Abstract
Background
Upper cervical spinal cord hemisection causes paralysis of the ipsilateral hemidiaphragm; however, the effect of C2 hemisection on the function of the intercostal muscles is not clear. We hypothesized that C2 hemisection would eliminate inspiratory intercostal activity ipsilateral to the injury and that some activity would return in a time-dependent manner.
Methods
Female Sprague Dawley rats were anesthetized with urethane and inspiratory intercostal electromyogram (EMG) activity was recorded in control rats, acutely injured C2 hemisected rats, and at 1 and 16 weeks post C2 hemisection.
Results
Bilateral recordings of intercostal EMG activity showed that inspiratory activity was reduced immediately after injury and increased over time. EMG activity was observed first in rostral spaces followed by recovery occurring in caudal spaces. Theophylline increased respiratory drive and increased intercostal activity, inducing activity that was previously absent.
Conclusion
These results suggest that there are crossed, initially latent, respiratory connections to neurons innervating the intercostal muscles similar to those innervating phrenic motor neurons.
Keywords: Intercostal muscles, Spinal cord injury, Theophylline, Latent respiratory synaptic pathways
Introduction
Spinal cord injury (SCI) results in a loss of muscle function below the site of injury. The primary cause of morbidity and mortality associated with all SCI is due to complications related to impaired respiratory function,1 despite the fact that some recovery of muscle function is known to occur in the respiratory muscles.2 In all mammals, inspiration is accomplished primarily by the contraction of the diaphragm and aided by the activity of the intercostal muscles. The effect of SCI on diaphragm function has been well characterized3–8; however, there are only a few examples of studies that have examined the effect of SCI on intercostal musculature.9,10 It is well known that upper cervical SCI interrupts the neural signals arising from medullary respiratory neurons that project down the spinal cord to innervate ipsilateral phrenic motor neurons located in the cervical cord (C3–C6 in rats) resulting in ipsilateral hemidiaphragmatic paralysis. Innervation of external intercostal muscles appears to be largely due to polysynaptic pathways, which cross the midline to affect motoneurons on the contralateral side of the spinal cord.11,12 Kirkwood et al.9 and Dougherty et al.10 showed that hemisection of the spinal cord caused an immediate reduction in ipsilateral intercostal nerve activity below the injury site. In both studies, intercostal activity returned; however, Dougherty et al.10 only examined the rostral intercostal activity of the first and second rib space after a C2 hemisection and Kirkwood et al.9 hemisected the thoracic spinal cord and also only looked at a few thoracic intercostal spaces below the injury. Therefore, the present study was designed to better characterize the effect of a C2 spinal cord hemisection on the 10 most rostral intercostal muscles after several time points following injury. We hypothesized that spinal cord hemisection would eliminate inspiratory intercostal activity ipsilateral to the injury and that we would see a time-dependent increase in restored activity throughout the intercostal musculature.
In addition, we examined the effect of theophylline, a known respiratory stimulant capable of activating latent crossed phrenic pathways, to determine whether similar increases in respiratory drive also activated latent pathways projecting to intercostal muscles. Dougherty et al.10 showed that hypercapnia increased intercostal activity after SCI and this correlated with increases in tidal volume. A case study examining the effect of theophylline on respiratory function after chronic SCI in a patient also reported that theophylline increased muscle activity not only in the diaphragm but also in the parasternal intercostal muscles.13 Thus, we further hypothesized that increasing respiratory drive by theophylline after C2 hemisection would not only increase hemidiaphragm muscle activity, but also intercostal muscle activity in the rat.
Methods
All experimental procedures were approved prior to experimentation by the Wayne State University Animal Investigative Committee. The C2 hemisection procedure has been previously published in detail.14 Briefly, adult Sprague Dawley female rats (Harlan retired breeders, ∼1 year old) were anesthetized with ketamine (70 mg/kg) and xylazine (20 mg/kg, intramuscularly). The spinous process and laminae of the C2 vertebra were removed and the C2 spinal cord was hemisected on either the left or right side of the spinal cord. The neck muscles were sutured (3.0 Vicryl) and the skin closed with wound clips. After surgery, the rats were injected with 10 ml of saline (subcutaneously (s.c.)) to prevent dehydration and buprenorphine (0.01 mg/kg s.c.), an analgesic for pain management. The rats were warmed on a heating pad until they aroused from anesthesia and then placed in cages and provided with food and water ad libitum, supplemented with apples for 1–16 weeks. Sham control rats were treated exactly as above except that during the surgery, the spinal cord was not cut.
Experimental protocol
On the day of experimentation, rats were prepared for a second non-survival surgical procedure. They were anesthetized with urethane (1.6 g/kg, intraperitoneally) and placed supine and maintained at 37°C using a rectal thermometer connected to a thermal-controlled heating pad. An incision was made in the skin down the midline from the clavicles to just below the xiphoid process. The superficial thoracic muscles, pectoralis major and minor, were cut and retracted to expose the external intercostal muscles. The diaphragm was also exposed by abdominal laparotomy. Bilateral electromyogram (EMG) recordings were taken first in the lateral costal region of the diaphragm and recorded on Chart 4.2 (ADInstruments Pty Ltd, Bella Vista, Australia). Then, external intercostal EMG recordings were obtained by placing one pair of bipolar electrodes ∼1 cm lateral to the sternum on each side in the 10th intercostal space. This procedure was followed by recording from each successive intercostal space rostrally to the first intercostal space. EMG recordings were taken for ∼30 seconds to 1 minute bilaterally and then the electrodes were moved to the next space for recording. This procedure was repeated until all of the intercostal spaces were recorded.
Four animal groups were examined: acute (within 1 hour following hemisection) (n = 7), 1 week following SCI (n = 7), 16 weeks following SCI (n = 6), and sham controls (n = 13). Acutely injured animals were hemisected under urethane anesthesia and EMG intercostal recordings were made immediately in these animals. Animals were euthanized after the completion of EMG recordings.
In some of the animals (controls, n = 4, 1 week SCI, n = 7), the effect of increasing respiratory drive was examined using a known respiratory stimulant of crossed phrenic activity, theophylline. After EMGs were recorded as described above, theophylline was injected (15 mg/kg, intravenously) via a cannula that was placed in the femoral vein. After 20 minutes, EMG recordings were again obtained from the diaphragm and intercostal spaces as described above.
Data analysis
To obtain a comparison of muscle activity ipsilateral and contralateral to SCI, right and left EMG activity from the diaphragm and intercostal muscles were full-wave rectified and integrated using Chart 4.2 software (ADInstruments Pty Ltd). First, we verified that the intercostal muscle activity was inspiratory by comparing the intercostal activity to the diaphragmatic activity. The integrated traces were assessed for breathing frequency, mean burst area, and peak amplitude. Mean burst area and peak amplitude of the EMG activity ipsilateral to injury (injured side) was then presented as a percentage of the activity contralateral to injury (non-injured side). The average burst area and peak amplitude were then calculated by taking activity from all animals, including animals that showed zero activity. To evaluate the effect of theophylline treatment in SCI animals, we looked at how many intercostal spaces had muscle activity before treatment and then again after theophylline treatment.
Statistics were performed using Systat software (Systat Software, Inc., San Jose, CA, USA). The differences in breathing frequency, and the mean burst area and peak amplitude of the EMG activity of individual intercostal muscles at various times after SCI were examined using a one-way analysis of variance between groups. The effect of theophylline was examined by comparing the activity before and after treatment using a paired t-test. Significance was determined when P-values were <0.05.
Results
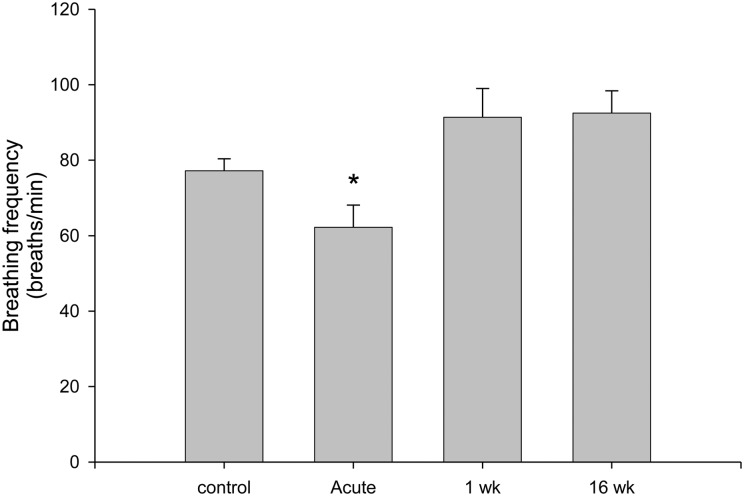
The initial assessment of diaphragm activity was determined at the time of the hemisection surgery to ensure that all hemidiaphragmatic activity ipsilateral to injury was initially absent. Spontaneous crossed phrenic activity was observed in the hemidiaphragm of all of our 16-week chronic SCI animals, but not in any other group (Fig. 1). In urethane-anesthetized, spontaneously breathing rats, the breathing frequency was significantly decreased immediately after acute SCI (Fig. 2). One week (P = 0.118) and 16 weeks (P = 0.054) post-SCI animals showed a slight, but non-significant elevation in breathing frequency during urethane anesthesia compared to the control animals (Fig. 2).
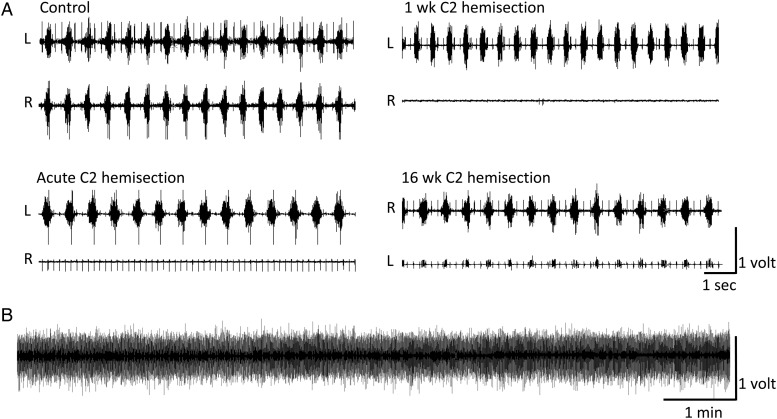
Figure 1 .
(A) Representative raw traces of EMG activity of the right (R) and left (L) hemidiaphragm of a control rat, immediately after C2 hemisection (acute), 1 week following C2 hemisection, and 16 weeks following C2 hemisection. Note the absence of EMG activity ipsilateral to SCI in animals immediately following hemisection (R) and 1 week following hemisection (R); only EKG activity is visible. By 16 weeks, spontaneous activation of crossed phrenic pathways occurs and activity is recorded in the hemidiaphragm ipsilateral to the SCI (L). (B) A 10-minute representative trace of the working hemidiaphragm from a 1-week hemisected rat showing the stability of the preparation over time.
Figure 2 .
The breathing frequency of urethane-anesthetized, spontaneously breathing Sprague Dawley rats was significantly decreased (*P = 0.041) immediately following a C2 SCI surgery (acute, n = 7) compared to sham control rats (n = 13). One week (n = 7) and 16 weeks (n = 6) following SCI, the respiratory frequency of urethane-anesthetized, spontaneously breathing rats was slightly higher than controls, but was not significantly different from control values (P = 0.054).
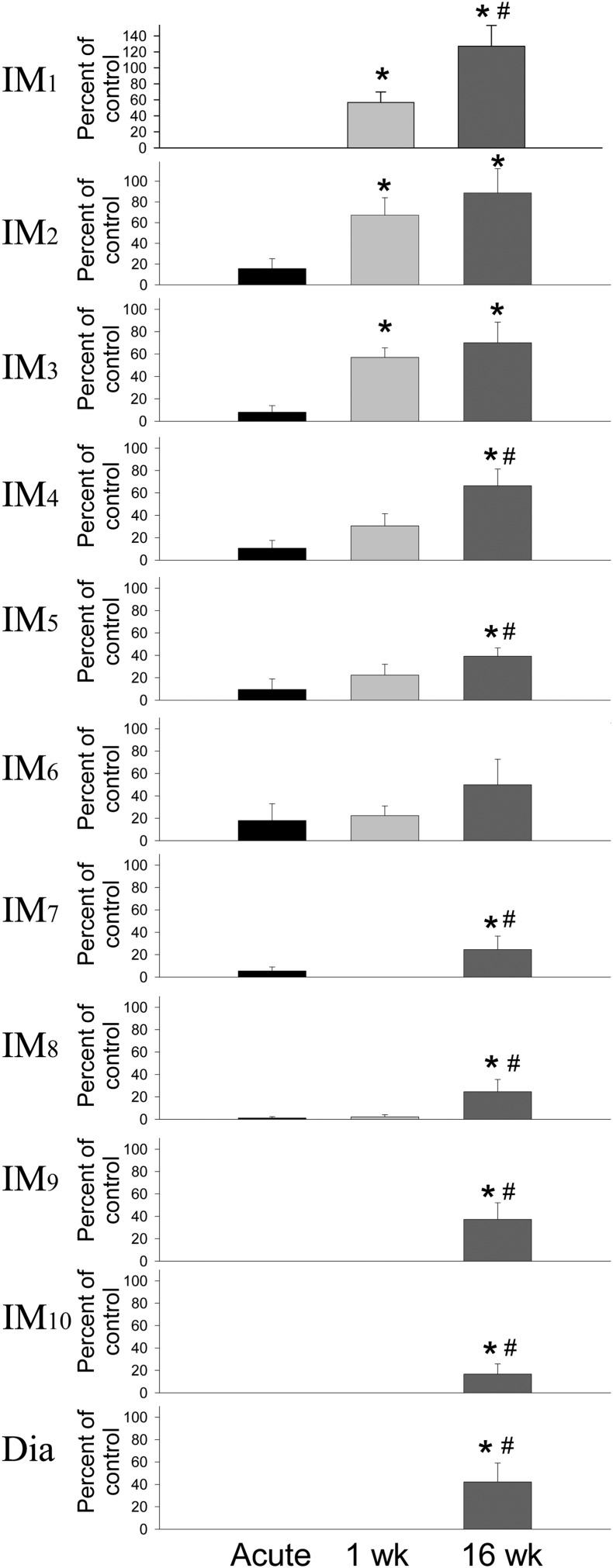
Immediately after C2 SCI surgery, there was a small amount of EMG activity recorded in five of seven animals between the second and the eighth intercostal spaces on the side of the injury. In the other two acute animals, there was no activity recorded in any intercostal space ipsilateral to injury. Measurement of the peak amplitude and the burst area of the EMG activity measured as a comparison between the injured side relative to the uninjured side revealed similar results; therefore, only peak amplitude data is reported here. Measurement of the peak amplitude of the EMG burst revealed a significant decrease in thoracic intercostal muscle activity ipsilateral to the hemisection (Figs. 3 and 4). At 1 week post-SCI, all animals showed motor activity in some ipsilateral intercostal muscles and EMG activity was noticeably increased compared to immediately after SCI. It is noteworthy that the motor activity observed in intercostal muscles at 1 week post-SCI was not accompanied by any recovery of the ipsilateral hemidiaphragm activity at the same time. The activity was more pronounced in the more rostral spaces with the first three intercostal spaces showing about half the activity of the non-injured side. At 16 weeks post-injury, there was a prominent increase in the amount of EMG activity in the intercostal spaces ipsilateral to the injury and activity was now observed in all intercostal spaces in all animals. The most obvious amount of motor activity was present in the two most rostral spaces (back to control levels); however, the EMG activity in most intercostal spaces was still well below normal levels.
Figure 3 .
Acute C2 spinal cord hemisection resulted in a significant reduction in the amount of inspiratory related intercostal muscle activity (IM) ipsilateral to the SCI as measured by the peak amplitude of the integrated EMG activity expressed as a percentage of the non-injured side (set at 100%). EMG activity was measured at each intercostal space between the first and the tenth space on the ventral thoracic surface. Spontaneous recovery of intercostal activity increased starting at 1 week following SCI and activity significantly increased compared to acute SCI rats (*P < 0.05). After 16 weeks post-SCI, all intercostal muscles showed some spontaneous recovery of intercostal activity and this activity was significantly greater than acute SCI rats (*P < 0.05). Many were significantly increased compared to 1 week (#P < 0.05). The spontaneous recovery of the hemidiaphragm ipsilateral to the hemisection is also expressed by 16 weeks after SCI.
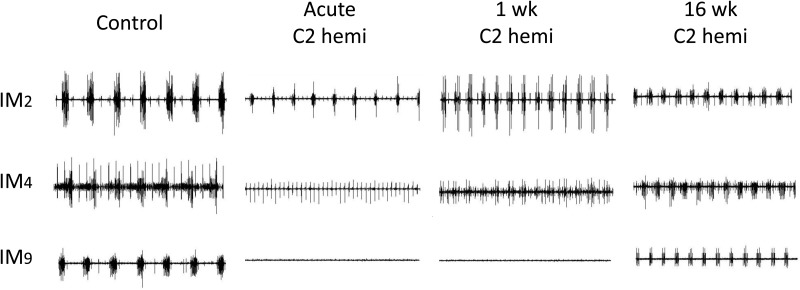
Figure 4 .
Representative raw traces of three intercostal spaces (IM 2, 4, and 9) of a control rat, acutely injured rat, and at 1 week and 16 weeks following C2 spinal cord hemisection injury. Note the prominent reduction in EMG activity immediately after injury. At 1 week following SCI, activity was increased in rostral thoracic intercostal spaces and we observed activity appearing over the middle thoracic spaces. By 16 weeks following SCI, EMG activity was observed in all 10 intercostal spaces, but the EMG activity was still reduced compared to control values in the caudal half of the thoracic region (IM7 – IM10). Each trace is 6 seconds in total length.
Theophylline treatment resulted in an increase in breathing frequency in controls (from 72.7 ± 3.9 to 86.3 ± 3.8) and in 1 week post-SCI animals (91.5 ± 1.3 to 105.6 ± 1.4) indicating increased central respiratory drive. At 1 week after SCI, theophylline treatment induced a significant increase in the number of intercostal spaces that expressed EMG activity (from 4.6 ± 0.48 to 6.1 ± 0.67); i.e. the muscles were quiescent prior to theophylline treatment.
Discussion
This study demonstrates that ipsilateral inspiratory intercostal muscle activity is initially interrupted by an acute upper cervical hemisection. Some intercostal activity gradually returned in a time-dependent manner. Activity in the most rostral regions of the thoracic intercostal spaces appeared first and returned to near or at control levels by 16 weeks, whereas activity in the more caudal spaces appeared much later after SCI and continued to remain significantly reduced compared to controls at 16 weeks post-injury. The functional significance of these results appears to be related to correlated increases in tidal volume.10 In addition, the return of intercostal activity in the more rostral intercostal spaces with increasing time after SCI may increase thoracic cage stability. Increasing respiratory drive with theophylline enhanced neural output of inspiratory intercostal muscle activity and turned some “silent” pathways on. Taken together, these results suggest that there are initially latent, crossed premotor respiratory synaptic connections to neurons innervating the intercostal muscles after SCI that can be turned on by increasing drive. Utilizing these neural pathways may be a useful alternative for pacing mechanisms when the diaphragm muscle is not an option.15–17
Effect of SCI on muscles controlling ventilation
The effect of C2 hemisection on diaphragm function has been examined extensively over the past 30 years.18–20 C2 hemisection results in complete paralysis of the ipsilateral hemidiaphragm due to the interruption of signals that arise primarily from descending monosynaptic, bulbospinal premotor neurons.4,21–23 This results in a decreased tidal volume and a compensatory increase in respiratory frequency which is observed consistently in unanesthetized rats.3,7,24 In the present study, respiratory frequency was not significantly increased. This is most likely due to the effect of the anesthetic and the fact that we did not maintain blood gases constant. In studies of anesthetized rats, frequency is not always significantly elevated after SCI.6,7,14,24
Activity in the paralyzed hemidiaphragm can be partially restored due to the activation of crossed phrenic pathways that have been localized in the spinal cord contralateral and below the site of SCI. These crossed phrenic pathways consist of latent bulbospinal pathways that cross below the site of SCI,4 and possibly inputs from propriospinal interneurons located within the cervical spinal cord.8,23 Multiple methods to restore function to the previously paralyzed hemidiaphragm have been examined in both males and females and, so far to date, enhancement of respiratory drive or synaptic input to phrenic motoneurons, or a decrease in inhibition, results in some restoration of phrenic activity below the site of SCI.6,25–29 Functionally, the activation of the crossed phrenic pathways appears to make a meaningful contribution to ventilation in anesthetized rats after SCI,7,24,30 which may contribute even more during augmented breaths or sighs.6 Spontaneous activation of the crossed phrenic pathway also occurs over time. Earlier studies by Goshgarian and colleagues3,4,14,26,28,29 performed on female retired breeders showed spontaneous activation between 12 and 16 weeks after SCI. More recent studies have showed that the spontaneous activation of the crossed phrenic phenomenon is also present in young male rats as early as 2–4 weeks after SCI.5–8,10,24,25,27 It is unknown whether this is due to age, sex, or individual rat strains.
The diaphragm is not the only respiratory muscle that is affected by upper SCI. Both inspiratory and expiratory intercostal muscles, as well as abdominal muscles are affected by SCI. Recent attention given to the expiratory system has shown that a reduction in expiratory maneuvers such as coughing,31 reduces the capacity to fight disease and contributes significantly to the morbidity and mortality associated with SCI.1 Information, however, regarding the effect of SCI on inspiratory intercostal musculature is still lacking.9,10 The present study examines and characterizes for the first time the effect of upper SCI on the EMG activity of the first 10 inspiratory intercostal muscles.
Studies in cats have shown that the primary drive to intercostal muscles was through descending inputs9 arising from the contralateral medulla.11 Like others,9–11 this study showed that C2 hemisection resulted in paralysis of ipsilateral inspiratory intercostal muscle activity confirming that the primary ipsilateral descending drive in rats is carried through projections that cross above C2 before descending through the ipsilateral spinal cord (Fig. 5A). The majority of descending inputs to intercostal muscles are polysynaptic involving interneurons. Monosynaptic connections to intercostal muscles are rare.11,12 The return of some activity in the most rostral intercostal muscles appeared rather quickly indicating that crossed inputs at the thoracic level must be rather common. However, activity in the lower half of the spinal cord did not return as quickly or as robust suggesting that the amount of input or drive is substantially less than the activity in the rostral sections.
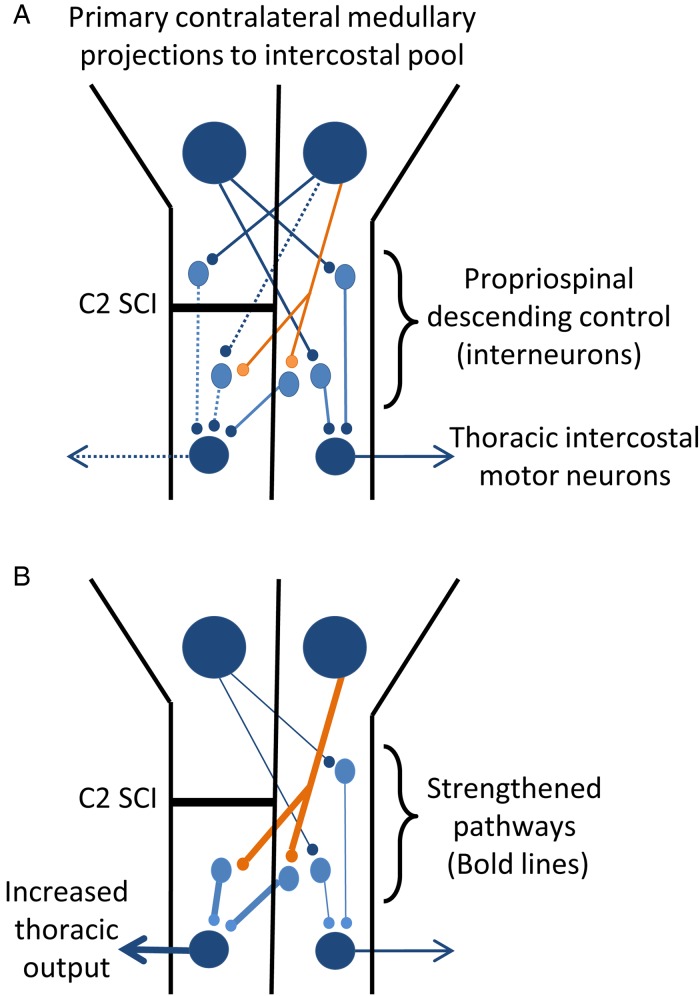
Figure 5 .
A model of neural pathways that project to thoracic motor neurons. The primary projection to intercostal motor neurons arises from contralateral medullary neurons and is polysynaptic. (A) A C2 SCI disrupts the primary signal descending to the thoracic motor neurons ipsilateral to the injury and the thoracic output is silenced. (B) Recovery of motor function most likely arises from the strengthening of established connections from the contralateral spinal cord below the site of injury. A possible pathway for this contralateral, propriospinal input is hypothesized in (B).
In cats, studies showed that at least 60% of respiratory interneurons projected contralaterally and at the same level of the spinal cord from which they arose.12,32 In the present study, this interneuronal pool did not sufficiently activate the side ipsilateral to the injury. It also does not appear to be a strong input on the side contralateral to the injury, since there were no differences in the contralateral activity in injured animals compared to controls. However, strengthening of the synapses onto and from these interneurons may be a possible substrate for the spontaneous activity that appears weeks after C2 hemisection (Fig. 5B) Recently, a population of interneurons was found throughout the cervical spinal cord in the rat that may be part of crossed phrenic pathways.8 There may be silent or low activity pathways in the thoracic cord, similar to the pathways in the cervical cord at the level of the phrenic nucleus that needs time for synaptic strengthening in order to be activated. More studies are needed to address the exact pathways that contribute to the appearance of crossed intercostal activity after SCI.
Immediately after SCI, breathing frequency was significantly reduced compared to control animals during urethane anesthesia. This is not an uncommon observation after surgery (in neonates;30 in adult female rats – personal observations). We speculate that the fall in frequency may be due to an abrupt decrease in respiratory drive associated with the removal of ascending afferent input. SCI removes many ascending inputs that would result in an altered integration of neural output in regions above the site of injury.33 In fact, there are findings which indicate that respiratory alterations occur above the site of SCI to affect respiratory function days to weeks after the injury.5,33
Increasing respiratory drive
Increasing respiratory drive with acute administration of theophylline resulted in an enhanced inspiratory motor output. Both acute and chronic administration of theophylline have been shown to induce the crossed phrenic phenomenon in the C2 hemisected rat.28,29 In this study, we found that acute theophylline administration significantly increased breathing frequency, and turned on intercostal activity in previously silent muscles. These results indicate that enhancing central neural output is sufficient to activate previously quiescent intercostal muscle activity similar to that observed with the crossed phrenic pathway. Increasing intercostal muscle activity enhances the stability of the chest wall during inspiratory and expiratory movements. DiMarco et al.15–17 have been examining the use of intercostal muscle pacing to aid in inspiratory and expiratory movements after SCI, especially when phrenic nerve pacing is not an option. Selective targeting of intercostal muscle function after SCI is proving to be a practical method to restore ventilation when the diaphragm is not effective.15 If thoracic muscle activity can be enhanced with drug therapy or other means, muscle pacing techniques may become even more effective. More studies are needed to fully understand the effect of SCI on intercostal muscle activity to aid in the treatment and care of SCI patients.
Disclaimer statements
Contributors MBZ was the postdoctoral student that helped design and set up the following experiment. She ran experiments, analyzed data, and wrote the first draft of the manuscript. JSG and AEA were medical students at the time and were responsible for carrying out the experiment. They helped analyze data and review manuscripts. HGG is the PI. He was instrumental in helping design and carry out the experiment. He helped write the manuscript and oversee the entire procedure.
Conflicts of interest None.
Ethics approval The following experiments were approved prior to experimentation by the Wayne State University Animal Investigative Committee.
Funding This work was supported by NIH grant HD 31550 to HGG.
References
- 1.National Spinal Cord Injury Statistical Center. Spinal cord injury: facts and figures at a glance. J Spinal Cord Med 2010;33(4):439–40. [PubMed] [Google Scholar]
- 2.Brown MD, DiMarco AF, Hoit JD, Garshick E. Respiratory dysfunction and management in spinal cord injury. Respir Care 2006;51(8):853–70. [PMC free article] [PubMed] [Google Scholar]
- 3.Goshgarian HG, Moran MF, Prcevski P. Effect of cervical spinal cord hemisection and hemidiaphragm paralysis on arterial blood gases, pH and respiratory rate in the adult rat. Exp Neurol 1986;93(2):440–5. [DOI] [PubMed] [Google Scholar]
- 4.Moreno DE, Yu XJ, Goshgarian HG. Identification of the axon pathways which mediate functional recovery of a paralyzed hemidiaphragm following spinal cord hemisection in the adult rat. Exp Neurol 1992;116(3):219–28. [DOI] [PubMed] [Google Scholar]
- 5.Golder FJ, Reier PJ, Bolser DC. Altered respiratory motor drive after spinal cord injury: supraspinal and bilateral effects of a unilateral lesion. J Neurosci 2001;21(21):8680–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golder FJ, Fuller DD, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J Neurosci 2003;23(6):2494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuller DD, Golder FJ, Olson EB, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol (1985) 2006;100(3):800–6. [DOI] [PubMed] [Google Scholar]
- 8.Lane MA, Lee KZ, Fuller DD, Reier PJ. Spinal circuitry and respiratory recovery following spinal cord injury. Respir Physiol Neurobiol 2009;169(2):123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirkwood PA, Sears TA, Westgaard RH. Restoration of function in external intercostal motoneurones of the cat following partial central deafferentation. J Physiol 1984;350:225–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dougherty BJ, Lee KZ, Gonzalez-Rothi EJ, Lane MA, Reier PJ, Fuller DD. Recovery of inspiratory intercostal muscle activity following high cervical hemisection. Resp Physiol Neurobiol 2012;183:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merrill EG, Lipski J. Inputs to intercostal motoneurons from ventrolateral medullary respiratory neurons in the cat. J Neurophysiol 1987;57(6):1837–53. [DOI] [PubMed] [Google Scholar]
- 12.Saywell SA, Ford TW, Meehan CF, Todd AF, Kirkwood PA. Electrophysiological and morphological characterization of propriospinal interneurons in the thoracic spinal cord. J Neurophysiol 2011;105(2):806–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson GT, Khanchandani N, Lattin CD, Goshgarian HG. Clinical effects of theophylline on inspiratory muscle drive in tetraplegia. Neurorehabil Neural Repair 1999;13:191–7. [Google Scholar]
- 14.Zimmer MB, Goshgarian HG. GABA, not glycine mediates inhibition of latent respiratory motor pathways after spinal cord injury. Exp Neurol 2007;203(2):493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiMarco AF, Kowalski KE. Intercostal muscle pacing with high frequency spinal cord stimulation in dogs. Respir Physiol Neurobiol 2010;171(3):218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiMarco AF. Restoration of respiratory muscle function following spinal cord injury: review of electrical and magnetic stimulation techniques. Respir Physiol Neurobiol 2005;147(2–3):273–87. [DOI] [PubMed] [Google Scholar]
- 17.DiMarco AF, Romaniuk JR, Kowalski KE, Supinski GS. Efficacy of combined inspiratory intercostal and expiratory muscle pacing to maintain artificial ventilation. Am J Resp Crit Care Med 1997;156(1):122–6. [DOI] [PubMed] [Google Scholar]
- 18.Goshgarian HG. The crossed phrenic phenomenon and recovery of function following spinal cord injury. Respir Phyiol Neurobiol 2009;169(2):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lane MA, Fuller DD, White TE, Reier PJ. Respiratory neuroplasticity and cervical spinal cord injury: translational perspectives. Trends Neurosci 2008;31(10):538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinit S, Kastner A. Descending bulbospinal pathways and recovery of respiratory motor function following spinal cord injury. Respir Physiol Neurobiol 2009;169(2):115–22. [DOI] [PubMed] [Google Scholar]
- 21.Ellenberger HH, Feldman JL. Monosynaptic transmission of respiratory drive to phrenic motoneurons from brainstem-bulbospinal neurons in rats. J Comp Neurol 1988;269(1):47–57. [DOI] [PubMed] [Google Scholar]
- 22.Ellenberger HH, Feldman JL, Goshgarian HG. Ventral respiratory group projections to phrenic motoneurons: electron-microscopic evidence for monosynaptic connections. J Comp Neurol 1990;302(4):707–14. [DOI] [PubMed] [Google Scholar]
- 23.Lipski J, Zhang X, Kruszewska B, Kanjhan R. Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Res 1994;640:171–84. [DOI] [PubMed] [Google Scholar]
- 24.Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol 2008;211(1):97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuller DD, Johnson SM, Olson RB Jr, Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci 2003;23(7):2993–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kajana S, Goshgarian HG. Spinal activation of the cAMP-PKA pathway induces respiratory motor recovery following high cervical spinal cord injury. Brain Res 2008;1232:206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee KZ, Sandhu MS, Dougherty BJ, Reier PJ, Fuller DD. Influence of vagal afferents on supraspinal and spinal respiratory activity following cervical spinal cord injury in rats. J Appl Physiol 2010;109(2):377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nantwi KD, El-Bohy A, Goshgarian HG. Actions of systemic theophylline on hemidiaphragmatic recovery in rats following cervical spinal cord hemisection. Exp Neurol 1996;140(1):53–9. [DOI] [PubMed] [Google Scholar]
- 29.Nantwi KD, Goshgarian HG. Effects of chronic systemic theophylline injections on recovery of hemidiaphragmatic function after cervical spinal cord injury in adult rats. Brain Res 1998;789(1):126–9. [DOI] [PubMed] [Google Scholar]
- 30.Dougherty BJ, Lee KZ, Lane MA, Reier PJ, Fuller DD. Contribution of the spontaneous crossed-phrenic phenomenon to inspiratory tidal volume in spontaneously breathing rats. J Appl Physiol (1985) 2012;112(1):96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kowalski KE, Romaniuk JR, DiMarco AF. Changes in expiratory muscle function following spinal cord section. J Appl Physiol (1985) 2007;102(4):1422–8. [DOI] [PubMed] [Google Scholar]
- 32.Schmid K, Kirkwood PA, Munson JB, Shen E, Sears TA. Contralateral projections of thoracic respiratory interneurones in the cat. J Physiol 1993;461:647–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmer MB, Goshgarian HG. Spinal cord injury in neonates alters respiratory motor output via supraspinal mechanisms. Exp Neurol 2007;206(1):137–45. [DOI] [PubMed] [Google Scholar]