objects | (n = 1) 6.66% | NA | |
| Myelin virus | (n = 2) 13.33% | NA | |
SD, standard deviation; NA, not applicable.
Passive pendulum test procedure
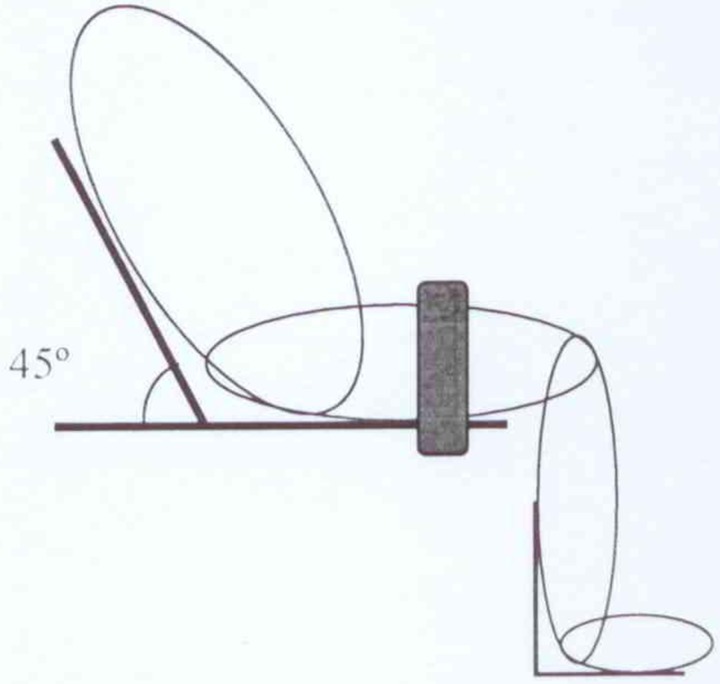
Passive pendulum testing is a means of acquiring passive viscous-elastic parameters of the knee. The subject was placed in a semi-upright sitting position (45°) with the lower legs hanging over the edge of a chair (Fig. 1). The thigh was tightened with a strap to make it stays in a stationary condition. To avoid any modification to the passive characteristic of the knee due to ankle movements, a plastic ankle foot orthosis was used to keep the ankle at 90°. The subject's shank was raised and held by the examiner with the knee in maximum extension until the knee muscle was completely relaxed. This may take about 10–15 seconds. Then the subject's leg was released and was allowed to swing and oscillate freely between flexion and extension and the leg movement was recorded until the shank reaches its final resting position and stopped. The viscous-elastic properties of the joint and surrounding tissues, together with the mass of the moving foot and leg, caused the leg to finally come to rest close to the vertical position. Using a flexible twin axis electronic goniometer (Model: SG110/A, Biometrics Ltd, Newport, UK), movements of the leg were recorded during a passive pendulum test. During testing procedure, the subject's eyes were kept close using sleep masks. The test was performed three times and the mean value of three measurements was taken for the analysis.
Figure 1 .
Passive pendulum test experiment.
Measurements and estimations
There are several variables that could be derived from kinematics of pendulum test. The following displacement and timing parameters were measured using the Wartenberg's technique8–12 (Fig. 2):
-
•
Start angle, onset angle (OA)
-
•
Resting angle (RA)
-
•
First three peak flexion angles (F1, F2, F3)
-
•
First three peak extension angles (E1, E2, E3)
-
•
Amplitude of initial flexion (F1Amp = F1−OA)
-
•
Amplitude of initial extension (E1Amp = F1−E1)
-
•
Plateau amplitude (PA = RA−OA)
-
•
Relaxation index (RI = F1Amp/PA)
-
•
Extension relaxation index , (ERI = E1Amp/PA)
-
•
Period of the first cycle (T)
Some kinematic data of knee motion and anthropometric measures were used to compute the viscosity and stiffness. Knee stiffness (K) and viscosity (B) were estimated by computing the damping ratio (ζ) and the natural frequency (ωn) obtained from the test data using the following equations reported by others8:
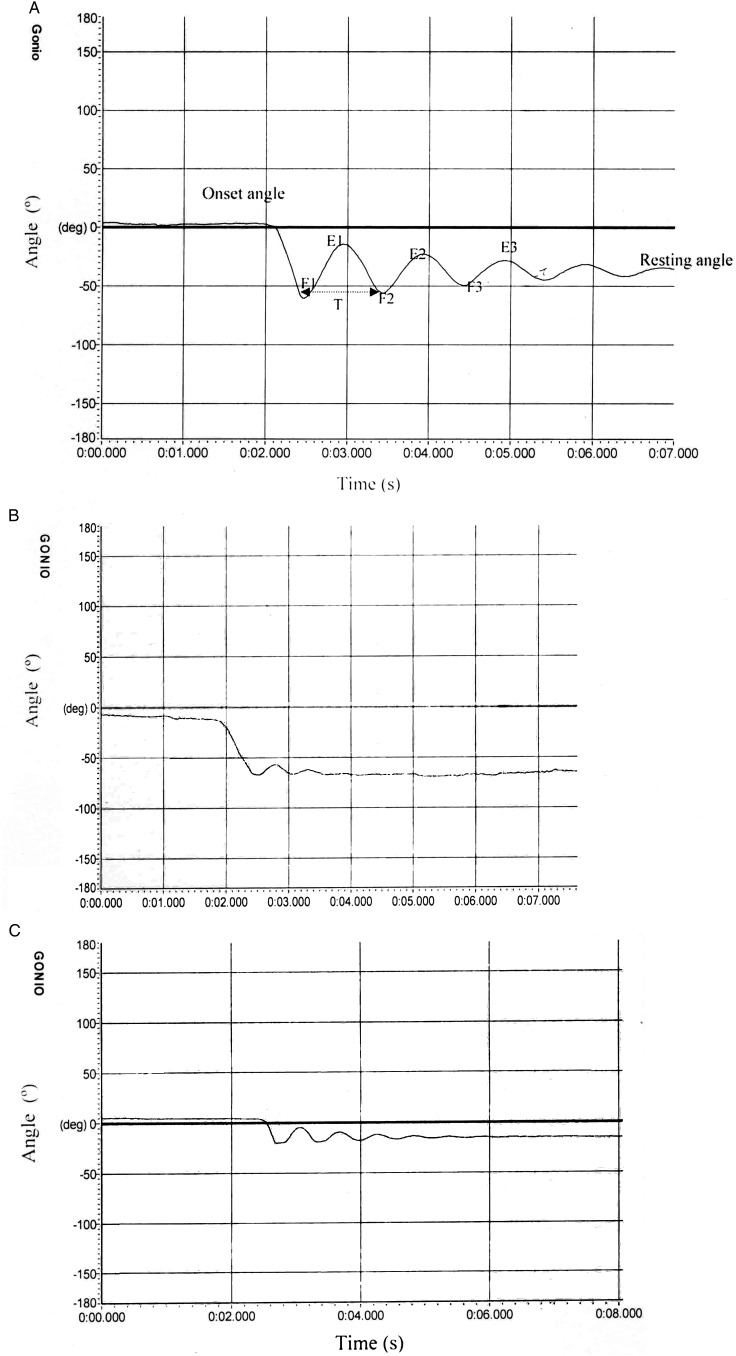
Figure 2 .
Pendulum test angular values for one healthy subject and two patients with paraplegic SCI. (A) Typical electrogoniometric output during pendulum test in a healthy subject. (B, C) Pendulum test angular response for two patients with paraplegia.
The estimation for J and mass characteristics (m and l) were obtained for the subjects according to Winter.18 The values of viscosity (B) and stiffness (K) were obtained as follows:
where “J” is the sagittal moment of inertia applied to the leg-foot complex rotation around the knee axis; “m” is the leg-foot complex mass; “g” is the gravity acceleration, and “l” is the leg-foot length from the knee axis; “θ1” is the peak angle of one cycle; “θ2” is the peak angle of the following cycle and “T” is the period of one cycle. To assure reliability of measurements, the same examiner tested all participants.
The data were normalized by dividing the results of moment of inertia, stiffness, and viscosity by the fifth power of body stature according to the procedure previously described by others.7,8
Statistical analysis
Statistical analysis was performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Multivariate analysis of variance (ANOVA) was used to compare the stiffness, viscosity, and kinematic data assessed during the pendulum test in healthy subjects and those with paraplegic SCI. The alpha level was set at 0.05.
Results
The demographic data for two individual groups are displayed in Table 1. There was no statistically significant difference in subjects’ age (P = 0.27), height (P = 0.87), and weight (P = 0.51) among the two groups. Of the subjects with SCI, nine were from motor vehicle accident, two from falls, one from gunshot wounds, one lifting heavy objects during a parade, and two from myelin degeneration secondary to, they claim, virus of unknown origin. Of subjects with paraplegia, eight were male and seven were female. Of the control group, eight were male and seven female.
Pendulum test angular values for one control subject and two subjects with paraplegic SCI as a sample are presented in Fig. 2. The plots for subjects with SCI showed that not all persons with SCI respond the same way during test. However, control subjects responded similarly.
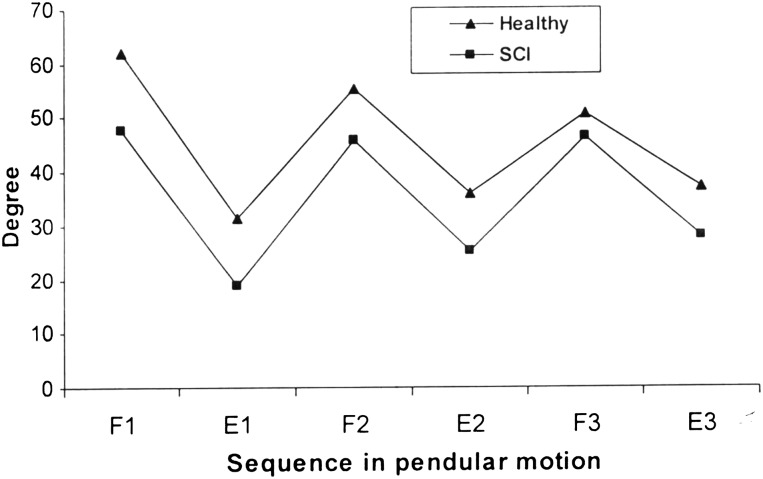
Fig. 3 depicts the mean values of the first three peak flexion and extension angles (F1, F2, F3, E1, E2, E3) in subjects with and without SCI.
Figure 3 .
The mean values of the first three peak flexion and extension angles (F1, F2, F3, E1, E2, E3) in patients with SCI and healthy subjects.
The results of the multivariate ANOVA for between-subject effect in tested variables is shown in Table 2. Descriptive statistics (mean ± SD) for some kinematic data assessed during the pendulum test and comparison between two groups are presented in Table 3.
Table 2 .
Multivariate ANOVA for between-subject effect in tested variables
| Variables | Type III sum of squares | DF | Mean square | F | P value |
|---|---|---|---|---|---|
| F1Amp (°) | 1055.68 | 1 | 1055.68 | 4.97 | 0.04 |
| E1Amp (°) | 68.43 | 1 | 68.43 | 0.35 | 0.56 |
| Plateau amplitude (PA) (°) | 237.16 | 1 | 237.16 | 1.25 | 0.28 |
| Relaxation index (RI) | 0.40 | 1 | 0.40 | 2.91 | 0.11 |
| Extension relaxation index (ERI) | 0.002 | 1 | 0.002 | 0.03 | 0.84 |
| Mass segment (kg) | 0.40 | 1 | 0.40 | 0.35 | 0.56 |
| Stiffness (N/rad m4) | 221.05 | 1 | 221.05 | 7.63 | 0.01 |
| Viscosity (Ns/rad m4) | 0.002 | 1 | 0.002 | 2.08 | 0.17 |
Table 3 .
Descriptive statistics (mean±SD) for the kinematic data assessed during the pendulum test and comparison between two groups
| Variables | Patients with paraplegia and SCI (n=15) | Healthy subjects (n=21) | P value |
|---|---|---|---|
| Leg-limb length (cm) | 42.55 ± 5.51 | 42.25 ± 2.27 | 0.88 |
| F1Amp (°) | 45.30 ± 17.28 | 62.42 ± 3.44 | 0.04 |
| E1Amp (°) | 25.44 ± 16.05 | 29.8 ± 5.69 | 0.56 |
| Plateau amplitude (PA) (°) | 37 ± 16.09 | 45.11 ± 4.53 | 0.28 |
| Relaxation index (RI) | 1.07 ± 0.19 | 1.41 ± 0.01 | 0.11 |
| Extension relaxation index (ERI) | 0.34 ± 0.23 | 0.31 ± 0.07 | 0.82 |
| Mass segment (kg) | 4.18 ± 1.19 | 3.84 ± 0.28 | 0.56 |
| Stiffness (N/rad m4) | 10.56 ± 6.43 | 2.72 ± 0.69 | 0.01 |
| Viscosity (Ns/rad m4) | 0.02 ± 0.01 | 0.04 ± 0.002 | 0.17 |
Significant values are shown in bold.
There was significant difference in the stiffness between healthy subjects and those with paraplegic SCI (P = 0.01) (Table 3). The results indicated that subjects with SCI have significantly greater stiffness compared to able-bodied subjects. However, no significant difference was found in the viscosity (P = 0.17) between two groups (Table 3).
Except for F1Amp, all other displacement parameters exhibited no statistically significant difference between subjects with and without SCI (Table 3).
Discussion
The passive pendulum test is a biomechanical method commonly used to measure viscous-elastic parameters (stiffness, viscosity). It is simple and easy to use, specific to the knee and influenced by quadriceps and antagonistic hamstring (important muscles for functional activities), and has shown to be reliable.7–11 Several studies have used this test to evaluate lower limbs spasticity and hypertonia in persons with neurological disorders.12,15,17–20 The trajectory of the oscillating leg from passive pendulum test presents a set of kinematic parameters such as peak angular values, useful to monitor the changes in the range of knee motion. This mechanical model is thought to be fairly well suited to describe the passive characteristics of the knee joint of both able-bodied subjects and those with paraplegia.21
Passive joint stiffness results from the physical properties of the muscles that span it. Since muscles are the only elements of a joint that can actively change their mechanical property, their role may be to alter the joint stiffness during a movement so as to optimize the joint stiffness for a given activity. Thus, stiffness is considered as a better measure of tissue contribution to function than more conventional measures of tissue integrity such as strength and ROM, which have been shown to be weakly associated to locomotor function.
The results of this study showed a difference in the stiffness between healthy subjects and those with paraplegic SCI (Table 2). Our data demonstrated a greater stiffness in patients with SCI compared to the able-bodied subjects. The kinematic outcome depends on a combination of forces acting at a joint. Stiffness and viscosity represent the passive resistances provided by tissues to the angular motion. Paraplegic patients commonly suffer from spasticity, which may include clonus and involuntary movements due to the altered electrical muscular activity, and altered muscle tone. Similar findings have been reported elsewhere in patients with spasticity.13–24
Previous studies have demonstrated that increased resistance to stretch in spastic muscle has both an active and passive component.15–24 Dietz et al.25 provided evidence that altered mechanical properties of muscle may contribute to hypertonia in spastic patients. Some investigators have demonstrated increased passive ankle stiffness in spastic patients.26–28 In contrast, Douglas et al.29 found that in patients with SCI resonant frequency and muscle stiffness were lower than values in control non-injured subjects. This controversy could arise from the fact that different approaches and samples have been used in the previous studies. With the use of different designs, testing procedures and samples, controversial results may be reported. In the studies conducted by Douglas et al.,29 the subjects with SCI all had clinically complete stable lesions between the neurological levels of C5 and T12. However, in this study, patients were classified as having incomplete injury (AIS-C) between the neurological levels of T4–L1. Douglas et al.29 used the torque-induced motion analysis system to measure muscle tone, stiffness, and resonant frequency around the knee while in our study; similar to other recent previous studies, we used passive pendulum test to assess viscous-elastic properties. As mentioned, in this method some anthropometric measures (such as leg-foot complex mass) and moment of inertia applied to the leg-foot complex were used to compute the viscosity and stiffness. Previous studies have shown muscle atrophy and loss of muscle and bone mass in patients with SCI.30–32 Because of the dependence of stiffness and viscosity measurement on the inertial estimates and considering this issue that individuals with SCI might have varying degrees of atrophy and this could affect the measurement of viscous-elastic properties, the measured stiffness and viscosity can be criticized and the results should be interpreted with caution.
Change in muscle tone, hyperactive reflexes, and involuntary movements are considered as common signs in paraplegics. In addition, it has been thought that there is a transformation of the muscle fibers from slow, type I to fast, type II in the paraplegic muscles,33–36 which may also contribute to altered tone because type II fibers can produce higher tension than slow fiber. Some investigators have attributed the increased stiffness found in spastic muscle to various factors such as disturbed attaching and detaching mechanism between filaments, changes in the moment arms at the joint, changes in the fascia that held these tissues in place, or transformations of the musculotendinous units following SCI.5 Greater stiffness in patients with SCI might be due to the increased muscle tone. Lakie et al.37 showed an increased muscle tone in the spastic wrist of patients with hemiplegia compared to the contralateral normal wrist. However, hemiplegia arises from cerebral injury and not spinal lesion as in paraplegia in patients with SCI. Some investigators attributed increased stiffness and passive resistance to the relative immobilization due to poor physical condition.38 It is also thought that passive stiffness is used to prevent injuries and to stabilize motion.39 It has been hypothesized that increases in joint stiffness aid individuals with SCI in their ability to resist an external disturbance. The increase in the joint stiffness required patients with SCI to expend more energy.
Considering muscle dysfunction in the patients with SCI with paraplegia, greater stiffness in patients with SCI seems reasonable.
The analysis of oscillating limb during passive pendulum test also indicated that the amplitudes of the first flexion movement (F1 Amp) were significantly reduced in the paraplegic patients. The reduction in knee angle ROM in patients with SCI during the first cycle depended on the increasing of stiffness. Considering muscle spasticity and hyper-activity in SCI, contracture represents a muscle adaptation in which the muscle increases passive stiffness such that ROM around a joint is limited. Thus, the persons with SCI are susceptible to the tightness and shortening of ligaments, stiffening of joint capsules, and limited joint ROM.
Considering the greater stiffness in subjects with SCI could be useful for clinicians to design the rehabilitation programs and to follow-up the effects of the interventions and rehabilitation programs. Lack of movement and decreased ROM are commonly associated with the stiffness and passive movements should be commonly performed by physical therapists to decrease muscles or joints stiffness. Furthermore, functional electrical stimulation has been widely used for muscular training in individuals with SCI. Modeling of the musculoskeletal system is used to facilitate the design of stimulation patterns that will produce the desired force and motion. Therefore, considering the greater stiffness in subjects with SCI could be useful for accurate modeling to design the stimulation patterns.
Although our data revealed significantly greater stiffness in subjects with SCI compared to normal subjects, no significant difference was found in the viscosity. Another study has shown change in stiffness and no difference in viscosity or damping characteristics between healthy subjects and those with different pathologies.9 Muscle activity has been more associated to changes in joint stiffness than viscosity.8 Stiffness of the ankle joint complex was shown to increase with increased contractions of the surrounding musculature with little or no change in the damping characteristic and viscosity.40
The total resistance produced by joint tissues may also depend on viscosity that is related to the frictional force between adjacent layers of tissues. We found no significant change of viscosity in paraplegic patients relative to healthy subjects. Thus, SCI would affect the overall ability to resist deformation of articular and periarticular tissues, with no significant contribution by resistance yielded by the friction between adjacent layers.
Limitations
However, we acknowledge several limitations. Electromyography (EMG) is needed to assess the muscular activity during the test. In this study, EMG and reflex loop time were not assessed. Further research is indicated to collectively investigate the EMG activity of the muscles and kinematic parameters in paraplegic patients compared with the healthy subjects.
Another area of concern in our study is reliability assessment. Although the reliability of the pendulum test has been previously established and has shown to be reliable, we did not assess the reliability in our study.
More clinical studies are needed to determine the therapeutic effect of different treatment methods or rehabilitation programs on stiffness or viscosity in patients with paraplegic SCI.
Conclusion
The results of this study indicated greater stiffness in patients with SCI with paraplegia compared with able-bodied subjects. Therefore, testing and estimation of the joint stiffness seems important for modeling and control of knee joint of patients with paraplegia and prescribing effective treatment strategy for patients with paraplegia due to SCI.
Disclaimer statements
Contributors All authors actively contributed in this study. MJ contributed to the conception, design, interpretation of data and drafting the manuscript. AMA contributed to the conception, design, analysis, interpretation of data and drafting the manuscript. HH carried out the data collection and was involved in drafting the manuscript. MTJ participated in analysis and interpretation of data. MOT contributed to conception and design.
Conflicts of interest None.
Ethics approval Not applicable; no intervention.
Funding The authors would like to thank Iran National Science Foundation (INSF) for their financial support towards the “Designing and Development of Functional Electrical Stimulation-assisted Rowing Machine and its Efficacy in Patients with Spinal Cord Injury” project.
References
- 1.Grundy D, Swain A. ABC of spinal cord injury. 4th ed London: BMJ Books; 2002. [Google Scholar]
- 2.Sisto SA, Forrest GF, Faghri PD. Technology for mobility and quality of life in spinal cord injury, analyzing a series of options available. IEEE Eng Med Biol Mag 2008;27(2):56–68. [DOI] [PubMed] [Google Scholar]
- 3.Skold C. Spasticity in spinal cord injury: intrinsic fluctuations and intervention-induced changes self and clinically rated. Arch Phys Med Rehabil 2000;81(2):144–9. [DOI] [PubMed] [Google Scholar]
- 4.Skold C, Levi R, Seiger A. Spasticity after traumatic spinal cord injury: nature, severity and location. Arch Phys Med Rehabil 1999;80(12):1548–57. [DOI] [PubMed] [Google Scholar]
- 5.Farmer SE, James M. Contractures in orthopaedic and neurological conditions: a review of causes and treatment. Disabil Rehabil 2001;23(13):549–58. [DOI] [PubMed] [Google Scholar]
- 6.Castro MJ, Apple DF Jr, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol 1999;80(4):373–8. [DOI] [PubMed] [Google Scholar]
- 7.Patrick JH, Farmer SE, Bromwich W. Muscle stretching for treatment and prevention of contracture in people with spinal cord injury. Spinal Cord 2002;40(8):421–2. [DOI] [PubMed] [Google Scholar]
- 8.Oatis CA. The use of a mechanical model to describe the stiffness and damping characteristics of the knee joint in healthy adults. Phys Ther 1993;73(11):740–9. [DOI] [PubMed] [Google Scholar]
- 9.Valle MS, Casabona A, Sgarlata R, Garozzo R, Vinci M, Cioni M. The pendulum test as a tool to evaluate passive knee stiffness and viscosity of patients with rheumatoid arthritis. BMC Musculoskelet Disord 2006;7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamstra-Wright KL, Buz Swanik C, Ennis TY, Swanik KA. Joint stiffness and pain in individuals with patellofemoral syndrome. J Orthop Sports Phys Ther 2005;35(8):495–501. [DOI] [PubMed] [Google Scholar]
- 11.Brown RA, Lawson DA, Leslie GC. Observations on the applicability of the Wartenberg pendulum test to healthy elderly subjects. J Neurol Neurosurg Psychiatry 1988;51(9):1171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wartenberg R. Pendulousness of the legs as a diagnostic test. Neurology 1951;1(1):18–24. [DOI] [PubMed] [Google Scholar]
- 13.Katz RT, Rymer WZ. Spastic hypertonia: mechanisms and measurement. Arch Phys Med Rehabil 1989;70(2):144–55. [PubMed] [Google Scholar]
- 14.Lin DC, Rymer WZ. A quantitative analysis of pendular motion of the lower leg in spastic human subjects. IEEE Trans Biomed Eng 1991;38(9):906–18. [DOI] [PubMed] [Google Scholar]
- 15.Fee JW, Foulds RA. Neuromuscular modeling of spasticity in cerebral palsy. IEEE Trans Neural Syst Rehabil Eng 2004;12(1):55–64. [DOI] [PubMed] [Google Scholar]
- 16.Jailani R, Tokhi MO, Ibrahim KK, Joghtaei M, Gharooni S, Huq MS. Estimation of passive stiffness and viscosity in paraplegic: a dynamic leg model using visual Nastran. A 4th International Conference on Methods and Models in Automation and Robotic; 2009. [Google Scholar]
- 17.Winter DA. Biomechanics and motor control of human movement. 2nd ed New York: Wiley-Interscience; 1990. [Google Scholar]
- 18.Nordmark E, Anderson G. Wartenberg pendulum test: objective quantification of muscle tone in children with spastic diplegia undergoing selective dorsal rhizotomy. Dev Med Child Neurol 2002;44(1):26–33. [DOI] [PubMed] [Google Scholar]
- 19.Fowler EG, Nwigwe A, Ho TW. Sensitivity of the pendulum test for assessing spasticity in persons with cerebral palsy. Dev Med Child Neurol 2000;42(3):182–9. [DOI] [PubMed] [Google Scholar]
- 20.Brown RA, Lawson DA, Leslie GC, McArthur A, MacLennan WJ, McMurdo ME, et al. Does the Wartenberg pendulum test differentiate quantitatively between spasticity and rigidity? A study in elderly stroke and Parkinsonian patients. J Neurol Neurosurg Psychiatry 1988;51(9):1178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kearney RE, Hunter IW. System identification of human joint dynamics. Cri Rev Biomed Eng 1990;18(1):55–87. [PubMed] [Google Scholar]
- 22.Lorentzen J, Grey MJ, Crone C, Mazevet D, Biering-Sorensen F, Nielsen JB. Distinguishing active from passive components of ankle plantar flexor stiffness in stroke, spinal cord injury and multiple sclerosis. Clin Neurophysiol 2010;121(11):1939–51. [DOI] [PubMed] [Google Scholar]
- 23.Mirbagheri MM, Barbeau H, Ladouceur M, Kearney RE. Intrinsic and reflex stiffness in normal and spastic, spinal cord injured subjects. Exp Brain Res 2001;141(4):446–59. [DOI] [PubMed] [Google Scholar]
- 24.Wiart L, Darrah J, Kembhavi G. Stretching with children with cerebral palsy: what do we know and where are we going? Pediatr Phys Ther 2008;20(2):173–8. [DOI] [PubMed] [Google Scholar]
- 25.Dietz V, Quintern J, Berger W. Electrophysiological studies of gait spasticity and rigidity. Evidence that altered mechanical properties of muscle contribute to hypertonia. Brain 1981;104(3):431–49. [DOI] [PubMed] [Google Scholar]
- 26.Halar EM, Stolov WC, Venkatesh B, Brozovich FV, Harley JD. Gastrocnemius muscle belly and tendon length in stroke patients and able-bodied persons. Arch Phys Med Rehabil 1978;59(10):476–84. [PubMed] [Google Scholar]
- 27.Dietz V, Berger W. Normal and impaired regulation of muscle stiffness in gait: a new hypothesis about muscle hypertonia. Exp Neurol 1983;79(3):680–87. [DOI] [PubMed] [Google Scholar]
- 28.Smith LR, Lee KS, Ward SR, Chambers HG, Lieber RL. Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J Physiol 2011;589:2625–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Douglas AJ, Walsh EG, Wright GW, Edmond P. Muscle tone around the human knee in paraplegia. Q J Exp Physiol 1989;74(6):897–905. [DOI] [PubMed] [Google Scholar]
- 30.Giangregorio L, McCartney N. Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med 2006;29(5):489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas CK, Grumbles RM. Muscle atrophy after human spinal cord injury. Biocybern Biomed Eng 2005;25(3):39–46. [Google Scholar]
- 32.Spungen AM, Adkins RH, Stewart CA, Wang J, Pierson RN, Waters RL, et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol 2003;95(6):2398–407. [DOI] [PubMed] [Google Scholar]
- 33.Burke RE. Motor units: anatomy, physiology, and functional organization. In: Brookes U, (ed.) Handbook of physiology, section I, The nervous system, vol. 2, motor control, part 1, Bethesda, MD, USA: American Physiology Society; 1981. pp. 345–422. [Google Scholar]
- 34.Scelsi R. Skeletal muscle pathology after spinal cord injury: our 20-year experience and results on skeletal muscle changes in paraplegics, related to functional rehabilitation. Basic Appl Myol 2001;11(2):75–85. [Google Scholar]
- 35.West SP, Roland RR, Reggie Edgerton V. Fibre type and fibre size in cat ankle, knee and hip extensor and flexors following low thoracic spinal cord transection in an early age. Exp Neurol 1986;91(1):174–82. [DOI] [PubMed] [Google Scholar]
- 36.Leiber R, Johansson CAB, Wahlsing HL, Hargens AR, Feringa ER. Long-term effects of spinal cord transection on fast and slow rat skeletal muscle. Exp Neurol 1986;91(3):423–34. [DOI] [PubMed] [Google Scholar]
- 37.Lakie M, Walsh EG, Wright GW. Assessment of human hemiplegic spasticity by a resonant frequency method. Clin Biomech 1988;3(3):173–8. [DOI] [PubMed] [Google Scholar]
- 38.Lin C-CK, Ju M-S, Huang H-W. Muscle tone in diabetic polyneuropathy evaluated by the quantitative pendulum test. Arch Phys Med Rehabil 2007;88(3):368–73. [DOI] [PubMed] [Google Scholar]
- 39.Migliore SA. The role of passive joint stiffness and active knee control in robotic leg swinging: application to dynamic walking [PhD thesis] Georgia Institute of Technology; 2008. [Google Scholar]
- 40.Weiss PL, Hunter IW, Kearney RE. Human ankle joint stiffness over the full range of muscle activation levels. J Biomech 1988;21(7):539–44. [DOI] [PubMed] [Google Scholar]