Abstract
Background
Curcumin, a polyphenolic compound extracted from the plant turmeric, has protective effects on spinal cord injury (SCI) through attenuation of inflammatory response. This study was designed to detect whether curcumin modulates toll-like receptor 4 (TLR4) and the nuclear factor-kappa B (NF-κB) inflammatory signaling pathway in the injured rat spinal cord following SCI.
Methods
Adult male Sprague–Dawley rats were subjected to laminectomy at T8–T9 and compression with a vascular clip. There were three groups: (a) sham group; (b) SCI group; and (g) SCI + curcumin group. We measured TLR4 gene and protein expression by real-time polymerase chain reaction and western blot analysis; NF-κB activity by electrophoretic mobility shift assay, inflammatory cytokines tumor necrosis factor-α, interleukin-1β, and interleukin-6 levels by enzyme-linked immunosorbent assay, hindlimb locomotion function by Basso, Beattie, and Bresnahan rating, spinal cord edema by wet/dry weight method, and apoptosis by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) analysis.
Results
The results showed that SCI induced the up-regulation of TLR4, NF-κB, and inflammatory cytokines in the injured rat spinal cord. Treatment with curcumin following SCI markedly down-regulated the levels of these agents related to the TLR4/NF-κB inflammatory signaling pathway. Administration of curcumin also significantly ameliorated SCI induced hind limb locomotion deficits, spinal cord edema, and apoptosis.
Conclusions
Post-SCI curcumin administration attenuates the TLR4/NF-κB inflammatory signaling pathway in the injured spinal cord, and this may be a mechanism whereby curcumin improves the outcome following SCI.
Keywords: Curcumin, Toll-like receptor 4, Nuclear factor-kappa B, Inflammation, Spinal cord injury
Introduction
Traumatic spinal cord injury (SCI) is a serious clinical problem connected with high morbidity and mortality throughout the world. SCI remains a prevalent and persistent clinical challenge due to unknown pathogenesis.1 A variety of pathomechanisms have been proposed for the development of secondary injury following SCI. However, it is accepted that spinal inflammation may be a critical pathway after SCI.2
Toll-like receptors (TLRs) are an ancient family of transmembrane receptors first discovered in drosophila.3 Activation of the TLRs can initiate innate immune defense. Ten mammalian TLRs have been found by sequence analysis.4 Among them, toll-like receptor 4 (TLR4) plays an important role in the recognition of endogenous agonists, such as the degradation products of macromolecules, heat shock protein, products of proteolytic cascades, intracellular components of ruptured cells, and products of genes that are activated by inflammation.4 There is accumulating evidence for the important role of TLR4 in initiating the spinal inflammation related to amyotrophic lateral sclerosis,5 ischemia reperfusion injury,6 neuropathic pain,7 and trauma.8 Activation of TLRs initiates signal transduction cascade that culminates in the activation of nuclear factor-kappa B (NF-κB) transcription factor and the mitogen-activated protein kinases (MAPKs). As one of the most important downstream molecules in the TLRs signaling pathway, NF-κB is a transcriptional factor required for the expression of many inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6).9
Curcumin is an active component of the rhizome, Curcuma longa. It has been reported that curcumin has various clinical and experimental beneficial effects. Curcumin exerts neuroprotective functions such as anti-oxidation, anti-inflammation, anti-cancer, anti-apoptosis, and immunomodulatory in animal models of ischemia, hemorrhage, and traumatic brain injury as well as SCI through crosstalk with multiple genes and cell-signaling pathways at multiple levels.10–13 In spite of the above facts, little is known about the anti-inflammatory mechanism of curcumin in spinal cord tissues after SCI. It has been demonstrated that curcumin inhibited the expression of NF-κB and the release of inflammatory cytokines.14 A recent investigation also reported the activation of TLR4 which contribute to the progression of inflammation following SCI.15 The purpose of this research was therefore to investigate the influence of curcumin administration in modulating spinal cord expressions of TLR4 and NF-κB inflammatory signaling pathway, and to determine the outcomes in terms of locomotion recovery, spinal cord edema, and number of apoptotic cells in a rat SCI model.
Materials and methods
Rats model of SCI
The protocols of animal use and care conformed to Guide for the Care and Use of Laboratory Animals from the National Institutes of Health, and were approved by the Animal Care and Use Committee of Nanjing University. Male Sprague–Dawley rats (250–300 g) were purchased from Animal Center of Chinese Academy of Sciences, Shanghai, China. The rats were housed under temperature-controlled conditions with a 12-hour light/dark cycle and ad libitum access to water and food.
Following intraperitoneal anesthesia with sodium pentobarbital (50 mg/kg), a 2-cm midline incision was made along the vertebrae T7–T10. The thoracolumbar fascia and paraspinal musculature were incised along the spinous processes and retracted. A T8–T9 laminectomy was performed using an operating microscope. Thirty seconds extradural compression with a vascular clip (30 g forces, Kent Scientific Corporation, INS 14120, USA) was performed around the exposed spinal cord to induce compression injury.16 Then, the spinal cord was irrigated with saline, the muscles and skin sutured. The site of the lesion was marked with a non-degradable suture. The animals were placed under a heating lamp for recovery.
Experimental protocol
The experimental groups consisted of sham group, SCI group, and SCI + curcumin group (n = 16 per group). Rats of SCI + curcumin group received intraperitoneal injections of curcumin at 100 mg/kg in phosphate buffered saline (PBS) including 1% dimethyl sulfoxide (DMSO) at 15 minutes after SCI. The dose of curcumin used in this study was based on previous experiments.17 Rats of sham and SCI groups received equal volume PBS including 1% DMSO at 15 minutes after the surgery.
At 72 hours following sham or injury, rats were sacrificed for sample collection by cervical dislocation, after being tested for locomotion deficits (n = 16). For real-time polymerase chain reaction (PCR), western blot analysis, electrophoretic mobility shift assay (EMSA) analysis, enzyme-linked immunosorbent assay (ELISA), and wet/dry weight (WW/DW) ratio, eight rats in each group were exsanguinated by cardiac puncture. The spinal cords spanning the injury site about 0.8 cm were rapidly excised. A portion of the tissue was harvested for WW/DW ratio detection, the remainder was immediately stored in liquid nitrogen until analysis. For terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) analysis, the other eight rats in each group were perfused via left ventricular puncture with cold saline (4 °C), followed by 4% neutral-buffered formalin. The injured spinal cords about 0.8 cm were excised, stored overnight in 4% neutral-buffered formalin, and then embedded in paraffin.
Quantitative real-time PCR
Total RNA was extracted from spinal cord sample using Trizol Reagents (Invitrogen Lift Technologies, Carlsbad, CA, USA) according to the manufacture's instruction. The cDNA synthesis from the isolated RNA was performed using oligo dT and MMLV Reverse Transcriptase (Promega, Madison, WI, USA). The primer sets of TLR4 (5′-TTGCCTTCATTACAGGGACTT-3′ and 5′-CAGAGCGGCTACTCAGAAACT-3′) and GAPDH (5′-GGAAAGCTGTGGCGTGAT-3′ and 5′-AAGGTGGAAGAATGGGAGTT-3′) were synthesized by Kangcheng Biotechnology (Shanghai, China). Quantitative real-time PCR analysis was performed by using the Rotor-Gene™ 3000 real-time DNA analysis system (Corbett Research, Sydney, Australia), applying real-time SYBR Green PCR technology. The reaction mixtures contained diluted cDNA, SYBR Green 1 Nucleic Acid Stain (Invitrogen Lift Technologies, Carlsbad, CA, USA), 20 µM of each gene-specific primer and nuclease-free water to a final volume of 25 µl. Test cDNA results were normalized to GAPDH measured on the same plate.
Western blot analysis
The frozen spinal cord tissue was mechanically lysed in 20 mM Tris, pH 7.6, which contains 0.2% sodium dodecyl sulfate, 1% Triton X-100, 1% deoxycholate, 1 mM phenylmethylsulphonyl fluoride, and 0.11 IU/ml aprotinin (Sigma-Aldrich, Inc., St. Luis, MO, USA). Lysates were centrifuged at 14 000g for 15 minutes at 4 °C. The protein concentration was estimated by the Bradford method using the protein assay kit (Kangcheng Biotechnology, Shanghai, China). The samples (50 µg per lane) were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and electro-transferred onto a polyvinylidene-difluoride membrane (Bio-Rad Lab, Hercules, CA, USA). The membrane was blocked with 5% skimmed milk for 2 hours at room temperature, incubated overnight at 4 °C with primary antibodies directed against the TLR4 protein (Santa Cruz Biotechnology, Santa Cruz, CA, USA) in PBS + Tween 20 (PBST) at a dilution of 1:3000, and GAPDH (Santa Cruz Biotechnology) diluted 1:10000 in PBST (Sigma-Aldrich) was used as a loading control. After the membrane was washed for 5 minutes each for three times in PBST, it was incubated in the appropriate HRP-conjugated secondary antibody at a dilution of 1:5000 for 1 hour. The blotted protein bands were visualized by enhanced chemiluminescence western blot detection reagents (Amersham, Arlington Heights, IL, USA) and were exposed to X-ray film. Developed films were digitized using an Epson Perfection 2480 scanner (Seiko Corp, Nagano, Japan). Optical densities were obtained using Glyko Bandscan software (Glyko, Novato, CA, USA) and the TLR4 protein expression levels were normalized to GAPDH.
Nuclear protein extract and EMSA
Nuclear protein was extracted and quantified as described previously.18 EMSA was performed using a commercial kit (Gel Shift Assay System; Promega) following the methods in our laboratory. A Consensus oligonucleotide probe for NF-κB (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) was end-labeled with [γ-32P]-ATP (Free Biotech., Beijing, China) with T4-polynucleotide kinase. EMSA was performed according to our previous study.18 NF-κB activity was quantified by computer-assisted densitometric analysis.
ELISA analysis
Spinal cord levels of inflammatory cytokines such as TNF-α, IL-1β, and IL-6 were quantified using ELISA kits specific for rats according to the manufacturers' instructions (TNF-α from Diaclone Research, France; IL-1β, IL-6 from Biosource Europe SA, Belgium) and our previous study.19 The cytokine contents were expressed as pg/mg protein.
Evaluation of locomotion deficit
The locomotion deficit of rats after SCI was scored in an open field according to Basso, Beattie, and Bresnahan (BBB) locomotion rating scale of 0 (complete paralysis) to 21 (normal locomotion) as previously described.16 The scale grossly assessed hind limb movements, body weight support, forelimb-hind limb coordination, and whole body movements. Each session was conducted for 5 minutes by two independent observers blinded to the experiments at 72 hours after sham or injury.
Spinal cord wet/dry weight ratio
Spinal cord water content was determined using the WW/DW method as previously described.20 Spinal cord samples were taken, and immediately weighted to obtain the WW. The samples were dried in an oven for 24 h at 110°C and weighted again to obtain the DW. Water content was calculated as [(WW − DW) × 100]/WW.
TUNEL staining
The formalin-fixed, paraffin-embedded tissue samples were sectioned at 4-μm thickness with a microtome. The sections were scored for apoptotic cells by TUNEL. An in situ cell death detection kit POD (ISCDD, Boehringer Mannheim, Germany) was used according to the kit protocol and our previous study.18 Microscopy of the stained tissue sections was performed by a pathologist blinded to the experimental condition. TUNEL-positive cells were counted in the section through the injury epicenter. The extent of spinal cord damage was evaluated by apoptotic index (AI) which was the average percentage of TUNEL-positive cells in each section counted in 10 cortical microscopic fields (at 200× magnifications).
Statistical analysis
Software SPSS 15.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. All data were expressed as mean ± SD. The measurements were subjected to one-way analysis of variance. Differences between experimental groups were determined by Fisher's LSD posttest. Significance was assigned at P < 0.05.
Results
mRNA expression of TLR4 in spinal cord
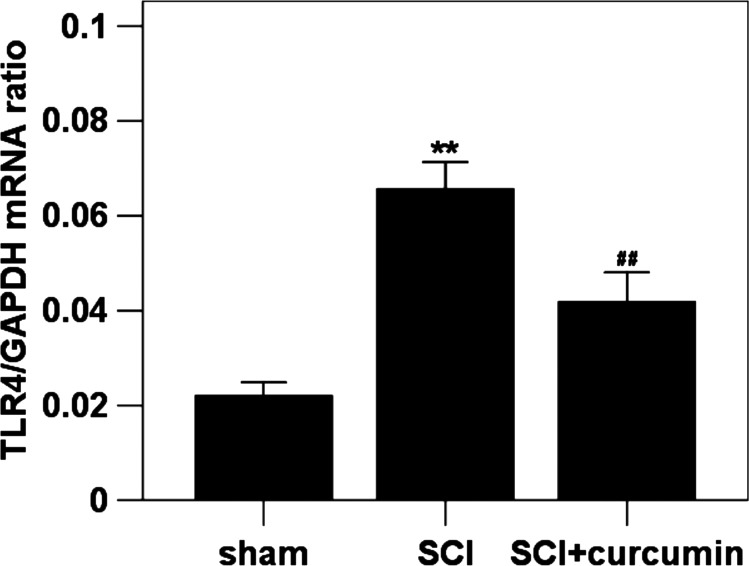
The mRNA expression level of TLR4 was detected by quantitative real-time PCR. The mRNA of TLR4 in the rat spinal cords of sham group was expressed at the lowest level of the three groups. Increased spinal cord mRNA expression level of TLR4 (298.6% increase relative to the sham group) was detected at 72 hours after SCI when compared with rats in sham group. The mRNA expression level of TLR4 (190.0% increase relative to the sham group) in the spinal cord samples was significantly down-regulated by curcumin administration as compared with SCI group (Fig. 1).
Figure 1 .
Spinal cord TLR4 mRNA expression in sham group, SCI group and SCI + curcumin group by quantitative real-time PCR (n = 8 per group). The figure shows that the TLR4 mRNA level in the spinal cords was significantly increased after SCI and could be suppressed when treated with curcumin. **P < 0.01 versus sham group; ##P < 0.01 versus SCI group.
Protein expression of TLR4 in spinal cord
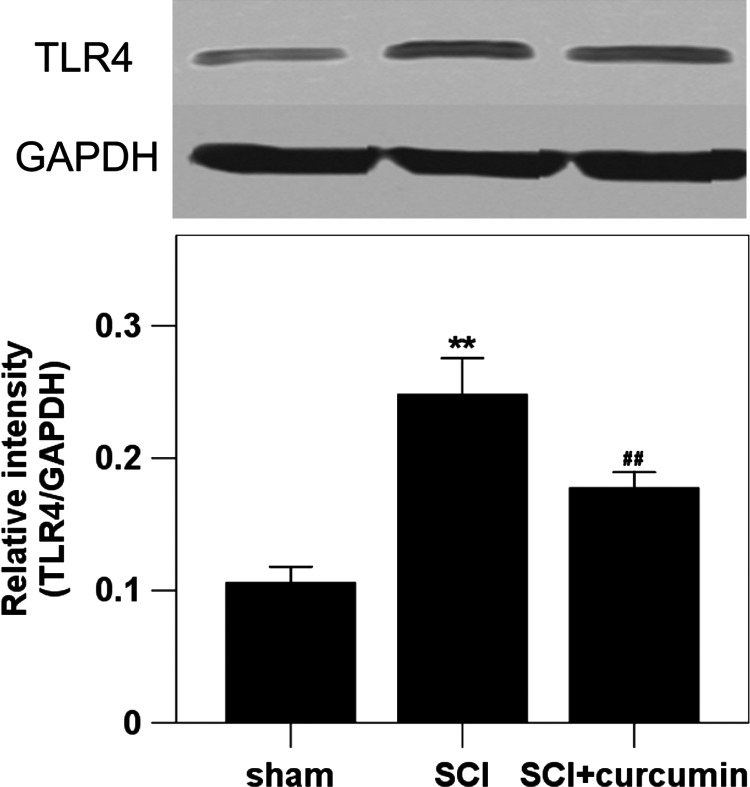
The levels of TLR4 protein expression were measured by western blot analysis. The protein expression of TLR4 in the rat spinal cords of sham group was the lowest of the three groups. Increased spinal cord protein expression level of TLR4 (234.3% increase relative to the sham group) was detected at 72 hours after SCI when compared with rats in sham group. After curcumin administration, the increased level of TLR4 was significantly suppressed (167.7% increase relative to the sham group) in the rat spinal cords of SCI+ curcumin group (Fig. 2).
Figure 2 .
Spinal cord TLR4 protein expression in sham group, SCI group and SCI + curcumin group by western blot analysis (n = 8 per group). Upper, Representative autoradiogram of the TLR4 protein expression by western blot. We detected TLR4 at 89 kDa and the loading control GAPDH at 36 kDa. Bottom, Quantitative analysis of the western blot results for the expression of TLR4. The figure shows that the protein expression of TLR4 in the spinal cords was significantly increased after SCI and could be suppressed when treated with curcumin. **P < 0.01 versus sham group; ##P < 0.01 versus SCI group.
NF-κB DNA-binding activity in spinal cord
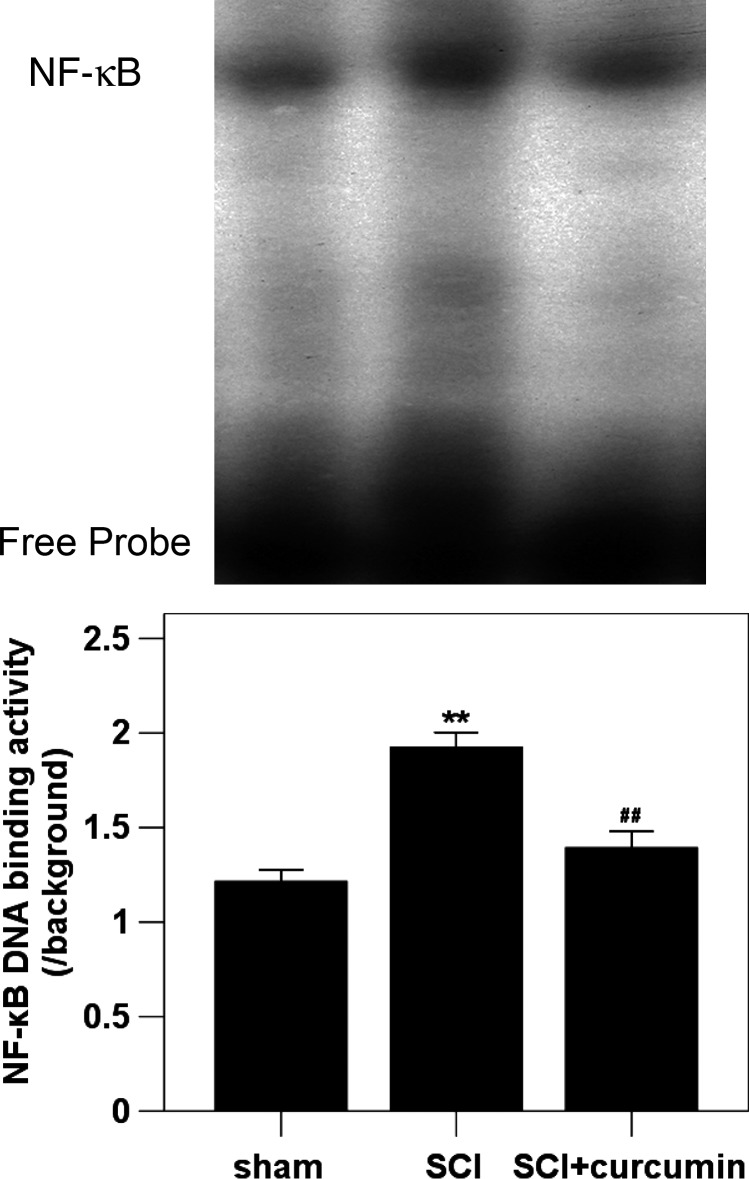
The DNA-binding activity of NF-κB in spinal cord was determined by EMSA. The NF-κB DNA-binding activity in the rat spinal cords of sham group was the lowest of the three groups. Compared with the sham group, NF-κB DNA-binding activity in the spinal cords was increased (162.9% increase relative to the sham group) at 72 hours postinjury. In SCI + curcumin group, the NF-κB DNA-binding activity was markedly down-regulated (117.9% increase relative to the sham group) in the rat spinal cords after SCI (Fig. 3).
Figure 3 .
Spinal cord NF-κB DNA-binding activity in sham group, SCI group and SCI group by EMSA (n = 8 per group). EMSA autoradiography of NF-κB DNA binding is shown on top of the graph, and the order of individual band correspond to that of graph bar. Curcumin treatment significantly down-regulated the NF-κB activation in the injured spinal cord following SCI. **P < 0.01 versus sham group; ##P < 0.01 versus SCI group.
Inflammatory cytokine concentrations in spinal cord
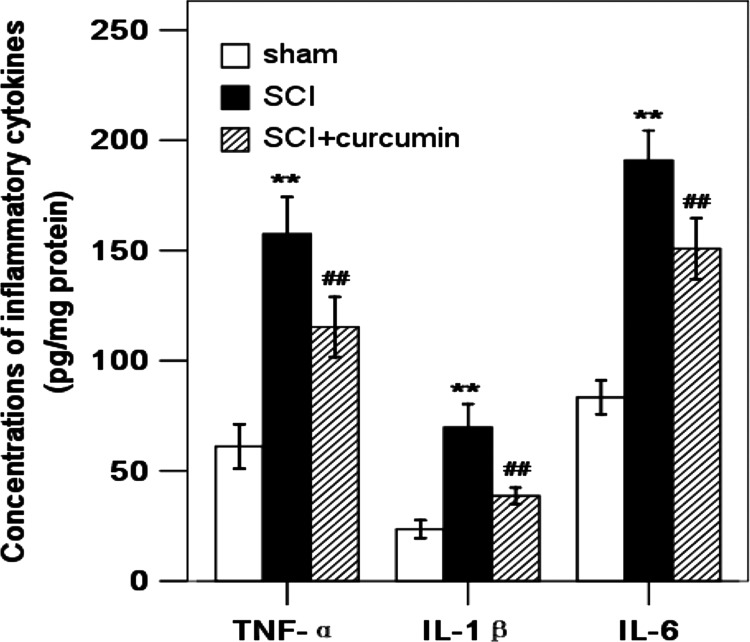
Concentrations of inflammatory cytokines including TNF-α, IL-1β, and IL-6 in the rat spinal cords of sham group were the lowest of the three groups. Compared with the sham group, the concentrations of TNF-α (257.9% increase relative to the sham group), IL-1β (296.3% increase relative to the sham group), and IL-6 (229.0% increase relative to the sham group) in the spinal cords were significantly increased at 72 hours after SCI. As shown in Fig. 4, the TNF-α (188.8% increase relative to the sham group), IL-1β (164.2% increase relative to the sham group), and IL-6 (181.0% increase relative to the sham group) concentrations were significantly decreased by curcumin administration after SCI.
Figure 4 .
Concentrations of inflammatory cytokines in the spinal cord in sham group, SCI group, and SCI + curcumin group by ELISA analysis (n = 8 per group). The figure indicates that concentrations of TNF-α, IL-1β, and IL-6 in the injured spinal cord were significantly increased after SCI and could be suppressed when treated with curcumin. **P < 0.01 versus sham group; ##P < 0.01 versus SCI group.
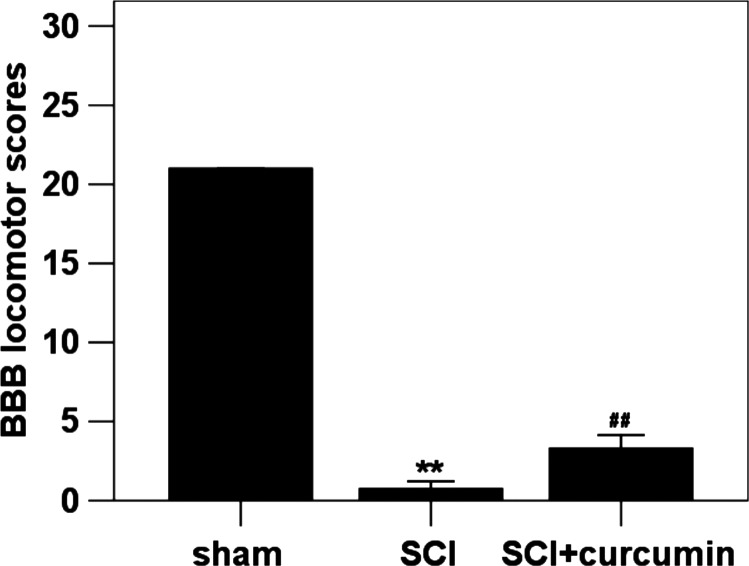
Locomotion deficit
The BBB locomotion rating scale was developed based on natural progression of locomotion recovery in rats with thoracic SCI. The BBB score was approximately 21 in the sham group. Immediately after surgery, BBB score of every rat was approximately 0, which meant that the SCI model was successful. At 72 hours after SCI, decreased BBB score, which reflected an impairment of locomotion function, was observed in SCI group (96.4% decrease relative to the sham group) in comparison with sham group. In SCI + curcumin group, when compared with the SCI group, the BBB score (87.5% decrease relative to the sham group) was significantly increased (Fig. 5).
Figure 5 .
BBB locomotion assessment in sham group, SCI group and SCI + curcumin group (n = 16 per group). The figure shows that the BBB locomotion score was significantly decreased after SCI and could be up-regulated when treated with curcumin. **P < 0.01 versus sham group; ##P < 0.01 versus SCI group.
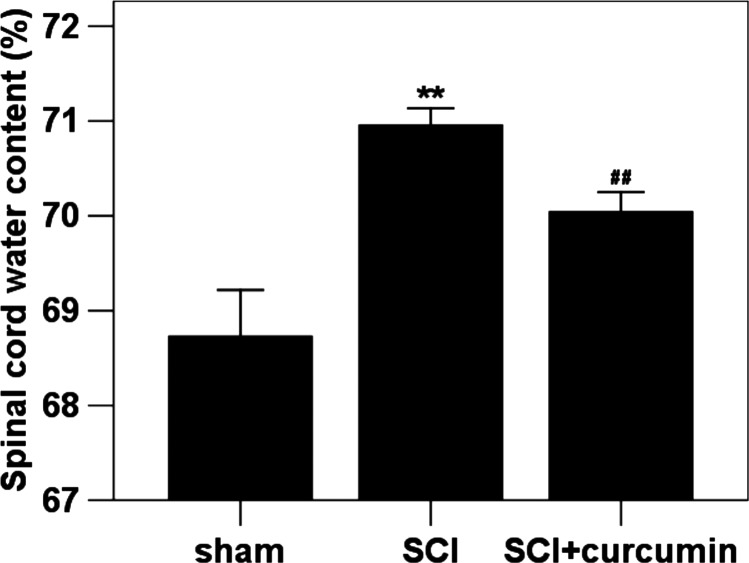
Spinal cord water content
We investigated the spinal cord water content using WW/DW ratio method. The water content in rats of sham group was the lowest of the three groups as shown in Fig. 6. Significant increase in water content (103.2% increase relative to the sham group) was detected in the spinal cord samples at 72 hours after SCI when compared with rats in sham group. The mean value of spinal cord water content was decreased (101.9% increase relative to the sham group) by curcumin administration as compared with in SCI + curcumin group.
Figure 6 .
Spinal cord water content in sham group, SCI group and SCI + curcumin group by wet/dry weight method (n = 8 per group). Curcumin treatment significantly attenuated SCI-induced spinal cord edema in rats. **P < 0.01 versus sham group; ##P < 0.01 versus SCI group.
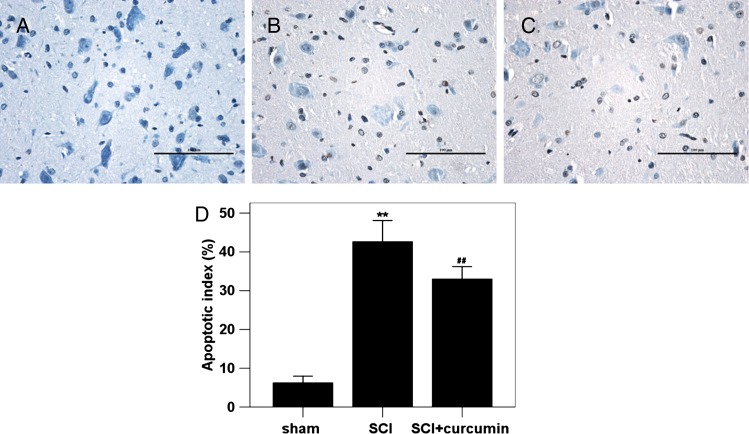
Apoptotic index
The AI in the sham group rat brain tissue was found to be the lowest of the three groups (6.25 ± 0.84 %), as shown in Fig. 7. In SCI group, the AI in the studied rat spinal cords was found to be significantly increased (682.1% increase relative to the sham group) compared with those in sham group animals. In SCI + curcumin group, the AI in the spinal cord tissue (528.0% increase relative to the sham group) was significantly decreased.
Figure 7 .
TUNEL immunohistochemistry staining in the spinal cord tissue in sham group, SCI group and SCI + curcumin group (n = 8 per group). (A) sham group rats showing few TUNEL-positive cells; (B) SCI group rats showing more TUNEL-positive cells; (C) SCI + curcumin group rats showing less TUNEL-positive cells than SCI group; (D) curcumin treatment significantly decreased the apoptotic index in the injured spinal cord following SCI. **P < 0.01 versus sham group; ##P < 0.01 versus SCI group.
Discussion
The main findings of this study were (i) SCI could induce significant up-regulation of TLR4 mRNA and protein expression in the spinal cord; (ii) the levels of NF-κB activity, TNF-α, IL-1β, and IL-6; concentrations in the injured spinal cord were significantly increased after trauma; (iii) the spinal cord levels of these agents related to TLR4/NF-κB inflammatory signaling pathway could be suppressed when treated with curcumin; (iv) after curcumin administration, SCI-induced hindlimb locomotion deficits, spinal cord edema, and apoptotic cell death were markedly ameliorated. These findings reported here suggest for the first time that curcumin may attenuate the SCI-induced the activation of TLR4/NF-κB inflammatory signaling pathway which may participate in the pathogenesis of secondary spinal cord damage following primary trauma in rat SCI model.
There have been several studies focusing on the neuroprotective effects of curcumin in SCI. As mentioned by Cemil et al.21 in their literature, administration of curcumin provided neuroprotection in an experimental rat model of SCI. Their results suggested that these effects were accompanied by decreased level of tissue MDA and increased levels of tissue GSH-Px, SOD, and CAT activity after SCI. Another research indicated that treatment of SCI with curcumin inhibited apoptosis and neuron loss, quenched astrocyte activation, and significantly improved neurologic deficit in rats.22 In a recent study by Ormond et al.,23 postinjury curcumin administration resulted in improved motor function recovery from SCI, correlating with no adverse effects noted in experimental animals. In this research, our data are consistent with the previous studies. We found in this study that curcumin administration following SCI could ameliorate locomotion deficit, spinal cord edema and apoptotic cell death, which played as indices of secondary spinal cord damage after SCI. However, despite the demonstrated mechanisms of curcumin in neuroprotection, none of the previous studies have focused on the TLR4/NF-κB signaling pathway mediated spinal cord inflammatory response in relation to cytotoxic damage after SCI.
The TLRs play key roles in controlling innate immunity that responds to a wide variety of pathogen-associated molecule patterns. The TLRs mediated intracellular signaling pathways converge to activate NF-κB and c-Jun N-terminal kinases (JNKs), which induce the transcription of a series of inflammatory cytokines that are involved in the initiation or regulation of the inflammatory response.24 A variety of TLRs have been identified in human cells and some other species' spinal cords.25 The TLRs family can also be activated by numerous endogenous ligands such as heat shock protein, necrotic cells, extracellular matrix, and oxidized lipids that are found in the injured spinal cord.26,27. Many of these ligands have been shown to activate TLR4, therefore our study focused on this receptor. In this study, we reported for the first time that both mRNA and protein expression of spinal cord TLR4 were significantly increased and could be inhibited by curcumin in the rats SCI model.
Activation of NF-κB signaling pathway has been shown to be central to the pathophysiology of spinal inflammatory response induced by SCI.28 NF-κB can be activated by lesion-induced oxidative stress, bacterial endotoxin, and cytokines.29 The functional importance of NF-κB in acute inflammation is based on its ability to regulate the promoters of multiple inflammatory genes whose products, such as TNF-α, IL-1β, and IL-6, are critical to inflammatory processes.30 NF-κB activation enhances the transcription of inflammatory cytokines, and the cytokines are known to in turn activate NF-κB.31 The positive feedback is believed to serve to amplify inflammatory signals and exacerbate SCI after trauma. In this study, we evaluated the effects of curcumin on SCI-induced activation of pro-inflammatory NF-κB signaling pathway in the spinal cord. The results showed that the levels of NF-κB, TNF-α, IL-1β, and IL-6 in the injured spinal cord were markedly up-regulated and could be suppressed by curcumin administration after trauma, which showed a positive relation with the TLR4 expression, suggesting the inhibitory effects of curcumin on the spinal cord activation of TLR4/NF-κB inflammatory signaling pathway following SCI.
There is compelling evidence that curcumin inhibits a number of inflammatory processes known to be important during SCI. Curcumin therapy has been shown to protects against sepsis-induced acute lung injury by counteracting inflammatory cells infiltration and ROS generation, and regulating cytokine effects.32 Curcumin also interferes with multiple steps of inflammatory kinases involving P13K-Akt, ERK1/2, P38, and JNK in regional myocardial ischemia/reperfusion injury rats.33 In other studies, curcumin has been suggested to engage the NF-κB signal pathway in streptozotocin-induced diabetes,34 caerulein-induced acute pancreatitis,35 and traumatic brain injury.36 However, whether these mechanisms actually apply in the setting of SCI remains elusive. Our present data extended these findings by showing that curcumin suppresses induction of TLR4/NF-κB inflammatory signaling pathway in the injured spinal cord after SCI. However, the mechanisms by which curcumin reducing SCI-induced TLR4/NF-κB signaling pathway and inflammatory response remain unclear. It is assured that further researches are needed and will be conducted in our laboratory.
Conclusion
The results of this research showed that SCI could induce an activation of TLR4/NF-κB inflammatory signaling pathway in the injured spinal cord, which might play a central role in the inflammatory response that leads to secondary insults after SCI. The therapeutic benefit of post-SCI curcumin administration might be due to its salutary effect on modulating TLR4/NF-κB inflammatory signaling pathway.
Disclaimer statements
Contributors All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; ZM, WL and JJ designed the study; HN, WJ, TZ, JW and BY performed the experiments. WJ examined related parameters. HN and WJ analyzed the data and wrote the manuscript.
Conflicts of interest None.
Ethics approval None.
Funding None.
Acknowledgments
This work was supported by grants from Drum Tower Hospital of China. We thank Dr Lizhi Xu and Dr Gengbao Feng for technical assistance.
References
- 1.Cadotte DW, Fehlings MG. Spinal cord injury: a systematic review of current treatment options. Clin Orthop Relat Res 2011;469(3):732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol 2008;209(2):378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson KV, Bokla L, Nusslein-Volhard C. Establishment of dorsal–ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell 1985;42(3):791–8. [DOI] [PubMed] [Google Scholar]
- 4.Johnson GB, Brunn GJ, Platt JL. Activation of mammalian Toll-like receptors by endogenous agonists. Crit Rev Immunol 2003;23(1–2):15–44. [DOI] [PubMed] [Google Scholar]
- 5.Casula M, Lyer AM, Spliet WG, Anink JJ, Steentjes K, Sta M, et al. Toll-like receptor signaling in amyotrophic lateral sclerosis spinal cord tissue. Neuroscience 2011;179:233–43. [DOI] [PubMed] [Google Scholar]
- 6.Arslan F, Keogh B, McGuirk P, Parker AE. TLR2 and TLR4 in ischemia reperfusion injury. Mediators Inflamm 2010;2010:704202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanga FY, Raghavendra V, Deleo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int 2004;45(2–3):397–407. [DOI] [PubMed] [Google Scholar]
- 8.Kigerl KA, Lai W, Rivest S, Hart RP, Satoskar AR, Popovich PG. Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. J Neurochem 2007;102(1):37–50. [DOI] [PubMed] [Google Scholar]
- 9.Takeda K, Akira S. TLR signaling pathways. Semin Immunol 2004;16(1):3–9. [DOI] [PubMed] [Google Scholar]
- 10.Strimpakos AS, Sharma RA. Curcumin:preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal 2008;10(3):511–45. [DOI] [PubMed] [Google Scholar]
- 11.Shukla PK, Khanna VK, Ali MM, Khan MY, Srimal RC. Anti-ischemic effect of curcumin in rat brain. Neurochem Res 2008;33(6):1036–43. [DOI] [PubMed] [Google Scholar]
- 12.Sun Y, Dai M, Wang Y, Wang W, Sun Q, Yang GY, et al. Neuroprotection and sensorimotor functional improvement by curcumin after intracerebral hemorrhage in mice. J Neurotrauma 2011;28(12):2513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu A, Ying Z, Schubert D, Gomez-Pinilla F. Brain and spinal cord interaction: a dietary curcumin derivative counteracts locomotor and cognitive deficits after brain trauma. Neurorehabil Neural Repair 2011;25(4):332–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, et al. Curcumin blocks cytokine-mediated NF-kB activation and proinflammatory gene expression by inhibiting inhibitory factor I-kB kinase activity. J Immunol 1999;163(6):3474–83. [PubMed] [Google Scholar]
- 15.Yao AH, Jia LY, Zhang YK, Ma QR, Cheng P, Liu L, et al. Early blockade of TLRs MyD88-dependent pathway may reduce secondary spinal cord injury in the rats. Evid Based Complement Alternat Med 2012;2012:591298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang WB, Han QQ, Jin W, Xiao ZF, Huang JC, Ni HB, et al. The promotion of neurological recovery in the rat spinal cord crushed injury model by collagen-binding BDNF. Biomaterials 2010;31(33):8634–41. [DOI] [PubMed] [Google Scholar]
- 17.Yang C, Zhang X, Fan H, Liu Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res 2009;1282:133–41. [DOI] [PubMed] [Google Scholar]
- 18.Jin W, Wang HD, Yan W, Zhu L, Hu ZG, Ding YS, et al. Role of Nrf2 in protection against traumatic brain injury in mice. J Neurotrauma 2009;26(1):131–9. [DOI] [PubMed] [Google Scholar]
- 19.Jin W, Kong J, Wang HD, Wu J, Lu TY, Jiang J, et al. Protective effect of tert-butylhydroquinone on cerebral inflammatory response following traumatic brain injury in mice. Injury 2011;42(7):714–8. [DOI] [PubMed] [Google Scholar]
- 20.Jin W, Kong J, Lu TY, Wang HD, Ni HB, Wu J, et al. Erythropoietin prevents secondary brain injury induced by cortical lesion in mice: possible involvement of Nrf2 signaling pathway. Ann Clin Lab Sci 2011;41(1):25–32. [PubMed] [Google Scholar]
- 21.Cemil B, Topuz K, Demircan MN, Kurt G, Tun K, Kutlay M, et al. Curcumin improves early functional results after experimental spinal cord injury. Acta Neurochir (Wien) 2010;152(9):1583–90; discussion 1590. [DOI] [PubMed] [Google Scholar]
- 22.Lin MS, Lee YH, Chiu WT, Hung KS. Curcumin provides neuroprotection after spinal cord injury. J Surg Res 2011;166(2):280–9. [DOI] [PubMed] [Google Scholar]
- 23.Ormond DR, Peng H, Zeman R, Das K, Murali R, Jhanwar-Uniyal M. Recovery from spinal cord injury using naturally occurring antiinflammatory compound curcumin. J Neurosurg Spine 2012;16(5):497–503. [DOI] [PubMed] [Google Scholar]
- 24.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol 2001;1(2):135–45. [DOI] [PubMed] [Google Scholar]
- 25.Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol 2002;61(11):1013–21. [DOI] [PubMed] [Google Scholar]
- 26.Loannou S, Voulgarelis M. Toll-like receptors, tissue injury, and tumourigenesis. Mediators Inflamm 2010;2010:581837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, et al. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem 2001;276(13):10229–33. [DOI] [PubMed] [Google Scholar]
- 28.Bethea JR, Castro M, Keane RW, Lee TT, Dietrich WD, Yezierski RP. Traumatic spinal cord injury induces nuclear factor-κB activation. J Neurosci 1998;18(9):3251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen F, Castranova V, Shi X, Dwmers LM. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin Chem 1999;45(1):7–17. [PubMed] [Google Scholar]
- 30.Hang CH, Shi JX, Tian J, Li JS, Wu W, et al. Effect of systemic LPS injection on cortical NF-kappaB activity and inflammatory response following traumatic brain injury in rats. Brain Res 2004;1026(1):23–32. [DOI] [PubMed] [Google Scholar]
- 31.Merrill JE, Benveniste EN. Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci 1996;19(8):331–8. [DOI] [PubMed] [Google Scholar]
- 32.Xiao X, Yang M, Sun D, Sun S. Curcumin protects against sepsis-induced acute lung injury in rats. J Surg Res 2012;176(1):e31–9. [DOI] [PubMed] [Google Scholar]
- 33.Jeong CW, Yoo KY, Lee SH, Jeong HJ, Lee CS, Kim SJ. Curcumin protects against regional myocardial ischemia/reperfusion injury through activation of RISK/GSK-3β and inhibition of p38 MAPK and JNK. J Cardiovasc Pharmacol Ther 2012;17(4):387–94. [DOI] [PubMed] [Google Scholar]
- 34.Soetikno V, Sari FR, Veeraveedu PT, Thandavarayan RA, Harima M, Sukumaran V, et al. Curcumin ameliorates macrophage infiltration by inhibiting NF-κB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr Metab (Lond) 2011;8(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu WG, Xu G, Ren GJ, Xu X, Yuan HQ, Qi XL, et al. Preventive action of curcumin in experimental acute pancreatitis in mouse. Indian J Med Res 2011;134(5):717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laird MD, Sukumari-Ramesh S, Swift AE, Meiler SE, Vender JR, Dhandapani KM. Curcumin attenuates cerebral edema following traumatic brain injury in mice: a possible role for aquaporin-4? J Neurochem 2010;113(3):637–48. [DOI] [PMC free article] [PubMed] [Google Scholar]