Abstract
Mutations of exon 2 of Kirsten rat sarcoma viral oncogene homologue (KRAS) (exon 2 codons 12/13) lead to constitutive activation of the EGFR (epidermal growth factor receptor) mediated signal transduction pathway and been shown to be a negative predictive biomarker for EGFR-directed monoclonal antibodies among patients with colorectal cancer (CRC). As selection of patients is very important for administration of anti-EGFR therapy, this lone biomarker has proved to be insufficient for selecting the appropriate patients as more patients lacking exon 2 KRAS mutation were resistant to anti-EGFR therapy. The results of various randomized clinical trials have confirmed the presence of other RAS mutation including additional RAS mutations (KRAS exons 3/4 and NRAS exon 1/2/3/4). Extended RAS analysis should be considered before initiating anti-EGFR therapy to patients of metastatic CRC. This can help in proper selection of patients leading to tailored individualistic treatment, decreasing cost of treatment and the adverse effects related to use of monoclonal antibody therapy. The new evidence is supporting the need to make ‘Extended RAS’ analysis essential before start of treatment with anti-EGFR monoclonal antibody therapy. Prior to this the Extended RAS testing should be standardized.
Keywords: Extended RAS analysis, metastatic colorectal cancer (CRC), monoclonal antibody therapy
Introduction
According to the American Cancer Society, the latest records of year 2012 showed that colorectal cancer is the third most commonly diagnosed cancer and the third leading cause of cancer death among both men and women in USA (1). The management of this widely prevalent cancer has also been evolving from being non-specific to being patient and target specific in the recent past. As a step towards targeted treatment, epidermal growth factor receptor (EGFR) was validated as a therapeutic target for chemotherapeutic agents (2).
Various randomized controlled trials (RCTs) proved the beneficial effects of anti-EGFR monoclonal antibodies as monotherapy as well as in combination therapy among patients with metastatic colorectal cancer (mCRC) in the last decade (3-7). Two anti-EGFR monoclonal antibodies (mAbs), cetuximab and panitumumab, were approved for use alone or with standard chemotherapy among patients with advanced CRC (8,9). As the mAbs are expensive and can be potentially toxic drugs, there was a need for proper selection of patients eligible for administration of the antibody based therapy. EGFR expression level was the first biomarker to be studied among patients likely to be prescribed anti-EGFR mAbs. But, no correlation could be established between the response to anti-EGFR mAbs and the EGFR expression levels (6,10). Later, an association between the occurrence of mutation of KRAS gene and the poor response with anti-EGFR mAbs was established (11). This was followed by the recommendation of testing of mutational status of KRAS gene before initiation of therapy with anti-EGFR mAbs among patients of mCRC (12,13).
EGFR and RAS signaling pathway
EGFR, a tyrosine kinase receptor involved in signal transduction mechanism, is one of the important molecular targets for drug therapy (14). Binding of EGF or any other ligand to EGFR activates signal transduction via various pathways. These include the RAS-RAF-BRAF-MAPK (mitogen activated protein kinase) pathway or phosphatidylinositol 3-kinase (PI3K)-Akt or phospholipase Cγ pathway (15). RAS is the most important superfamily of proteins, which includes mainly KRAS and NRAS proteins. KRAS is a guanosine triphosphate cleaving enzyme (GTPase). The signaling through KRAS-RAF-BRAF-MAPK pathway controls gene transcription, cell proliferation, apoptosis, angiogenesis, invasion and migration (16-18).
Although EGFR is a molecular target for anti-EGFR mAbs and is also over expressed among approximately 80% of CRCs, it could not be established as a predictive biomarker in the management of CRC (16,19). Positive EGFR protein expression proved to be a poor biomarker for response with anti-EGFR mAbs (18). Thus, other effectors in the downstream signal transduction pathway were evaluated for their predictive value. It was observed that mutation in KRAS, NRAS, BRAF or PI3KCA genes result in constitutive activation of signaling pathway. Approximately 30-50% CRCs carry a mutation at codon 12 or 13 of exon 2 of the KRAS gene, followed by mutations of NRAS, PI3KCA and BRAF (20,21). These mutations are responsible for constitutive activation of EGFR downstream pathways which disrupt the normal signaling pathway independent of EGFR (15,18). Mutations in BRAF lead to uncontrolled BRAF activation independent of EGFR and RAS (17).
KRAS mutant status as a predictive biomarker
After the approval of cetuximab and panitumumab for use among patients with mCRC, various studies demonstrated that these drugs were effective among patients with KRAS exon 2 wild type tumors only and not among those with KRAS exon 2 mutant tumors (22,23). The median progression free survival (PFS) and overall survival (OS) significantly improved among the KRAS exon 2 wild type group with anti-EGFR antibody therapy when used either in monotherapy or combination therapy as compared to the basic support care group or standard chemotherapy regimen respectively (22,23). On the other hand, the KRAS exon 2 mutant group did not show any difference in efficacy with the addition of anti-EGFR mAbs as compared to the standard chemotherapy regimen (22-25). In addition, somewhat unexpected detrimental effects were observed in the mutant KRAS groups in the PRIME (panitumumab randomized trial in combination with chemotherapy for metastatic colorectal cancer to determine efficacy) and OPUS (oxaliplatin and cetuximab in first-line treatment of mCRC) studies (26,27). Both prospective and retrospective analysis of the clinical studies concluded that mutation of codon 12 or 13 of exon 2 of KRAS is a negative predictive biomarker for therapy with anti-EGFR antibody therapy (11,22-27).
This led to the recommendation for routine KRAS exon 2 mutational testing. The American Society of Clinical Oncology and National Comprehensive Cancer Network (NCCN) recommended that all patients with mCRC who are candidates for anti-EGFR antibody therapy should have their tumor tested for KRAS mutations. If KRAS mutation in codon 12 or 13 is detected, then patients with mCRC should not receive anti-EGFR antibody therapy as part of their treatment due to the predicted lack of response (12,13,28). This recommendation restricted the use of anti-EGFR mAbs to about 60% of all patients with KRAS wild type tumors (20). A meta-analysis of 45 clinical studies (29) concluded that KRAS mutations are predictive of survival, disease progression, and treatment failure in patients with advanced colorectal cancer treated with anti-EGFR antibodies. The benefits of anti-EGFR therapy were largely limited to KRAS wild type patients (29).
Unfortunately, not all patients with KRAS wild type status respond to anti-EGFR mAbs. The presence of KRAS mutations has low sensitivity and relatively high negative likelihood for determining non-responsiveness among the patients (30). One hypothesis to explain this could be the simultaneous or isolated presence of genetic aberrations of genes encoding the other downstream effectors of the EGFR mediated signal transduction pathway (31-34). This hypothesis was proven by the results of the following clinical studies which show that additional RAS mutation (KRAS exons 3 and 4 and NRAS exon 1, 2, 3, 4) analysis can help in further refining the treatment modalities.
Clinical evidence of presence of other genetic mutations in patients resistant to anti-EGFR therapy
In the era of personalized medicine various retrospective and prospective analyses are being conducted to search for more predictive biomarkers in the treatment protocol for various malignancies specially mCRC. As KRAS wild type status was not sufficient to ensure response to anti-EGFR mAbs, other predictive biomarkers (KRAS, NRAS, BRAF mutations, PIK3CA mutations and PTEN loss) from the signaling pathway were analyzed. Although the results are favorable for the predictive strength of some other genomic biomarkers, till now no recommendation has been made for extensive genotypic analysis before initiation of anti-EGFR antibody therapy (34-36).
A systematic review and meta-analysis by Yang et al. explored the association of BRAF, PIK3CA mutations and/or loss of PTEN expression with PFS, OS and objective response rate (ORR) among patients with KRAS wild type tumors treated with anti-EGFR mAbs were included. The authors concluded that BRAF mutations, PIK3CA mutations and loss of PTEN are promising biomarkers and can help in identifying the appropriate patients (37). In contrast, the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group (EWG) discouraged the testing of BRAF, NRAS or PIK3CA, and/or loss of expression of PTEN or AKT proteins for taking decisions regarding the administration of anti-EGFR antibody therapy among patients with mCRC (38).
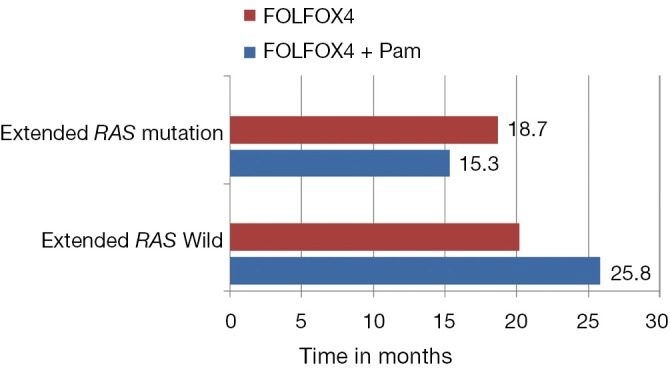
These contradictory statements could not help in establishing the status of other biomarkers in the algorithm of mCRC management. Later, the retrospective analysis of PRIME study by Douillard et al. initiated the concept of Extended RAS analysis (39). The prospective-retrospective analysis of PRIME study assessed the efficacy and safety of panitumumab plus FOLFOX4 (oxaliplatin, fluorouracil and leucovorin) as compared with FOLFOX4 alone, according to RAS (KRAS or NRAS) or BRAF mutation status. Of the study population, 48% patients had tumors with non mutated RAS (no KRAS or NRAS mutations in exons 2, 3, or 4) and rest had mutations in RAS (any KRAS or NRAS mutations in exon 2, 3, or 4). The administration of panitumumab-FOLFOX4 led to a significant improvement in PFS and OS (Table 1). In the subgroup of patients without RAS mutations, there was a significant improvement in PFS (P=0.004) and OS (P=0.04) with panitumumab-FOLFOX4, as compared with FOLFOX4 alone (39). Another subset (17%), consisting of those patients with wild type KRAS tumors, but with mutations in other RAS exons [non KRAS exon 2 codon (12 and 13) mutation, KRAS exon 3 (at codon 61) and exon 4 (at codons 117 and 146); NRAS exon 2 (at codons 12 and 13), exon 3 (at codon 61), and exon 4 (at codons 117 and 146); and BRAF exon 15 (at codon 600)], showed a non-significantly shorter PFS and OS in the panitumumab-FOLFOX4 group than in the FOLFOX4-alone group (Figure 1). These results were similar to those observed in the subgroup of patients with KRAS mutations in exon 2 in tumors (Table 1). Another important observation of the study was that the treatment effects were different between the subgroups of patients without RAS mutations and those without KRAS mutations in exon 2 but with other RAS (KRAS or NRAS mutations in exons 2, 3, 4) mutations. This might suggest that RAS mutations, in addition to KRAS mutations in exon 2 codon (12 and 13), were negative predictive factors. The results suggest that presence of RAS mutations was a negative predictive factor. Further analysis showed that in the nonmutated RAS and nonmutated BRAF subgroup, panitumumab-FOLFOX4 was associated with a 1.6-month improvement in PFS and a 7.4-month improvement in OS, as compared with FOLFOX4 alone. Analysis of the prognostic effect of BRAF mutations showed that BRAF mutations were associated with reduced OS among patients without KRAS mutations in exon 2 and among those with NRAS mutations in exon 3. The safety profile for patients with RAS mutations was similar to that reported for patients with KRAS mutations in exon 2 (39).
Table 1. PRIME study, primary end points of (PFS and OS) efficacy results according to RAS mutation status.
| Variable | FOLFOX4 + panitumumab (months) | FOLFOX4 (months) | P value |
|---|---|---|---|
| PFS | |||
| Extended RAS wild | 10.1 | 7.9 | 0.004 |
| No KRAS exon 2 (12+13) mutation | 9.6 | 8.0 | 0.02 |
| Non KRAS exon 2 (12+13) mutation but other RAS mutation present | 7.3 | 8.0 | 0.04 |
| Extended RAS mutation | 7.3 | 8.7 | 0.001 |
| OS | |||
| Extended RAS wild | 25.8 | 20.2 | 0.009 |
| No KRAS exon 2 (12+13) mutation | 23.8 | 19.4 | 0.03 |
| Non KRAS exon 2 (12+13) mutation | 17.1 | 17.8 | 0.01 |
| Extended RAS mutation | 15.3 | 18.7 | 0.001 |
Abbreviations: PFS, progression free survival; OS, overall survival.
Figure 1.
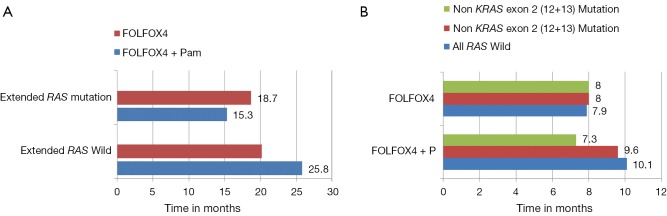
(A) PRIME study, in the wild KRAS exon 2 group FOLFOX + P improved progression free survival (PFS) when compared with FOLFOX only group (9.6 vs. 8 months respectively), the absolute magnitude of improvement of PFS is more pronounced when Extended RAS analysis is used to determine the RAS status with PFS 10.1 m in FOLFOX + P compared with 7.9 m in FOLFOX only group (P=0.004). The absolute improvement in PFS has increased from 1.6 months with wild KRAS exon 2 analysis to 2.2 months when Extended KRAS analysis is utilized; (B) PRIME study, clinically and statistically significant improvement in survival in FOLFOX and panitumumab in Extended RAS wild group when compared to FOLFOX alone, the presence of RAS mutation (any KRAS or NRAS mutations in exon 2, 3, or 4) in this population had detrimental effect on survival and did not drive any survival benefit from the addition of panitumumab in contrast to Extended RAS wild population where significant improvement in survival with FOLFOX and panitumumab comparing with FOLFOX only population (25.8 vs. 20.2 months respectively) (P=0.009).
Similarly, Soeda et al. while studying the response with cetuximab among irinotecan- and oxaliplatin-refractory Japanese patients with mCRC, found that the KRAS, BRAF, and PIK3CA wild type group had a better response rate and PFS than did the wild-type KRAS exon 2 subgroup (40). In the GERCOR efficacy, tolerance and translational molecular study, Andre et al. also studied BRAF, NRAS mutations and EGFR copy number in addition to the KRAS mutant status. Patients with BRAF mutations had a poorer prognosis and lower response rates to anti-EGFR antibody therapy as compared to other groups. Evidence for rare KRAS, NRAS and PIK3CA mutations was poor because of small number of patients in these groups. The response was highly dependent on the mutant status of the patients and thus recommended an extended genotyping including rare KRAS and NRAS mutants (41).
The PEAK [panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6] study also assessed the treatment effect with an extended RAS analysis including exons 2, 3, 4 of both KRAS and NRAS among patients with previously untreated, unresectable, wild type KRAS exon 2 mCRC. Patients with wild type RAS tumors had better PFS (P=0.029) and median OS (P=0.058) with anti-EGFR therapy. PFS was similar and OS was better in the panitumumab group among the patients with wild type KRAS exon 2 tumors (42).
New evidence was presented at the American Society of Clinical Oncology 2014 and European Cancer Congress 2013 (25,43). Peeters et al. assessed the effect of second line treatment of panitumumab plus FOLFIRI (continuous infusion fluorouracil, oxaliplatin, and irinotecan) vs. FOLFIRI based on RAS mutation status in the population of the earlier study conducted in 2010. Mutations detected included KRAS exon 3, 4 and NRAS exons 2, 3, 4 in patients with known KRAS wild type exon 2 mCRC. About 18% of the wild type KRAS patients had additional RAS mutations. The PFS and OS were better in the RAS wild type group as compared to RAS mutant group. Bokemeyer et al. studied KRAS exon 2 wild type patients from the OPUS study for 26 mutations (referred as new RAS) and additional KRAS, NRAS codons. New RAS mutations were present among 26% of patients. The patients from RAS wild type group showed significant improvement with addition of cetuximab to FOLFOX4 therapy. The distinctive observation of this study is that there was a trend towards worse outcome among patients with RAS mutation with the addition of cetuximab (26,44). Tejpar et al. (45) presented another set of results from the OPUS study about the patients which were tested for KRAS exons 3 and 4 and NRAS exons 2, 3 and 4. The tumor status was available for 31% of patients and there was benefit among RAS wild type population with addition of cetuximab to FOLFOX4. There was a less favorable outcome and no benefit among RAS mutant population with addition of cetuximab (45). Ciardiello et al. studied the new RAS mutations among KRAS wild type exon 2 tumors from CRYSTAL study patients and RAS mutations were present in 15% of the patients. There was a significant benefit in all end points among RAS wild type patients with the addition of cetuximab to FOLFIRI regimen. Also, there was no benefit among the RAS mutant group with the addition of cetuximab (46). Stintzing et al. evaluated the effect of mutations in exon 3 (codon 61), and exon 4 (codon 146), and NRAS exon 2 (codons 12 and 13), exon 3 (codons 59 and 61), and exon 4 (codons 117 and 146) on the ORR, PFS and OS among the KRAS (exon 2) codon 12/13 wild type patients. The ORR and OS were increased among RAS wild type patients with the addition to cetuximab to FOLFIRI regimen as compared to addition of bevacizumab to FOLFIRI regimen (Table 2, Figure 2) (47).
Table 2. FIRE 3 study, primary end points of (PFS and OS) efficacy results according to RAS mutation status.
| Variable | FOLFIRI + cetuximab (months) | FOLFIRI + bevacizumab (months) | P value |
|---|---|---|---|
| PFS | |||
| KRAS exon 2 (12+13) wild | 10.0 | 10.3 | 0.55 |
| Extended RAS wild-type [Excluding all non KRAS exon 2 (12+13) mutation]* | 10.4 | 10.2 | 0.54 |
| OS | |||
| KRAS exon 2 (12+13) wild | 28.7 | 25.0 | 0.017 |
| Extended RAS wild-type [Excluding all non KRAS exon 2 (12+13) mutation]* | 33.1 | 25.6 | 0.011 |
*exon 3 (codon 61), and exon 4 (codon 146), and NRAS exon 2 (codons 12 and 13), exon 3 (codons 59 and 61), and exon 4 (codons 117 and 146). Abbreviations: PFS, progression free survival; OS, overall survival.
Figure 2.
FIRE 3 study, clinically and statistically significant improvement in survival in FOLFIRI and cetuximab KRAS wild group in the preplanned analysis when compared to FOLFIRI and bevacizumab, the overall survival was more pronounced among Extended RAS wild type patients with the addition to cetuximab to FOLFIRI regimen as compared to addition of bevacizumab to FOLFIRI group.
A recent systematic review and meta-analysis on alterations in KRAS exons 3 and 4, NRAS, BRAF and PIK3CA and PTEN and the outcome with anti-EGFR antibody therapy suggests that mutations in KRAS exons 3 and 4 and NRAS predict resistance to anti-EGFR mAbs. The ORR was significantly poor among those with KRAS mutation in exon 3 and 4 (odds ratio 0.26). The PFS was also significantly shorter due to mutations in KRAS exons 3 and 4 and NRAS (48). Sorich et al. have included all the above mentioned clinical trials assessing the role of anti-EGFR mAbs for tumors harboring RAS mutations. They divided the patients from various RCTs into three subgroups. First group was the “KRAS exon 2” mutant group; second consisted of “new RAS mutant” (wild-type for KRAS exon 2, but with a KRAS mutation in exons 3 or 4 and/or a NRAS mutation in exons 2, 3 or 4) patients and third consisted of “Extended RAS wild type” patients. Tumors without any RAS mutations (either KRAS exon 2 or new RAS mutations) had significantly superior response [PFS (P<0.001) and OS (P=0.008)] with anti-EGFR mAb treatment as compared to tumors with any of the new RAS mutations. There was no PFS and OS benefit with anti-EGFR mAbs for tumors with any RAS mutations (P>0.05) (49).
Discussion
Although in the initial years of use of anti-EGFR mAbs for mCRC, testing of KRAS exon 2 mutation helped in individualizing the treatment with anti-EGFR mAbs, yet, even after this analysis, a subset population of KRAS exon 2 wild type patients showed continues resistance to anti-EGFR agents. Since the isolation of KRAS mutant status as a lone negative predictor marker few years back to the present day scenario each and every step has been corroborated by evidence from clinical studies. The results of the above mentioned RCTs, systematic reviews and meta-analysis show that patients with tumors that are KRAS exon 2 wild-type (which includes both the “Extended RAS wild-type” and “new RAS mutant” subgroups) should not be considered to represent a single homogenous group for efficacy or resistance to anti-EGFR mAbs. The “Extended RAS wild-type” subgroup is distinct and has a significantly better response to anti-EGFR mAbs as compared to other patients. The response is indistinguishable among the KRAS exon 2 mutant patients and those with newly identified RAS mutations which include KRAS mutation in exons 3 or 4 and/or a NRAS mutation in exons 2, 3 or 4. Although the beneficial effects of anti-EGFR mAbs are explicit in the Extended RAS wild type group, results are still limited regarding the detrimental effects of anti-EGFR mAbs among RAS mutant groups (39,44). A broader analysis of mutant status can help in tailoring patient specific regimen and achieving maximum benefit. Thus based on the emerging benefit Extended RAS analysis, beyond KRAS exon 2, should be utilized in practice for predicting the benefit from the anti-EGFR mAbs among patients with mCRC.
Conclusions
The additional analysis of KRAS and NRAS genes as predictive markers can allow more accurate selection of patients who are more likely to benefit from anti-EGFR antibody therapy. Treatment with anti-EGFR mAbs should only be initiated after screening tumors for mutations in exon 2, 3 and 4 of both KRAS and NRAS genes. This will help in preventing unnecessary drug toxicity and associated expenses. Prior to the implantation of such recommendation there is a need to establish a standardized acceptable expanded RAS mutant status testing.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.American Cancer Society. Colorectal Cancer Facts & Figures 2011-2013. American Cancer Society, Atlanta. 2011. Available online: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-028312.pdf. Accessed 19th September 2014.
- 2.Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer 2004;4:361-70. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004;351:337-45. [DOI] [PubMed] [Google Scholar]
- 4.Tabernero J, Van Cutsem E, Díaz-Rubio E, et al. Phase II trial of cetuximab in combination with fluorouracil, leucovorin, and oxaliplatin in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 2007;25:5225-32. [DOI] [PubMed] [Google Scholar]
- 5.Saltz LB, Meropol NJ, Loehrer PJ, Sr, et al. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 2004;22:1201-8. [DOI] [PubMed] [Google Scholar]
- 6.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007;25:1658-64. [DOI] [PubMed] [Google Scholar]
- 7.Jonker DJ, O'Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med 2007;357:2040-8. [DOI] [PubMed] [Google Scholar]
- 8.US FDA. FDA Approves Erbitux for Colorectal Cancer. 2004. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2004/ucm108244.htm. Accessed 29th August, 2014.
- 9.US FDA. FDA Approves a New Drug for Colorectal Cancer, Vectibix. 2006. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108745.htm. Accessed 29th August, 2014.
- 10.Chung KY, Shia J, Kemeny NE, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol 2005;23:1803-10. [DOI] [PubMed] [Google Scholar]
- 11.Lièvre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 2006;66:3992-5. [DOI] [PubMed] [Google Scholar]
- 12.Engstrom PF, Arnoletti JP, Benson AB, 3rd, et al. NCCN Clinical Practice Guidelines in Oncology: colon cancer. J Natl Compr Canc Netw 2009;7:778-831. [DOI] [PubMed] [Google Scholar]
- 13.Engstrom PF, Arnoletti JP, Benson AB, 3rd, et al. NCCN Clinical Practice Guidelines in Oncology: rectal cancer. J Natl Compr Canc Netw 2009;7:838-81. [DOI] [PubMed] [Google Scholar]
- 14.Prenen H, Tejpar S, Van Cutsem E.New strategies for treatment of KRAS mutant metastatic colorectal cancer. Clin Cancer Res 2010;16:2921-6. [DOI] [PubMed] [Google Scholar]
- 15.Wicki A, Herrmann R, Christofori G.Kras in metastatic colorectal cancer. Swiss Med Wkly 2010;140:w13112. [DOI] [PubMed] [Google Scholar]
- 16.Yokota T.Are KRAS/BRAF Mutations Potent Prognostic and/or Predictive Biomarkers in colorectal cancers? Anti-Cancer Agents in Medicinal Chemistry 2012;12:163-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Domagała P, Hybiak J, Sulżyc-Bielicka V, et al. Kras mutation testing in colorectal cancer as an example of the pathologist’s role in personalized targeted therapy: a practical approach. Pol J Pathol 2012;63:145-64. [DOI] [PubMed] [Google Scholar]
- 18.Siena S, Sartre-Bianchi A, Di Nicolantonio F, et al. Biomarkers Predicting Clinical Outcome of Epidermal Growth Factor Receptor—Targeted Therapy in Metastatic Colorectal Cancer. J Natl Cancer Inst 2009;101:1308-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan C, Du X.KRAS mutation testing in metastatic colorectal cancer. World J Gastroenterol 2012;18:5171-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bekaii-Saab T.Moving Forward With Expanding to an "All- RAS Mutational Analysis" in Metastatic Colorectal Cancer: Beyond KRAS mutations. J Natl Compr Canc Netw 2014;12:299-300. [DOI] [PubMed] [Google Scholar]
- 21.Samowitz WS, Curtin K, Schaffer D, et al. Relationship of Ki-ras mutations in colon cancers to tumor location, stage, and survival: a population-based study. Cancer Epidemiol Biomarkers Prev 2000;9:1193-7. [PubMed] [Google Scholar]
- 22.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008;359:1757-65. [DOI] [PubMed] [Google Scholar]
- 23.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:1626-34. [DOI] [PubMed] [Google Scholar]
- 24.Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408-17. [DOI] [PubMed] [Google Scholar]
- 25.Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared to FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:4706-13. [DOI] [PubMed] [Google Scholar]
- 26.Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 2009;27:663-71. [DOI] [PubMed] [Google Scholar]
- 27.Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluoruracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697-705. [DOI] [PubMed] [Google Scholar]
- 28.Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 2009;27:2091-6. [DOI] [PubMed] [Google Scholar]
- 29.Dahabreh IJ, Terasawa T, Castaldi PJ, et al. Systematic Review: Anti–Epidermal Growth Factor Receptor Treatment Effect Modification by KRAS Mutations in Advanced Colorectal Cancer. Ann Intern Med 2011;154:37-49. [DOI] [PubMed] [Google Scholar]
- 30.Linardou H, Dahabreh I, Kanaloupiti D, et al. Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol 2008;9:962-72. [DOI] [PubMed] [Google Scholar]
- 31.Bardelli A, Siena S.Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J Clin Oncol 2010;28:1254-61. [DOI] [PubMed] [Google Scholar]
- 32.Peeters M, Oliner KS, Parker A, et al. Massively parallel tumor multigene sequencing to evaluate response to panitumumab in a randomized phase 3 study of metastatic colorectal cancer. Clin Cancer Res 2013;19:1902-12. [DOI] [PubMed] [Google Scholar]
- 33.Janakiraman M, Vakiani E, Zeng Z, et al. Genomic and biological characterization of exon 4 KRAS mutations in human cancer. Cancer Res 2010;70:5901-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol 2010;11:753-62. [DOI] [PubMed] [Google Scholar]
- 35.Loupakis F, Ruzzo A, Cremolini C, et al. KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br J Cancer 2009;101:715-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molinari F, Felicioni L, Buscarino M, et al. Increased detection sensitivity for KRAS mutations enhances the prediction of anti-EGFR monoclonal antibody resistance in metastatic colorectal cancer. Clin Cancer Res 2011;17:4901-14. [DOI] [PubMed] [Google Scholar]
- 37.Yang ZY, Wu XY, Huang YF, et al. Promising biomarkers for predicting the outcomes of patients with KRAS wild-type metastatic colorectal cancer treated with anti-epidermal growth factor receptor monoclonal antibodies: a systematic review with meta-analysis. Int J Cancer 2013;133:1914-25. [DOI] [PubMed] [Google Scholar]
- 38.Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: can testing of tumor tissue for mutations in EGFR pathway downstream effector genes in patients with metastatic colorectal cancer improve health outcomes by guiding decisions regarding anti-EGFR therapy? Genet Med 2013;15:517-27. [DOI] [PubMed] [Google Scholar]
- 39.Douillard JY, Oliner KS, Siena S, et al. Panitumumab–FOLFOX4 Treatment and RAS Mutations in Colorectal Cancer. N Engl J Med 2013;369:1023-34. [DOI] [PubMed] [Google Scholar]
- 40.Soeda H, Shimodaira H, Watanabe M, et al. Clinical usefulness of KRAS, BRAF, and PIK3CA mutations as predictive markers of cetuximab efficacy in irinotecan- and oxaliplatin-refractory Japanese patients with metastatic colorectal cancer. Int J Clin Oncol 2013;18:670-7. [DOI] [PubMed] [Google Scholar]
- 41.André T, Blons H, Mabro M, et al. Panitumumab combined with irinotecan for patients with KRAS wild-type metastatic colorectal cancer refractory to standard chemotherapy: a GERCOR efficacy, tolerance, and translational molecular study. Annals of Oncology 2013;24:412-9. [DOI] [PubMed] [Google Scholar]
- 42.Schwartzberg LS, Rivera F, Karthaus M, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol 2014;32:2240-7. [DOI] [PubMed] [Google Scholar]
- 43.Peeters M, Oliner KS, Price TJ, et al. Analysis of KRAS/NRAS mutations in phase 3 study 20050181 of panitumumab (pmab) plus FOLFIRI versus FOLFIRI for second-line treatment (tx) of metastatic colorectal cancer (mCRC). Available online: http://meetinglibrary.asco.org/content/122548-143. Accessed 20th August, 2014.
- 44.Bokemeyer C, Kohne CH, Ciardiello F, et al. Treatment outcome according to tumor RAS mutation status in OPUS study patients with metastatic colorectal cancer (mCRC) randomized to FOLFOX4 with/without cetuximab. Available online: http://meetinglibrary.asco.org/content/127861-144. Accessed 20th August, 2014.
- 45.Tejpar S, Lenz HJ, Köhne CH, et al. Effect of KRAS and NRAS mutations on treatment outcomes in patients with metastatic colorectal cancer (mCRC) treated first-line with cetuximab plus FOLFOX4: New results from the OPUS study. Available online: http://meetinglibrary.asco.org/content/121584-143. Accessed 20th August, 2014.
- 46.Ciardiello F, Lenz HJ, Kohne CH, et al. Effect of KRAS and NRAS mutational status on first-line treatment with FOLFIRI plus cetuximab in patients with metastatic colorectal cancer (mCRC): New results from the CRYSTAL trial. Available online: http://meetinglibrary.asco.org/content/121586-143. Accessed 20th August, 2014.
- 47.Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:1065-75. [DOI] [PubMed] [Google Scholar]
- 48.Therkildsen C, Bergmann TK, Henrichsen-Schnack T, et al. The predictive value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment in metastatic colorectal cancer: A systematic review and meta-analysis. Acta Oncol 2014;53:852-64. [DOI] [PubMed] [Google Scholar]
- 49.Sorich MJ, Wiese MD, Rowland A, et al. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta-analysis of randomized, controlled trials. Ann Oncol 2015;26:13-21. [DOI] [PubMed] [Google Scholar]


