Abstract
Background
Sorafenib is the only FDA-approved systemic therapy for advanced hepatocellular carcinoma (HCC). In clinical practice, dose reductions are often required, although there are limited efficacy data related to dose modifications. Given the prevalence of HCC in South Texas, we assessed the efficacy and safety of sorafenib therapy in relation to dose and Child Pugh (CP) score.
Methods
A retrospective analysis was done of advanced HCC patients, starting sorafenib at 400 mg twice daily, or at physician discretion at 400 mg daily, with the goal of titrating to twice daily. Overall survival (OS) and progression-free survival (PFS) were assessed.
Results
Among 107 patients, median OS (mOS) was 10.2 months; median PFS (mPFS) was 5.2 months. mOS for sorafenib 400 mg/day was 6.6 vs. 800 mg/day was 12.8 months [hazard ratio (HR), 0.59; P=0.04]; mPFS was 3.5 vs. 5.9 months, respectively (HR, 0.66; P=0.07). For Child Pugh A class (CP-A) patients, mOS was 15.8 months for 400 mg/day vs. 12.8 months for 800 mg/day (HR, 1.48; P=0.35); mPFS was 9.0 vs. 5.9 months, respectively (HR, 1.23; P=0.56). For Child Pugh B class (CP-B) patients, mOS was 5.0 months for 400 mg/day vs. 11.2 months for 800 mg/day (HR, 0.33; P=0.002); mPFS was 2.1 vs. 5.6 months, respectively (HR, 0.41; P=0.006). No differences in adverse events (AEs) were observed in CP-A vs. CP-B.
Conclusions
Patients with CP-A or CP-B advanced HCC should be offered sorafenib at 400 mg twice daily with optimal management of AEs in order to improve survival.
Keywords: Liver cancer, hepatobiliary, Child Pugh (CP) cirrhosis, metastatic
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer in men and seventh most common in women worldwide, but the third most common cause of cancer-related mortality. At the present, limited treatment options are available for this challenging disease. To date, sorafenib, a multi-kinase inhibitor with anti-proliferative, anti-angiogenic, and pro-apoptotic properties is the first and only systemic therapy FDA-approved for treating advanced HCC. The approval was based on improvement in median overall survival (mOS) from 7.9 months with placebo to 10.7 months with sorafenib 400 mg twice daily (1). The median time to radiologic progression was 5.5 months with sorafenib vs. 2.8 months with placebo (1). In the Asia-Pacific study, patients with advanced HCC who received sorafenib had a mOS of 6.5 vs. 4.2 months with placebo (2). The median time to progression (TTP) also improved from 1.4 months with placebo to 2.8 months with sorafenib (2).
The majority of patients in these studies had an ECOG performance status of 0-1 with Child Pugh A class (CP-A) cirrhosis (95-97%) (1,2). However, in the United States (US), a higher percentage of patients with HCC present with Child Pugh B class (CP-B) cirrhosis (3). Despite the observation that less than 5% of patients treated with sorafenib in the clinical trials were CP-B (1), sorafenib has become the first-line of therapy in patients with CP-B, with the recommended daily dose of 800 mg.
In clinical practice dosing of sorafenib varies from the recommended daily dose, which is often dictated by baseline CP status, performance status, co-morbidities and/or physician preference. A global, prospective, non-interventional study undertaken to evaluate the safety of sorafenib in patients with unresectable HCC in real-life practice, the GIDEON study, reported a variation in patient and disease characteristics and treatment patterns for US patients compared to rest of the world (4,5). In the US, like Latin America, a proportionally higher percentage of CP-B patients receive sorafenib than in European and Asian regions (4). The mOS was higher for CP-A than CP-B, with a mOS of 13.6 vs. 5.2 months, respectively, but with similar median TTP, 4.7 vs. 4.4 months, respectively (6). Although a similar proportion of patients received 800 mg initial dose, the median daily dose was 721 mg in CP-B vs. 680 mg in CP-A (5). Despite the higher average dose received in CP-B patients, their OS was worse than CP-A patients, possibly due to shorter median duration of sorafenib in patients with CP-B (8.6 weeks) vs. CP-A (13.7 weeks) (5). Nonetheless, studies have not yet reported data on efficacy of sorafenib in relation to dosing in advanced HCC.
The variation in clinical characteristics and treatment patterns for US patients compared to the rest of the world may be related to differences in demographic factors. In the United States, Latinos have higher rates of HCC than the general population (7), and more importantly, the incidence of HCC among Latinos in South Texas is significantly higher than other regions of the country (8). Given the relatively large number of HCC cases in South Texas, we assessed the clinical efficacy of sorafenib therapy in relation to dose, CP score and safety in our advanced HCC patient population.
Materials and methods
Patients
We conducted a retrospective cohort analysis of patients diagnosed with advanced HCC according to the American Association for the Study of Liver Diseases practice guidelines who received sorafenib at our tertiary referral center in South Texas from 2008-2013 (9). From our database management system, a total of 107 patients with advanced HCC were identified as unresectable, either with metastatic disease and/or residual disease after failure of all local therapies. Once started on sorafenib, if patients continued to have disease stability or liver-only progression, patients may be permitted to receive additional local therapies, such as radiofrequency ablation (RFA), transarterial chemoembolization (TACE), radiation therapy, and radioembolization with Yttrium-90 based on multi-disciplinary tumor board recommendation. All patients had baseline laboratory values, including complete blood cell counts, liver function test, coagulation studies and serum alpha-fetoprotein. All patients had a baseline imaging with either triple-phase CT or MRI of the liver. Liver reserve was calculated based on CP score (10). CLIP score and Barcelona Clinic Liver Cancer (BCLC) staging classification were documented for each patient at first evaluation by the oncologist (11,12). Patients were assessed clinically at four-week intervals with history, physical exam and laboratory evaluation. Blood pressures were obtained every visit. Dose reductions were documented during visits.
Treatment
Treatment with sorafenib was started at a dose of 800 mg orally (400 mg twice daily). At the discretion of the oncologist, if frail, elderly, or with signs of liver dysfunction, some patients were started at 400 mg daily, with the goal of titrating up to 800 mg/day. Treatment was continued until disease progression, unacceptable toxicity, lost to follow-up or withdrawal of patient consent.
Efficacy
Clinical response and radiological responses were documented by providers, with imaging usually done at 12-week intervals, unless indicated sooner. Physicians relied upon radiologist’s reading of the CT or MRI compared to baseline.
Toxicity
Adverse events (AEs) were graded according to the Common Terminology Criteria for Adverse Events version 4.0. Toxicities at 1, 3, and 6 months of treatment were reviewed and documented.
Statistical analysis
The association between demographic, clinical and laboratory variables with OS and progression-free survival (PFS) were assessed by comparison of Kaplan-Meier curves. Groups were statistically compared by the log rank test and the magnitude of association with outcome was summarized as the hazards ratio (HR) with 95% confidence interval (CI). The SAS statistical package (version 9.2, SAS Institute, Cary, USA) was used for all statistical analyses.
Ethics
This study was based on our patient database retrospectively. Patient records and information were de-identified and anonymized prior to analysis. Therefore, IRB approval and patient consent were not required.
Results
Patient characteristics
From 2008-2013, 107 patients were evaluated at the Cancer Therapy and Research Center (CTRC), University of Texas Health Science Center at San Antonio. The median age was 57 (range, 41-93) years. These patients were 83% male, and 72% Hispanic, 24% White, 1.9% Asian, and 1.9% Black. ECOG performance status was as follows: zero—32 (29.9%), one—67 (62.6%), two—7 (6.5%). Causes for cirrhosis included: hepatitis C—71 (66.4%), hepatitis B—5 (4.7%), alcohol—63 (58.9%), fatty liver—3 (2.8%), other—20 (18.7%). Thirty-four (31.8%) patients had portal vein thrombosis, 42 (39.3%) had extrahepatic disease, and 16 (15.0%) had ascites. Prior to sorafenib, patients received local treatments for HCC: TACE—37 (34.6%), RFA—16 (15.0%), radiation—15 (14.0%), Yttrium-90—13 (12.1%). After the initiation of sorafenib, and at least stable disease was maintained in patients without extrahepatic disease, 45 (42.1%) patients received concurrent local treatments with one or more of the following: TACE—21 (19.6%), RFA—9 (8.4%), radiation—15 (14.0%), Yttrium-90—13 (12.1%).
Sixty (56.6%) patients had CP-A cirrhosis, 46 (43.4%) had CP-B cirrhosis, and one had a missing CP score. When available, 70 (65.4%) patients had a CLIP score of 0-2, and 35 (32.7%) had a CLIP score of 3+. Based on the BCLC staging classification, 13 (12.1%) patients were class B and 94 (87.9%) patients were class C. Median duration of treatment with sorafenib was 3.9 months (mean 6.8; range, 0.7-46.2 months) for all patients.
Overall, 102 patients had dosing data available for review, of which 33 (32.4%) patients were started at sorafenib 400 mg/day and 69 (67.6%) at 800 mg/day. Of the 97 patients with dose reduction information available, 66 (68.0%) patients required dose reductions. Among CP-A patients, 12 (21.8%) received sorafenib 400 mg/day vs. 43 (78.2%) received 800 mg/day. Among CP-B patients, 21 (45.7%) received sorafenib 400 mg/day vs. 25 (54.3%) received 800 mg/day. Patient characteristics based on CP score are in Table 1.
Table 1. Patient characteristics in advanced HCC.
| Characteristics | Child Pugh class |
All subjects | P value | |
|---|---|---|---|---|
| A | B | |||
| Age (years) | <0.001 | |||
| N | 60 | 46 | 107 | |
| Mean (SD) | 61.5 (9.9) | 55.8 (8.2) | 59.2 (9.6) | |
| Median | 60 | 54 | 57 | |
| Min, max | 43, 93 | 41, 75 | 41, 93 | |
| Duration (months) | 0.05 | |||
| N | 60 | 46 | 107 | |
| Mean (SD) | 7.3 (6.5) | 5.4 (6.6) | 6.8 (7.6) | |
| Median | 4.8 | 3.3 | 3.9 | |
| Min, max | 0.7, 26.9 | 0.7, 40.7 | 0.7, 46.2 | |
| Dose/day, n (%) | 0.02 | |||
| N | 55 | 46 | 101 | |
| 400 mg | 12 (21.8) | 21 (45.7) | 33 (32.7) | |
| 800 mg | 43 (78.2) | 25 (54.3) | 68 (67.3) | |
P value for association using Fisher’s exact test. HCC, hepatocellular carcinoma.
Treatment outcome
Among 107 patients, the mOS was 10.2 months (95% CI: 7.8-13.5) (Figure 1A). The mPFS was 5.2 months (95% CI: 3.8-7.2) (Figure 1B). Of the 106 patients with CP score available, CP-A patients (n=60) had mOS of 13.1 months (95% CI: 8.1-15.9), compared to CP-B patients (n=46) with mOS of 6.6 months (95% CI: 4.1-10.8) (HR, 1.57; 95% CI: 1.0-2.47; P=0.05) (Figure 2A). CP-A patients had mPFS of 7.1 months (95% CI: 4.6-9.0), compared to CP-B patients with mPFS of 3.6 months (95% CI: 2.8-5.2) (HR, 1.61; 95% CI: 1.06-2.44; P=0.03) (Figure 2B).
Figure 1.
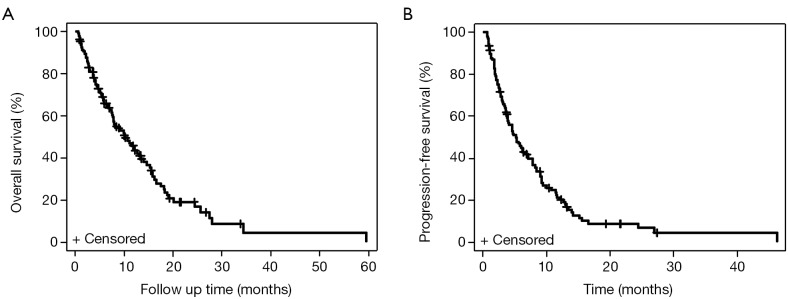
Kaplan-Meier curves for survival for all patients with HCC treated with sorafenib. (A) mOS for all HCC patients; (B) mPFS for all HCC patients. HCC, hepatocellular carcinoma; mOS, median overall survival; mPFS, median progression-free survival.
Figure 2.
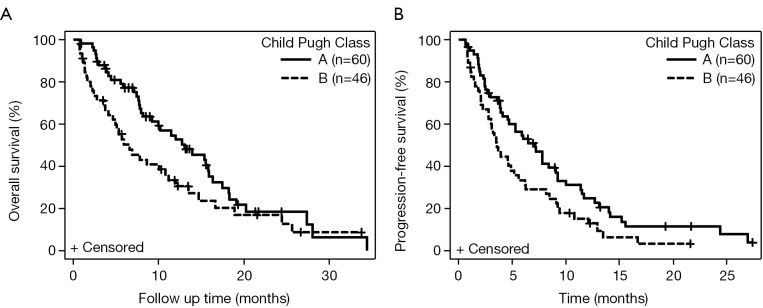
Kaplan-Meier curves for survival for patients with HCC based on Child-Pugh score. (A) mOS for CP-A versus CP-B cirrhosis; (B) mPFS for CP-A versus CP-B cirrhosis. HCC, hepatocellular carcinoma; mOS, median overall survival; mPFS, median progression-free survival.
Dose
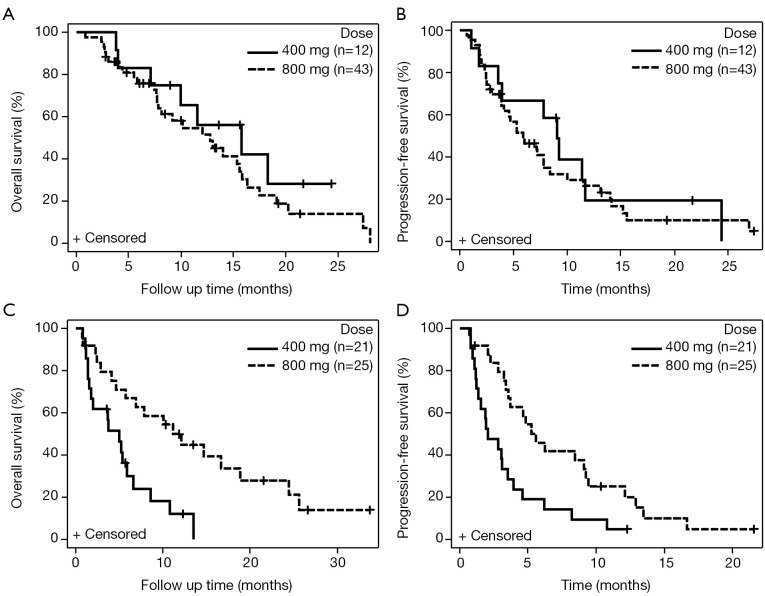
Among 102 patients with dosing data available, mOS for patients starting sorafenib at a total of 400 mg/day (n=33) was 6.6 months (95% CI: 3.8-10.8) vs. those starting at 800 mg/day (n=69) was 12.8 months (95% CI: 7.8-15.9) (HR, 0.59; 95% CI: 0.36-0.97, P=0.04). The mPFS was 3.5 months for 400 mg/day (95% CI: 1.8-6.2) vs. 5.9 months (95% CI: 4.6-8.4) for 800 mg/day (HR, 0.66; 95% CI: 0.42-1.03; P=0.07). Among patients with CP-A cirrhosis, patients receiving 400 mg/day (n=12) had mOS of 15.8 vs. 12.8 months for 800 mg/day (n=43) CP-A patients (HR, 1.48; 95% CI: 0.65-3.39; P=0.35) (Figure 3A). The mPFS was 9.0 months for 400 mg/day vs. 5.9 months for 800 mg/day CP-A patients (HR, 1.23; 95% CI: 0.61-2.51; P=0.56) (Figure 3B). Among patients with CP-B, mOS was 5.0 months for 400 mg/day (n=21) vs. 11.2 months for 800 mg/day (n=25) (HR, 0.33; 95% CI: 0.16-0.70; P=0.002) (Figure 3C). The mPFS was 2.1 months for 400 mg/day vs. 5.6 months for 800 mg/day CP-B patients (HR, 0.41; 95% CI: 0.22-0.79; P=0.006) (Figure 3D).
Figure 3.
Kaplan-Meier curves for survival based on sorafenib dose in HCC patients with CP-A and CP-B cirrhosis. (A) mOS for HCC patients with CP-A cirrhosis given 400 vs. 800 mg sorafenib/day; (B) mPFS for HCC patients with CP-A cirrhosis given 400 vs. 800 mg sorafenib/day; (C) mOS for HCC patients with CP-B cirrhosis given 400 vs. 800 mg sorafenib/day; (D) mPFS for HCC patients with CP-B cirrhosis given 400 vs. 800 mg sorafenib/day. HCC, hepatocellular carcinoma; mOS, median overall survival; mPFS, median progression-free survival.
CLIP score
Among CP-A patients, the mOS based on CLIP score of 0-2 (n=51) was 14.0 vs. 7.9 months for CLIP score of 3+ (n=9) (HR, 1.68; 95% CI: 0.73-3.88; P=0.22), and the mPFS for CLIP score of 0-2 was 7.1 vs. 6.5 months for CLIP score of 3+ (HR, 1.40; 95% CI: 0.65-3.04; P=0.38). Among CP-B patients, the mOS based on CLIP score of 1-2 (n=20) was 8.5 vs. 5.9 months for CLIP score of 3+ (n=26) (HR, 1.12; 95% CI: 0.57-2.18; P=0.74), and the mPFS for CLIP score of 1-2 was 4.6 vs. 3.5 months for CLIP score of 3+ (HR, 1.62; 95% CI: 0.85-3.09; P=0.14).
Ethnicity
The mOS for Hispanics (n=78) was 11.2 (95% CI: 7.2-18.4) vs. 8.7 months (95% CI: 7.8-19.1) (HR, 1.18; 95% Cl: 0.70-2.01; P=0.53) for non-Hispanics (n=28). The mPFS for Hispanics was 4.9 (95% Cl: 3.1-9.1) vs. 6.0 months (95% CI: 3.6-9.4) for non-Hispanics (HR, 0.86; 95% Cl: 0.53-1.37, P=0.57).
Age
The mOS for patients 70+ years of age was 9.2 months (95% CI: 5.-59.5) compared to <70 years with mOS of 10.2 months (95% CI: 7.7-13.5) (P=0.28). The mPFS for patients 70+ years of age was 4.7 months (95% CI: 2.1-9.0) compared to patients <70 years of age with mPFS of 5.3 months (95% CI: 3.6-7.2) (P=0.48).
Response rates (RR)
If the patient did not have progressive disease, best response was documented. Of the 87 patients with response data available, no patients had a complete response, 48 (45.7%) had stable disease, 18 (17.1%) had partial response (PR), and 39 (37.1%) had progressive disease.
Toxicity
The highest grade toxicity observed from baseline to 6 months was documented. Nausea occurred in 31 (29.2%) patients, with grade 3 in 1 (0.9%). Fatigue occurred in 90 (84.9%) patients, with grade 3 in 6 (5.7%). Diarrhea occurred in 45 (42.5%) patients, with grade 2 in 1 (0.9%). Hand-foot syndrome occurred in 39 (36.8%) patients, with grade 3 in 7 (6.7%). No grade 4 or 5 AEs were observed. AEs based on CP score are in Table 2.
Table 2. Adverse events in advanced HCC based on Child Pugh class at baseline.
| Side effect | Child Pugh class |
All subjects (N=106) | P value | |
|---|---|---|---|---|
| A (N=60) | B (N=46) | |||
| Nausea, n (%) | 0.94 | |||
| 0 | 41 (68.3) | 34 (73.9) | 75 (70.8) | |
| 1 | 13 (21.7) | 8 (17.4) | 21 (19.8) | |
| 2 | 5 (8.3) | 4 (8.7) | 9 (8.5) | |
| 3 | 1 (1.7) | 0 (0) | 1 (0.9) | |
| Fatigue, n (%) | 0.32 | |||
| 0 | 11 (18.3) | 5 (10.9) | 16 (15.1) | |
| 1 | 31 (51.7) | 21 (45.7) | 52 (49.1) | |
| 2 | 14 (23.3) | 18 (39.1) | 32 (30.2) | |
| 3 | 4 (6.7) | 2 (4.3) | 6 (5.7) | |
| Diarrhea, n (%) | 0.41 | |||
| 0 | 33 (55.0) | 28 (60.9) | 61 (57.5) | |
| 1 | 24 (40.0) | 14 (30.4) | 38 (35.8) | |
| 2 | 2 (3.3) | 4 (8.7) | 6 (5.7) | |
| 3 | 1 (1.7) | 0 (0) | 1 (0.9) | |
| Hand foot, n (%) | 0.08 | |||
| 0 | 34 (56.7) | 33 (71.7) | 67 (63.2) | |
| 1 | 13 (21.7) | 9 (19.6) | 22 (20.8) | |
| 2 | 6 (10.0) | 4 (8.7) | 10 (9.4) | |
| 3 | 7 (11.7) | 0 (0) | 7 (6.6) | |
Highest grade of toxicity observed from baseline to 6 months; Child Pugh Class at baseline. Adverse events were graded according to the Common Terminology Criteria for Adverse Events version 4.0. Statistical testing was performed using Fisher's Exact Test. HCC, hepatocellular carcinoma.
Discussion
In our retrospective analysis of South Texas patients with unresectable advanced HCC, who were treated with sorafenib, we found mOS of 10.2 months. Sorafenib therapy appeared to be well tolerated in both CP-A (57%) and CP-B (43%) cirrhosis, which was predominantly due to hepatitis C (66%) and alcohol (59%). Differences in survival based on age and ethnicity were not observed.
Although our reported mOS of 10.2 months appeared similar to the reported mOS of 10.7 months in the SHARP study, our population had a higher percentage of CP-B patients (43%) vs. the 5% in the SHARP study. Our patient population receiving sorafenib also had advanced disease, with 88% of patients with BCLC score C and 12% BCLC score B who had failed local therapies, similar to the SHARP study. However, when subgroup analysis of the SHARP study was performed, among HCC patients with hepatitis C etiology, a reported improvement in mOS of 14 vs. 7.4 months, sorafenib compared to placebo (13). Conversely, among HCC patients with hepatitis B etiology, a more modest improvement of mOS of 9.7 vs. 6.1 months in the sorafenib compared to placebo was noted in the SHARP study (13). Whether these differences are related to patient imbalances relative to etiology of HCC or truly represent differences in outcome based on geography remain to be further elucidated (13).
Patients receiving sorafenib were permitted to be re-challenged with local treatments (42.1%), such as TACE or RFA, based on recommendations from a multi-disciplinary tumor board. This may account for the higher RR with 17% PR documented in our study relative to that reported in the SHARP study. A further explanation on improved efficacy is our multi-disciplinary management, in which patients with residual HCC are offered sorafenib soon after initial failure of local therapies. To confirm our results, results from ongoing prospective randomized studies evaluating the benefit of adding local treatments to sorafenib are eagerly awaited. In addition, stratification of outcome analyses based on HCC etiologies and/or geography should be incorporated into future HCC studies.
Currently, we have limited data on the effect of dosing on efficacy of sorafenib, especially in patients with liver dysfunction. To date, the large global prospective study, GIDEON, has not yet reported final efficacy analysis in relation to sorafenib dosing. Our cohort had a higher proportion of CP-B patients (43%) and showed a difference in survival between CP-A and CP-B patients but with similar tolerability. A higher percentage of CP-A patients (78%) were started at 800 mg/day when compared to CP-B patients (54%) However, 68% of patients required dose reduction, with CP-A 22% vs. CP-B 46%. When stratified based on dose, patients receiving sorafenib at 800 mg/day had a better OS than 400 mg/day (12.8 vs. 6.6 months, respectively). Although the efficacy analysis did not show improved survival among patients with CP-A treated with 800 mg/day, we observed that CP-B patients had a statistically significant survival advantage over lower dose sorafenib (P=0.002). The association between dosing and efficacy demonstrates that adequate dosing of sorafenib does matter in the treatment of CP-B patients with advanced HCC. The improved OS with higher dosing seen in the CP-B group, and not the CP-A group, may be due to patients with CP-B cirrhosis having worse prognosis than those with CP-A, and a larger cohort of patients is likely needed to detect a statistical difference. Although this retrospective data should be further validated through larger prospective trials, our study demonstrates that full dosing of sorafenib in patients with liver dysfunction did not cause more toxicity. Therefore, while we await prospective data, in clinical practice, patients with CP-B cirrhosis should be considered for goal dose of sorafenib 800 mg/day. Furthermore, correlation of sorafenib dosing with other prognostic scoring systems that take into account the extent of the tumor, such as CLIP scoring system or BCLC staging system, may be more useful in identifying subsets of patients who will benefit from sorafenib.
In clinical practice, dose reductions are often instituted due to a patient’s performance status, age, comorbidities and concerns of perceived toxicities often without optimal management, but this may lead to decreased efficacy of costly therapy. From other tumor types, we have learned that dosing is critical for improving efficacy. For example, in metastatic gastrointestinal stromal tumors with an activating KIT mutation in exon 9, the higher dose of imatinib at 800 mg/day has a superior PFS when compared to imatinib 400 mg/day (14,15). Similarly, dosing of targeted therapies appears to be important in renal cell carcinoma (RCC). Recently, continuous dosing of sunitinib at 37.5 mg/day has been compared to the 6-week dosing schedule (50 mg/day for 4 weeks, 2 weeks off), with preliminary data showing at trend toward decreased TTP (7.1 vs. 9.9 months, respectively; P=0.090) (16). The 4/2 schedule was superior to continuous dosing in time to deterioration, a composite end point of death, progression, and disease-related symptoms (P=0.034). Another agent, axitinib was evaluated in a randomized phase II study of 213 treatment-naïve advanced RCC patients, who were treated with axitinib 5 mg orally twice daily for 4 weeks, and then randomized to stepwise dose titration (from 5 to 7 to 10 mg twice daily) or placebo dose titration. This study showed that higher axitinib exposure was related to higher overall RR (59% vs. 40%) and better PFS (14 vs. 11 months) (17). The effects on OS have not been reported yet for this study.
Our study shows that the full dose of sorafenib is safe in patients with CP-B cirrhosis but dose reductions compromises efficacy. Given limited treatment options many novel agents are being evaluated among patients with advanced HCC, some with a lower dosage compared to known maximum tolerated dose or approved dose in other tumor types secondary to drug intolerance. EVOLVE-1, a phase III study of HCC patients with BCLC B or C and CP-A cirrhosis, which compared everolimus 7.5 mg daily orally versus placebo after progression on sorafenib or sorafenib intolerance showed no difference in mOS (7.6 vs. 7.3 months; P=0.675) (18). It would remain unknown if this drug may have had clinical efficacy in HCC if dosed at the standard dose of 10 mg daily or in a pre-specified subgroup of patients. A phase II study of first-line gefitinib 250 mg orally daily in advanced HCC demonstrated lack of efficacy (19). Similarly, it will remain unclear if the higher dosage of gefitinib at 500 mg daily would have improved activity in HCC.
Other studies have shown that agents can be safely tolerated at full doses in patients with advanced HCC with liver dysfunction. For example, lenalidomide was given second-line to 40 advanced HCC patients (19 CP-A, 16 CP-B, and 5 CP-C) at 25 mg orally daily (21 days of 28-day cycle), which is the same dose for multiple myeloma (20). Grade 3 fatigue (n=3) and rash (n=4) were the most common non-hematologic toxicities; grade 4 neutropenia occurred in only 2.5% patients. Currently, lenalidomide is being evaluated for efficacy in a phase II second-line study in advanced HCC at the above dose (ClinicalTrials.gov, NCT01545804). Also, other studies of bevacizumab (10 mg/kg intravenously every 2 weeks) with erlotinib (150 mg orally daily) have been tolerated at the traditional full doses in patients with advanced HCC, although efficacy is conflicting (21,22). Second-line axitinib 5 mg twice daily is also well tolerated in advanced HCC patients, with preliminary results demonstrating efficacy (23).
Sorafenib is currently the only systemic treatment approved for advanced HCC; and therefore, patients with CP-A and CP-B cirrhosis should be considered for therapy with the aim of treating at the approved dose of 400 mg twice daily in order to improve survival. Unlike CP-A patients, we observed that CP-B patients had a statistically significant survival advantage when they received 800 mg/day sorafenib, and dose reductions should be conservatively done such that efficacy is not compromised. Although, we have evidence of the need for adequate dosing to improve efficacy, this has to be balanced with toxicities that may develop, which should be optimally managed prior to considering dose reductions. Further validation through prospective trials is also needed to assess whether altered or lower dosing in advanced HCC patients does have a detrimental effect on overall survival.
Acknowledgements
The authors thank Subrata Haldar, PhD for help with scientific writing.
Disclosure: Devalingam Mahalingam is on the Advisory Board/Speaker Bureau for Bayer Pharmaceuticals and Onyx Pharmaceuticals. The authors declare no conflict of interest.
References
- 1.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [DOI] [PubMed] [Google Scholar]
- 2.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [DOI] [PubMed] [Google Scholar]
- 3.Mair RD, Valenzuela A, Ha NB, et al. Incidence of hepatocellular carcinoma among US patients with cirrhosis of viral or nonviral etiologies. Clin Gastroenterol Hepatol 2012;10:1412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lencioni R, Kudo M, Ye SL, et al. First interim analysis of the GIDEON (Global Investigation of therapeutic decisions in hepatocellular carcinoma and of its treatment with sorafeNib) non-interventional study. Int J Clin Pract 2012;66:675-83. [DOI] [PubMed] [Google Scholar]
- 5.Lencioni R, Kudo M, Ye SL, et al. GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib): second interim analysis. Int J Clin Pract 2014;68:609-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marrero JA, Lencioni R, Ye SL, et al. Final analysis of GIDEON (Global Investigation of Therapeutic Decisions in Hepatocellular Carcinoma [HCC] and of Its Treatment with Sorafenib [Sor]) in >3000 Sor-treated patients (pts): clinical findings in pts with liver dysfunction. J Clin Oncol 2013;31:abstr 4126.
- 7.Howe HL, Wu X, Ries LA, et al. Annual report to the nation on the status of cancer, 1975-2003, featuring cancer among U.S. Hispanic/Latino populations. Cancer 2006;107:1711-42. [DOI] [PubMed] [Google Scholar]
- 8.Ramirez AG, Weiss NS, Holden AE, et al. Incidence and risk factors for hepatocellular carcinoma in Texas Latinos: implications for prevention research. PLoS One 2012;7:e35573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruix J, Sherman M, American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646-9. [DOI] [PubMed] [Google Scholar]
- 11.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 1998;28:751-5. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-38. [DOI] [PubMed] [Google Scholar]
- 13.Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 2012;57:821-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debiec-Rychter M, Sciot R, Le Cesne A, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer 2006;42:1093-103. [DOI] [PubMed] [Google Scholar]
- 15.Carew JS, Medina EC, Esquivel JA, 2nd, et al. Autophagy inhibition enhances vorinostat-induced apoptosis via ubiquitinated protein accumulation. J Cell Mol Med 2010;14:2448-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motzer RJ, Hutson TE, Olsen MR, et al. Randomized phase II trial of sunitinib on an intermittent versus continuous dosing schedule as first-line therapy for advanced renal cell carcinoma. J Clin Oncol 2012;30:1371-7. [DOI] [PubMed] [Google Scholar]
- 17.Rini BI, Grünwald V, Fishman MN, et al. Axitinib for first-line metastatic renal cell carcinoma (mRCC): Overall efficacy and pharmacokinetic (PK) analyses from a randomized phase II study. J Clin Oncol 2012;30:abstr 4503.
- 18.Zhu AX, Kudo M, Assenat E, et al. EVOLVE-1: Phase 3 study of everolimus for advanced HCC that progressed during or after sorafenib. J Clin Oncol 2014;32:abstr 172.
- 19.O’Dwyer PJ, Giantonio BJ, Levy DE, et al. Gefitinib in advanced unresectable hepatocellular carcinoma: Results from the Eastern Cooperative Oncology Group’s Study E1203. J Clin Oncol 2006;24:abstr 4143.
- 20.Safran H, Charpentier KP, Kaubisch A, et al. Lenalidomide for Second-line Treatment of Advanced Hepatocellular Cancer: A Brown University Oncology Group Phase II Study. Am J Clin Oncol 2015;38:1-4. [DOI] [PubMed] [Google Scholar]
- 21.Kaseb AO, Garrett-Mayer E, Morris JS, et al. Efficacy of bevacizumab plus erlotinib for advanced hepatocellular carcinoma and predictors of outcome: final results of a phase II trial. Oncology 2012;82:67-74. [DOI] [PubMed] [Google Scholar]
- 22.Philip PA, Mahoney MR, Holen KD, et al. Phase 2 study of bevacizumab plus erlotinib in patients with advanced hepatocellular cancer. Cancer 2012;118:2424-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNamara MG, Horgan AM, Aspinall A, et al. A phase II trial of second-line axitinib following prior antiangiogenic therapy in advanced hepatocellular carcinoma (HCC). J Clin Oncol 2012;30:abstr 314. [DOI] [PubMed]