Abstract
Objective
Since European Society for Medical Oncology (ESMO) recommendations and French guidelines, pelvic lymphadenectomy should not be systematically performed for women with early-stage endometrioid endometrial cancer (EEC) preoperatively assessed at presumed low- or intermediate-risk. The aim of our study was to evaluate the change of our surgical practices after ESMO recommendations, and to evaluate the rate and morbidity of second surgical procedure in case of understaging after the first surgery.
Methods
This retrospective single-center study included women with EEC preoperatively assessed at presumed low- or intermediate-risk who had surgery between 2006 and 2013. Two periods were defined the times before and after ESMO recommendations. Demographics characteristics, surgical management, operative morbidity, and rate of understaging were compared. The rate of second surgical procedure required for lymph node resection during the second period and its morbidity were also studied.
Results
Sixty-one and sixty-two patients were operated for EEC preoperatively assessed at presumed low-or intermediate-risk before and after ESMO recommendations, respectively. Although immediate pelvic lymphadenectomy was performed more frequently during the first period than the second period (88.5% vs. 19.4%; p<0.001), the rate of postoperative risk-elevating or upstaging were comparable between the two periods (31.1% vs. 27.4%; p=0.71). Among the patients requiring second surgical procedure during the second period (21.0%), 30.8% did not undergo the second surgery due to their comorbidity or old age. For the patients who underwent second surgical procedure, mean operative time of the second procedure was 246.1±117.8 minutes. Third operation was required in 33.3% of them because of postoperative complications.
Conclusion
Since ESMO recommendations, second surgical procedure for lymph node resection is often required for women with EEC presumed at low- or intermediate-risk. This reoperation is not always performed due to age/comorbidity of the patients, and presents a significant morbidity.
Keywords: Complications, Endometrial Neoplasms, Lymph Node Excision, Retrospective Studies, Risk Factors
INTRODUCTION
Endometrial cancer is the most common gynecological malignancies in developed countries, and its incidence is rising. Patients are commonly diagnosed at an early stage, when the tumor is confined to the body of uterus, with an overall survival ranging from 85% to 91% [1,2].
Surgical management of endometrial cancer is nonetheless a challenge. Until recently, many hospitals use extensive surgery with pelvic lymph node resection for all women. Two recent multicenter randomized controlled trials found that this procedure provided no benefits in terms of overall or disease-specific and recurrence-free survival in women with early-stage endometrioid endometrial cancer (EEC) [3,4]. It is thus widely accepted now that pelvic lymphadenectomy should not be performed for low-risk disease. Instead, the risks and benefits of each surgical option must be balanced to avoid both over- and under-treatment. Changes in practices including fewer pelvic lymphadenectomies led to the publication of new guidelines in Europe by the European Society for Medical Oncology (ESMO) [5] and by French institutions [6].
Guidelines by the ESMO [5] and by French institutions [6] classify women with early-stage endometrial cancer in three groups: low-risk (The International Federation of Gynecology and Obstetrics [FIGO] [7] stage IA, grade 1 or 2 histology, endometrioid type); intermediate-risk (stage IA and grade 3, endometrioid type, or FIGO IB, grade 1 or 2, endometrioid type); and high-risk (stage IB and grade 3, endometrioid type, or stage IA-B non-endometrioid type, or stage I with lymphovascular invasion). The staging and risk assessment is determined preoperatively on histological analysis (biopsy or curettage) and imaging (ultrasound or magnetic resonance imaging [MRI]). Because recurrence is unlikely in women in the lowand intermediate-risk groups, lymph node resection is not recommended for both of them in French recommendations. In ESMO's guidelines, systematic pelvic lymphadenectomy should not be performed for low-risk EEC and is debated for intermediate-risk EEC. In this case, preoperative staging must be highly accurate, for the lack of lymph node resection may increase the risk of recurrence and necessitate further surgery, while unnecessary lymph node resection may increase the risk of surgical complications. Indeed, if high risk is diagnosed on the surgical specimen, lymph node status should be known to adapt the adjuvant treatment (pelvis radiation therapy or extended aortic field radiation if para-aortic lymph node resection is positive).
Since the publication of new French guidelines in November 2010, our surgical practices have changed, and in parallel, the number of reoperations has increased because of discrepancy between preoperative and final histology results.
The aim of our study is, first, to analyze the evolution of our surgical practices regarding management of early-stage EEC since the new recommendations. We secondly evaluate the issues of this new two-step surgical approach-rate of reoperation, morbidity-in cases of preoperative understaging.
MATERIALS AND METHODS
This retrospective observational before-after study was performed at Lille University Hospital from January 2006 to December 2013. Two periods were defined: 2006 to 2010 (before ESMO recommendations) and 2011 to 2013 (after ESMO recommendations), in order to have a similar number of patients in the two groups.
After approval by a National Ethic Committee (CEROG 2013-GYN-0901), we used computerized medical records to identify all women who underwent surgery for EEC assessed preoperatively at low- or intermediate-risk and conducted a retrospective analysis of the data retrieved from their hospital charts. The study excluded women with type 2 histology, high-risk, or a FIGO stage >1 and those for whom surgery was contraindicated due to severe comorbidities or for whom a conservative treatment was performed in order to preserve fertility.
Preoperative staging was based-according to FIGO classification-on preoperative MRI and histology (either endometrial biopsy or hysteroscopy-curettage). Preoperative and postoperative risk levels (low, intermediate, or high) were attributed a posteriori according to the ESMO recommendations: low-risk (FIGO stage IA, grade 1 or 2 histology, endometrioid type); intermediate-risk (FIGO stage IA and grade 3, endometrioid type; or FIGO IB, grade 1 or 2, endometrioid type); and high-risk (stage IB and grade 3, endometrioid type; or stage IA-B non-endometrioid type; or stage I with lymphovascular invasion). The preoperative low/intermediate-risk assessment was compared with the final risk assessment based on the final pathology diagnosis of the resected uterus as the reference. The preoperative assessment was considered understaged if the postoperative FIGO stage was more than I or the patient was classified as high risk.
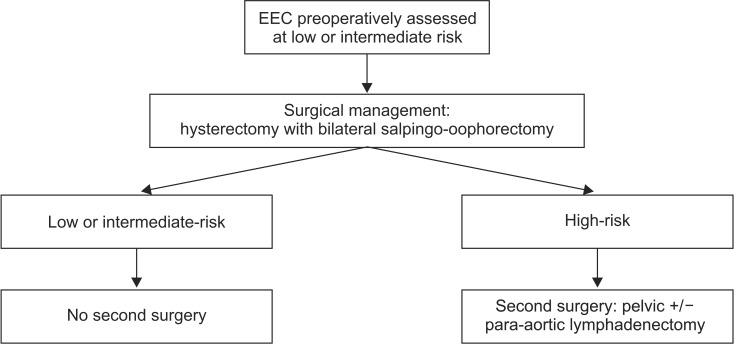
Demographic and histological characteristics of patients were studied to verify that two groups were comparable. The surgical management (incision, rate of pelvic lymphadenectomy, mean operative time), the operative morbidity and mortality (blood loss, rate of perioperative complications), and the rate of preoperative understaging were compared between the two periods. The complications were described using the Dindo classification [8]. In the second time, the rate of reoperation during the second period for lymph node resection-theoretically necessary and really performed-and the morbidity of this reoperation were analyzed. Our surgical management for the second period is summarized in a flow diagram (Fig. 1).
Fig. 1. Flow diagram for patient's management after 2010. EEC, endometrioid endometrial cancer.
Statistical analysis was based on Student t-test and the Mann-Whitney test for parametric and non-parametric continuous variables, respectively, and the chi-square test or the Fisher exact test, as appropriate, for categorical variables. Results are presented as percentages or as mean±SD. Values of p<0.05 were considered to denote significant differences. Data were managed with Graph Pad Software (Graph Pad Software Inc., La Jolla, CA, USA).
RESULTS
1. Demographic and preoperative histological characteristics of overall population
In total, 123 women were consecutively treated for EEC preoperatively assessed at low- or intermediate-risk at our institution between January 1, 2006, and December 31, 2013: 61 were included between 2006 and 2010 and 62 between 2011 and 2013. Demographic characteristics (age at diagnosis, body mass index) were comparable between the two groups. Risk factors for endometrial cancer (diabetes, hormonal therapy after breast cancer) were also present similarly between the two groups, except the postmenopausal hormone replacement therapy which has been most widely prescribed during the first period (14.8% vs. 3.2%, p=0.03). Preoperative tumor characteristics (histological grade, myometrial invasion on pelvic MRI, and presumed risk level) were also comparable (Table 1).
Table 1. Demographic and preoperative histological characteristics of overall population.
| Characteristic | Period 1: 2006-2010 (n=61) | Period 2: 2011-2013 (n=62) | p-value |
|---|---|---|---|
| Age (yr) | 63.4±10.5 | 63.4±9.6 | 0.84 |
| Body mass index (kg/m2) | 32.2±11.4 | 31.5±8.5 | 0.75 |
| Menopause | 55 (90.2) | 55 (88.7) | >0.99 |
| Diabetes | 11 (18.0) | 13 (21.0) | 0.68 |
| Hormone replacement therapy | 9 (14.8) | 2 (3.2) | 0.03 |
| Tamoxifen treatment | 2 (3.3) | 4 (6.5) | 0.68 |
| Histological grade | 0.21 | ||
| 1 | 48 (78.7) | 52 (83.9) | |
| 2 | 10 (16.4) | 10 (16.1) | |
| 3 | 3 (4.9) | 0 | |
| Myometrial invasion on MRI | 0.17 | ||
| <1/2 | 48 (78.7) | 42 (67.7) | |
| ≥1/2 | 13 (21.3) | 20 (32.3) | |
| Presumed risk | 0.46 | ||
| Low | 45 (73.4) | 42 (67.7) | |
| Intermediate | 16 (26.2) | 20 (32.3) |
Values are presented as mean±SD or number (%).
FIGO, International Federation of Gynecology and Obstetrics; MRI, magnetic resonance imaging.
2. Surgical management (first procedure) of overall population
Surgery was performed by laparoscopy equivalently during the two periods (78.7% vs. 80.6%) (Table 2). In addition, we noticed a development of the robot-assisted laparoscopic surgery, which is not significant (8.2% vs. 16.1%, p=0.07).
Table 2. Surgical characteristics of overall population for the first procedure.
| Characteristic | Period 1: 2006-2010 (n=61) | Period 2: 2011-2013 (n=62) | p-value |
|---|---|---|---|
| Surgical approach | 0.070 | ||
| Laparoscopic surgery | 48 (78.7) | 50 (80.6) | |
| Robot-assisted laparoscopic surgery | 5 (8.2) | 10 (16.1) | |
| Laparotomy | 8 (13.1) | 2 (3.2) | |
| Surgical protocol | |||
| Total number of surgical procedures | 1.1±0.2 | 1.2±0.4 | 0.030 |
| Hysterectomy+BSO | 61 (100) | 62 (100) | NS |
| Pelvic lymphadenectomy | 54 (88.5) | 12 (19.4) | <0.001 |
| Para-aortic lymphadenectomy | 3 (4.9) | 1 (8.3) | 0.360 |
| Mean operative time (min) | 237.3±97.2 | 184.8±90.2 | 0.001 |
| Length of hospital stay (day) | 5.6±1.6 | 4.4±2.3 | <0.001 |
| Complications (Dindo classification*) | 20 (32.8) | 6 (9.7) | <0.001 |
| I | 6 (30.0) | 5 (83.3) | |
| II | 3 (15.0) | 0 | |
| IIIa | 4 (20.0) | 1 (16.7) | |
| IIIb | 7 (35.0) | 0 | |
| Mean blood loss (mL) | 500±650 | 190±170 | 0.003 |
| Transfusion | 3 (4.9) | 0 | 0.120 |
Values are presented as number (%) or mean±SD.
BSO, bilateral salpingo-oophorectomy; NS, not significant.
*Dindo classification: classification of surgical complications: Grade I, any deviation from the normal postoperative course without the need for pharmacological treatment or surgical, endoscopic and radiological interventions (allowed therapeutic regimens are: drugs as antiemetics, antipyretics, analgesics, diuretics and electrolytes and physiotherapy. This grade also includes wound infection opened at the bedside); Grade II, requiring pharmacological treatment with drugs other than such allowed for grade 1 complications. Blood transfusions and total parenteral nutrition are also included; Grade III, requiring surgical, endoscopic or radiological intervention (IIIa, intervention not under general anesthesia; IIIb, intervention under general anesthesia); Grade IV, life-threatening complication; Grade V, death of a patient.
Pelvic lymphadenectomy at the first surgery was performed significantly less frequently in the second period than the first period (19.4% vs. 88.5%, p < 0.001). Mean operative time has also decreased (237.3±97.2 and 184.8±90.2 minutes, p=0.001), together with the length of hospital stay (5.6±1.6 days vs. 4.4±2.3 days, p<0.001).
We noticed that the rate of complications decreased during the second period (32.8% vs. 9.7%, p<0.001), in particular for severe complications (complications Dindo IIIb: 35.0% vs. 0%, p<0.001).
3. Final histology and rate of understaging of overall population
For the first period, of 61 women initially considered at low- or intermediate-risk, 19 patients were upstaged to high-risk or FIGO >I. Thus, the rate of discrepancies between preoperative risk group and final histology diagnosis was 31.1% (20% and 62.5% for the presumed low- and intermediate-risk, respectively) (Table 3).
Table 3. Final pathology.
| Variable | Period 1: 2006-2010 (n=61) | Period 2: 2011-2013 (n=62) | p-value |
|---|---|---|---|
| Histology | 0.37 | ||
| Endometrioid adenocarcinoma | 50 (82.0) | 57 (91.9) | |
| Mixed adenocarcinoma | 4 (6.6) | 1 (1.6) | |
| Type 2 | 4 (6.6) | 2 (3.2) | |
| No residual tumour | 3 (4.9) | 2 (3.2) | |
| Histological grade | 0.22 | ||
| 1 | 40/54 (74.1) | 44/58 (75.9) | |
| 2 | 11/54 (20.4) | 13/58 (22.4) | |
| 3 | 3/54 (5.5) | 1/58 (1.7) | |
| Lymphovascular space invasion | 16 (26.2) | 12 (19.4) | 0.36 |
| Positive pelvic nodes | 5/54 (9.3) | 2/12 (16.7) | 0.60 |
| Risk for recurrence | 0.71 | ||
| Low-risk | 32 (52.5) | 37 (59.7) | |
| Intermediate-risk | 10 (16.4) | 8 (12.9) | |
| High-risk or FIGO stage >I* | 19 (31.1) | 17 (27.4) |
Values are presented as number (%).
*It indicates understaging.
FIGO, International Federation of Gynecology and Obstetrics.
For the second period, of 62 women initially considered at low- or intermediate-risk, 17 patients were upstaged to high-risk or FIGO >I. Thus, the rate of discrepancies between preoperative risk group and final histology diagnosis was 27.4% (19% and 45% for the presumed low- and intermediate-risk respectively). The histological discrepancies between preoperative risk group and final histology for understaged women are described in the Table 4.
Table 4. Histological characteristics of the "understaged" patients of the second period.
| Patient no. | Preoperative risk | Surgery | Postoperative risk | Final histology | Tumor size (mm) | FIGO stage | Lymphovascular invasion | Reintervention |
|---|---|---|---|---|---|---|---|---|
| 1 | Low | HT+PL | High | Endometrioid | 50 | II | No | NI |
| 2 | Low | HT+PL | High | Endometrioid | 45 | IIIC | No | NI |
| 3 | Low | HT+PL | High | Mucinous | 73 | IIIC | No | NI |
| 4 | Intermediate | HT+PL | High | Carcinosarcoma | 63 | IA | No | NI |
| 5 | Low | HT | High | Mixed adenocarcinoma | 35 | IA | Yes | Yes |
| 6 | Low | HT | High | Endometrioid | 20 | IB | Yes | Yes |
| 7 | Low | HT | High | Endometrioid | 30 | IB | Yes | Yes |
| 8 | Low | HT | High | Endometrioid | 35 | IB | Yes | Yes |
| 9 | Low | HT | High | Endometrioid | 30 | IA | Yes | Yes |
| 10 | Low | HT | High | Endometrioid | NA | IA | Yes | Yes |
| 11 | Low | HT | High | Endometrioid | 70 | II | Yes | Yes |
| 12 | Low | HT | High | Endometrioid | 65 | IIIB | Yes | No |
| 13 | Low | HT | High | Endometrioid | 27 | IB | Yes | No |
| 14 | Intermediate | HT | High | Clear cell adenocarcinoma | 43 | IA | No | Yes |
| 15 | Intermediate | HT | High | Endometrioid | 20 | IA | Yes | Yes |
| 16 | Intermediate | HT | High | Endometrioid | 35 | IA | Yes | No |
| 17 | intermediate | HT | High | Endometrioid | 40 | IIIB | Yes | No |
FIGO, International Federation of Gynecology and Obstetrics; HT, hysterectomy; NA, not available; NI, non indicated; PL, pelvic lymphadenectomy.
The understaging rate was comparable between the two periods (31.1% vs. 27.4%, p=0.71).
4. Surgical management of patients whose risk was understaged (2011 to 2013)
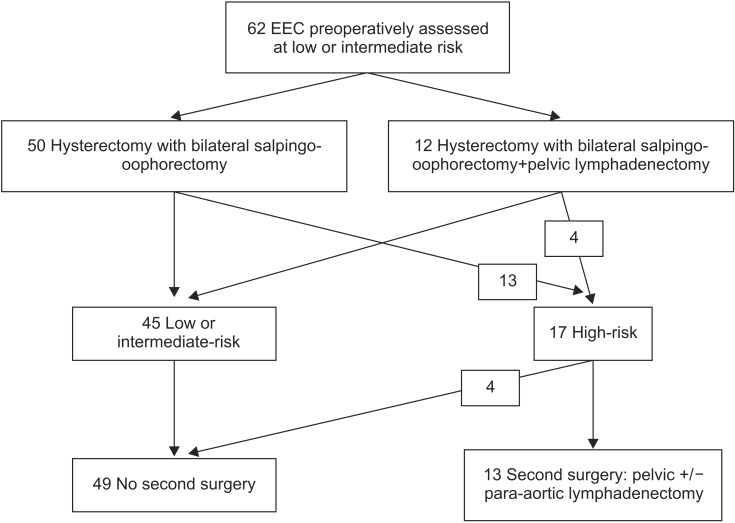
Among 17 patients whose risk was underestimated preoperatively, four had received pelvic lymphadenectomy concomitant with total hysterectomy (one patient had pelvic MRI which evoke a carcinosarcoma-confirmed on final histology-and one patient had a stage IB grade 2 disease) (Table 4, Fig. 2). Thus, 13 patients (21.0%) required second surgical procedure for lymph node staging. Among them, four patients (30.8%) didn't undergo this surgery unlike guidelines, due to age and/or comorbidities (stroke with hemiplegia and severe cardiac history).
Fig. 2. Flow chart of patients with endometrioid endometrial cancer (EEC) of the period 2 (2011 to 2013).
Nine patients (69.2%) underwent a second-line surgery with para-aortic lymphadenectomy (+/- pelvic lymphadenectomy, omentectomy, appendectomy if type 2 histology) according to ESMO recommendations. The surgical approach was laparoscopy in seven cases, robot-assisted laparoscopy in one case and laparotomy in one case. The average para-aortic lymph node yield was 11.5±7.2 (range, 0 to 24). In one patient, the para-aortic lymphadenectomy wasn't feasible due to obesity and peritoneal adhesion on the operative site. The mean operative time of this second procedure was 246.1±117.8 minutes. The length of hospital stay was 4.4±2.7 days. 33.3% of these patients had a postoperative complication requiring a third operation (grade IIIb of Dindo classification). One patient had a hematoma of the rectus abdominis muscle secondary infected and drained surgically, one patient a lymphocele causing ureteral compression requiring ureteral catheters, and one patient had an infected retroperitoneal hematoma with mass effect on the urinary tract surgically drained. Pathology was positive for two patients: one patient had para-aortic lymph node metastasis, and one had omentum metastasis. Consequently, these two patients underwent a more aggressive postoperative treatment (radiation therapy with an extended aortic field of radiation).
DISCUSSION
Our study allowed to highlight the changes in surgical practices since the new recommendations. These guidelines are followed in our center, as pelvic lymphadenectomy rate has significantly decreased after 2010 (88.5% vs. 19.4%, p<0.0001). The majority of the patients were operated by laparoscopic surgery and the rate of robot-assisted laparoscopic surgery has doubled during the second period. We noticed that perioperative morbidity and mean blood loss decreased after the recommendations for overall population. We also found a longer operative time and a longer length of stay during the first period. These differences can be explained, firstly by the learning curve of the laparoscopic approach (longer operative time) and secondly, by the morbidity of the pelvic lymph node resection (increased risk of bleeding, postoperative lymphedema).
Since 15 years, laparoscopy has become the gold standard surgical approach for the management of patients with presumed early-stage endometrial cancer [9]. Several randomized studies [10,11] and a Cochrane Review [9] published in 2012 have compared these two surgical approaches, without bringing out any differences in term of intraoperative morbidity or disease-free survival, but a decrease of mean blood loss, postoperative complications and mean length of stay. For all these reasons, minimally invasive surgical techniques have become established as standard therapy for treating women with early-stage EEC, as notified in the different guidelines [5,6]. However, the presence-as other surgeries-of a learning curve can explain longer operative times, more blood loss, or even a higher rate of intraoperative complications [12,13]. So we cannot ignore in our series the increasing experience of surgeons to explain the improvement observed between the two periods. Indeed, the generalization of the laparoscopic approach for the surgical treatment of endometrial cancer occurred in our center during the 2000s, with a rate of laparoscopy only about 53.8% between 2000 and 2005.
Moreover, pelvic lymphadenectomy has its own morbidity, already described in literature [3,4,14]. Although systematic pelvic lymphadenectomy statistically significantly improved surgical staging, it did not improve disease-free or overall survival for the confirmed early-stage EEC at low-risk. Postoperative complications (essentially lymphedema and deep venous thrombosis) occurred statistically significantly more frequently in patients who had received pelvic systematic lymphadenectomy, as it has been demonstrated in the two large randomized trials on the subject [3,4]. Dowdy et al. [14] and Mariani et al. [15] have also demonstrated that lymphadenectomy increases morbidity and cost of care without discernible benefits in this low-risk EEC. According to these data on morbidity, the sentinel lymph node procedure has emerged as a possible alternative to systematic pelvic lymphadenectomy for some authors [16,17,18]. In fact, the advantage of this method is to perform a less invasive surgical procedure, while benefiting from the nodal staging to choose adjuvant therapy or other surgical management. Since ASTEC trial [4], the debate was revived concerning the benefit of lymphadenectomy in case of intermediate-risk EEC. For example, the SEPAL study [19] showed that complete lymphadenectomy improves the overall survival in intermediate and high risk EEC. Therapeutic management of the intermediate-risk group remains a hot topic.
Indeed, we should balance the morbidity of pelvic lymphadenectomy with the necessity of reoperation in case of understaging preoperative risk. A second surgery for a complete node staging is not without its own complications [20] and it can increase the cost of medical care. A cost-effectiveness analysis should be performed in the second time to evaluate this point. In our study, this second surgical procedure had a mean operative time around 4 hours and a high rate of morbidity. Indeed 33.3% of patients had a third surgery for serious postoperative complication. Besides, surgical staging has not been fully realized for one patient due to obesity and peritoneal adhesion due to the first operation.
Moreover, in our study, 30.8% of patients didn't undergo the second staging due to their age or comorbidities. In fact, women with endometrial cancer, who are often old and overweight and have frequent comorbidities (hypertension and/or diabetes) [21,22] cannot always benefit from the second procedure due to anesthetic or surgical contraindications. In these cases, they didn't undergo the optimal treatment.
In summary, there are two issues raised by these new recommendations for women with presumed early-stage disease. Indeed, the therapeutic de-escalation proposed for these women exposes them to: (1) a higher rate of morbidity in case of reintervention; and (2) a risk of recurrence due to inadequate initial staging if the second surgical procedure is not performed.
Our study showed an important-and stable over time-understaging rate for women whose preoperative risk was assessed as low or intermediate. More than one quarter of the patients were finally upstaged as high risk or FIGO >I on final pathology. Moreover, the understaging rate during the second period is probably slightly underestimated because few pelvic lymphadenectomy were performed (2% of the patients during the first period were upstaged only due to lymph node metastasis). This rate, although important, is similar to those in other studies [23,24,25]. Preoperative histology samples often differ from the final pathology finding. In a study of 291 endometrial cancer patients, Goudge et al. [23] found that 18% of tumors were upstaged. Similarly, Ben-Shachar et al. [24] reported that the final pathology finding led to upstaging for 19% of 181 patients with a preoperative grade 1 tumor.
Nevertheless, since the new guidelines the preoperative risk staging has become the cornerstone of optimal management of EEC. The risk assessment is actually based on a simple histological analysis (either endometrial biopsy or hysteroscopy-curettage) associated with MRI. But several trials are developing news preoperative independent markers of lymph-node metastasis and poor prognosis of the disease, such as the dosage of serum human epididymis secretory protein E4 (HE4) and CA-125 [26]. Other studies support that status for estrogen receptor, progesterone receptor and the tumor suppressor p53 in primary EEC are independent prognostic markers [27,28]. These biomarkers analyze can be performed on curettage samples [28]. These new perspectives concerning the evaluation of the extra-uterine dissemination risk and the prognosis would be one way to better define patients requiring surgical lymph node staging. This would overcome the consequences of histological and/or radiological preoperative understaging which occur frequently. Other trials are developing intra-operative markers of lymph node metastasis, such as sentinel lymph node procedure [16,17,18], and the "Mayo criteria" [15,29,30] or other risk scoring system [31].
Our study has some limits, mainly related to its retrospective nature, and the short follow-up for the patients of the second period. Indeed, it was not possible to compare the recurrence-free survival between the two periods because they were not comparable in terms of postoperative follow-up. Consequently, we can't assess the impact of the new guidelines on disease-free survival for EEC presumed at low- or intermediate-risk. The consequences of these new guidelines on the perioperative morbidity were difficult to establish too, due to a concomitant evolution of our practices (learning curve of the laparoscopic surgery). However it's important to note that few publications evaluating the new recommendations in clinical practice are available in the literature. Moreover, the quality of the methodology and the large effective are two strengths of our work.
In conclusion, our study highlights two major difficulties related to the clinical application of these new guidelines. First, we found an important preoperative understaging rate, which implies in theory that many patients will undergo second procedure. Then, these second-line surgeries are not always performed due to the significant comorbidity of this kind of patients. They also have a perioperative morbidity which must be balanced to the expected benefits of the absence of systematic pelvic lymphadenectomy. Prospective studies with sufficient follow-up are necessary to assert the absence of impact of these new recommendations on recurrence rate or disease-free survival. Waiting for these new data, it seems reasonable to perform immediately pelvic lymphadenectomy for presumed intermediate-risk EEC to avoid the risk of undertreatment. New preoperative or intraoperative technics need to be developed to improve the evaluation of risk of lymph node dissemination (biomarkers, sentinel lymph node biopsy).
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S105–S143. doi: 10.1016/S0020-7292(06)60031-3. [DOI] [PubMed] [Google Scholar]
- 3.Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 4.ASTEC study group. Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125–136. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C, et al. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(Suppl 6):vi35–vi39. doi: 10.1093/annonc/mdr374. [DOI] [PubMed] [Google Scholar]
- 6.L'Institut National du Cancer (INCa) Cancer de l'endomètre. Collection Recommandations & référentiels. Boulogne-Billancourt: INCa; 2010. [Google Scholar]
- 7.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galaal K, Bryant A, Fisher AD, Al-Khaduri M, Kew F, Lopes AD. Laparoscopy versus laparotomy for the management of early stage endometrial cancer. Cochrane Database Syst Rev. 2012;9:CD006655. doi: 10.1002/14651858.CD006655.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Lu Q, Liu H, Liu C, Wang S, Li S, Guo S, et al. Comparison of laparoscopy and laparotomy for management of endometrial carcinoma: a prospective randomized study with 11-year experience. J Cancer Res Clin Oncol. 2013;139:1853–1859. doi: 10.1007/s00432-013-1504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tinelli R, Litta P, Meir Y, Surico D, Leo L, Fusco A, et al. Advantages of laparoscopy versus laparotomy in extremely obese women (BMI>35) with early-stage endometrial cancer: a multicenter study. Anticancer Res. 2014;34:2497–2502. [PubMed] [Google Scholar]
- 12.Eltabbakh GH. Effect of surgeon\'s experience on the surgical outcome of laparoscopic surgery for women with endometrial cancer. Gynecol Oncol. 2000;78:58–61. doi: 10.1006/gyno.2000.5828. [DOI] [PubMed] [Google Scholar]
- 13.Holub Z, Jabor A, Bartos P, Hendl J, Urbanek S. Laparoscopic surgery in women with endometrial cancer: the learning curve. Eur J Obstet Gynecol Reprod Biol. 2003;107:195–200. doi: 10.1016/s0301-2115(02)00373-1. [DOI] [PubMed] [Google Scholar]
- 14.Dowdy SC, Borah BJ, Bakkum-Gamez JN, Weaver AL, McGree ME, Haas LR, et al. Prospective assessment of survival, morbidity, and cost associated with lymphadenectomy in low-risk endometrial cancer. Gynecol Oncol. 2012;127:5–10. doi: 10.1016/j.ygyno.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 15.Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-risk corpus cancer: is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000;182:1506–1519. doi: 10.1067/mob.2000.107335. [DOI] [PubMed] [Google Scholar]
- 16.Ballester M, Dubernard G, Bats AS, Heitz D, Mathevet P, Marret H, et al. Comparison of diagnostic accuracy of frozen section with imprint cytology for intraoperative examination of sentinel lymph node in early-stage endometrial cancer: results of Senti-Endo study. Ann Surg Oncol. 2012;19:3515–3521. doi: 10.1245/s10434-012-2390-7. [DOI] [PubMed] [Google Scholar]
- 17.Fersis N, Gruber I, Relakis K, Friedrich M, Becker S, Wallwiener D, et al. Sentinel node identification and intraoperative lymphatic mapping: first results of a pilot study in patients with endometrial cancer. Eur J Gynaecol Oncol. 2004;25:339–342. [PubMed] [Google Scholar]
- 18.Raimond E, Ballester M, Hudry D, Bendifallah S, Darai E, Graesslin O, et al. Impact of sentinel lymph node biopsy on the therapeutic management of early-stage endometrial cancer: results of a retrospective multicenter study. Gynecol Oncol. 2014;133:506–511. doi: 10.1016/j.ygyno.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 2010;375:1165–1172. doi: 10.1016/S0140-6736(09)62002-X. [DOI] [PubMed] [Google Scholar]
- 20.Chan JK, Kapp DS, Cheung MK, Shin JY, Stieglitz D, Husain A, et al. Prognostic factors and risk of extrauterine metastases in 3867 women with grade 1 endometrioid corpus cancer. Am J Obstet Gynecol. 2008;198:216.e1–216.e5. doi: 10.1016/j.ajog.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 21.Benedetti Panici P, Basile S, Salerno MG, Di Donato V, Marchetti C, Perniola G, et al. Secondary analyses from a randomized clinical trial: age as the key prognostic factor in endometrial carcinoma. Am J Obstet Gynecol. 2014;210:363.e1–363.e10. doi: 10.1016/j.ajog.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 22.Fader AN, Arriba LN, Frasure HE, von Gruenigen VE. Endometrial cancer and obesity: epidemiology, biomarkers, prevention and survivorship. Gynecol Oncol. 2009;114:121–127. doi: 10.1016/j.ygyno.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 23.Goudge C, Bernhard S, Cloven NG, Morris P. The impact of complete surgical staging on adjuvant treatment decisions in endometrial cancer. Gynecol Oncol. 2004;93:536–539. doi: 10.1016/j.ygyno.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Shachar I, Pavelka J, Cohn DE, Copeland LJ, Ramirez N, Manolitsas T, et al. Surgical staging for patients presenting with grade 1 endometrial carcinoma. Obstet Gynecol. 2005;105:487–493. doi: 10.1097/01.AOG.0000149151.74863.c4. [DOI] [PubMed] [Google Scholar]
- 25.Frederick PJ, Straughn JM., Jr The role of comprehensive surgical staging in patients with endometrial cancer. Cancer Control. 2009;16:23–29. doi: 10.1177/107327480901600104. [DOI] [PubMed] [Google Scholar]
- 26.Angioli R, Plotti F, Capriglione S, Montera R, Damiani P, Ricciardi R, et al. The role of novel biomarker HE4 in endometrial cancer: a case control prospective study. Tumour Biol. 2013;34:571–576. doi: 10.1007/s13277-012-0583-0. [DOI] [PubMed] [Google Scholar]
- 27.Salvesen HB, Haldorsen IS, Trovik J. Markers for individualised therapy in endometrial carcinoma. Lancet Oncol. 2012;13:e353–e361. doi: 10.1016/S1470-2045(12)70213-9. [DOI] [PubMed] [Google Scholar]
- 28.Trovik J, Wik E, Werner HM, Krakstad C, Helland H, Vandenput I, et al. Hormone receptor loss in endometrial carcinoma curettage predicts lymph node metastasis and poor outcome in prospective multicentre trial. Eur J Cancer. 2013;49:3431–3441. doi: 10.1016/j.ejca.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 29.Sala P, Morotti M, Menada MV, Cannavino E, Maffeo I, Abete L, et al. Intraoperative frozen section risk assessment accurately tailors the surgical staging in patients affected by early-stage endometrial cancer: the application of 2 different risk algorithms. Int J Gynecol Cancer. 2014;24:1021–1026. doi: 10.1097/IGC.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 30.Hahn HS, Song HS, Lee IH, Kim TJ, Lee KH, Shim JU, et al. Magnetic resonance imaging and intraoperative frozen sectioning for the evaluation of risk factors associated with lymph node metastasis in endometrial cancer. Int J Gynecol Cancer. 2013;23:1411–1416. doi: 10.1097/IGC.0b013e3182a580d3. [DOI] [PubMed] [Google Scholar]
- 31.Bendifallah S, Canlorbe G, Huguet F, Coutant C, Hudry D, Graesslin O, et al. A risk scoring system to determine recurrence in early-stage type 1 endometrial cancer: a French multicentre study. Ann Surg Oncol. 2014;21:4239–4245. doi: 10.1245/s10434-014-3864-6. [DOI] [PubMed] [Google Scholar]