Abstract
Objective
To examine the effect of low-magnitude whole body vibration on bone density and microstructure in women and men with chronic motor complete paraplegia.
Methods
We studied nine subjects (four women and five men) with motor complete paraplegia of 2 years duration or more, age 20–50 years. Subjects were instructed to stand on a low-magnitude vibration plate within a standing frame for 20 minutes per day, 5 days a week, and for 6 months. Bone density at the proximal femur by dual-energy X-ray absorptiometry and bone microstructure at the distal tibia by high-resolution peripheral quantitative computed tomography were assessed at four timepoints over 12 months (baseline, at 3 months and 6 months while on intervention, and after 6 months off intervention).
Results
Standing on the low-magnitude vibration plate with a standing frame was well tolerated by participants. However, most subjects did not show an improvement in bone density or microstructure after 6 months of intervention, or any relevant changes 6 months following the discontinuation of the low-magnitude vibration.
Conclusion
We were unable to identify an improvement in either bone density or microstructure following 6 months use of a low-magnitude vibration plate in women or men with chronic motor complete paraplegia. Longer duration of use may be necessary, or it is possible that this intervention is of limited benefit following chronic spinal cord injury.
Keywords: Bone density, Bone microstructure, Paraplegia, Spinal cord injuries, Low-magnitude vibration
Introduction
Bone loss is a universal complication of spinal cord injury (SCI), with increased bone resorption beginning soon after injury1 resulting in an accelerated loss of bone density, particularly in the first 2 years.2–5 Acutely, the rate of bone loss is greater than that observed following exposure to microgravity or bedrest alone.4,6,7 Although the pathogenesis for bone loss following SCI is considered multifactorial, and includes disruption of neuronal control of bone metabolism as well as hormonal and nutritional deficiencies,8 the loss of gravity-loaded mechanical strain on bone is considered a key mechanism.9,10 Bone loss following SCI increases the risk for fracture, notably in the lower extremities.11,12 The incidence of fracture increases with longer duration of injury13 and fracture prevalence is reported as being as high as 21%.14 Lower limb fractures can be a serious complication in persons with chronic SCI, resulting in joint stiffness, loss of range of motion of the limb, pressure ulcers, pain, and increased spasticity in some cases.12
There is no clear consensus or standard of care for the treatment of low bone density in persons with either acute or chronic SCI. Interventions to both preserve and improve bone health are needed. In both, the acute and chronic setting following SCI, bisphosphonates have been reported to be effective for bone health.15–19 However, there have been recent questions raised on the possible risks of long-term bisphosphonate therapy for osteoporosis,20 which may be particularly relevant in this population whose average age at injury is currently in the fourth decade (http://www.NSCISC.UAB.edu). There have been some non-pharmacological interventions, such as passive standing and functional electrical stimulation, that have shown promise for bone health if implemented in the acute phase following SCI.21–24 However, similar interventions have not demonstrated consistent improvement in bone density when studied in individuals who have chronic SCI.25–29 Additional treatment options for improving bone health in women and men living with chronic SCI are therefore needed.
Given the recognized importance of loading on bone metabolism, even in the general population, there is growing interest in identifying different delivery forms of mechanical stimulation that will improve bone health. One form of mechanical stimuli that has shown some benefit for bone density is low-magnitude whole body vibration. Mechanical stimuli that affect bone metabolism are transmitted to bone cells through alterations in fluid flow or sheer forces and changes in intramedullary pressure within the lacuna-canalicular network, the porous spaces of bone.30,31 Low-magnitude vibration provides mechanical stimuli to bone in the form of vertical oscillation, which is sufficient to increase fluid flow in bone and produce an osteogenic signal.32,33 Indeed, in animal studies, low-magnitude whole body vibration is anabolic to bone.34 Clinical studies using low-magnitude whole body vibration during weight bearing, administered as 10 or 20 minute sessions in a day, have reported improvement of bone mass in children and adolescents,35,36 although in studies involving post-menopausal women, the effects have been conflicting.37,38
The mechanical stimuli of a low-magnitude vibration plate combined with passive standing regimens using a standing frame may provide a promising non-pharmacological strategy for preventing bone loss and potentially improving bone density in the lower extremities of women and men following chronic SCI. Thus, the primary goal of this study was to examine the effect of low-magnitude whole body vibration in persons with chronic motor complete paraplegia on bone density, microstructure, and metabolism.
Methods
Study participants
The study was approved by the Institutional Review Board and all subjects provided written informed consent. Women and men between the ages of 20 and 50 years with traumatic, complete motor paraplegia (American Spinal Injury Association Impairment Scale (AIS) A or B)39 for at least 2 years were recruited from the clinical practice of an academic SCI center and with assistance from the EasyStand company. Study participants were unable to come to a stand without bracing or a standing frame. Persons with a history of long bone fracture since onset of SCI, existing pressure ulcer greater than stage 2, lower extremity contractures, or inability to tolerate passive standing for any reason were excluded. The subjects could have been previously using a standing frame regularly. None of the women studied were post-menopausal and none of the participants were on chronic oral glucocorticoids or receiving osteoporosis management, other than calcium and/or vitamin D supplementation.
Study protocol
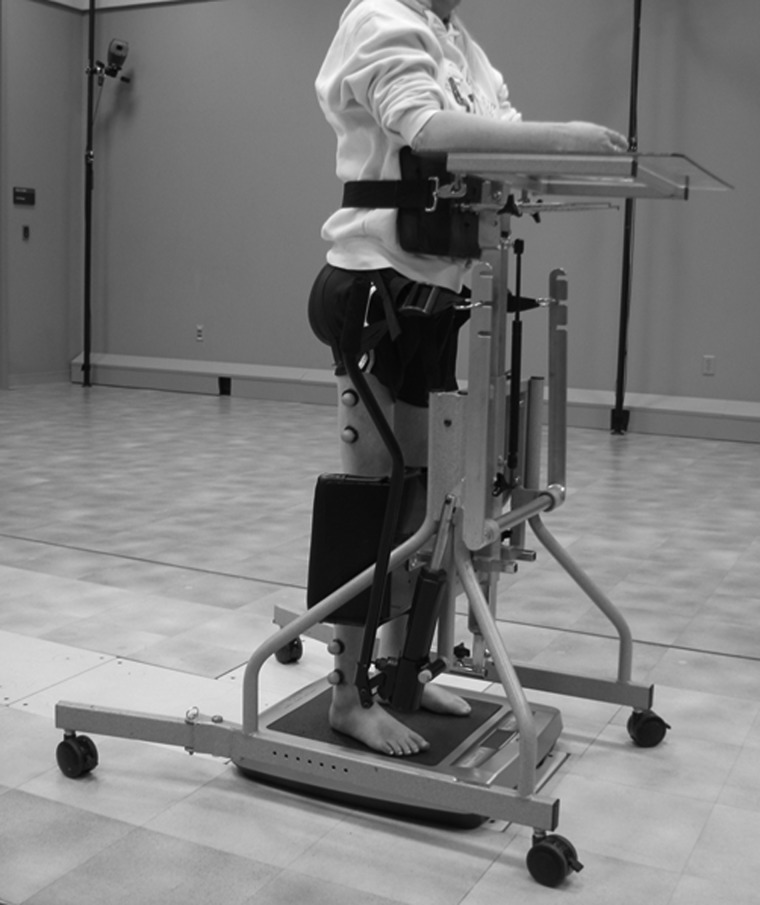
All participants had baseline bone mineral density (BMD) measurements at the proximal femur by dual-energy absorptiometry (DXA) and bone microstructure at the distal tibia by high-resolution peripheral quantitative computed tomography (HRpQCT). Measurements of bone turnover were also assessed at baseline. Subjects were then provided a low-magnitude vibrating platform (Juvent Medical, Somerset, NJ, USA; model Juvent 1000) for home use during the study and were instructed to stand on the vibrating platform with the aid of a standing frame, for 20 minutes per day, 5 days per week, and for 6 months. All were also asked to maintain a log of the duration of time they were able to stand per day on the machine, which was reviewed at each study visit. Subjects were advised by a rehabilitation physical therapist on proper positioning while in the standing frame, with hips and knees extended and bare feet flat on the vibrating plate surface (Fig. 1). Foot plates of the standing frames were removed to accommodate the vibrating plate. For subjects not accustomed to standing, abdominal binding, and compressive leg wraps were applied as deemed necessary by the study participant and therapist to ensure orthostatic homeostasis during the intervention. Subjects then had follow-up bone density, microstructure, and turnover markers measured at 3 and 6 months while on intervention, and then again 6 months after stopping the intervention.
Figure 1 .
Subject standing on a low-magnitude vibrating plate using a standing frame. (Reproduced with permission).40
Low-magnitude whole body vibration
The Juvent 1000 is a commercially available vibrating plate that provides a 0.3 g, 34 Hz vertical sinusoidal movement of ∼50 µm. We previously determined that women and men with chronic motor complete paraplegia bear the majority of their weight (86 ± 10% body weight) through their lower limbs when using a standing frame and that supporting their arms on the tray reduces the ground reaction forces by only ∼10% body weight.40 We also observed that low-magnitude vibration provided additional oscillation of the load-bearing forces and was proportionally similar regardless of arm position.40 Subjects were instructed to stand in their standing frames on the Juvent device without shoes. It was recommended that they keep their arms at their side to maximize ground reaction forces; however, most subjects preferred resting their arms on the tray for stability and comfort.
DXA and HRpQCT measurements
We measured areal BMD (aBMD) at the proximal femur (total hip and femoral neck) by DXA using the Lunar Prodigy system (GE Healthcare, Madison, WI, USA). The short-term coefficients of variation (CV) for the total hip and femoral neck measurements were 0.9 and 2.7%, respectively.
We evaluated the non-dominant distal tibia by HRpQCT (XtremeCT, Scanco Medical AG, Brüttisellen, Switzerland) for total, trabecular, and cortical volumetric BMD (vBMD) (CV: 0.3, 0.4, and 0.4%, respectively) and microstructure. As described elsewhere,41 trabecular bone volume/total volume (BV/TV) fraction (CV: 0.4%) was derived from trabecular vBMD. A thickness-independent structure extraction was used to identify three-dimensional ridges (centers of the trabeculae), and trabecular number (Tb.N) (CV: 4.7%) was then taken as the inverse of the mean spacing of the ridges.42 Analogous with standard histomorphometry,43 trabecular thickness (Tb.Th) (CV: 4.0%) was calculated as (BV/TV)/Tb.N, and trabecular spacing (Tb.Sp) (CV: 4.1%) as (1 − BV/TV)/Tb.N. Validation studies show excellent correlation (R ≥ 0.96) of these parameters with gold standard ex vivo μCT.44 The distal tibia cortex was segmented from the gray-scale image with a Gaussian filter and threshold.42 Cortical vBMD and area were measured directly and the periosteal circumference calculated from the contour; cortical thickness (Ct.Th) (CV: 0.5%) was then calculated as area/circumference. Excellent correlation (R = 0.98) has also been shown with Ct.Th measurements by μCT.45
Bone turnover markers
A serum bone resorption marker, C-terminal telopeptide of type I collagen (CTX), was measured by a two-site immunoenzymatic sandwich assay on the Roche Cobas e411 (Roche Diagnositics, Indianapolis, IN, USA). Intra-assay CVs are 7.8, 2.7, 3.2, and 1.9% at 0.046, 0.292, 0.709, and 2.94 ng/ml, respectively. Inter-assay CVs are 7.7, 8.5, and 7.8% at 0.291, 0.679, and 2.77 ng/ml, respectively. A serum bone formation marker, amino-terminal pro-peptide of type I collagen (PINP) was measured by a double antibody radioimmunoassay (Orion Diagnostica, Espoo, Finland; distributed by Diasorin, Stillwater, MN, USA). Intra-assay CVs are 2.3% at 44.5 µg/l and 12.7% at 103 µg/l. Inter-assay CVs are 3.8% at 28.0 µg/l and 9.2% at 165 µg/l. Serum sclerostin, an antagonist of bone formation, was measured using a validated enzyme-linked immunosorbent assay (Biomedica, Wien, Germany; distributed in USA by ALPCO, Salem, NH, USA). Intra-assay CVs are 5% at 54 pmol/l and 5% at 154 pmol/l. Inter-assay CVs are 6% at 44 pmol/l, 3% at 127 pmol/l, and 3% at 150 pmol/l.
Body composition measurements
We assessed body composition longitudinally also using the Lunar Prodigy instrument. Total body lean mass (kg) and total body fat mass (kg) was determined from the whole body scan (CV: 1.6 and 2.6%, respectively). From these scans, separate assessments of the lean and fat mass of the lower extremities could be determined.
Body mass index (BMI) (kg/m2) was calculated from the height and weight of each subject. Each subject with SCI was weighed in their wheelchair, and then the wheelchair alone was weighed, with the difference being the body mass (kg) of the subject. Self-reported height prior to their injury was recorded for subjects with paraplegia.
Statistical analyses
Trends over time using the repeated measurements were analyzed with a linear mixed effects model. Means and standard deviations were used to summarize values at each timepoint and plots were used to show the changes over time for each subject. We also determined the minimal detectable change (MDC) for the parameters being studied, based on CVs, in order to determine the proportion of subjects with relevant changes that could be detected over follow-up. Analyses were performed using SAS 9.3 and R 2.14 and the significance level was set at 0.05.
Results
For our intervention study, we enrolled six women and six men with traumatic, motor complete paraplegia, all of whom were white, non-Hispanic; however, two women and one man withdrew before their first follow-up visit. Drop-out was reported by subjects as due to inability to commit the time to the study, and not due to any adverse effects. We therefore studied four women and five men who had at least one follow-up visit while using the low-magnitude vibrating platform, of whom, four women and four men returned for all study visits. Individual SCI characteristics of the nine subjects who had at least one follow-up visit are presented in Table 1. At baseline, the mean ± SD for age, BMI, weight, and height of subjects (and for women and men, respectively) was 42 ± 8 years (41 ± 5 years and 43 ± 10 years), 22.3 ± 4.1 kg/m2 (21.2 ± 5.2 and 23.4 ± 3.3 kg/m2), 71.0 ± 13.3 kg (61.6 ± 13.0 and 78.5 ± 8.0 kg), and 177.2 ± 10.5 m (169.0 ± 5.7 and 183.8 ± 8.6 m). The results did not ultimately differ by sex, so results in Table 2 are shown for women and men combined.
Table 1 .
Characteristics of nine subjects (four women and five men) with motor complete paraplegia who had at least one follow-up visit
| Subjects | Age (years) | Level of injury | AIS* | Spastic/flaccid | Duration of injury (years) |
|---|---|---|---|---|---|
| Women | |||||
| 34 | T3 | A | Spastic | 17 | |
| 41 | T11 | A | Flaccid | 2 | |
| 43 | T12 | A | Spastic | 23 | |
| 44 | T7 | A | Flaccid | 3 | |
| Men | |||||
| 25 | T5 | A | Spastic | 7 | |
| 45 | T9 | A | Flaccid | 6 | |
| 46 | T6 | A | Spastic | 27 | |
| 48 | T11 | B | Spastic | 3 | |
| 50 | T5 | A | Spastic | 15 | |
*American Spinal Injury Association Impairment Scale (AIS).39
Table 2 .
Baseline (visit 1) and follow-up results (mean ± SD) at 3 months (visit 2) and 6 months (visit 3) on intervention and after 6 months off intervention (visit 4) for the subjects with motor complete paraplegia who had at least one follow-up visit
| Characteristics | Baseline | Visit 2 | Baseline* | Visit 3 | Baseline* | Visit 4 |
|---|---|---|---|---|---|---|
| No. of Subjects | 9 | 9 | 8 | 8 | 8 | 8 |
| Total hip aBMD (g/cm2) | 0.71 ± 0.22 | 0.71 ± 0.22 | 0.68 ± 0.22 | 0.69 ± 0.21 | 0.68 ± 0.22 | 0.70 ± δ0.20 |
| Femur neck aBMD (g/cm2) | 0.75 ± 0.20 | 0.74 ± 0.19 | 0.73 ± 0.20 | 0.74 ± 0.18 | 0.73 ± 0.20 | 0.75 ± 0.16 |
| Tibia total vBMD (mg/cm3) | 167.69 ± 64.73 | 163.02 ± 61.45 | 168.39 ± 69.16 | 163.25 ± 64.18 | 168.39 ± 69.16 | 159.98 ± 59.32 |
| Tibia trabecular vBMD (mg/cm3) | 67.53 ± 54.58 | 66.17 ± 51.83 | 69.13 ± 58.12 | 64.66 ± 52.68 | 69.13 ± 58.12 | 63.99 ± 49.95 |
| Tibia cortical vBMD (mg/cm3) | 809.84 ± 52.87 | 788.81 ± 73.43 | 811.18 ± 56.36 | 804.23 ± 66.80 | 811.18 ± 56.36 | 793.51 ± 62.48 |
| Tibia trabecular number (1/mm) | 1.09 ± 0.51 | 1.10 ± 0.53 | 1.10 ± 0.54 | 1.04 ± 0.48 | 1.10 ± 0.54 | 1.02 ± 0.51 |
| Tibia trabecular separation (mm) | 1.15 ± 0.82 | 1.25 ± 1.12 | 1.18 ± 0.87 | 1.27 ± 1.05 | 1.18 ± 0.87 | 1.36 ± 1.15 |
| Tibia: trabecular thickness (mm) | 0.04 ± 0.03 | 0.04 ± 0.03 | 0.04 ± 0.03 | 0.04 ± 0.03 | 0.04 ± 0.03 | 0.04 ± 0.04 |
| Tibia cortical thickness (mm) | 0.80 ± 0.28 | 0.78 ± 0.29 | 0.79 ± 0.30 | 0.78 ± 0.31 | 0.79 ± 0.30 | 0.76 ± 0.31 |
| Tibia cortical area (mm2) | 86.47 ± 30.49 | 83.63 ± 31.40 | 83.44 ± 31.11 | 81.98 ± 31.76 | 83.44 ± 31.11 | 80.53 ± 32.17 |
| C-terminal peptide (CTX) (ng/ml) | 0.27 ± 0.16 | 0.34 ± 0.19 | 0.25 ± 0.15 | 0.26 ± 0.12 | 0.25 ± 0.15 | 0.23 ± 0.17 |
| Procollagen type 1 (P1NP) (µg/l) | 56.90 ± 25.55 | 54.57 ± 26.00 | 52.86 ± 24.05 | 48.38 ± 20.53 | 52.86 ± 24.05 | 54.95 ± 23.00 |
| Sclerostin (pmol/l) | 28.27 ± 12.52** | 30.69 ± 15.81** | 27.04 ± 13.24** | 29.43 ± 10.88** | 27.04 ± 13.24** | 31.98 ± 16.98** |
| Total body fat mass (kg) | 27.0 ± 12.4 | 26.9 ± 12.4 | 27.7 ± 13.1 | 28.0 ± 12.0 | 25.2 ± 11.9*** | 25.4 ± 10.5*** |
| Lower extremity fat mass (kg) | 9.1 ± 4.3 | 9.0 ± 3.9 | 9.4 ± 4.5 | 9.5 ± 4.0 | 8.0 ± 2.2*** | 8.1 ± 1.9*** |
| Total body lean mass (kg) | 39.6 ± 10.9 | 39.7 ± 10.5 | 38.3 ± 11.0 | 38.8 ± 11.3 | 39.5 ± 11.3*** | 39.8 ± 11.8*** |
| Lower extremity lean mass (kg) | 10.1 ± 3.4 | 10.2 ± 3.3 | 9.6 ± 3.3 | 9.7 ± 3.5 | 9.7 ± 3.5*** | 10.2 ± 4.0*** |
None of the differences were statistically significant.
*Baseline results for the eight corresponding subjects available at follow-visits.
**Sclerostin measurements were available for seven subjects at baseline, so baseline vs. visit 2 values correspond to seven subjects, while baseline vs. visits 3 and 4 values correspond to six subjects.
***Body composition variables were available for seven subjects instead of eight at visit 4, so baseline values are for the corresponding seven subjects.
Of subjects who returned for their study visits, they self-reported standing between 20–60 minutes per day, 5 days per week. Although some stood on the plate for longer than 20 minutes, the vibrating plate would shut off at 20 minutes. Overall, the low-magnitude whole body vibration was well tolerated by participants who completed the study. One subject noted a mild increase in neuropathic pain, but did not require a change in medication regimen, nor did he stop the study. Several others noted slight increases in spasticity following standing, but again, did not change their medication regimen, nor stop the study protocol. One subject, who only completed the first 3 months of the study, did subsequently undergo revision surgery due to loose hardware in the spine. It is unknown if this was study related or not, as he had neither follow-up for his SCI nor imaging to assess his hardware in recent years and was followed up elsewhere.
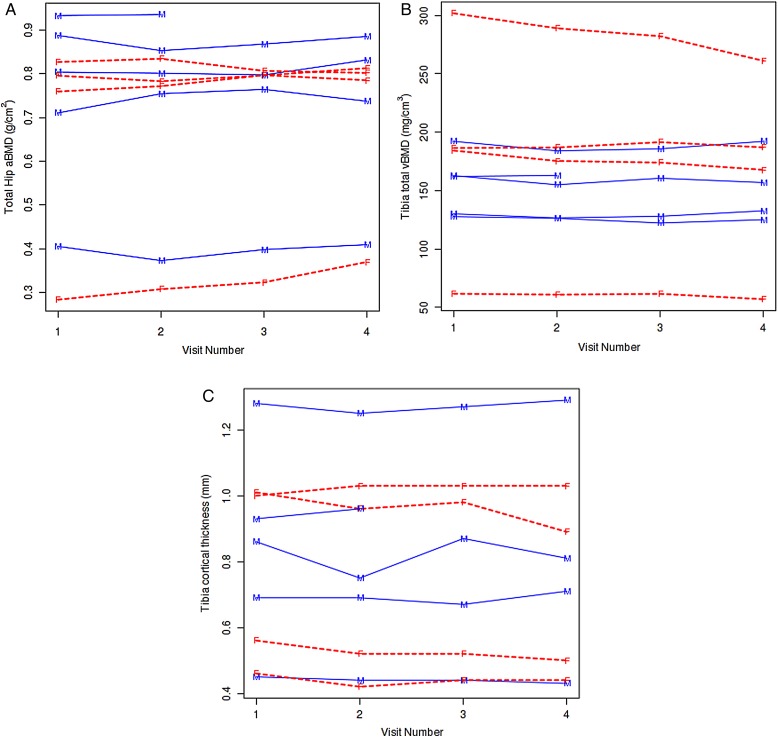
There was no significant change in aBMD at the proximal femur sites in subjects following either 6 months on intervention or 6 months off (Table 2 and Fig. 2A). Although our number of subjects limited statistical power, after 6 months on intervention, only three of the subjects had an increase in total hip aBMD that was greater than the MDC, while the remainder had no detectable changes. None had any changes in femoral neck aBMD that were greater than the MDC at 6 months on intervention. Similarly, when we examined tibia vBMD and bone microstructure parameters by HRpQCT, there were again no significant differences noted over follow-up in either the trabecular or cortical compartments (Table 2 and Fig. 2B and C). Almost all subjects either had no changes or worsening of tibia vBMD and bone microstructure parameters that were greater than the MDC by 6 months on intervention. There was one female subject who, at baseline, had a considerable loss of trabecular bone at the distal tibia, resulting in very low trabecular microstructure estimates which were considered outlier values, and contributed to the large standard deviations observed around means for some parameters; however, these values were consistent over the study period. We did not observe any relevant trends in the CTX, PINP, or sclerostin measurements over follow-up that would have suggested a beneficial change in bone metabolism over the period of intervention, or any detrimental changes once it was discontinued (Table 2). Finally, we did not observe any clinically relevant changes in total, or lower extremity, lean mass or fat mass over follow-up (Table 2).
Figure 2 .
This figure shows individual values for each subject at baseline (visit 1), 3 month (visit 2), 6 month (visit 3), and 12 month (visit 4) timepoints for (A) total hip aBMD, (B) total tibia vBMD, and (C) cortical thickness. The first 6 months (up to visit 3) were on intervention, and the last 6 months (visits 3–4) were off intervention. The men are indicated with solid lines with an “M” for each measured value and the women are indicated with dashed lines with an “F” for each measured value.
Discussion
This is the first study to examine the effects of combining passive standing and low-magnitude whole body vibration on bone density, microstructure, and metabolism in persons with chronic motor complete paraplegia. Although well tolerated, we did not observe any relevant improvement in bone density or microstructure after 6 months of combined standing and low-magnitude vibration. Nor did we note any significant changes in bone density or microstructure 6 months following completion of the intervention. There were no clinically relevant changes in bone turnover markers observed over follow-up. Total, and lower extremity, lean and fat mass were also unchanged.
Although we did not see any improvement in bone density with low-magnitude vibration in our study subjects with chronic SCI, it has shown beneficial effects in other populations, although not all. In a clinical trial by Gilsanz et al.35 of young women ages 15–20 years with low bone mass who were randomized to using vertical oscillation 10 minutes per day for 1 year or no intervention, there was a statistically significant increase in bone density at the femoral midshaft and lumbar spine for women on intervention. Furthermore, the young women who were compliant with utilizing the device showed a 3.9% greater increase in lumbar spine trabecular density and a 2.9% greater increase in femoral shaft cortical area when compared with the pooled group of controls and women who were non-compliant with the intervention. Ward et al.36 reported that non-ambulatory children with cerebral palsy, who were randomized to standing on a low-magnitude vibrating plate for 10 minutes per day, 5 days per week, and for 6 months, had an increase in volumetric tibial bone density by a mean of 6.3%, while the control group, who stood on a placebo device, experienced a mean decrease in bone density of 11.9% (P = 0.003). These significant differences were noted despite compliance at 44% (standing on the device 4.4 minutes per day instead of the prescribed 10 minutes per day).36 These studies suggest that low-magnitude whole body vibration may be effective at improving bone density when bone accrual is actively occurring, such as in the growing skeleton. On the other hand, studies involving post-menopausal women using low-magnitude vibration have not demonstrated a clear benefit. Rubin et al.37 studied 70 early post-menopausal women randomized to either receiving vertical oscillation from a low-magnitude vibrating plate or placebo while standing for two 10 minute sessions, three times per week, and for 1 year. In the intention-to-treat analysis, there was no significant difference observed in bone density between the two groups.37 However, in post hoc analyses of women who were in the highest quartile of compliance with standing on the vibrating platform or placebo (86% compliant), the intervention group showed little change in bone density at the hip or spine, while the control group demonstrated a 2.13% loss at the hip and a 1.6% loss at the lumbar spine (P = 0.06 and P = 0.09, respectively, for difference between the groups).37 In a more recent randomized trial involving 202 post-menopausal women by Slatkovska et al.38 daily low-magnitude whole body vibration, at either of two frequencies, had no measurable benefit on bone density when compared with controls receiving no intervention.
In our own study, even though the intervention was well tolerated and study participants self-reported they were compliant, the majority of our subjects did not show an improvement in lower extremity bone density or microstructure after 6 months of using the vibrating plate. It is possible that the duration of the intervention was not long enough to produce an increase in bone density. On the other hand, since we did not have a control group, it may be that low-magnitude vibration prevented greater than anticipated losses of bone in our subjects with chronic motor complete paraplegia, even though it could not reverse the loss already present. However, there are two recent longitudinal studies of 30 and 60 months duration in individuals with chronic SCI, measuring bone density and/or microstructure at the lower extremities, that have tended to suggest that bone turnover may reach a new steady state following the acute accelerated loss observed in the first few years after injury, with minimal annualized change in either bone density or bone microstructure parameters in the chronic phase, and for some, there may even be an increase in lower extremity bone density.46,47 We believe, therefore, that it is unlikely that vibration was efficacious in the prevention of bone loss, but this is difficult to say with any degree of certainty because of the absence of a well-matched control group; this is especially the case with regard to prevention of bone loss for those subjects who were injured within 3 years of being recruited for study. We also evaluated the distal tibia using HRpQCT in order to determine whether there were positive trends in bone microstructure that would suggest a potential beneficial effect of low-magnitude whole body vibration, but we observed none. Finally, we measured markers of bone turnover as well as sclerostin levels over follow-up. Sclerostin is an inhibitor of bone formation through its effect as a Wnt signaling antagonist.48 It is produced by osteocytes, cells which are considered mechanosensors responsible for signaling the adaptive response of bone to mechanical stimuli.49 In acute unloading, such as following SCI, sclerostin levels are increased, although in chronic SCI, levels are decreased, likely reflecting the severity of bone loss and reduction in osteocyte number.50 Sclerostin expression is downregulated by mechanical stimuli and is reported to be an obligatory step in mechanotransduction inducing osteogenesis.49 If low-magnitude vibration were producing a sufficient mechanical stimuli that would induce bone formation through an effect of Wnt signaling, we would expect sclerostin levels to decrease over 6 months of intervention, but we observed no relevant trends in either sclerostin or bone turnover markers in our study population.
It may be that mechanical stimuli as an intervention for improving bone density in chronic SCI is of limited efficacy, especially once significant bone mass is already lost. Among studies examining other loading strategies to bone, such as passive standing and functional electronic stimulation of muscles, many have failed to show a beneficial effect to bone when evaluated in those with chronic SCI25–29 even though similar interventions have shown promise in the acute setting.21–24 Education and modalities for maintaining bone health after SCI need to become a priority acutely, as there may be a window of time where treatment has the best opportunity in preserving bone mass. Use of low-magnitude vibration and passive standing may prove to have benefits in the acute phase following SCI, but further study is necessary; currently, a clinical trial is underway (http://www.clinicaltrials.gov; NCT00886145).51 If beneficial, timing the use of vibration after any surgical fusions with instrumentation would need to be considered. More recently, ambulation with exoskeleton systems offer new treatment modalities for mechanical loading through the lower extremities of persons with complete paraplegia52; however, the effect of exoskeleton use for bone preservation has yet to be reported.
Our study was limited by the small number of subjects, which precluded our ability to see significant changes over follow-up. Nevertheless, we saw no apparent trends over time when we plotted the data, suggesting there was little relevant change in bone density or microstructure either on or off intervention. Our study was initially designed to include a control group, but due to challenges in recruitment, we were required to change our design. Our lack of a control group limits our ability to determine if the intervention was completely ineffective. It remains possible that it helped to prevent greater than anticipated bone loss over the 6 months on intervention, although we believe this may be less likely based on the more recent literature available on longitudinal bone density changes in those with chronic SCI, as discussed earlier.46,47 Furthermore, based on these longitudinal data that are now available, we estimate that 72 subjects per group would have been needed to detect a difference of 0.0137 over the baseline total hip bone density value of 0.684, which is a 2% difference, at a significance level of 0.05 and 80% power. Nonetheless, a robust conclusion on the lack of benefit of low-magnitude whole body vibration in chronic paraplegia is not possible. The intervention was only 6 months, with standing compliance self-reported by study subjects. It remains possible that longer duration of use, with a larger cohort, may yield different findings. Compliance was self-reported, but because most of our participants had a strong desire to stand as part of their therapy, this inclination was probably the reason why their logs reported a level of compliance that was so markedly better than that previously observed in which children and ambulatory post-menopausal women were studied with a similar vibratory intervention. While we did not limit recruitment other than by age, our SCI population was entirely white, non-Hispanic, reflecting the local community population. However, we did study women as well as men with SCI, and findings were similar for both sexes. It is not known how low-magnitude vibration would affect bone density and microstructure in children or older age groups, including post-menopausal women, with chronic motor complete paraplegia.
Conclusion
In summary, we found that use of low-magnitude vibration is tolerable, low risk, and relatively easy to use with standing frames for persons with chronic motor complete paraplegia. However, 6 months of this intervention did not appear to improve their bone density or microstructure. A larger and controlled study, with potentially a longer intervention timeframe, would be required to determine if low-magnitude vibration is effective in improving bone density and structure following chronic SCI.
Disclaimer statements
Contributors All authors have made a substantial contribution to either the concept and design, acquisition of data or analysis and interpretation of data. All authors have either drafted the article or revised it critically for important intellectual content. All authors approved the version to be published.
Conflicts of interest All other authors state that they have no conflicts of interest with respect to this work.
Ethics approval The study was approved by the Institutional Review Board and all subjects provided written informed consent.
Funding Craig H. Neilsen Foundation grant. Research grants UL1-RR024150 (Center for Translational Science Activities) from the National Institutes of Health, U.S. Public Health Service.
Acknowledgments
The authors wish to thank the men and women in this study for their participation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Roberts D, Lee W, Cuneo RC, Wittmann J, Ward G, Flatman R, et al. Longitudinal study of bone turnover after acute spinal cord injury. J Clin Endocrinol Metab 1998;83(2):415–22. [DOI] [PubMed] [Google Scholar]
- 2.Biering-Sorensen F, Bohr HH, Schaadt OP. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Invest 1990;20(3):330–5. [DOI] [PubMed] [Google Scholar]
- 3.Garland DE, Stewart CA, Adkins RH, Hu SS, Rosen C, Liotta FJ, et al. Osteoporosis after spinal cord injury. J Orthop Res 1992;10(3):371–8. [DOI] [PubMed] [Google Scholar]
- 4.Wilmet E, Ismail AA, Heilporn A, Welraeds D, Bergmann P. Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia 1995;33(11):674–7. [DOI] [PubMed] [Google Scholar]
- 5.de Bruin ED, Dietz V, Dambacher MA, Stussi E. Longitudinal changes in bone in men with spinal cord injury. Clin Rehabil 2000;14(2):145–52. [DOI] [PubMed] [Google Scholar]
- 6.Bloomfield SA. Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc 1997;29(2):197–206. [DOI] [PubMed] [Google Scholar]
- 7.LeBlanc AD, Spector ER, Evans HJ, Sibonga JD. Skeletal responses to space flight and the bed rest analog: a review. J Musculoskelet Neuronal Interact 2007;7(1):33–47. [PubMed] [Google Scholar]
- 8.Jiang S-D, Jiang L-S, Dai L-Y. Mechanisms of osteoporosis in spinal cord injury. Clin Endocrinol 2006;65(5):555–65. [DOI] [PubMed] [Google Scholar]
- 9.Judex S, Carlson KJ. Is bone's response to mechanical signals dominated by gravitational loading? Med Sci Sports Exerc 2009;41(11):2037–43. [DOI] [PubMed] [Google Scholar]
- 10.Amin S. Mechanical factors and bone health: effects of weightlessness and neurologic injury. Curr Rheumatol Rep 2010;12(3):170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vestergaard P, Krogh K, Rejnmark L, Mosekilde L. Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord 1998;36(11):790–6. [DOI] [PubMed] [Google Scholar]
- 12.Fattal C, Mariano-Goulart D, Thomas E, Rouays-Mabit H, Verollet C, Maimoun L. Osteoporosis in persons with spinal cord injury: the need for a targeted therapeutic education. Arch Phys Med Rehabil 2011;92(1):59–67. [DOI] [PubMed] [Google Scholar]
- 13.Zehnder Y, Luthi M, Michel D, Knecht H, Perrelet R, Neto I, et al. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int 2004;15(3):180–9. [DOI] [PubMed] [Google Scholar]
- 14.Giangregorio L, McCartney N. Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med 2006;29(5):489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bubbear JS, Gall A, Middleton FRI, Ferguson-Pell M, Swaminathan R, Keen RW. Early treatment with zoledronic acid prevents bone loss at the hip following acute spinal cord injury. Osteoporos Int 2011;22(1):271–9. [DOI] [PubMed] [Google Scholar]
- 16.Gilchrist NL, Frampton CM, Acland RH, Nicholls MG, March RL, Maguire P, et al. Alendronate prevents bone loss in patients with acute spinal cord injury: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 2007;92(4):1385–90. [DOI] [PubMed] [Google Scholar]
- 17.Moran de Brito CM, Battistella LR, Saito ET, Sakamoto H. Effect of alendronate on bone mineral density in spinal cord injury patients: a pilot study. Spinal Cord 2005;43(6):341–8. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro J, Smith B, Beck T, Ballard P, Dapthary M, BrintzenhofeSzoc K, et al. Treatment with zoledronic acid ameliorates negative geometric changes in the proximal femur following acute spinal cord injury. Calcif Tissue Int 2007;80(5):316–22. [DOI] [PubMed] [Google Scholar]
- 19.Zehnder Y, Risi S, Michel D, Knecht H, Perrelet R, Kraenzlin M, et al. Prevention of bone loss in paraplegics over 2 years with alendronate. J Bone Miner Res 2004;19(7):1067–74. [DOI] [PubMed] [Google Scholar]
- 20.McClung M, Harris ST, Miller PD, Bauer DC, Davison KS, Dian L, et al. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med 2013;126(1):13–20. [DOI] [PubMed] [Google Scholar]
- 21.Shields RK, Dudley-Javoroski S, Law LAF. Electrically induced muscle contractions influence bone density decline after spinal cord injury. Spine 2006;31(5):548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alekna V, Tamulaitiene M, Sinevicius T, Juocevicius A. Effect of weight-bearing activities on bone mineral density in spinal cord injured patients during the period of the first two years. Spinal Cord 2008;46(11):727–32. [DOI] [PubMed] [Google Scholar]
- 23.Lai C-H, Chang WH-S, Chan WP, Peng C-W, Shen L-K, Chen J-JJ, et al. Effects of functional electrical stimulation cycling exercise on bone mineral density loss in the early stages of spinal cord injury. J Rehabil Med 2010;42(2):150–4. [DOI] [PubMed] [Google Scholar]
- 24.Groah SL, Lichy AM, Libin AV, Ljungberg I. Intensive electrical stimulation attenuates femoral bone loss in acute spinal cord injury. PMR 2010;2(12):1080–7. [DOI] [PubMed] [Google Scholar]
- 25.Kunkel CF, Scremin AM, Eisenberg B, Garcia JF, Roberts S, Martinez S. Effect of “standing” on spasticity, contracture, and osteoporosis in paralyzed males. Arch Phys Med Rehabil 1993;74(1):73–8. [PubMed] [Google Scholar]
- 26.Hjeltnes N, Aksnes AK, Birkeland KI, Johansen J, Lannem A, Wallberg-Henriksson H. Improved body composition after 8 week of electrically stimulated leg cycling in tetraplegic patients. Am J Physiol 1997;273(3 Pt 2):R1072–9. [DOI] [PubMed] [Google Scholar]
- 27.Giangregorio LM, Webber CE, Phillips SM, Hicks AL, Craven BC, Bugaresti JM, et al. Can body weight supported treadmill training increase bone mass and reverse muscle atrophy in individuals with chronic incomplete spinal cord injury? Appl Physiol Nutr Metab 2006;31(3):283–91. [DOI] [PubMed] [Google Scholar]
- 28.Shields RK, Dudley-Javoroski S. Musculoskeletal adaptations in chronic spinal cord injury: effects of long-term soleus electrical stimulation training. Neurorehabil Neural Repair 2007;21(2):169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffin L, Decker MJ, Hwang JY, Wang B, Kitchen K, Ding Z, et al. Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J Electromyogr Kinesiol 2009;19(4):614–22. [DOI] [PubMed] [Google Scholar]
- 30.Burger EH, Klein-Nulend J. Mechanotransduction in bone – role of the lacuno-canalicular network. FASEB 1999;13Suppl:S101–12. [PubMed] [Google Scholar]
- 31.Gurkan UA, Akkus O. The mechanical environment of bone marrow: a review. Ann Biomed Eng 2008;36(12):1978–91. [DOI] [PubMed] [Google Scholar]
- 32.Rubin C, Turner AS, Mallinckrodt C, Jerome C, McLeod K, Bain S. Mechanical strain, induced noninvasively in the high-frequency domain, is anabolic to cancellous bone, but not cortical bone. Bone 2002;30(3):445–52. [DOI] [PubMed] [Google Scholar]
- 33.Rubin C, Pope M, Fritton JC, Magnusson M, Hansson T, McLeod K. Transmissibility of 15-hertz to 35-hertz vibrations to the human hip and lumbar spine: determining the physiologic feasibility of delivering low-level anabolic mechanical stimuli to skeletal regions at greatest risk of fracture because of osteoporosis. Spine 2003;28(23):2621–7. [DOI] [PubMed] [Google Scholar]
- 34.Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Anabolism. Low mechanical signals strengthen long bones. Nature 2001;412(6847):603–4. [DOI] [PubMed] [Google Scholar]
- 35.Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res 2006;21(9):1464–74. [DOI] [PubMed] [Google Scholar]
- 36.Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res 2004;19(3):360–9. [DOI] [PubMed] [Google Scholar]
- 37.Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res 2004;19(3):343–51. [DOI] [PubMed] [Google Scholar]
- 38.Slatkovska L, Alibhai SMH, Beyene J, Hu H, Demaras A, Cheung AM. Effect of 12 months of whole-body vibration therapy on bone density and structure in postmenopausal women: a randomized trial. [Erratum appears in Ann Intern Med. 2011;155(12):860]. Ann Intern Med. 2011;155(10):668–79, W205. [DOI] [PubMed] [Google Scholar]
- 39.Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med 2011;34(6):535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernhardt KA, Beck LA, Lamb JL, Kaufman KR, Amin S, Wuermser L-A. Weight bearing through lower limbs in a standing frame with and without arm support and low-magnitude whole-body vibration in men and women with complete motor paraplegia. Am J Phys Med Rehabil 2012;91(4):300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirmani S, Christen D, van Lenthe GH, Fischer PR, Bouxsein ML, McCready LK, et al. Bone structure at the distal radius during adolescent growth. J Bone Miner Res 2009;24(6):1033–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laib A, Hauselmann HJ, Ruegsegger P. In vivo high resolution 3D-QCT of the human forearm. Technol Health Care 1998;6(5–6):329–37. [PubMed] [Google Scholar]
- 43.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 1987;2(6):595–610. [DOI] [PubMed] [Google Scholar]
- 44.Laib A, Ruegsegger P. Calibration of trabecular bone structure measurements of in vivo three-dimensional peripheral quantitative computed tomography with 28-μm-resolution microcomputed tomography. Bone 1999;24(1):35–9. [DOI] [PubMed] [Google Scholar]
- 45.MacNeil JA, Boyd SK. Accuracy of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys 2007;29(10):1096–105. [DOI] [PubMed] [Google Scholar]
- 46.Frotzler A, Berger M, Knecht H, Eser P. Bone steady-state is established at reduced bone strength after spinal cord injury: a longitudinal study using peripheral quantitative computed tomography (pQCT). Bone 2008;43(3):549–55. [DOI] [PubMed] [Google Scholar]
- 47.Garland DE, Adkins RH, Stewart CA. Five-year longitudinal bone evaluations in individuals with chronic complete spinal cord injury. J Spinal Cord Med 2008;31(5):543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baron R, Kneissel M. WNTsignaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 2013;19(2):179–92. [DOI] [PubMed] [Google Scholar]
- 49.Tu X, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D, et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone 2012;50(1):209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Battaglino RA, Sudhakar S, Lazzari AA, Garshick E, Zafonte R, Morse LR. Circulating sclerostin is elevated in short-term and reduced in long-term SCI. Bone 2012;51(3):600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bauman W.(Principal Investigator) Effect of using low-magnitude high frequency mechanical stimulation of bone in persons with subacute SCI [database on the Internet]. Available from: http://www.clinicaltrials.gov/ct2/show/study/NCT00886145. [Google Scholar]
- 52.Fineberg DB, Asselin P, Harel NY, Agranova-Breyter I, Kornfeld SD, Bauman WA, et al. Vertical ground reaction force-based analysis of powered exoskeleton-assisted walking in persons with motor-complete paraplegia. J Spinal Cord Med 2013;36(4):313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]