Abstract
Objectives
To demonstrate reduction in detrusor overactivity using surface electrical stimulation of posterior tibial nerve (PTN) or dorsal penile nerve (DPN) in patients with spinal cord injury (SCI).
Design
Patients with SCI with symptoms of urinary urgency/leaks, with cystometrogram (CMG) proven detrusor overactivity were recruited in this study. Ten persons with observable F-wave from tibial nerve were included in the PTN group. Five persons who had F-wave absent but preserved bulbocavernosus reflex were included in the DPN group. Stimulation was given at 20 Hz, 10–40 mA for 20 minutes/session/day for 14 consecutive days. Detrusor overactivity was recorded using CMG on days 1 and 15.
Settings
Rehabilitation Institute, Department of Physical Medicine and Rehabilitation, Christian Medical College and Hospital, Vellore, TN, India.
Participants
Patients with SCI.
Interventions
Surface stimulation of peripheral nerves for reduction of detrusor overactivity.
Outcome measures
Qualitative analysis using voiding diary data and quantitative analysis using CMG data comparing pre- and post-intervention.
Results
P value obtained from voiding chart was 0.021 for PTN and 0.062 for DPN. P value obtained from CMG data was not significant in both groups. In one subject, treatment was extended to 4 weeks and further improvement in voiding diary was seen.
Conclusions
In this pilot study of 15 patients, voiding chart data showed statistically significant improvement following PTN stimulation and trend of improvement following DPN stimulation. However, the CMG data were not statistically significant in this sample population. Further studies with larger, appropriately powered sample size would be helpful to demonstrate the associations of symptoms with CMG data.
Trial registration
CTRI no.; CTRI/2012/12/003234; CMCH Approval no.: CMC/IRB/6735/2008/12/18.
Keywords: Neuromodulation, Detrusor overactivity, Posterior tibial nerve, Dorsal penile nerve, Electrical stimulation therapy
Introduction
An important sequel to spinal cord injury (SCI) is urinary bladder dysfunction. Bladder dysfunction not only includes problems in storage and voiding of urine, but also development of high intravesical pressure due to detrusor overactivity. High pressure inside the bladder can lead to (a) reflux of urine which can lead to renal failure and (b) urinary incontinence, if the bladder neck resistance cannot withstand the developed high pressure. These symptoms may significantly reduce the quality of life. Patients with SCI have expressed the need for research on urinary incontinence as the highest priority in a recent study.1
Current medical management for detrusor overactivity is the use of long-term anti-cholinergic drugs, periodic botulinum toxin injections or surgical procedures like posterior rhizotomy, sacral neuromodulation, and bladder augmentation. Surgical procedures are irreversible with variable and unpredictable success rates. Medical management can be expensive and some of them require expert medical practitioners. An alternative to the procedures mentioned above is the use of electrical stimulation which has been reported in the literature over the last four decades for modulation of the detrusor reflex arc to reduce detrusor overactivity. Various stimulation sites have been identified for neuromodulation of detrusor overactivity such as sacral root, posterior tibial nerve (PTN) and dorsal penile nerve (DPN).2–4 Previous studies were performed on mixed groups of patients with conditions like multiple sclerosis, non-obstructive urinary retention or interstitial cystitis.5–8 Improvement in bladder function was assessed by using either patient's own record of fluid intake, urine output and leaks (voiding diary), cystometrogram (CMG) or quality-of-life questionnaires.5,6,8,9 In a multi-center study conducted using PTN stimulation by Govier et al.,3 urodynamics was used at the time of screening the patient for detrusor overactivity, the analyses of the results were based on voiding diary and quality of life questionnaire. They observed a 71% treatment success rate by considering multiple parameters such as reduction in voiding frequency, urge incontinence, improvement in quality of life and improvement in pain. Klingler et al. demonstrated significant improvement in cystometric capacity with P value of 0.008.7 The study was conducted on 15 subjects with detrusor overactivity syndrome. The subjects were given needle stimulation therapy at PTN site for 3 weeks, 30 minutes per day, 4 times per week. The data were analyzed based on CMG and reduction in number of voids per day. Vandoninck's study on 46 subjects showed significant improvement in cystometric capacity and reflex volume after 12 PTN needle stimulation sessions with P values of 0.043 and 0.012, respectively. The stimulation was given weekly once for a period of 12 weeks.10
Most of the earlier studies used needle electrodes for stimulation especially for PTN.2,3,5,6,11,12 Needle electrodes require sterile environment and are not suitable for home-based therapy.
This pilot study on a small number of patients demonstrates the effects of surface electrical stimulation for the treatment of detrusor overactivity in a uniform well-defined population of patients with SCI. PTN and DPN were chosen for stimulation because of ease of access for surface stimulation. Patients with SCI were included only after confirming integrity of the reflex arc formed by PTN (by testing F-wave by stimulating PTN at supramaximal stimulus and recording from abductor hallucis) and DPN (by testing for bulbocavernosus reflex (BCR) by squeezing the glans penis and observing the response at anal sphincter). The patients were asked to maintain a voiding diary during the treatment period. Pre- and post-treatment CMGs were performed. Information from voiding diary and CMG data were analyzed.
Methods
Setting
The study was conducted in the Rehabilitation Institute of the Department of Physical Medicine and Rehabilitation (PMR) in the Christian Medical College and Hospital (CMCH), Vellore, Tamil Nadu, India. The Department of PMR in CMCH has an inpatient capacity of about 115 beds, and the average outpatient attendance is approximately 100 persons per day.
Study design
This pilot study was approved by the Institutional Ethics Committee. The study was conducted in two phases. In the first phase, 10 patients were enrolled for PTN stimulation during September 2009 to January 2011. After completion of the first phase, five patients were enrolled for the DPN stimulation during February 2011 to December 2011. Informed consent was obtained from all of the patients. Patients who had urinary urgency or leaks, with detrusor overactivity evident from cystometrogram and intact F-wave from the PTN were recruited in the PTN study. F-wave was elicited by giving supramaximal stimuli to the PTN to assess the integrity of sacral efferents from spinal cord. Male patients with SCI who had absent F-wave from PTN but with intact BCR present were recruited for the DPN study. Patients with urinary tract infections, bladder/renal calculi, psychiatric illness, neurodegenerative disease, lower motor neuron lesions, or those with pacemaker were excluded from the study. Pregnant women and patients younger than 18 years were also excluded from the study. The International Standards of Neurological Classification of SCI (ISNCSCI) and its American Spinal Injury Association Impairment Scale (AIS) were used to describe neurological level and completeness of injury.13,14
Patients were asked to maintain a voiding diary throughout the treatment period. The voiding diary recorded the volume of fluid intake per day, urine output and record of any leaks. Patients who were on intermittent clean catheterization (ICC) were instructed to continue regular ICC every 4 hours while self-voiding patients were instructed to void urine according to their usual schedule. All medications related to neurogenic detrusor overactivity were withdrawn 1 week prior to the procedure.
Cystometrogram was performed to record detrusor activity using Life-Tech UroLab machine, Life-Tech Inc., Houston, TX, USA (Software: opus version 1.71) on days 1 and 15 of the study period. Stimulation was given by using a custom made nerve stimulator with stimulation parameters of 200 μs pulse width with rectangular pulses 10–40 mA of current strength at a frequency of 20 Hz. The parameters of the stimulation (frequency 20 Hz, up to 40 mA current strength, pulse width 200 µs) were consistent with the literature.5,7,15 Integrity of the PTN was checked by using F-wave test which was done by using a standard clinical nerve conduction machine (RMS EMG EP MKII). Self-adhesive pad electrodes were used for stimulation. The site of the tibial nerve was identified by using a nerve conduction machine and the location and polarity of electrode placement were marked at the ankle, posterior to the medial malleolus (anode placed 2 cm distal to the cathode posterior to the medial malleolus). For DPN stimulation, the anode was placed 2 cm distal to the cathode over the base of the penis. The electrodes were customized to fit the site of stimulation for each patient. Stimulation was given for 20 minutes per session each day for 14 consecutive days. In one particular case, the stimulation protocol was extended to 4 weeks on the patient's request.
The parameters evaluated in the voiding diary were the amount of fluid intake per day, volume of urine voided per day, number of leaks, number of ICC per day and maximum volume of urine voided in a day as per ICC. Other factors that contribute to urine production such as water content in food and perspiration could not be measured. The trend lines of these parameters were used for the analysis of voiding data.
Reduction in the number of leaks despite increase in the amount of fluid intake and increase in the maximum volume of urine voided was considered to signal improvement. No change or an increase in number of leaks and decrease in maximum volume of urine voided were considered to denote lack of improvement. The parameters available from CMG are reflex volume, cystometric capacity and maximum detrusor pressure. Reflex volume is defined as the volume at which the onset of detrusor contraction is first observed. As per the International Continence Society guidelines, cystometric capacity is the bladder volume at the end of filling cystometrogram when “permission to void” is given.16 However, patients with SCI cannot sense bladder fullness and do not have the ability to initiate micturition. In our study, cystometric capacity was estimated similar to the study conducted by Dalmose et al.4 when one of the following events occurred first during the CMG: (i) volume at which first leak was observed during cystometrogram procedure, (ii) volume of infused saline at which the patient felt discomfort or (iii) 500 ml of saline infused into the bladder. Maximum detrusor pressure is the highest detrusor pressure recorded during the CMG. Increase in reflex volume, increase in cystometric capacity and decrease in maximum pressure between the pre- and post-treatment cystometrogram were considered improvement.
Data analysis
Statistical analysis was performed by using SPSS (SPSS for Windows®, version 16.0; SPSS Inc., Chicago, IL, USA). Paired t-test was used to access the pre- and post-intervention difference on data available from CMG recording (i.e. reflex volume, maximum pressure, and cystometric capacity) for both PTN and DPN. Binomial distribution test was used on the voiding diary data where the outcome was the proportion of number of patients who had improved or not improved following the intervention in both PTN and DPN. P value of < 0.05 was considered significant at 95% confidence interval.
Results
PTN study
In this pilot study, 179 patients with SCI were screened, out of which 10 met the key inclusion criteria for the PTN study. Of 169 patients who were excluded, 96 were areflexic, 27 had occasional leaks, 22 had low compliance bladder (probably due to fibrosis that was seen as a rise in pressure without detrusor overactivity during filling phase), 9 had lower motor neuron lesion, 8 had other neurological disorders, 5 were minors and 2 patients refused participation. The characteristics of the 10 participants are shown in Table 1. Out of the 10 participants 9 were male and 1 female, among whom 6 had complete and 4 had incomplete spinal cord lesion. The mean age of the participants was 32 years and the time since injury ranged from 4 to 35 months. The neurological level of the participants ranged from C6–T3 (1 subject), T4–T10 (6 subjects) and T11–L3 (3 subjects). Table 1 also shows the AIS grade of the patients who participated in the study: five were AIS grade A, 1 was AIS grade B, 3 were AIS grade C and 1 was AIS grade D.
Table 1 .
Patient characteristic for PTN and DPN study
| Baseline parameters | PTN (no. = 10) | DPN (no. = 5) |
|---|---|---|
| Age (years), mean ± SD | 32.3 ± 6.18 | 42.8 ± 7.8 |
| Duration of injury (months), median (range) | 9.5 (4–35) | 88 (56–210) |
| no. (%) | no. (%) | |
| Male | 9 (90) | 5 (100) |
| Female | 1 (10) | — |
| Time since injury | ||
| 3 months to 1 year | 5 (50) | — |
| 1 to 5 years | 5 (50) | 1 (20) |
| 5 to 10 years | — | 2 (40) |
| > 10 years | — | 2 (40) |
| Injury type | ||
| Complete | 6 (60) | 5 (100) |
| Incomplete | 4 (40) | — |
| Neurological level | ||
| C6–T3 | 1 (10) | 0 |
| T4–T10 | 6 (60) | 3 (60) |
| T11–L3 | 3 (30) | 2 (40) |
| AIS grade | ||
| A | 5 (50) | 4 (40) |
| B | 1 (10) | 1 (10) |
| C | 3 (30) | — |
| D | 1 (10) | — |
PTN, posterior tibial nerve; DPN, dorsal penile nerve.
The pre- and post-intervention CMG data (i.e. reflex volume, maximum pressure, and cystometric capacity) are shown in Table 2. Following treatment of the 10 patients, 6 patients showed increase in reflex volume and reduction in maximum pressure and 5 had increased cystometric capacity. Reflex volume increased by 37.9 ± 98.9 ml (mean ± sd), maximum pressure decreased by −8 ± 23.7 cmH2O and cystometric capacity increased by 56 ± 169.5 ml (Table 3). However, P value for all of the three parameters obtained from the paired t-test was not statistically significant. The voiding diary data showed significant improvement with a P value of 0.021 (Table 4).
Table 2 .
Pre and post CMG data for PTN* and DPN** group
| Subject no. | Reflex volume (ml) |
Max. pressure (cmH2O) |
Cystometric capacity (ml) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTN |
DPN |
PTN |
DPN |
PTN |
DPN |
|||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| 1 | 28 | 218 | 56 | 219 | 93 | 41 | 225 | 119 | 80 | 245 | 63 | 311 |
| 2 | 159 | 319 | 170 | 200 | 56 | 53 | 113 | 72 | 178 | 395 | 238 | 240 |
| 3 | 90 | 241 | 140 | 166 | 82 | 44 | 104 | 91 | 201 | 503 | 143 | 171 |
| 4 | 117 | 185 | 45 | 53 | 50 | 55 | 123 | 127 | 412 | 401 | 45 | 57 |
| 5 | 215 | 225 | 68 | 75 | 55 | 26 | 132 | 76 | 270 | 502 | 69 | 77 |
| 6 | 108 | 114 | — | — | 73 | 79 | — | — | 147 | 184 | — | — |
| 7 | 165 | 136 | — | — | 53 | 64 | — | — | 326 | 210 | — | — |
| 8 | 208 | 169 | — | — | 42 | 40 | — | 225 | 225 | — | ||
| 9 | 104 | 60 | — | — | 58 | 81 | — | — | 500 | 266 | — | — |
| 10 | 146 | 52 | — | — | 42 | 41 | — | — | 204 | 172 | — | — |
| Mean | 134.0 | 171.9 | 95.8 | 142.6 | 60.4 | 52.4 | 139.4 | 97.0 | 254.3 | 310.3 | 111.6 | 171.2 |
| Std. Dev. | 56.6 | 83.7 | 55.7 | 74.6 | 16.9 | 17.8 | 49.0 | 24.9 | 126.7 | 128.3 | 79.9 | 107.5 |
| Std. Err. | 17.9 | 26.5 | 24.9 | 33.4 | 5.4 | 5.6 | 21.9 | 11.1 | 40.0 | 40.5 | 35.8 | 48.0 |
*PTN = Posterior tibial nerve.
**DPN = Dorsal penile nerve.
Table 3 .
Paired t-test on CMG data for PTN and DPN group
| CMG | Mean |
Standard deviation |
95% CI range |
t-value |
P value* |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| PTN | DPN | PTN | DPN | PTN | DPN | PTN | DPN | PTN | DPN | |
| RV | 37.90 | 46.80 | 98.90 | 65.80 | −32 to 108 | −35 to 128 | 1.21 | 1.59 | 0.25 | 0.18 |
| Max. P | −8.00 | −42.40 | 23.70 | 42.60 | −24 to 9 | −95 to 10 | −1.06 | −2.20 | 0.31 | 0.08 |
| CC | 56.00 | 59.60 | 169.50 | 105.8 | −65 to 177 | −72 to 191 | 1.04 | 1.26 | 0.32 | 0.27 |
*P value = Signed 2-tailed.
PTN, posterior tibial nerve; DPN, dorsal penile nerve; RV, reflex volume in ml; max. P, maximum pressure in cmH20; CC, cystometric capacity in ml.
Table 4 .
Binomial distribution test using voiding diary data for PTN and DPN group
| Group | Voiding diary |
Test prop. | P value | |
|---|---|---|---|---|
| Improved no. (%) | Not improved no. (%) | |||
| PTN | 9 (90%) | 1 (10%) | 0.5 | 0.021 |
| DPN | 5 (100%) | 0 (0%) | 0.5 | 0.062 |
PTN, posterior tibial nerve; DPN, dorsal penile nerve.
DPN study
In this pilot study, 170 patients with SCI were screened, out of which 5 met the key inclusion criteria. Of the 165 who were excluded, 114 had no symptoms of leaks, 15 participants had leaks due to stress incontinence (e.g. with coughing), 11 had F-wave present on the tibial nerve, 9 had lower motor neuron lesion, 6 had only occasional leak (e.g. once in a week or so), 3 practiced different modes of voiding other than ICC (e.g. Crede's method), 2 were areflexic, 2 patients refused to participate and the remaining 3 patients had BCR absent. The characteristics of the five subjects who participated in the study are shown in Table 1. All had complete motor spinal cord lesion. Time since injury ranged from 56 to 210 months. The neurological levels of the participant ranged from T4–T10 (3 subjects) and T11–L3 (2 subjects). Table 1 also shows the AIS grade of the patients who participated in the study: 4 were AIS grade A and 1 was AIS grade B.
The pre- and post-intervention CMG data (i.e. reflex volume, maximum pressure, and cystometric capacity) are shown in Table 2. The data show that in all five patients reflex volume had increased by 46.8 ± 65.8 ml (mean ± SD) after treatment, four patients had reduction in maximum pressure by −42.4 ± 42.6 cmH2O, and all five patients had increased cystometric capacity by 59.6 ± 105.8 ml (Table 3). However, P value obtained from paired t-test for all of the three parameters is not statistically significant. The voiding chart data suggest a trend of improvement with a P value of 0.062 (Table 4).
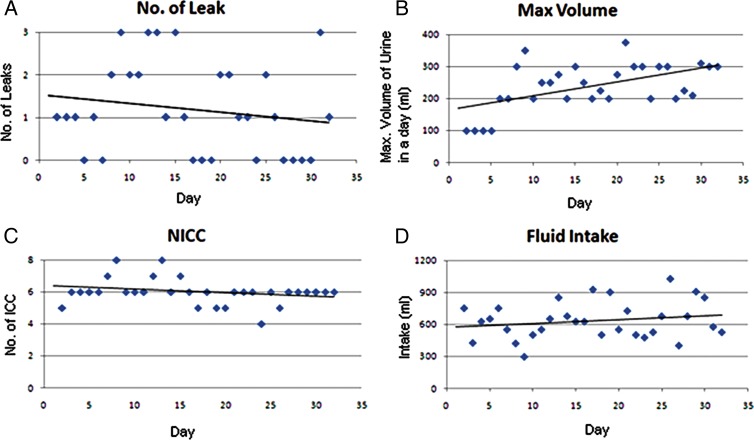
Fig. 1 shows the voiding chart data of a patient who had frequent leaks and had low capacity bladder (50–75 ml/ICC) before treatment. With 2–4 weeks of stimulation, the patient had a reduction in the frequency of leaks and improvement in bladder capacity (Fig. 1A) with linear regression trend-line with r = −0.17. The improvement in bladder capacity was around 200 ml at the end of 2 weeks of treatment and 300–350 ml at the end of 4 weeks of treatment (Fig. 1B) with linear regression trend-line with r = 0.53. The frequency of ICC (Fig. 1C) remained the same with r = −0.25. The fluid intake is shown in Fig. 1D with r = 0.197. The line in each figure is a linear regression trend-line.
Figure 1 .
Example of a voiding diary (one person who was given PTN stimulation for a month): (A) no. of leaks; (B) maximum volume of urine discharged in a day per ICC; (C) no. of time ICC done; and (D) fluid Intake.
Discussion
Stimulation of somatic afferents (e.g. PTN or DPN) which have the same root level as the urinary bladder (i.e. S2–S4) can result in neuromodulation leading to a reduction in spasticity of the detrusor muscle. Such a stimulation given over a period of several days can adaptively alter the synaptic loop gain of spinal neurons which in turn leads to reduction of detrusor overactivity. This pilot study of 15 patients showed a trend (although not statistically significant) in reduction of detrusor spasticity following stimulation of PTN or DPN.
We chose surface stimulation over percutaneous stimulation to avoid an invasive procedure. Most of the studies mentioned in the literature use percutaneous stimulation for PTN with a needle electrode (anode) being inserted posterior to the medial malleolus and a surface electrode (cathode) over the ipsilateral calcaneus.3,9,17 Current strength up to 10 mA is sufficient for percutaneous stimulation. In our study, both the cathode and the anode were gelled self-adhesive surface pad electrodes. The use of pad electrodes was found to be cost-effective and convenient. Surface pad electrodes are easy to use by patients for home-based therapy. The current strength used was up to 40 mA for transcutaneous stimulation. Surface stimulation requires higher current strength than percutaneous stimulation for obvious reasons of intervening skin impedance. The required current strength was determined from the motor conduction study which further confirms the stimulation reaching the tibial nerve.
However, it should be noted that certain factors such as urinary tract infections and autonomic dysfunction can affect the CMG especially in patients with neurogenic detrusor overactivity. Homma et al. studied the reproducibility of CMG in 30 subjects with detrusor overactivity.18 They conducted repeat CMG, 2–4 weeks after initial baseline CMG. They concluded that repeat cystometry in the overactive bladder is not highly reproducible.
In our study, the P value obtained from the paired t-test performed on pre- and post-intervention based on reflex volume, maximum pressure and cystometric capacity was found to be insignificant. However, the voiding chart data showed significant improvement in PTN study and trend of improvement in DPN study. For conclusive results, the study has to be conducted on a larger sample size.
The additional benefit noticed by one patient on commencement of DPN stimulation therapy was reduction in time taken for bowel management with digital evacuation. This is possible as the stimulation of the penile nerve reaches the S2–S4 spinal root which also has neuronal connection to the bowel.
Two patients recruited under the DPN study had progressive improvement in bladder capacity day by day as is evident from the voiding diary. However, they had persistent symptoms of stress leaks. On examination with transrectal ultrasound, bladder neck was found to be open. This suggested that patients with open bladder neck should be excluded from the study.
The voiding chart data of the patient showed that the dose is an important factor for neuromodulation (Fig. 1). However, further study on the long-term effects of the stimulation and carry over effect of stimulation will be helpful.
Conclusion
In this pilot study of 15 patients' voiding chart data showed statistically significant improvement following PTN stimulation and trend of improvement following DPN stimulation. However, the CMG data were not statistically significant in this sample population.
This study shows further support for the management of neurogenic bladder in patients with SCI through a simple, non-pharmacological, non-invasive intervention namely, neuromodulation by transcutaneous surface electrical stimulation of the PTN or DPNs. No adverse effect of the stimulation was observed suggesting that it is a safe treatment and has the potential to become an alternative treatment option for patients with detrusor overactivity.
However, further research on a larger sample is needed to provide statistically conclusive results regarding the efficacy of this treatment and optimal dosage.
Disclaimer statements
Contributors RO: Principal Investigator, Stimulator Design, Study Design, Stimulation therapy sessions, data analysis, F-wave test and manuscript writing. JG: Patient recruitment, CMG procedure, medical guidance and study design. BC: Patient recruitment, CMG procedure, medical guidance and manuscript writing. GT: Patient recruitment, medical guidance, study design and manuscript writing. SD: Technical guidance, study design, data analysis and manuscript writing.
Conflicts of interest None.
Ethics approval Clinical Trial Registry- India Approval no. CTRI/2012/12/003234 Christian Medical College. Vellore, Institutional Review Board Approval no. CMC/IRB/6735/2008/12/18.
Funding Fluid Research Grant Christian Medical College, Vellore, India. Departmental fund from Department of PMR and Bioengineering, CMC Vellore.
Acknowledgments
The authors thank the doctors of the Department of Physical Medicine and Rehabilitation, Christian Medical College, Vellore, India for helping in patient recruitment. We also thank Mr Saravanan, Department of Biostatistics, Christian Medical College, Vellore, India, for the statistical analysis. Finally, we are grateful to the Institute Fluid research grant from Christian Medical College in Vellore for supporting the study.
References
- 1.Nagarajan G, Arumugam E, Tharion G, Bhattacharji S. Perceptions of patients with spinal cord injury on future research in South India. Soc Care Neurodisabil 2012;3(1):20–6. [Google Scholar]
- 2.Geirsson G, Wang YH, Lindstrom S, Fall M. Traditional acupuncture and electrical stimulation of the posterior tibial nerve: a trial in chronic interstitial cystitis. Scand J Urol Nephrol 1993;27(1):67–70. [DOI] [PubMed] [Google Scholar]
- 3.Govier FE, Litwiller S, Nitti V, Kreder JKJ, Rosenblatt P. Percutaneous afferent neuromodulation for the refractory overactive bladder: results of a multicenter study. J Urol 2001;165(4):1193–8. [PubMed] [Google Scholar]
- 4.Dalmose AL, Rijkhoff NJ, Kirkeby HJ, Nohr M, Sinkjaer T, Djurhuus JC. Conditional stimulation of the dorsal penile/clitoral nerve may increase cystometric capacity in patients with spinal cord injury. Neurourol Urodyn 2003;22(2):130–7. [DOI] [PubMed] [Google Scholar]
- 5.van Balken MR, Vandoninck V, Gisolf KW, Vergunst H, Kiemeney LA, Debruyne FM, et al. Posterior tibial nerve stimulation as neuromodulative treatment of lower urinary tract dysfunction. J Urol 2001;166(3):914–8. [DOI] [PubMed] [Google Scholar]
- 6.Peters KM, Carrico DJ, Perez-Marrero RA, Khan AU, Wooldridge LS, Davis GL, et al. Randomized trial of percutaneous tibial nerve stimulation versus Sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol 2010;183(4):1438–43. [DOI] [PubMed] [Google Scholar]
- 7.Klingler HC, Pycha A, Schmidbauer J, Marberger M. Use of peripheral neuromodulation of the S3 region for treatment of detrusor overactivity: a urodynamic-based study. Urology 2000;56(5):766–71. [DOI] [PubMed] [Google Scholar]
- 8.de Sèze M, Raibaut P, Gallien P, Even-Schneider A, Denys P, Bonniaud V, et al. Transcutaneous posterior tibial nerve stimulation for treatment of the overactive bladder syndrome in multiple sclerosis: Results of a multicenter prospective study. Neurourol Urodyn 2011;30(3):306–11. [DOI] [PubMed] [Google Scholar]
- 9.MacDiarmid SA, Peters KM, Shobeiri SA, Wooldridge LS, Rovner ES, Leong FC, et al. Long-term durability of percutaneous tibial nerve stimulation for the treatment of overactive bladder. J Urol 2010;183(1):234–40. [DOI] [PubMed] [Google Scholar]
- 10.Vandoninck V. Posterior Tibial Nerve Stimulation: a new treatment option for lower urinary tract dysfunction [PhD thesis] Nijmegen, The Netherlands: UMC, St Radboud; 2007. [Google Scholar]
- 11.Finazzi-Agro E, Petta F, Sciobica F, Pasqualetti P, Musco S, Bove P. Percutaneous tibial nerve stimulation effects on detrusor overactivity incontinence are not due to a placebo effect: a randomized, double-blind, placebo controlled trial. J Urol 2010;184(5):2001–6. [DOI] [PubMed] [Google Scholar]
- 12.Zhao J, Bai J, Zhou Y, Qi G, Du L. Posterior tibial nerve stimulation twice a week in patients with interstitial cystitis. Urology 2008;71(6):1080–4. [DOI] [PubMed] [Google Scholar]
- 13.Kirshblum SC, Waring W, Biering-Sorensen F, Burns SP, Johansen M, Schmidt-Read M, et al. Reference manual for the 2011 revision of the international standards for neurological classification of spinal cord injury. J Spinal Cord Med 2011;34(6):547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Spinal cord Injury Statistical Center. Data collection syllabus for the national spinal cord injury database: 2006–2011 project period [data on internet]. Birmingham;2011. pp. 1–633. Available from: https://www.nscisc.uab.edu/PublicDocuments/data_collection_syllabus/Syllabus%202006-2011%20Revised%20B-E%209-10.pdf
- 15.Congregado RB, Pena OXM, Campoy MP, Leon DE, Leal LA. Peripheral afferent nerve stimulation for treatment of lower urinary tract irritative symptoms. Eur Urol 2004;45(1):65–9. [DOI] [PubMed] [Google Scholar]
- 16.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):167–78. [DOI] [PubMed] [Google Scholar]
- 17.Peters KM, Macdiarmid SA, Wooldridge LS, Leong FC, Shobeiri SA, Rovner ES, et al. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol 2009;182(3):1055–61. [DOI] [PubMed] [Google Scholar]
- 18.Homma Y, Kondo Y, Takahashi S, Kitamura T, Kawabe K. Reproducibility of cystometry in overactive detrusor. Eur Urol 2000;38(6):681–5. [DOI] [PubMed] [Google Scholar]